Abstract
Objective
To review the effectiveness of antibiotic stewardship interventions in hospitals in low- and middle-income countries.
Methods
We searched MEDLINE®, Embase®, Cochrane Central Register of Controlled Trials and regional indexes for studies of interventions to improve appropriate prescribing and use of antibiotics for hospitalized patients in low- and middle-income countries. We included controlled trials, controlled before-and-after studies and interrupted time-series studies published up to December 2017. We report prescribing, clinical and microbiological outcomes using a narrative approach.
Findings
We screened 7342 original titles and abstracts, assessed 241 full-text articles and included 27 studies from 2 low-income and 11 middle-income countries. We found a medium (11 studies) or high risk (13 studies) of bias. Generally, all types of interventions (structural, persuasive and enabling) and intervention bundles were reported to improve prescribing and clinical outcomes. However, the studied interventions and reported outcomes varied widely. The most frequent intervention was procalcitonin-guided antibiotic treatment (8 of 27 studies, all randomized controlled trials). The intervention was associated with a relative risk for patients receiving antibiotics ranging between 0.40 and 0.87.
Conclusion
The majority of studies reported a positive effect of hospital antibiotic stewardship interventions. However, we cannot draw general conclusions about the effectiveness of such interventions in low- and middle-income countries because of low study quality, heterogeneity of interventions and outcomes, and under-representation of certain settings. To strengthen the evidence base, action needs to be taken to address these shortcomings.
Résumé
Objectif
Étudier l'efficacité des interventions visant un usage plus rationnel des antibiotiques dans les hôpitaux de pays à revenu faible et intermédiaire.
Méthodes
Nous avons consulté MEDLINE®, Embase®, le registre central Cochrane des essais contrôlés ainsi que des index régionaux afin de rechercher des études portant sur des interventions menées pour améliorer la prescription et l'usage des antibiotiques pour les patients hospitalisés, dans des pays à revenu faible et intermédiaire. Nous avons inclus des essais contrôlés, des études contrôlées avant/après et des études en séries temporelles interrompues, publiés jusqu'à décembre 2017. Nous évoquons ici, en adoptant une approche narrative, les résultats obtenus en termes de prescription et aux niveaux clinique et microbiologique.
Résultats
Nous avons sélectionné 7342 résumés et titres originaux, évalué 241 articles dans leur version intégrale et inclus 27 études, pour 2 pays à faible revenu et 11 pays à revenu intermédiaire. Nous avons identifié un risque de biais moyen (11 études) ou élevé (13 études). En règle générale, ces publications indiquent que tous les types d'interventions (structurelles, persuasives et capacitantes) ainsi que toutes les interventions combinées ont permis d'améliorer les résultats en termes de prescription et au niveau clinique. Cependant, les interventions étudiées et les résultats publiés sont extrêmement variés. L'intervention la plus fréquente a consisté à guider les antibiothérapies en utilisant la procalcitonine (8 études sur 27; toutes correspondent à des essais contrôlés randomisés). Pour les patients, cette intervention a été associée à un risque relatif de prescription d’antibiotiques compris entre 0,40 et 0,87.
Conclusion
La majorité des études font état d'un effet positif des interventions visant à promouvoir l'usage rationnel des antibiotiques en milieu hospitalier. Néanmoins, nous ne pouvons pas tirer de conclusions générales sur l'efficacité de ces interventions dans les pays à revenu faible ou intermédiaire, compte tenu de la mauvaise qualité des études, de l'hétérogénéité des interventions et des résultats ainsi que de la sous-représentation de certains contextes. Pour consolider les données disponibles, des actions doivent être entreprises afin de combler ces lacunes.
Resumen
Objetivo
Revisar la eficacia de las intervenciones de administración de antibióticos en hospitales de países con ingresos medios y bajos.
Métodos
Se realizaron búsquedas en MEDLINE®, Embase®, en el Registro Central de Ensayos Controlados Cochrane y en índices regionales en relación a estudios de intervenciones para mejorar la prescripción y el uso adecuado de antibióticos para pacientes hospitalizados en países con ingresos medios y bajos. Incluimos ensayos controlados, estudios controlados de tipo antes y después y estudios de series de tiempo interrumpido publicados hasta diciembre de 2017. Informamos acerca de los resultados de prescripción, clínicos y microbiológicos usando un enfoque narrativo.
Resultados
Revisamos 7342 títulos originales y resúmenes, evaluamos 241 artículos de texto completos, incluidos 27 estudios de 2 países con ingresos bajos y 11 con ingresos medios. Encontramos riesgo medio de sesgo (11 estudios) o riesgo alto (13 estudios). Por lo general, se informó de que todos los tipos de intervenciones (estructurales, persuasivas y permisivas) y conjuntos de intervenciones mejoran los resultados de prescripción y los resultados clínicos. Sin embargo, las intervenciones estudiadas y los resultados sobre los que se informó varían considerablemente. La intervención más frecuente fue el tratamiento antibiótico guiado de procalcitonina (8 de 27 estudios, todos ellos ensayos controlados aleatorizados). La intervención se asoció a un riesgo relativo para pacientes que recibían antibióticos que oscilan entre 0,40 y 0,87.
