Abstract
Over 90% of prostate cancers over-express prostate specific membrane antigen (PSMA) and these tumor cells may be accurately targeted for diagnosis by 68Ga-PSMA-positron emission tomography/computed tomography (68Ga-PSMA-PET/CT) imaging. This novel molecular imaging modality appears clinically to have superseded CT, and appears superior to MR imaging, for the detection of metastatic disease. 68Ga-PSMA PET/CT has the ability to reliably stage prostate cancer at presentation and can help inform an optimal treatment approach. Novel diagnostic applications of 68Ga-PSMA PET/CT include guiding biopsy to improve sampling accuracy, and guiding surgery and radiotherapy. In addition to facilitating the management of metastatic castrate resistant prostate cancer (mCRPC), 68Ga-PSMA can select patients who may benefit from targeted systemic radionuclide therapy. 68Ga-PSMA is the diagnostic positron-emitting theranostic pair with the beta emitter Lutetium-177 PSMA (177Lu-PSMA) and alpha-emitter Actinium-225 PSMA (225Ac-PSMA) which can both be used to treat PSMA-avid metastases of prostate cancer in the molecular tumor-targeted approach of theranostic nuclear oncology.
Keywords: theranostics, nuclear oncology, molecular imaging, 68Ga-PSMA PET/CT, prostate cancer
1. Introduction
Gallium-68-prostate specific membrane antigen (68Ga-PSMA) positron emission tomography/computed tomography (PET/CT) imaging is revolutionizing the management of prostate cancer since its advent in 2013 [1]. Within 1 year, centers in every state in Australia were performing 68Ga-PSMA PET/CT and in less than 4 years, this form of targeted molecular imaging is not only being incorporated into routine clinical management of prostate cancer patients serviced by 40 centers country-wide, but arguably has become the standard of care throughout Australia in the diagnosis, selective staging and monitoring of therapeutic response of prostate cancer. Apart from the significantly higher diagnostic sensitivity that 68Ga-PSMA PET/CT offers, it also constitutes the diagnostic complementing the 177Lutetium-PSMA [2] and more recently the 225Actinium-PSMA [3], theranostic pairs, both currently being investigated for the therapy of metastatic castrate resistant prostate cancer (mCRPC). Why and how did this revolutionizing phenomenon occur in Australia and what significance does it have for oncological clinical practice throughout the world?
Australia has a record of early adoption of novel imaging technology. Within 4 months of Roentgen’s discovery of X-rays in Germany in November 1895, Walter Drowley Filmer, an amateur scientist and electrician, used X-rays to localize a foreign object within a patient in Newcastle, New South Wales [4]. The introduction of CT scans took a little longer with Australia waiting 2 years from the installation of the first whole body CT scanner at Northwick Park Hospital in London in 1975 [5] to the installation of the first CT scanner in a Sutherland hospital in Sydney [6]. The first human magnetic resonance imaging (MRI) scan was performed in 1977 and in 1986 the first MRI was installed in the private sector, in Sydney [7].
Cancer is a molecular disease and the advent of theranostic nuclear oncology is supplanting the traditional anatomic and functional imaging approaches typified by CT and MRI. Cancer is a disease of uncontrolled growth and proliferation whereby cells have escaped the body’s normal growth control mechanisms and have gained the ability to divide indefinitely. It is a multi-step evolutionary process that requires the accumulation of multiple genetic changes over time. Cell-signaling pathways, both external, and internal, are altered, driving the proliferation of neoplastic cells. We are beginning to understand the underlying genomics and proteomics underpinning neoplastic transformation and are now altering our treatment strategies to target and mitigate the changes driving tumor growth and development. Thus the assessment of molecular receptors such as human epidermal growth factor receptor 2 (HER2) in breast cancer and epidermal growth factor receptor (EGFR) in non-small cell lung cancer is now routine to guide treatment regimens [8]. The overexpression of PSMA in prostate cancer, which in cell lines has been demonstrated to increase angiogenesis, and enhance metabolism of polyglutamated folates and uptake of monoglutamated folates, thus imparting a proliferative advantage [9], may be similarly exploited.
Despite the move toward molecular diagnostics, our clinical imaging paradigms for diagnosing cancer and for monitoring cancer therapy have largely remained at the anatomical rather than the cellular or molecular level. We continue to use Response Evaluation Criteria in Solid Tumors (RECIST) criteria [10] based on anatomical size for monitoring treatment response even though, as we now appreciate, measurement of tumor diameter is at best a crude instrument for understanding what is happening at a cellular level within the tumor.
Prostate cancer is one of the leading causes of morbidity and death in the Western world. It is the second most common cancer in men worldwide [11]. With the aging population more men are being diagnosed with prostate cancer, as has been demonstrated in the UK with a 44% increase in prostate cancer incidence rates since the early 1990’s [12]. In Australia and the UK, more men now die from prostate cancer each year than women die of breast cancer [13]. Unlike in breast cancer and other tumor types such as colon cancer, there is no well-defined screening program for prostate cancer apart from the somewhat controversial use of prostate specific antigen (PSA) as a potential screening tool [14,15,16]. Also, unlike the major advances seen with treatments in breast cancer and colon cancer relatively little progress has been made in patients with prostate cancer which has spread beyond the confines of the prostate gland at the time of surgery. The failure rate after primary therapy for prostate cancer is between 20–60% of patients treated [17,18] and the 5-year survival in patients presenting with high volume metastatic disease is less than 30% [19].
The mainstays of diagnostic investigations for over 30 years have been physical examination (i.e., the digital rectal examination—DRE), blood investigation in the form of PSA and biopsy, be it trans-rectal, trans-perineal, blind or image-directed. Imaging investigations, predominantly CT and more recently MRI, and in particular multiparametric MRI, have contributed additional value to the diagnostic algorithm [20,21]. Occasionally bone scintigraphy with technetium-99 based compounds may allude to, or increase the likelihood of, a diagnosis of prostate cancer, particularly in the setting of an incidental high PSA result in an otherwise unsuspecting patient. Ultimately, the diagnosis is established by pathological confirmation, most commonly from prostatic biopsy, but occasionally from biopsy material obtained from extra-prostatic metastatic deposits.
Diagnosis by DRE, PSA and anatomical imaging has a number of flaws. The positive predictive value of DRE, in settings of low PSA, has been shown to be 5–30% [22]. DRE not only misses a number of clinically important prostate cancers but also has a high false positive rate [23]. PSA is a non-specific blood marker and may be raised in non-malignant clinical scenarios such as prostatitis and benign prostatic hypertrophy. Furthermore, a low PSA does not necessarily exclude the presence of prostatic malignancy [24]. Anatomical imaging with CT has poor sensitivity and specificity within the prostate gland itself and has been used predominantly to stage the disease after a diagnosis has been made. CT may show the evidence of metastatic spread to pelvic lymph nodes, or the seminal vesicles, and occasionally demonstrates sclerotic deposits characteristic of the osseous metastases from prostate adenocarcinoma. Unfortunately, CT can only give a suggestion of potential metastatic disease based on changes to anatomy, particularly with regard to size, rather than giving information germane to actual tumor involvement of a tissue. This limitation in early stage disease is well documented. A recent meta-analysis of 24 studies showed an acceptable specificity of 82% for lymph node diagnosis but an unacceptable sensitivity of only 42% with CT [25].
Multiparametric MRI is being utilized with increasing frequency worldwide due to its higher sensitivity, specificity and predictive value in assessing the prostate gland [21,26]. Apart from detecting changes in prostatic architecture and anatomy, this technique gives insights into potential malignant transformation via parameters such as diffusion restriction. The use of multiparametric MRI also appears to be increasing the proportion of clinically relevant diagnosed prostate cancers [27]. This technique is also more accurate than CT in assessing the lymph nodes within the pelvis [28]. However MRI, although a major step forward, is limited by issues such as claustrophobia, cost and the field of view limited usually to the pelvis alone.
In the 1990’s attempts were made to specifically target tumor characteristics with molecular imaging techniques based upon single photon emission computed tomography (SPECT) and positron emission tomography (PET). The objective was to improve the accuracy of detecting prostate cancer at an earlier time point than with anatomical imaging techniques such as CT. Prostate cancer cells overexpress a surface marker known as Prostate Specific Membrane Antigen (PSMA). Antibodies were initially developed, followed by peptides, to target this antigen as the basis for prostate cancer specific molecular imaging agents.
PSMA is a 750-amino acid transmembrane protein. In benign prostatic tissue, it is found within the apical epithelium of secretory ducts. While the physiologic role of PSMA in the prostate remains unclear, its enzymatic role is in the cleavage of α-linked glutamate from N-acetylaspartyl glutamate and γ-linked glutamates from polyglutamated folates [9]. During malignant transformation PSMA is translocated to the luminal surface of the ducts [29]. There an overexpression is seen. This overexpression has not been found in benign disease such as prostatic hyperplasia [30]. Several functions, including roles in cell migration, cellular nutrition, transport, and signal transduction, have been ascribed to PSMA [31]. Once a ligand binds to the PSMA protein it is internalized into the cell. However, PSMA is not prostate specific as receptors are found within lacrimal and salivary glands, the kidneys, small intestine, liver and spleen [32]. It is also expressed in tumor associated angiogenesis, and has been described in other tumors, such as glioblastoma, thyroid cancer, gastric, breast, renal and colorectal cancers [32].
Despite its presence in other tissues, PSMA has been found to be an excellent agent for targeted imaging and therapy. It is overexpressed one-hundred to one thousand-fold in 95% of prostate cancer cells [32]. The PSMA-ligand complex is internalized after binding via clathrin coated pits and endosome accumulation [33], which leads to enhanced retention important for both image quality and therapeutic efficacy. PSMA expression appears to correlate with advanced disease, castrate resistant disease, Gleason score and PSA level [34,35].
A variety of monoclonal antibodies (mAb) have been developed targeting both the intracellular and extracellular epitopes of PSMA. The typical issues related to antibodies, such as long circulating half-life, background activity and poor target tissue uptake have impeded commercial development of antibody based imaging and therapeutic agents. Despite this, two mAb variants have demonstrated high affinity, and specific and efficient targeting in vivo. These include the murine mAb 7E11, which binds an intracellular domain of PSMA, and the humanized mAb hJ591, which binds to an extracellular domain of PSMA [36]. 111Indium-7E11 has been investigated clinically as a SPECT imaging agent for recurrent and metastatic prostate cancer [37,38] (111In-7E11, ProstaScint™). The therapeutic concomitant 90Yttrium radiolabelled conjugate has also been studied [39]. But the high myelotoxic rate seen with the Y-90 conjugate also limited further development. Improvements in other imaging modalities and the overall poor sensitivity has lead to the virtual demise of ProstaScint™ as an imaging tool in most parts of the world. The J591 mAb has been clinically investigated for PET/CT imaging as 89Zr-hJ591 [40] and for therapy as 177Lu-hJ591 [41] and clinical development of radioimmunotherapy of mCRPC continues.
Small molecule PSMA-peptide inhibitor (also known as ligand) molecules have been developed which show high binding and these are the mainstay of current PSMA imaging techniques. Several PSMA ligands, differing slightly in chemical structure, are commercially available. Three peptide ligands have become the dominant agents in clinical use; one of these may be labelled only with 68Ga, while two may be labelled with either 68Ga or 177Lu, allowing, where desired, a theranostic approach to prostate cancer. A rational approach has underpinned the development of these agents, with PSMA affinity (and therefore tumor uptake) and rapid blood clearance being key parameters targeted.
Glu–NH–CO–Lys–(Ahx)–[68Ga-HBED-CC] (HBED CC: N,N′-Bis(2-hydroxy-5-(ethylene-beta-carboxy)benzyl)ethylenediamine N,N′-diacetic acid (known also as 68Ga-PSMA-11) is perhaps the most widely used agent for prostate cancer PET/CT imaging. The HBED chelator forms a thermodynamically stable complex with Ga, and 68Ga-PSMA-11 shows fast blood clearance, low liver uptake and high uptake in PSMA-expressing tissues [40]. The PSMA-617 agent, a 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) conjugated molecule suitable for labelling with 68Ga and 177Lu, allows the use of a theranostic pair. Numerous studies have evaluated the role of this agent in both imaging and therapy; some have found that this, and other DOTA-conjugated agents, have lower tumor uptake relative to HBED-CC chelated agents [42] although the agent is commonly used due to its possible therapeutic applications. The lower tumor affinity of DOTA-conjugated compounds led to the development of 1,4,7,10-tetraazacyclododececane,1-(glutaric acid-4,7,10-triacetic acid (DOTAGA)-conjugated compounds [43], including PSMA-I&T, which also incorporates a lipophilic peptidic linker to improve the interaction with PSMA, and therefore enhance tumor uptake. This agent, like PSMA-617, allows a theranostic approach to prostate cancer diagnosis and treatment. The clinical efficacy of 68Ga-PSMA-I&T appears equivalent to 68Ga-PSMA-HBED though imaging characteristics appear slightly better for the latter imaging agent [44].
Technetium [45], iodine [46] and fluorine-18 [47,48,49] labelled PSMA analogues have also been developed, and although potentially promising, particularly due to their longer half-life, this paper will concentrate on the Gallium-68 agents which could be considered now almost part of routine clinical practice in Australia. F-18 labelled PSMA ligands have recently been reviewed elsewhere [50,51]. Choline, though initially considered a potential diagnostic tool [52], although established and reimbursed in Europe, has not been well established in Australia and may now possibly be relegated to the history books with the advent of 68Ga PSMA PET/CT [51,53,54].
A clear advantage of 68Ga-labelled diagnostic radiopharmaceuticals is on-site synthesis, with gallium-68 produced and eluted from a 68Ge/68Ga generator. This process is analogous to the 99Mo/99mTc generator, the “workhorse” of nuclear medicine for the past 50 years. In-house synthesis allows, within the limits of 68Ge decay and 68Ga ingrowth, on demand radiopharmaceutical production, without the need for a medical cyclotron and its supporting infrastructure. These benchtop generators have a footprint such that they can be easily housed in most imaging facility supporting laboratories without significant, if any, modification to infrastructure. In addition, ongoing developments by the various manufacturers mean that they are becoming increasingly user-friendly, with manufacturer efforts focusing, importantly, on radiation safety.
There are now several GMP-compliant 68Ge/68Ga generators available which yield highly chemically pure 68Ga on elution, with minimal breakthrough of parent 68Ge and other metal impurities [55], which may interfere significantly with peptide radiolabelling. These generators differ principally in the stationary phase to which the parent radioisotope (68Ge) is adsorbed. This phase determines the concentration, ranging from 0.05 to 1.0 M, of HCl mobile phase that must be used to separate daughter 68Ga from 68Ge during elution. In turn this mobile phase has an effect on the chemistry of radiolabelling. The various approaches to elution and subsequent chemistry of radiolabelling have been nicely summarized by Mueller et al. [56].
PSMA labelling with 68Ga is most often performed with modular synthesis units; these use sterile, single use cassettes and GMP compliant reagents and are accommodated in a hot cell, or similarly shielded workspace. Such units have the advantage of automation and minimal operator radiation exposure, but have lengthy synthesis times (up to 35 min), during which period, of course, 68Ga decays, meaning that the efficiency of the generator, in terms of patient doses produced, is decreased. Furthermore, these units are costly, and the design of some is not failsafe, leading to unsuccessful syntheses, and consequences in terms of workflow, patient scheduling and inconvenience.
Recently, several one- or two-step GMP compliant kit-based synthesis cold kits have become available. While these are not compatible with all 68Ge/68Ga generators, they have short reaction times at room temperature, with minimal risk of operator (or instrument) error. They are similar in principle to cold kits for 99mTc labelling, are well-accepted by technologists and laboratory staff, do not have high associated consumables costs, and do not require a hot cell environment. As with 68Ga-labelled peptides produced by synthesis units, they require quality control by well-established instant thin layer chromatography methods, but are, overall, superior in terms of generator efficiency (for the 68Ge/68Ga generator with which they are compatible) and simplicity for the user. Several PET/CT facilities in Sydney and Perth attest to the efficiency, high yield and ease of use of kit technology in providing 68Ga-PSMA-11 for routine clinical prostate cancer imaging [57]. Other kit formulations of 68Ga-PSMA are in preclinical [58] and early clinical development [59]. This evolution of simple kits will be instrumental in the ongoing widespread adoption of 68Ga-PSMA PET/CT imaging in staging and restaging of prostate cancer.
2. 68Ga PSMA PET/CT in Recurrent Disease
The earliest, and most extensive, experience with 68Ga-PSMA PET/CT imaging has been in the most common clinical scenario of biochemical relapse post-definitive primary therapy [60]. Up until the advent of 68Ga-PSMA PET/CT the mainstay of imaging included CT +/− bone scintigraphy and in some cases MRI [20]. The largest retrospective study of 1007 patients, reported detection rates for 68Ga-PSMA-11 PET/CT of 79.5%, in the setting of biochemical recurrence [61]. A recent meta-analysis revealed detection rates with 68Ga-PSMA PET/CT of 58% in patients with PSA between 0.2–1.0 ng/mL, 76% for PSA between 1 and 2 ng/mL and 95% for PSA > 2.0 ng/mL [62]. These findings reflect our own single institutional dataset utilizing 68Ga-PSMA I&T in 150 consecutive patients, where we have seen PSMA-avid disease in the setting of biochemical relapse in 25% of patients with PSA < 0.5 ng/mL. 67% of patients with PSA 0.5–1.5 ng/mL and in 92% of patients with PSA > 1.5 ng/mL [63]. Importantly, 68Ga-PSMA-I&T PET/CT reveals, in many cases, metastatic disease that is considered occult on CT, as demonstrated in Figure 1. In our dataset, statistically significant differences were seen between recurrent disease demonstrated by 68Ga-PSMA I&T and diagnostic contrast CT detection rates [63]. When compared with histological diagnosis, specificities of up to 100% have been detected in pre-surgical nodal assessment prior to nodal salvage surgery using 68Ga-PSMA [64,65]. A recent prospective Australian multi-center trial has shown that 68Ga-PSMA PET/CT leads to a change in management intent in 62% of patients with biochemical relapse, based upon the PSMA PET/CT scan result [66]. A separate study of 131 patients showed 68Ga-PSMA HBED PET/CT had a clinical impact in 76% of patients imaged [67].
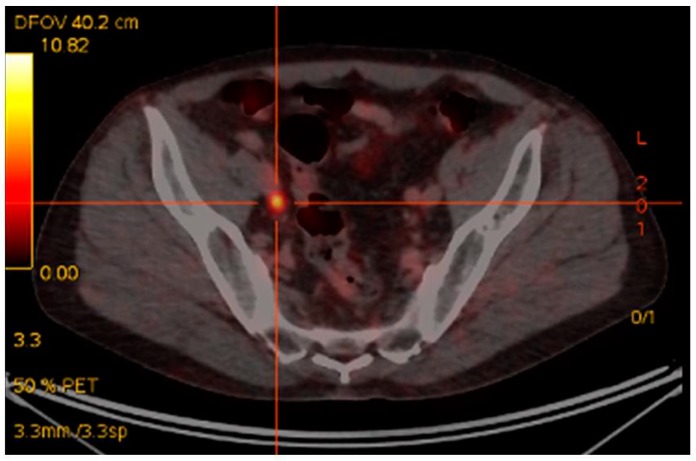
Figure 1.
Rising PSA post-prostatectomy. PSA 0.9 ng/mL. CT unremarkable. 68Ga-PSMA-avid right pelvic lymph node.
68Ga-PSMA PET/CT has been shown in multiple studies to be superior to choline-based PET/CT imaging in the setting of biochemical relapse [53,68,69]. Choline PET/CT imaging is now effectively no longer offered clinically in Australia for this indication. Fluorinated PSMA derivatives appear to show equivalence in the setting of mCRPC to that of 68Ga-PSMA ligands [50,51,70,71], but are not yet routinely available, and do not have a true theranostic therapeutic pair.