Conclusión
La mayoría de los estudios informaron sobre un efecto positivo de las intervenciones hospitalarias con administración de antibióticos. Sin embargo, no podemos extraer conclusiones generales acerca de la efectividad de tales intervenciones en países con ingresos medios y bajos debido a la baja calidad del estudio, a la heterogeneidad de las intervenciones y los resultados y a la baja representación de ciertas regiones. Para fortalecer la base de las evidencias, es necesario tomar medidas para abordar estas deficiencias.
الملخص
الغرض
مراجعة فاعلية تدخلات الإشراف على المضادات الحيوية داخل المستشفيات في البلدان محدودة ومتوسطة الدخل.
الطريقة
قمنا بإجراء بحث في MEDLINE®، وEmbase®، وسجل Cochrane المركزي الخاص بالتجارب المضبطة بالشواهد والمؤشرات الإقليمية الخاصة بدراسات التدخلات بهدف تحسين وصف الأدوية المناسبة واستخدام المضادات الحيوية للمرضى الذين تم إدخالهم المستشفيات في البلدان محدودة ومتوسطة الدخل. وقمنا بتضمين التجارب المضبطة بالشواهد، والدراسات القبلية والبعدية المضبطة بالشواهد ودراسات السلاسل الزمنية المتقطعة التي تم نشرها حتى شهر ديسمبر/كانون الأول 2017. ولقد أورد تقريرنا على محصلات مكروبيولوجية وسريرية ووصفية باستخدام النهج السردي.
النتائج
كما قمنا بفحص 7342 من العناوين والملخصات الأصلية، وقمنا بتقييم 241 من المقالات النص الكامل، وتم تضمين 27 دراسة من اثنتين من البلدان محدودة الدخل و11 بلدًا من البلدان متوسطة الدخل. أظهرت (11 دراسة) مستوى متوسط من اختطار التحيز، أو (13 دراسة) مستوى مرتفع من اختطار التحيز. بشكل عام، تم الإبلاغ عن كل أشكال التدخلات (الهيكلية، والمقنعة، والتمكينية) ومجموعة التدخلات بهدف تحسين المحصلات السريرية والوصفية. ومع ذلك، فقد تباينت التدخلات والمحصلات المبلغ عنها بشكل كبير. وكان أكثر تدخل متكرر هو العلاج بالمضاد الحيوي الموجه بروكلسيتونين (8 من 27 دراسة، كل التجارب المعشاة المضبطة بشواهد). وارتبط التدخل بخطر نسبي على المرضى الذين يتناولون المضادات الحيوية تتراوح نسبته ما بين 0.40 و0.87.
الاستنتاج
أبلغت معظم الدارسات عن وجود أثر إيجابي فيما يخص تدخلات الإشراف على المضادات الحيوية في المستشفيات. ومع ذلك، لا يمكننا الوصول إلى نتائج نهائية تخص فاعلية تلك التدخلات في البلدان محدودة ومتوسطة الدخل، بسبب الجودة النوعية المتدنية للدراسات، وعنصر عدم التجانس في التدخلات والمحصلات، والتمثيل غير الكافي لمناطق معينة. وحرصًا على تعزيز قاعدة الأدلة، فإننا نحتاج إلى اتحاذ الإجراءات اللازمة لتعويض هذا القصور.
摘要
目的:
评价低收入和中等收入国家的医院对抗生素的管理干预措施的有效性。
方法:
我们分别在 MEDLINE®、Embase®、Cochrane 临床对照试验中心注册数据库中进行检索,用于研究干预措施,以改善低收入和中等收入国家住院患者适当的开具处方和使用抗生素。我们包括了发表至 2017 年 12 月的对照试验、前后对照研究和断续时间序列研究。我们使用叙述性方法来报告处方、临床和微生物结果。
结果
:我们筛选了 7342 条原标题和摘要,评估了 241 篇全文文献,其中包括来自 2 个低收入国家和 11 个中等收入国家的 27 项研究。我们发现了中等(11 项研究)或高等(13 项研究)偏倚风险。普遍来说,所有类型的干预措施(结构型、劝导型和授权型)和干预组合措施据报告可改善处方和临床结果。然而,研究的干预措施和报告的结果差异很大。最常见的干预措施是降钙素原指导的抗生素治疗(所有 27 例随机对照试验研究中有 8 例采用此干预措施)。此干预措施对接受抗生素治疗患者的相对危险范围为 0.40 至 0.87。
结论:
大多数研究报告了医院抗生素管理干预措施的积极效果。然而,由于研究质量较低、干预措施及其结果的异质性以及某些背景的代表性不足,我们不能就这些干预措施在低收入和中等收入国家的有效性得出一般性结论。为了加强证据基础,需要采取行动来解决这些不足。
Резюме
Цель
Провести анализ эффективности введения стратегии рационального использования антибактериальных препаратов в больницах в странах с низким и средним уровнем доходов населения.