On the strength of the growing body of evidence in this setting of biochemical relapse post definitive primary therapy [60,61,62,63,64,65,66,67,72] the use of 68Ga-PSMA PSMA PET/CT been incorporated into commonly accepted imaging algorithms in Australia such as the evidence-based Western Australian Health Diagnostic Imaging Pathways [21]. This clinical acceptance is despite the lack of reimbursement in Australia for 68Ga-PSMA PET/CT imaging.
3. 68Ga-PSMA PET/CT in Primary Disease
68Ga-PSMA PET/CT has recently been investigated as a potential staging modality in primary prostate cancer. Given that cellular PSMA expression has been shown to correlate with PSA and Gleason score [73] and 68Ga-PSMA PET/CT has been shown to be superior to standard staging modalities, such as CT, the use of 68Ga-PSMA PET/CT would seem to be a logical clinical extension in staging of primary disease. Early data appear to confirm this contention, with several studies [74,75], including our own [76], showing high rates of detection of CT occult early metastatic disease (Figure 2). In our recent study there was very good correlation between PSA and Gleason score and the chance of PSMA-avid metastatic disease. This finding correlates with other studies indicating a role in intermediate-to-high risk primary prostate cancer, with often occult nodal, and occasionally osseous, metastases identified. In assessment of potential bone metastases, 68Ga-PSMA PET/CT has been shown to be superior to bone scintigraphy in several studies [77,78].
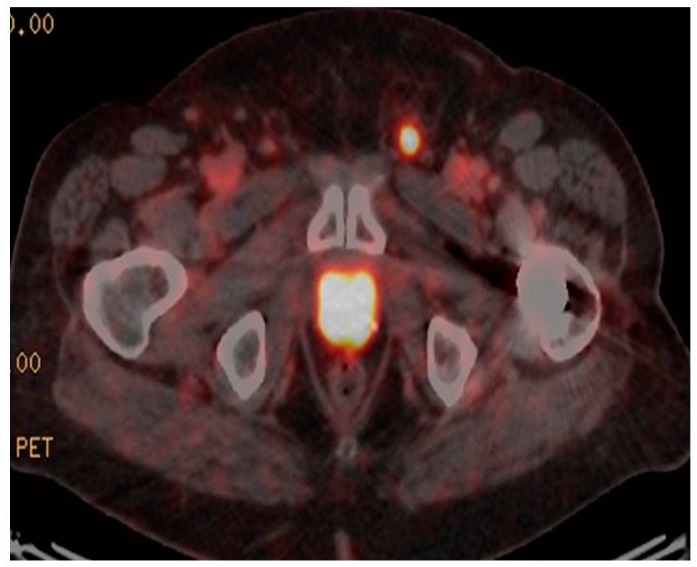
Figure 2.
Staging 68Ga-PSMA PET/CT. Gleason 4 + 5. PSA 19 ng/mL. Intense 68Ga-PSMA avid primary disease in prostate with 68Ga-PSMA avid superficial left inguinal node metastasis.
In assessment of intra-prostatic tumor 68Ga-PSMA-11 PET/CT has been compared with multi-parametric MRI, the current standard method for intra-prostatic tumor localization. Interestingly, just as with 68Ga-PSMA PET/CT, multiparametric MRI, which is accepted as the most sensitive imaging method for assessing the prostate gland itself [21], is also not reimbursed in Australia. Giesel et al. revealed concordance between positive findings on each modality [79]. Combined 68Ga-PSMA-11 PET/CT/MRI was analyzed in 53 patients with intermediate- and high-risk prostate cancer [80], and indicated a superior accuracy of the hybrid approach than with either modality alone. The sensitivities and specificities were 76% and 97%, for hybrid 68Ga-PSMA-11 PET/CT/MRI; 58% and 82% for multi-parametric MRI (p = 0.003); and 64% and 94%, for 68Ga-PSMA-11 PET/CT.
The greatest clinical importance of 68Ga-PSMA PET/CT in the staging of primary prostate cancer is the resulting change in management intent in one-fifth of all patients imaged [66]. The prognostic implication of treatment replanning in these patients is substantial. It is hoped that this will be reinforced by the outcome of the ProPSMA study (ACTRN12617000005358), which is currently recruiting in Australia, and a number of studies in the United States (e.g., NCT02611882 and NCT03388346).
4. New Clinical Applications
4.1. 68Ga-PSMA PET/CT and PET/MRI Guided Biopsy
The high sensitivity of 68Ga-PSMA PET/CT, coupled with the increased expression in higher grade tumors, guided intra-prostatic biopsy has shown in several small studies that 68Ga-PSMA avidity correlates well with gross tumor volume as detected by multiparametric MRI and voxel based determinants directly matched to histopathological specimens [81,82]. This correlation supports the concept of utilizing these data to potentially reduce sampling error and improve diagnostic accuracy of targeted biopsy. Current prostatic biopsy is often performed blind, although MRI directed biopsy based on Prostate Imaging Reporting and Data System (PI-RADS) is being increasingly utilized [83,84,85,86]. The addition of 68Ga-PSMA PET/MRI may further improve the utility and accuracy of this technique and direct biopsy to the most relevant area within the prostate. This is exquisitely demonstrated in Figure 3.
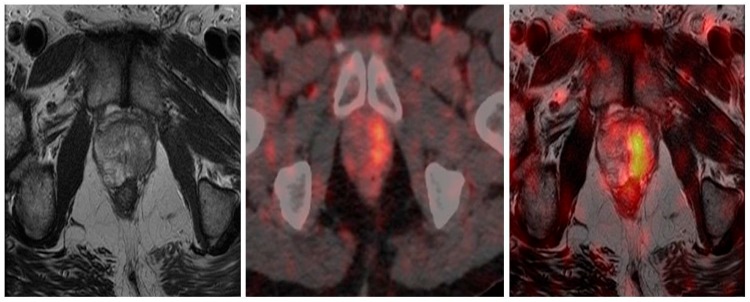
Figure 3.
Primary prostate cancer. PIRADS 4/5 left lobe of prostate on multiparametric MRI. Fused to 68Ga-PSMA PET/CT images for MRI in-bore guided targeted biopsy.
4.2. 68Ga-PSMA PET/CT-Directed Surgery and Radiotherapy
The visualization of previously unappreciated PSMA-avid small lymph nodes in the pelvis, in both primary staging of prostate cancer and in the setting of biochemical recurrence post-definitive primary therapy, has raised interest in extended or targeted primary lymphadenectomy at time of primary prostatic surgery or as salvage lymphadenectomy. The results, however, of 68Ga-PSMA PET/CT targeted nodal surgery, either in the primary or salvage setting, have been relatively poor, with an increase in morbidity and no significant improvement in biochemical remission [65,87,88]. Although the technique shows high specificity (>90%), the sensitivity is only moderate (60–80%), thus the nodes visualized resemble the tip of the iceberg. Micro-metastatic, or very small volume metastatic nodal disease, will be unlikely to be detected even with the resolving capacity of the most modern PET/CT imaging technology.
Attempts at improving surgical detection by probe techniques, similar to those described with sentinel node biopsy, have been attempted, to improve lymphadenectomy both in the primary and salvage settings. Although data are limited, the use of a gamma probe to detect 111In-PSMA injected pre-operatively appears promising in detecting PSMA-avid occult small volume/micro-metastatic nodal disease [89,90,91,92]. This technique also exemplifies the utility of the I&T PSMA compound, which allows successful binding of other radio-isotopes to allow this novel radio-guided surgical approach.
The results with 68Ga-PSMA PET/CT directed external beam radiotherapy in the primary and salvage setting have been encouraging. Several studies have shown a change in radiotherapy planning from 20–60% of patients imaged with 68Ga-PSMA PET/CT prior to external beam radiotherapy [93,94,95]. 68Ga-PSMA PET/CT also has a role post-definitive radiotherapy in detecting site of biochemical relapse. In an Australian study of 419 men treated with external beam radiotherapy, 68Ga-PSMA PET/CT identified all cases of biochemical relapse [96]. This allows potential salvage therapy if the recurrence is outside the previous radiotherapy field, as was the case for the patient in Figure 4. A negative 68Ga-PSMA PET/CT scan in the setting of biochemical recurrence also appears to have prognostic implications for salvage radiotherapy. In a study of 164 Australian men, a negative 68Ga-PSMA PET/CT showed treatment response benefit compared with patients with a positive 68Ga-PSMA PET/CT scan [97]. Although seemingly counter-intuitive, this again reinforces the likely scenario that very small volume/micro-metastatic nodal recurrence is more likely to be amenable to pelvic radiotherapy than disease that is visible on 68Ga-PSMA PET/CT. By this stage the chance of disease having spread outside the confines of the area of the salvage pelvic radiotherapy field, is likely to be high. Whether systemic therapy, combined with either chemotherapy or targeted radiopeptide therapy, together with salvage pelvic radiotherapy can improve response rates in this setting has yet to be elucidated. The long-term outcome of altering treatment planning based on 68Ga-PSMA PET/CT has not yet been addressed in any large longitudinal studies.
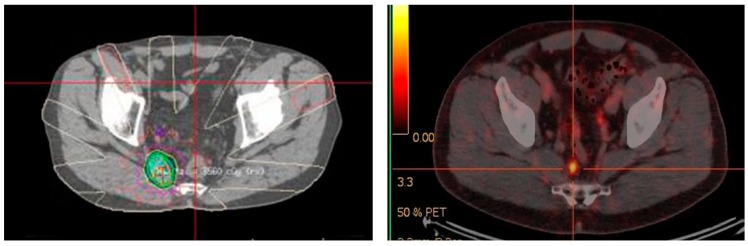
Figure 4.
Targeted radiotherapy to recurrent 68Ga-PSMA avid right pre-sacral lymph node. June 2015.
4.3. Monitoring Treatment Response
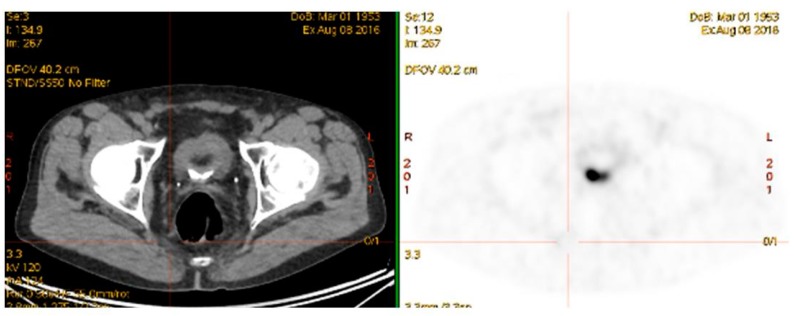
Serial 68Ga-PSMA PET/CT scans have been used to assess the intermediate term outcomes of radiotherapy in recurrent nodal disease [98,99] (Figure 5). These studies show reductions in SUV in most lesions treated, indicating response, however, the responses based on 68Ga-PSMA PET/CT may take several months to be fully realized [99]. Therapeutic response of chemotherapy based on 68Ga-PSMA PET/CT imaging has been poorly studied with only a single paper showing a potential role of this imaging modality with monitoring the effectiveness of docetaxel chemotherapy [100]. This is, however, a growing area of interest as 68Ga-PSMA PET/CT imaging starts taking over the traditional role that CT and bone scintigraphy has had in monitoring chemotherapeutic response in prostate cancer patients [27]. The poor correlation of RECIST to recognized measures of evaluating molecular responses to cancer treatments, such as the European Organization for Research and Treatment of Cancer (EORTC) criteria has been documented [101]. There is a consensus movement within the nuclear medicine community to harmonize quantitative methods to more accurately compare tumor response to treatment, such as with standard uptake values, and using established criteria such as those found in the EORTC [102], and the PERCIST criteria [103]. More specific definitions for interpreting and evaluating responses 68Ga-PSMA PET/CT have also been described [104]. These molecular based evaluation systems will likely replace the antiquated RECIST criteria for assessing therapeutic response in the era of targeted therapies and molecular imaging.
Figure 5.
Monitoring response of targeted radiotherapy by 68Ga-PSMA PET/CT August 2016. (Previous 68Ga-PSMA avid right pre-sacral lymph node treated June 2015—see Figure 4).
4.4. 68Ga-PSMA PET/CT Guided Theranostic Pair Therapy with 177Lu-PSMA or 225Ac-PSMA
Given that cancer is a molecular disease characterized by alterations in molecular epitopes, we can target specific biomarkers, such as PSMA, for imaging. Such imaging agents can be radiolabelled with diagnostic gamma emitters or positron emitters and their theranostic pair with beta and alpha radiometals such as lutetium-177 and actinium-225 for therapeutic purposes. The power of this theranostic paradigm has been well demonstrated in gastroentero-pancreatic neuroendocrine tumors (GEP-NETs) over many years [105,106,107,108,109,110,111] and recently confirmed in the randomized controlled NETTER-1 trial which showed a five-fold improvement in response with 177Lu-DOTATATE (Lutathera™) compared with conventional treatment [112]. The theranostic principle allows us “to see what we treat and treat what we see” [113]. The use of molecular imaging agents to target specific therapy is directly analogous to the use of Herceptin in patients with breast cancer where treatment is only given on the prior demonstration of the specific HER-2 receptor on tumor cells [114]. 68Ga-PSMA PET/CT imaging performs the same gate keeping function by in vivo demonstration of the upregulation of the PSMA receptor in prostate cancer.
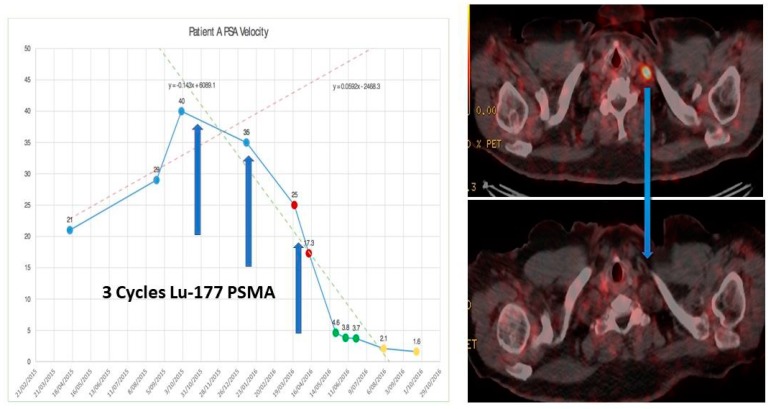
68Ga-PSMA PET/CT has been used over the last 4–5 years in its theranostic capacity to guide therapy using the labelled therapeutic radiopharmaceutical 177Lu-PSMA (Figure 6). This targeted radiopeptide therapy has been shown to decrease PSA levels, objectively decrease tumor volumes and tumor activity and improve progression free survival in 40–70% of mCRPC patients who have failed previous treatment modalities including chemotherapy [2,115,116,117,118,119,120]. This has been shown to be a well-tolerated treatment with minimal acute or medium term side-effects, similar to our experience with 177Lu-DOTATATE in NET’s. More recently targeted alpha therapy 225Ac-PSMA has also been shown, in much smaller studies, to also decrease PSA levels, tumor volumes and tumor activity in patients who have failed 177Lu-PSMA [3,121]. A more pronounced effect on salivary gland function has been identified following 225Ac-PSMA therapy. Longer term toxicities, if any, are yet to be determined.
Figure 6.
Before (top) and after (bottom) 3 cycles of 177Lu-PSMA therapy for progressive metastatic castrate resistant prostate cancer. [X axis—time in months/; Y axis—PSA (μg/L)].
5. Conclusions
68Ga-PSMA PET/CT imaging has become in a relatively short period of time, the gold standard for restaging recurrent prostate cancer in clinical centers in which this imaging modality is available. It is likely to become the standard imaging modality in the staging of intermediate-to-high risk primary prostate cancer. The potential to guide therapy, and to facilitate more accurate prostatic biopsy is being explored. In the theranostic paradigm, 68Ga-PSMA PET/CT imaging is critical for detecting PSMA-avid disease which may then respond to targeted 177Lu-PSMA or 225Ac-PSMA therapies. The 68Ga-PSMA PET/CT is being investigated as a method to monitor the therapeutic response to all treatment modalities.
Acknowledgments
Giuseppe Cardaci Diagnostic Nuclear Imaging. Andrew Henderson and Julie Crouch Perth Oceanic Molecular Hollywood PET Center/Perth Radiological Clinic.
Conflicts of Interest
Theranostics Australia is the Australian distributor of ANMI products.
References
- 1.Afshar-Oromieh A., Malcher A., Eder M., Eisenhut M., Linhart H.G., Hadaschik B.A., Holland-Letz T., Giesel F.L., Kratochwil C., Haufe S., et al. PET/CT imaging with a [68Ga] gallium-labelled PSMA ligand for the diagnosis of prostate cancer: Biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med. Mol. Imaging. 2013;40:486–495. doi: 10.1007/s00259-012-2298-2. [DOI] [PubMed] [Google Scholar]
- 2.Fendler W.P., Rahbar K., Herrmann K., Kratochwil C., Eiber M. (177)Lu-PSMA Radioligand Therapy for Prostate Cancer. J. Nucl. Med. 2017;58:1196–1200. doi: 10.2967/jnumed.117.191023. [DOI] [PubMed] [Google Scholar]
- 3.Kratochwil C., Bruchertseifer F., Rathke H., Bronzel M., Apostolidis C., Weichert W., Haberkorn U., Giesel F.L., Morgenstern A. Targeted α-Therapy of Metastatic Castration-Resistant Prostate Cancer with (225)Ac-PSMA-617: Dosimetry Estimate and Empiric Dose Finding. J. Nucl. Med. 2017;58:1624–1631. doi: 10.2967/jnumed.117.191395. [DOI] [PubMed] [Google Scholar]
- 4.Smith T. A short history of the origins of radiography in Australia. Radiography. 2009;15(Suppl. 1):e42–e47. doi: 10.1016/j.radi.2009.07.005. [DOI] [Google Scholar]
- 5.Robb W.L. Perspective on the first 10 years of the CT scanner industry. Acad. Radiol. 2003;10:756–760. doi: 10.1016/S1076-6332(03)80121-6. [DOI] [PubMed] [Google Scholar]
- 6.Imagination at Work. [(accessed on 28 November 2017)]; Available online: https://www.ge.com/au/company/history/1971–1985.
- 7.Australian Government Policy Regarding MRI Services. [(accessed on 28 November 2017)]; Available online: http://www.users.on.net/~spinupdownunder/misc/medicare.htm.
- 8.Mahipal A., Kothari N., Gupta S. Epidermal growth factor receptor inhibitors: Coming of age. Cancer Control. 2014;21:74–79. doi: 10.1177/107327481402100111. [DOI] [PubMed] [Google Scholar]
- 9.Yao V., Berkman C.E., Choi J.K., O’Keefe D.S., Bacich D.J. Expression of Prostate-Specific Membrane Antigen (PSMA) Increases Cell Folate Uptake and Proliferation and Suggests a Novel Role for PSMA in the Uptake of the Non-Polyglutamated Folate, Folic Acid. Prostate. 2010;70:305–316. doi: 10.1002/pros.21065. [DOI] [PubMed] [Google Scholar]
- 10.Therasse P., Arbuck S.G., Eisenhauer E.A., Wanders J., Kaplan R.S., Rubinstein L., Verweij J., Van Glabbeke M., van Oosterom A.T., Christian M.C., et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 11.World Cancer Research Fund International. [(accessed on 28 November 2017)]; Available online: http://www.wcrf.org/int/cancer-facts-figures/data-specific-cancers/prostate-cancer-statistics.
- 12.Cancer Research UK. [(accessed on 28 November 2017)]; Available online: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer/incidence.
- 13.Cancer Australia Prostate Cancer. [(accessed on 28 November 2017)]; Available online: https://prostate-cancer.canceraustralia.gov.au/statistics.
- 14.Sadi M.V. PSA screening for prostate cancer. Rev. Assoc. Med. Bras. 1992. 2017;63:722–725. doi: 10.1590/1806-9282.63.08.722. [DOI] [PubMed] [Google Scholar]
- 15.Ilic D., Neuberger M.M., Djulbegovic M., Dahm P. Screening for prostate cancer. Cochrane Database Syst. Rev. 2013:CD004720. doi: 10.1002/14651858.CD004720.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loeb S. Guideline of guidelines: Prostate cancer screening. BJU Int. 2014;114:323–325. doi: 10.1111/bju.12854. [DOI] [PubMed] [Google Scholar]
- 17.Pound C.R., Partin A.W., Eisenberger M.A., Chan D.W., Pearson J.D., Walsh P.C. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal P.K., Sadetsky N., Konety B.R., Resnick M.I., Carroll P.R. Cancer of the Prostate Strategic Urological Research Endeavor (CaPSURE). Treatment failure after primary and salvage therapy for prostate cancer: Likelihood, patterns of care, and outcomes. Cancer. 2008;112:307–314. doi: 10.1002/cncr.23161. [DOI] [PubMed] [Google Scholar]
- 19.Ang M., Rajcic B., Foreman D., Moretti K., O’Callaghan M.E. Men presenting with prostate-specific antigen (PSA) values of over 100 ng/mL. BJU Int. 2016;117(Suppl. 4):68–75. doi: 10.1111/bju.13411. [DOI] [PubMed] [Google Scholar]
- 20.Guidelines on Prostate Cancer European Association of Urology. [(accessed on 28 November 2017)];2015 Available online: http://uroweb.org/wp-content/uploads/09-Prostate-Cancer_LR.pdf.
- 21.Diagnostics Imaging Pathways. [(accessed on 28 November 2017)]; Available online: http://www.imagingpathways.health.wa.gov.au/index.php/imaging-pathways/urological/staging-of-prostate-cancer#pathway.
- 22.Carvalhal G.F., Smith D.S., Mager D.E., Ramos C., Catalona W.J. Digital rectal examination for detecting prostate cancer at prostate specific antigen levels of 4 ng/mL or less. J. Urol. 1999;161:835–839. doi: 10.1016/S0022-5347(01)61785-3. [DOI] [PubMed] [Google Scholar]
- 23.Cui T., Kovell R.C., Terlecki R.P. Is it time to abandon the digital rectal examination? Lessons from the PLCO Cancer Screening Trial and peer-reviewed literature. Curr. Med. Res. Opin. 2016;4:1–7. doi: 10.1080/03007995.2016.1198312. [DOI] [PubMed] [Google Scholar]
- 24.Thompson I.M., Pauler D.K., Goodman P.J., Tangen C.M., Lucia M.S., Parnes H.L., Minasian L.M., Ford L.G., Lippman S.M., Crawford E.D., et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4.0 ng per milliliter. N. Engl. J. Med. 2004;350:2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 25.Hövels A.M., Heesakkers R.A., Adang E.M., Jager G.J., Strum S., Hoogeveen Y.L., Severens J.L., Barentsz J.O. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: A meta-analysis. Clin. Radiol. 2008;63:387–395. doi: 10.1016/j.crad.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 26.Radtke J.P., Teber D., Hohenfellner M., Hadaschik B.A. The current and future role of magnetic resonance imaging in prostate cancer detection and management. Transl. Androl. Urol. 2015;4:326–341. doi: 10.3978/j.issn.2223-4683.2015.06.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlaw K.R., Woo H.H. Evaluation of the changing landscape of prostate cancer diagnosis and management from 2005 to 2016. Prostate Int. 2017;5:130–134. doi: 10.1016/j.prnil.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saokar A., Islam T., Jantsch M., Saksena M.A., Hahn P.F., Harisinghani M.G. Detection of lymph nodes in pelvic malignancies with Computed Tomography and Magnetic Resonance Imaging. Clin. Imaging. 2010;34:361–366. doi: 10.1016/j.clinimag.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Bouchelouche K., Turkbey B., Choyke P.L. PSMA PET/CT and Radionuclide Therapy in Prostate Cancer. Semin. Nucl. Med. 2016;46:522–535. doi: 10.1053/j.semnuclmed.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Birtle A.J., Freeman A., Masters J.R., Payne H.A., Harland S.J. BAUS Section of Oncology Cancer Registry. Tumour markers for managing men who present with metastatic prostate cancer and serum prostate-specific antigen levels of <10 ng/mL. BJU Int. 2005;96:303–307. doi: 10.1111/j.1464-410X.2005.05619.x. [DOI] [PubMed] [Google Scholar]
- 31.Rajasekaran A.K., Anilkumar G., Christiansen J.J. Is prostate-specific membrane antigen a multifunctional protein? Am. J. Physiol. Cell Physiol. 2005;288:C975–C981. doi: 10.1152/ajpcell.00506.2004. [DOI] [PubMed] [Google Scholar]
- 32.Silver D.A., Pellicer I., Fair W.R., Heston W.D., Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 1997;3:81–85. [PubMed] [Google Scholar]
- 33.Rajasekaran S.A., Anilkumar G., Oshima E., Bowie J.U., Liu H., Heston W., Bander N.H., Rajasekaran A.K. A novel cytoplasmic tail MXXXL motif mediates the internalization of prostate-specific membrane antigen. Mol. Biol. Cell. 2003;14:4835–4845. doi: 10.1091/mbc.E02-11-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mannweiler S., Amersdorfer P., Trajanoski S., Terrett J.A., King D., Mehes G. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol. Oncol. Res. 2009;15:167–172. doi: 10.1007/s12253-008-9104-2. [DOI] [PubMed] [Google Scholar]
- 35.Chang S.S. Overview of prostate-specific membrane antigen. Rev. Urol. 2004;6(Suppl. 10):S13–S18. [PMC free article] [PubMed] [Google Scholar]
- 36.Fung E.K., Cheal S.M., Fareedy S.B., Punzalan B., Beylergil V., Amir J., Chalasani S., Weber W.A., Spratt D.E., Veach D.R., et al. Targeting of radiolabeled J591 antibody to PSMA-expressing tumors: Optimization of imaging and therapy based on non-linear compartmental modeling. EJNMMI Res. 2016;6:7. doi: 10.1186/s13550-016-0164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sodee D.B., Malguria N., Faulhaber P., Resnick M.I., Albert J., Bakale G. Multicenter ProstaScint imaging findings in 2154 patients with prostate cancer. The ProstaScint Imaging Centers. Urology. 2000;56:988–993. doi: 10.1016/S0090-4295(00)00824-4. [DOI] [PubMed] [Google Scholar]
- 38.PET/Ctronis J.D., Regan F., Lin K. Indium-111 capromab pendetide (ProstaScint) imaging to detect recurrent and metastatic prostate cancer. Clin. Nucl. Med. 1998;23:672–677. doi: 10.1097/00003072-199810000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Deb N., Goris M., Trisler K., Fowler S., Saal J., Ning S., Becker M., Marquez C., Knox S. Treatment of hormone-refractory prostate cancer with 90Y-CYT-356 monoclonal antibody. Clin. Cancer Res. 1996;2:1289–1297. [PubMed] [Google Scholar]
- 40.Pandit-Taskar N., O’Donoghue J.A., Beylergil V., Lyashchenko S., Ruan S., Solomon S.B., Durack J.C., Carrasquillo J.A., Lefkowitz R.A., Gonen M., et al. ⁸⁹Zr-huJ591 immuno-PET/CT imaging in patients with advanced metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging. 2014;41:2093–2105. doi: 10.1007/s00259-014-2830-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tagawa S.T., Milowsky M.I., Morris M., Vallabhajosula S., Christos P., Akhtar N.H., Osborne J., Goldsmith S.J., Larson S., Taskar N.P., et al. Phase II study of lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2013;19:5182–5191. doi: 10.1158/1078-0432.CCR-13-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eder M., Schafer M., Bauder-Wust U., Hull W.E., Wangler C., Mier W., Haberkorn U., Eisenhut M. 68Ga-complex lipophilicity and the targeting property of urea-based PSMA inhibitor for PET/CT imaging. Bioconjug. Chem. 2012;23:688–697. doi: 10.1021/bc200279b. [DOI] [PubMed] [Google Scholar]
- 43.Lutje S., Heskamo S., Cornelissen A.S., Poeppel T.D., van den Broek S.A.M.W., Rosenbaum-Krumme S., Bockisch A., Gotthardt M., Rijpkema M., Boerman O.C. PSMA ligands for radionuclide imaging and therapy of prostate cancer: Clinical status. Theranostics. 2015;5:1388–1401. doi: 10.7150/thno.13348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCarthy M., Langton T., Kumar D., Campbell A. Comparison of PSMA-HBED and PSMA-I&T as diagnostic agents in prostate carcinoma. Eur. J. Nucl. Med. Mol. Imaging. 2017;44:1455–1462. doi: 10.1007/s00259-017-3699-z. [DOI] [PubMed] [Google Scholar]
- 45.Vallabhajosula S., Nikolopoulou A., Babich J.W., Osborne J.R., Tagawa S.T., Lipai I., Solnes L., Maresca K.P., Armor T., Joyal J.L., et al. 99mTc-labeled small-molecule inhibitors of prostate-specific membrane antigen: Pharmacokinetics and biodistribution studies in healthy subjects and patients with metastatic prostate cancer. J. Nucl. Med. 2014;55:1791–1798. doi: 10.2967/jnumed.114.140426. [DOI] [PubMed] [Google Scholar]
- 46.Zechmann C.M., Afshar-Oromieh A., Armor T., Stubbs J.B., Mier W., Hadaschik B., Joyal J., Kopka K., Debus J., Babich J.W., et al. Radiation dosimetry and first therapy results with a (124)I/ (131)I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur. J. Nucl. Med. Mol. Imaging. 2014;41:1280–1292. doi: 10.1007/s00259-014-2713-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cho S.Y., Gage K.L., Mease R.C., Senthamizhchelvan S., Holt D.P., Jeffrey-Kwanisai A., Endres C.J., Dannals R.F., Sgouros G., Lodge M., et al. Biodistribution, tumor detection, and radiation dosimetry of 18F-DCFBC, a low-molecular-weight inhibitor of prostate-specific membrane antigen, in patients with metastatic prostate cancer. J. Nucl. Med. 2012;53:1883–1891. doi: 10.2967/jnumed.112.104661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Giesel F.L., Hadaschik B., Cardinale J., Radtke J., Vinsensia M., Lehnert W., Kesch C., Tolstov Y., Singer S., Grabe N., et al. F-18 labelled PSMA-1007: Biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging. 2017;44:678–688. doi: 10.1007/s00259-016-3573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Szabo Z., Mena E., Rowe S.P., Plyku D., Nidal R., Eisenberger M.A., Antonarakis E.S., Fan H., Dannals R.F., Chen Y., et al. Initial Evaluation of [(18)F]DCFPyL for Prostate-Specific Membrane Antigen (PSMA)-Targeted PET/CT Imaging of Prostate Cancer. Mol. Imaging Biol. 2015;17:565–574. doi: 10.1007/s11307-015-0850-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Evans J.D., Jethwa K.R., Ost P., Williams S., Kwon E.D., Lowe V.J., Davis B.J. Prostate cancer-specific PET radiotracers: A review on the clinical utility in recurrent disease. Pract. Radiat. Oncol. 2018;8:28–39. doi: 10.1016/j.prro.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 51.Eiber M., Fendler W.P., Rowe S.P., Calais J., Hofman M.S., Maurer T., Schwarzenboeck S.M., Kratowchil C., Herrmann K., Giesel F.L. Prostate-Specific Membrane Antigen Ligands for Imaging and Therapy. J. Nucl. Med. 2017;58:67S–76S. doi: 10.2967/jnumed.116.186767. [DOI] [PubMed] [Google Scholar]
- 52.McCarthy M., Siew T., Campbell A., Lenzo N., Spry N., Vivian J., Morandeau L. ¹⁸F-Fluoromethylcholine (FCH) PET imaging in patients with castration-resistant prostate cancer: Prospective comparison with standard imaging. Eur. J. Nucl. Med. Mol. Imaging. 2011;38:14–22. doi: 10.1007/s00259-010-1579-x. [DOI] [PubMed] [Google Scholar]
- 53.Afshar-Oromieh A., Zechmann C.M., Malcher A., Eder M., Eisenhut M., Linhart H.G., Holland-Letz T., Hadaschik B.A., Giesel F.L., Debus J., et al. Comparison of PET imaging with a 68Ga-labelled PSMA ligand and 18F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging. 2014;41:11–20. doi: 10.1007/s00259-013-2525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Witkowska-Patena E., Mazurek A.J., Dziuk M. 68Ga-PSMA PET/CT imaging in recurrent prostate cancer: Where are we now? Cent. Eur. J. Urol. 2017;70:37–43. doi: 10.5173/ceju.2017.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Velikyan I. Prospective of 68Ga-radiopharmaceutical development. Theranostics. 2014;4:47–80. doi: 10.7150/thno.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mueller D., Breeman W.A., Klette I., Gottschaldt M., Odparlik A., Baehre M., Tworowska I., Schultz M.K. Radiolabelling of DOTA-like conjugated peptides with generator-produced 68Ga and using NaCl-based cationic elution method. Nat. Protoc. 2016;11:1057–1066. doi: 10.1038/nprot.2016.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meyrick D., Lenzo N.P., Crouch J., Trifunovic M., Henderson A., Turner J.H. Gallium-68-PSMA-11 kit formulation in routine clinical management of prostate cancer. Cancer Biother. Radiopharm. 2017 in review. [Google Scholar]
- 58.Young J.D., Abbate V., Imberti C., Meszaros L.K., Ma M.T., Terry S.Y.A., Hider R.C., Mullen G.E., Blower P.J. 68Ga-THP-PSMA: A PET imaging agent for prostate cancer offering rapid, room temperature, one-step kit-based radiolabeling. J. Nucl. Med. 2017;58:1270–1277. doi: 10.2967/jnumed.117.191882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hofman M.S., Eu P., Jackson P., Hong E., Binns D., Iravani A., Murphy D., Mitchell C., Siva S., Hicks R.J., et al. Cold Kit PSMA PET Imaging: Phase I study of (68)Ga-THP-PSMA PET/CT in patients with prostate cancer. J. Nucl. Med. 2017 doi: 10.2967/jnumed.117.199554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Afshar-Oromieh A., Haberkorn U., Eder M., Eisenhut M., Zechmann C.M. [68Ga]Gallium-labelled PSMA ligand as superior PET/CT tracer for the diagnosis of prostate cancer: Comparison with 18F-FECH. Eur. J. Nucl. Med. Mol. Imaging. 2012;39:1085–1086. doi: 10.1007/s00259-012-2069-0. [DOI] [PubMed] [Google Scholar]
- 61.Afshar-Oromieh A., Holland-Letz T., Giesel F.L., Kratochwil C., Mier W., Haufe S., Debus N., Eder M., Eisenhut M., Schäfer M., et al. Diagnostic performance of (68Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: Evaluation in 1007 patients. Eur. J. Nucl. Med. Mol. Imaging. 2017;44:1258–1268. doi: 10.1007/s00259-017-3711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perera M., Papa N., Christidis D., Wetherell D., Hofman M.S., Murphy D.G., Bolton D., Lawrentschuk N. Sensitivity, Specificity, and Predictors of Positive (68)Ga-Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2016;70:926–937. doi: 10.1016/j.eururo.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 63.Asokendaran M., Meyrick D., Henderson A., Turner J.H., Lenzo N.P. A comparison of (68)Ga-PSMA-PET/CT with diagnostic CT alone in relapsed prostate cancer. J. Mol. Imaging Dyn. 2018 in review. [Google Scholar]
- 64.Jilg C.A., Drendel V., Rischke H.C., Beck T., Vach W., Schaal K., Wetterauer U., Schultze-Seemann W., Meyer P.T. Diagnostic Accuracy of 68Ga-HBED-CC-PSMA-Ligand-PET/CT before Salvage Lymph Node Dissection for Recurrent Prostate Cancer. Theranostics. 2017;7:1770–1780. doi: 10.7150/thno.18421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rauscher I., Maurer T., Beer A.J., Graner F.P., Haller B., Weirich G., Doherty A., Gschwend J.E., Schwaiger M., Eiber M. Value of 68Ga-PSMA HBED-CC PET/CT for the Assessment of Lymph Node Metastases in Prostate Cancer Patients with Biochemical Recurrence: Comparison with Histopathology After Salvage Lymphadenectomy. J. Nucl. Med. 2016;57:1713–1719. doi: 10.2967/jnumed.116.173492. [DOI] [PubMed] [Google Scholar]
- 66.Roach P.J., Francis R., Emmett L., Hsiao E., Kneebone A., Hruby G., Eade T., Nguyen Q., Thompson B., Cusick T., et al. The impact of 68Ga-PSMA PET/CT on management intent in prostate cancer: Results of an Australian prospective multicenter study. J. Nucl. Med. 2018;59:82–88. doi: 10.2967/jnumed.117.197160. [DOI] [PubMed] [Google Scholar]
- 67.Albisinni S., Artigas C., Aoun F., Biaou I., Grosman J., Gil T., Hawaux E., Limani K., Otte F.X., Peltier A., et al. Clinical impact of 68Ga-prostate-specific membrane antigen (PSMA) positron emission tomography/computed tomography (PET/CT) in patients with prostate cancer with rising prostate-specific antigen after treatment with curative intent: Preliminary analysis of a multidisciplinary approach. BJU Int. 2017;120:197–203. doi: 10.1111/bju.13739. [DOI] [PubMed] [Google Scholar]
- 68.Morigi J.J., Stricker P.D., van Leeuwen P.J., Tang R., Ho B., Nguyen Q., Hruby G., Fogarty G., Jagavkar R., Kneebone A., et al. Prospective Comparison of 18F-Fluoromethylcholine Versus 68Ga-PSMA PET/CT in Prostate Cancer Patients Who Have Rising PSA After Curative Treatment and Are Being Considered for Targeted Therapy. J. Nucl. Med. 2015;56:1185–1190. doi: 10.2967/jnumed.115.160382. [DOI] [PubMed] [Google Scholar]
- 69.Schwenck J., Rempp H., Reischl G., Kruck S., Stenzl A., Nikolaou K., Pfannenberg C., la Fougère C. Comparison of 68Ga-labelled PSMA-11 and (11)C-choline in the detection of prostate cancer metastases by PET/CT. Eur. J. Nucl. Med. Mol. Imaging. 2017;44:92–101. doi: 10.1007/s00259-016-3490-6. [DOI] [PubMed] [Google Scholar]
- 70.Dietlein M., Kobe C., Kuhnert G., Stockter S., Fischer T., Schomäcker K., Schmidt M., Dietlein F., Zlatopolskiy B.D., Krapf P., et al. Comparison of [(18)F]DCFPyL and 68Ga-PSMA-HBED-CC for PSMA-PET/CT Imaging in Patients with Relapsed Prostate Cancer. Mol. Imaging Biol. 2015;17:575–584. doi: 10.1007/s11307-015-0866-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rowe S.P., Macura K.J., Ciarallo A., Mena E., Blackford A., Nadal R., Antonarakis E.S., Eisenberger M.A., Carducci M.A., Ross A.E., et al. Comparison of Prostate-Specific Membrane Antigen-Based 18F-DCFBC PET/CT to Conventional Imaging Modalities for Detection of Hormone-Naïve and Castration-Resistant Metastatic Prostate Cancer. J. Nucl. Med. 2016;57:46–53. doi: 10.2967/jnumed.115.163782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maurer T., Eiber M., Schwaiger M., Gschwend J.E. Current use of PSMA-PET in prostate cancer management. Nat. Rev. Urol. 2016;13:226–235. doi: 10.1038/nrurol.2016.26. [DOI] [PubMed] [Google Scholar]
- 73.Uprimny C., Kroiss A.S., Decristoforo C., Fritz J., von Guggenberg E., Kendler D., Scarpa L., di Santo G., Roig L.G., Maffey-Steffan J., et al. 68Ga-PSMA-11 PET/CT in primary staging of prostate cancer: PSA and Gleason score predict the intensity of tracer accumulation in the primary tumour. Eur. J. Nucl. Med. Mol. Imaging. 2017;44:941–949. doi: 10.1007/s00259-017-3631-6. [DOI] [PubMed] [Google Scholar]
- 74.Herlemann A., Wenter V., Kretschmer A., Thierfelder K.M., Bartenstein P., Faber C., Gildehaus F.J., Stief C.G., Gratzke C., Fendler W.P. 68Ga-PSMA Positron Emission Tomography/Computed Tomography Provides Accurate Staging of Lymph Node Regions Prior to Lymph Node Dissection in Patients with Prostate Cancer. Eur. Urol. 2016;70:553–557. doi: 10.1016/j.eururo.2015.12.051. [DOI] [PubMed] [Google Scholar]
- 75.Bailey J., Piert M. Performance of 68Ga-PSMA PET/CT for Prostate Cancer Management at Initial Staging and Time of Biochemical Recurrence. Curr. Urol. Rep. 2017;18:84. doi: 10.1007/s11934-017-0736-1. [DOI] [PubMed] [Google Scholar]
- 76.Meyrick D.P., Asokendaran M., Skelly L.A., Lenzo N.P., Henderson A. The role of 68Ga-PSMA-I&T PET/CT in the pretreatment staging of primary prostate cancer. Nucl. Med. Commun. 2017;38:956–963. doi: 10.1097/MNM.0000000000000738. [DOI] [PubMed] [Google Scholar]
- 77.Pyka T., Okamoto S., Dahlbender M., Tauber R., Retz M., Heck M., Tamaki N., Schwaiger M., Maurer T., Eiber M. Comparison of bone scintigraphy and 68Ga-PSMA PET/CT for skeletal staging in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging. 2016;43:2114–2121. doi: 10.1007/s00259-016-3435-0. [DOI] [PubMed] [Google Scholar]
- 78.Janssen J.C., Meißner S., Woythal N., Prasad V., Brenner W., Diederichs G., Hamm B., Makowski M.R. Comparison of hybrid 68Ga-PSMA-PET/CT and (99m)Tc-DPD-SPECT/CT for the detection of bone metastases in prostate cancer patients: Additional value of morphologic information from low dose CT. Eur. Radiol. 2018;28:610–619. doi: 10.1007/s00330-017-4994-6. [DOI] [PubMed] [Google Scholar]
- 79.Giesel F.L., Sterzing F., Schlemmer H.P., Holland-Letz T., Mier W., Rius M., Afshar-Oromieh A., Kopka K., Debus J., Haberkorn U., et al. Intra-individual comparison of 68Ga-PSMA-11-PET/CT and multi-parametric MR for imaging of primary prostate cancer. Eur. J. Nucl. Med. Mol. Imaging. 2016;43:1400–1406. doi: 10.1007/s00259-016-3346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Eiber M., Weirich G., Holzapfel K., Souvatzoglou M., Haller B., Rauscher I., Beer A.J., Wester H.J., Gschwend J., Schwaiger M., et al. Simultaneous 68Ga-PSMA HBED-CC PET/CT/MRI Improves the Localization of Primary Prostate Cancer. Eur. Urol. 2016;70:829–836. doi: 10.1016/j.eururo.2015.12.053. [DOI] [PubMed] [Google Scholar]
- 81.Zamboglou C., Drendel V., Jilg C.A., Rischke H.C., Beck T.I., Schultze-Seemann W., Krauss T., Mix M., Schiller F., Wetterauer U., et al. Comparison of 68Ga-HBED-CC PSMA-PET/CT and multiparametric MRI for gross tumour volume detection in patients with primary prostate cancer based on slice by slice comparison with histopathology. Theranostics. 2017;7:228–237. doi: 10.7150/thno.16638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zamboglou C., Schiller F., Fechter T., Wieser G., Jilg C.A., Chirindel A., Salman N., Drendel V., Werner M., Mix M., et al. 68Ga-HBED-CC-PSMA PET/CT Versus Histopathology in Primary Localized Prostate Cancer: A Voxel-Wise Comparison. Theranostics. 2016;6:1619–1628. doi: 10.7150/thno.15344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Del Monte M., Costantino L., Salvo V., Grompone M.D., Pecoraro M., Stanzione A., Campa R., Vullo F., Sciarra A., Catalano C., et al. MRI/US fusion-guided biopsy: Performing exclusively targeted biopsies for the early detection of prostate cancer. Radiol. Med. 2017 doi: 10.1007/s11547-017-0825-8. [DOI] [PubMed] [Google Scholar]
- 84.Bastian-Jordan M. Magnetic resonance imaging of the prostate and targeted biopsy, Comparison of PIRADS and Gleason grading. J. Med. Imaging Radiat. Oncol. 2017 doi: 10.1111/1754-9485.12678. [DOI] [PubMed] [Google Scholar]
- 85.Elkjær M.C., Andersen M.H., Høyer S., Pedersen B.G., Borre M. Prostate cancer: In-bore magnetic resonance guided biopsies at active surveillance inclusion improve selection of patients for active treatment. Acta Radiol. 2017:284185117723372. doi: 10.1177/0284185117723372. [DOI] [PubMed] [Google Scholar]
- 86.Monni F., Fontanella P., Grasso A., Wiklund P., Ou Y.C., Randazzo M., Rocco B., Montanari E., Bianchi G. Magnetic resonance imaging in prostate cancer detection and management: A systematic review. Minerva Urol. Nefrol. 2017;69:567–578. doi: 10.23736/S0393-2249.17.02819-3. [DOI] [PubMed] [Google Scholar]
- 87.Herlemann A., Kretschmer A., Buchner A., Karl A., Tritschler S., El-Malazi L., Fendler W.P., Wenter V., Ilhan H., Bartenstein P., et al. Salvage lymph node dissection after (68)Ga-PSMA or (18)F-FEC PET/CT for nodal recurrence in prostate cancer patients. Oncotarget. 2017;8:84180–84192. doi: 10.18632/oncotarget.21118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Van Leeuwen P.J., Emmett L., Ho B., Delprado W., Ting F., Nguyen Q., Stricker P.D. Prospective evaluation of 68Gallium-prostate-specific membrane antigen positron emission tomography/computed tomography for preoperative lymph node staging in prostate cancer. BJU Int. 2017;119:209–215. doi: 10.1111/bju.13540. [DOI] [PubMed] [Google Scholar]
- 89.Siriwardana A., Thompson J., van Leeuwen P.J., Doig S., Kalsbeek A., Emmett L., Delprado W., Wong D., Samaratunga H., Haynes A.M., et al. Initial multicentre experience of (68) gallium-PSMA PET/CT guided robot-assisted salvage lymphadenectomy: Acceptable safety profile but oncological benefit appears limited. BJU Int. 2017;120:673–681. doi: 10.1111/bju.13919. [DOI] [PubMed] [Google Scholar]
- 90.Maurer T., Weirich G., Schottelius M., Weineisen M., Frisch B., Okur A., Kübler H., Thalgott M., Navab N., Schwaiger M., et al. Prostate-specific membrane antigen-radioguided surgery for metastatic lymph nodes in prostate cancer. Eur. Urol. 2015;68:530–534. doi: 10.1016/j.eururo.2015.04.034. [DOI] [PubMed] [Google Scholar]
- 91.Schottelius M., Wirtz M., Eiber M., Maurer T., Wester H.J. [(111)In]PSMA-I&T: Expanding the spectrum of PSMA-I&T applications towards SPECT and radioguided surgery. EJNMMI Res. 2015;5:68. doi: 10.1186/s13550-015-0147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rauscher I., Düwel C., Wirtz M., Schottelius M., Wester H.J., Schwamborn K., Haller B., Schwaiger M., Gschwend J.E., Eiber M., et al. Value of (111)In-prostate-specific membrane antigen (PSMA)-radioguided surgery for salvage lymphadenectomy in recurrent prostate cancer: Correlation with histopathology and clinical follow-up. BJU Int. 2017;120:40–47. doi: 10.1111/bju.13713. [DOI] [PubMed] [Google Scholar]
- 93.Bluemel C., Linke F., Herrmann K., Simunovic I., Eiber M., Kestler C., Buck A.K., Schirbel A., Bley T.A., Wester H.J., et al. Impact of (68)Ga-PSMA PET/CT on salvage radiotherapy planning in patients with prostate cancer and persisting PSA values or biochemical relapse after prostatectomy. EJNMMI Res. 2016;6:78. doi: 10.1186/s13550-016-0233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Calais J., Czernin J., Cao M., Kishan A.U., Hegde J.V., Shaverdian N., Sandler K.A., Chu F.I., King C.R., Steinberg M.L., et al. (68)Ga-PSMA PET/CT mapping of prostate cancer biochemical recurrence following radical prostatectomy in 270 patients with PSA < 1.0 ng/mL: Impact on Salvage Radiotherapy Planning. J. Nucl. Med. 2017 doi: 10.2967/jnumed.117.201749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Habl G., Sauter K., Schiller K., Dewes S., Maurer T., Eiber M., Combs S.E. (68)Ga-PSMA-PET for radiation treatment planning in prostate cancer recurrences after surgery: Individualized medicine or new standard in salvage treatment. Prostate. 2017;77:920–927. doi: 10.1002/pros.23347. [DOI] [PubMed] [Google Scholar]
- 96.Hruby G., Eade T., Kneebone A., Emmett L., Guo L., Ho B., Hsiao E., Schembri G., Hunter J., Kwong C. Delineating biochemical failure with (68)Ga-PSMA-PET following definitive external beam radiation treatment for prostate cancer. Radiother. Oncol. 2017;122:99–102. doi: 10.1016/j.radonc.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 97.Emmett L., van Leeuwen P.J., Nandurkar R., Scheltema M.J., Cusick T., Hruby G., Kneebone A., Eade T., Fogarty G., Jagavkar R., et al. Treatment Outcomes from (68)Ga-PSMA PET/CT-Informed Salvage Radiation Treatment in Men with Rising PSA After Radical Prostatectomy: Prognostic Value of a Negative PSMA PET. J. Nucl. Med. 2017;58:1972–1976. doi: 10.2967/jnumed.117.196683. [DOI] [PubMed] [Google Scholar]
- 98.Zschaeck S., Wust P., Beck M., Wlodarczyk W., Kaul D., Rogasch J., Budach V., Furth C., Ghadjar P. Intermediate-term outcome after PSMA-PET guided high-dose radiotherapy of recurrent high-risk prostate cancer patients. Radiat. Oncol. 2017;12:140. doi: 10.1186/s13014-017-0877-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Baumann R., Koncz M., Luetzen U., Krause F., Dunst J. Oligometastases in prostate cancer: Metabolic response in follow-up PSMA-PET-CTs after hypofractionated IGRT. Strahlenther. Onkol. 2017 doi: 10.1007/s00066-017-1239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Seitz A.K., Rauscher I., Haller B., Krönke M., Luther S., Heck M.M., Horn T., Gschwend J.E., Schwaiger M., Eiber M., et al. Preliminary results on response assessment using (68)Ga-HBED-CC-PSMA PET/CT in patients with metastatic prostate cancer undergoing docetaxel chemotherapy. Eur. J. Nucl. Med. Mol. Imaging. 2017 doi: 10.1007/s00259-017-3887-x. [DOI] [PubMed] [Google Scholar]
- 101.Kim J.H., Kim B.J., Jang H.J., Kim H.S. Comparison of the RECIST and EORTC PET criteria in the tumor response assessment: A pooled analysis and review. Cancer Chemother. Pharmacol. 2017;80:729–735. doi: 10.1007/s00280-017-3411-9. [DOI] [PubMed] [Google Scholar]
- 102.Aide N., Lasnon C., Veit-Haibach P., Sera T., Sattler B., Boellaard R. EANM/EARL harmonization strategies in PET quantification: From daily practice to multicentre oncological studies. Eur. J. Nucl. Med. Mol. Imaging. 2017;44:17–31. doi: 10.1007/s00259-017-3740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wahl R.L., Jacene H., Kasamon Y., Lodge M.A. From RECIST to PERCIST: Evolving Considerations for PET Response Criterai in Solid Tumours. J. Nucl. Med. 2009;50:122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eiber M., Herrmann K., Calais J., Hadaschihk B., Giesel F.L., Hartenbach M., Hope T.A., Reiter R., Maurer T., Weber W.A., et al. PROstate cancer Molecular Imaging Standardized Evaluation (PROMISE): Proposed miTNM classification for the interpretation of PSMA-ligand PET/CT. J. Nucl. Med. 2017 doi: 10.2967/jnumed.117.198119. [DOI] [PubMed] [Google Scholar]
- 105.Claringbold P.G., Brayshaw P.A., Price R.A., Turner J.H. Phase II study of radiopeptide 177Lu-octreotate and capecitabine therapy of progressive disseminated neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging. 2011;38:302–311. doi: 10.1007/s00259-010-1631-x. [DOI] [PubMed] [Google Scholar]
- 106.Claringbold P.G., Price R.A., Turner J.H. Phase I-II study of radiopeptide 177Lu-octreotate in combination with capecitabine and temozolomide in advanced low-grade neuroendocrine tumors. Cancer Biother. Radiopharm. 2012;27:561–569. doi: 10.1089/cbr.2012.1276. [DOI] [PubMed] [Google Scholar]
- 107.Claringbold P.G., Turner J.H. Pancreatic Neuroendocrine Tumor Control: Durable Objective Response to Combination 177Lu-Octreotate-Capecitabine-Temozolomide Radiopeptide Chemotherapy. Neuroendocrinology. 2016;103:432–439. doi: 10.1159/000434723. [DOI] [PubMed] [Google Scholar]
- 108.Claringbold P.G., Turner J.H. NeuroEndocrine Tumor Therapy with Lutetium-177-octreotate and Everolimus (NETTLE): A Phase I Study. Cancer Biother. Radiopharm. 2015;30:261–269. doi: 10.1089/cbr.2015.1876. [DOI] [PubMed] [Google Scholar]
- 109.Baum R.P., Kluge A.W., Kulkarni H., Schorr-Neufing U., Niepsch K., Bitterlich N., van Echteld C.J. [(177)Lu-DOTA](0)-D-Phe(1)-Tyr(3)-Octreotide ((177)Lu-DOTATOC) For Peptide Receptor Radiotherapy in Patients with Advanced Neuroendocrine Tumours: A Phase-II Study. Theranostics. 2016;6:501–510. doi: 10.7150/thno.13702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Van Vliet E.I., van Eijck C.H., de Krijger R.R., Nieveen van Dijkum E.J., Teunissen J.J., Kam B.L., de Herder W.W., Feelders R.A., Bonsing B.A., Brabander T., et al. Neoadjuvant Treatment of Nonfunctioning Pancreatic Neuroendocrine Tumors with [177Lu-DOTA0,Tyr3]Octreotate. J. Nucl. Med. 2015;56:1647–1653. doi: 10.2967/jnumed.115.158899. [DOI] [PubMed] [Google Scholar]
- 111.Brabander T., van der Zwan W.A., Teunissen J.J.M., Kam B.L.R., Feelders R.A., de Herder W.W., van Eijck C.H.J., Franssen G.J.H., Krenning E.P., Kwekkeboom D.J. Long-Term Efficacy, Survival, and Safety of [(177)Lu-DOTA(0),Tyr(3)]octreotate in Patients with Gastroenteropancreatic and Bronchial Neuroendocrine Tumors. Clin. Cancer Res. 2017;23:4617–4624. doi: 10.1158/1078-0432.CCR-16-2743. [DOI] [PubMed] [Google Scholar]
- 112.Strosberg J., El-Haddad G., Wolin E., Hendifar A., Yao J., Chasen B., Mittra E., Kunz P.L., Kulke M.H., Jacene H., et al. NETTER-1 Trial Investigators. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017;376:125–135. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Turner J.H. Recent advances in Theranostics and challenges for the future. BJR. 2017 doi: 10.1259/bjr.20170893. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Duffy M.J., Harbeck N., Nap M., Molina R., Nicolini A., Senkus E., Cardoso F. Clinical use of biomarkers in breast cancer: Updated guidelines from the European Group on Tumor Markers (EGTM) Eur. J. Cancer. 2017;75:284–298. doi: 10.1016/j.ejca.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 115.Boegemann M., Schrader A.J., Rahbar K. (177)Lu-PSMA therapy: Current evidence for use in the treatment of patients with metastatic prostate cancer. Urologe A. 2017;56:1440–1444. doi: 10.1007/s00120-017-0510-5. [DOI] [PubMed] [Google Scholar]
- 116.Calopedos R.J.S., Chalasani V., Asher R., Emmett L., Woo H.H. Lutetium-177-labelled anti-prostate-specific membrane antigen antibody and ligands for the treatment of metastatic castrate-resistant prostate cancer: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2017;20:352–360. doi: 10.1038/pcan.2017.23. [DOI] [PubMed] [Google Scholar]
- 117.Emmett L., Willowson K., Violet J., Shin J., Blanksby A., Lee J. Lutetium (177) PSMA radionuclide therapy for men with prostate cancer: A review of the current literature and discussion of practical aspects of therapy. J. Med. Radiat. Sci. 2017;64:52–60. doi: 10.1002/jmrs.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fendler W.P., Kratochwil C., Ahmadzadehfar H., Rahbar K., Baum R.P., Schmidt M., Pfestroff A., Lützen U., Prasad V., Heinzel A., et al. 177Lu-PSMA-617 therapy, dosimetry and follow-up in patients with metastatic castration-resistant prostate cancer. Nuklearmedizin. 2016;55:123–128. [PubMed] [Google Scholar]
- 119.Weineisen M., Schottelius M., Simecek J., Baum R.P., Yildiz A., Beykan S., Kulkarni H.R., Lassmann M., Klette I., Eiber M., et al. 68Ga- and 177Lu-Labeled PSMA I&T: Optimization of a PSMA-Targeted Theranostic Concept and First Proof-of-Concept Human Studies. J. Nucl. Med. 2015;56:1169–1176. doi: 10.2967/jnumed.115.158550. [DOI] [PubMed] [Google Scholar]
- 120.Kesavan M., Turner J.H., Meyrick D., Yeo S., Cardaci G., Lenzo N.P. Salvage Radiopeptide Therapy of Advanced Castrate Resistant Prostate Cancer with Lutetium-177 labelled Prostate Specific Membrane Antigen (177Lu-PSMA): Efficacy and safety in Routine Practice. Cancer Biother. Radiopharm. 2017 doi: 10.1089/cbr.2017.2403. in review. [DOI] [PubMed] [Google Scholar]
- 121.Kratochwil C., Bruchertseifer F., Giesel F.L., Weis M., Verburg F.A., Mottaghy F., Kopka K., Apostolidis C., Haberkorn U., Morgenstern A. 225Ac-PSMA-617 for PSMA-Targeted α-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016;57:1941–1944. doi: 10.2967/jnumed.116.178673. [DOI] [PubMed] [Google Scholar]