Методы
Авторы провели поиск в базах данных MEDLINE®, Embase®, Центральном Кокрановском реестре контролируемых испытаний и региональных предметных указателях на предмет исследований вмешательств с целью оптимизации назначения и использования антибиотиков для госпитализированных пациентов в странах с низким и средним уровнем доходов населения. Авторы включили контролируемые испытания, контролируемые исследования до и после вмешательства и исследования прерванных временных рядов, опубликованные до декабря 2017 года. Авторы сообщают о назначениях, результатах клинических и микробиологических исследований, используя описательный подход.
Результаты
Авторы просмотрели 7342 оригинальных периодических издания и резюме, проверили 241 полнотекстовую статью и включили 27 исследований из 2 стран с низким уровнем доходов населения и 11 стран со средним уровнем. Был обнаружен средний (11 исследований) и высокий риск (13 исследований) систематической ошибки. В целом было отмечено, что все виды вмешательств (структурные вмешательства, убеждение и создание благоприятных условий), а также их комплексы оптимизировали назначение антибактериальных препаратов и клинические результаты. Тем не менее изученные вмешательства и зарегистрированные результаты сильно различались. Наиболее частым вмешательством было управление антибиотикотерапией на основе уровня прокальцитонина в крови (8 из 27 исследований, все — рандомизированные контролируемые исследования). Вмешательство было связано с относительным риском для получавших антибиотики пациентов, показатель которого находился в диапазоне от 0,40 до 0,87.
Вывод
В большинстве исследований сообщалось о положительном эффекте введения стратегии рационального использования антибактериальных препаратов в больницах. Однако авторы не могут сделать общих выводов об эффективности таких вмешательств в странах с низким и средним уровнем доходов населения из-за низкого качества исследования, неоднородности вмешательств и результатов и недостаточного представления определенных параметров. Для укрепления доказательной базы необходимо принять меры для устранения этих недостатков.
Introduction
Antibiotic resistance is a problem of global importance.1 Representative data on the extent of the problem in low-and middle-income countries are relatively scarce, but high levels of resistance are increasingly being reported worldwide.2–4 Misuse and overuse of antibiotics in humans and animals is one of the main drivers of antibiotic resistance.5,6 Antibiotic stewardship, that is, interventions designed to optimize use of antibiotics, is therefore one of the key actions of the World Health Organization (WHO) Global Action Plan to contain antibiotic resistance.5,7 Stewardship interventions are typically classified as structural (such as the introduction of new diagnostic tests to guide antibiotic treatment), persuasive (such as expert audit of prescriptions and feedback advice to prescribers), enabling (such as guidelines or education on antibiotic use) or restrictive (such as expert approval for use of certain antibiotics).8 Often, different interventions are combined in antibiotic stewardship bundles.
Several systematic reviews showed that antibiotic stewardship interventions for hospitalized patients increased compliance with local antibiotic policies and improved clinical patient outcomes.8–10 These reviews included mainly or exclusively papers from high-income countries. Whether these results also apply to low- and middle-income countries is unclear. The organization of health-care system, availability of diagnostic testing and appropriate antibiotics, infection prevention and control practices and prescribing practices (such as over-the-counter availability of antibiotics) differs markedly between high-income countries and low- and middle-income countries.11 These differences may affect the implementation and effectiveness of antibiotic stewardship interventions in these settings.
Many hospitals in low- and middle-income countries are setting up antibiotic stewardship programmes.12 To better inform the selection of antibiotic stewardship interventions, we systematically reviewed the literature for studies that describe the effect of these interventions on clinical, microbiological or antibiotic prescribing outcomes in hospitalized patients in low- and middle-income countries.
Methods
The review protocol including the complete search strategy has been registered at the PROSPERO international prospective register of systematic reviews (CRD42016042019).13
We included studies on antibiotic stewardship interventions for hospitalized patients in low- and middle-income countries. Stewardship interventions were defined as any intervention aiming to improve appropriate prescribing of antibiotics. A summary of the search strategy is shown in Box 1. Low- and middle-income countries were defined according to the World Bank criteria.14 To be included, studies had to report at least one prescribing outcome (such as defined daily doses per 100 bed-days), clinical outcome (such as mortality) or microbiological outcome (such as proportion of bacterial isolates with antibiotic resistance). We included (non)randomized controlled trials, cluster randomized controlled trials, controlled before‒after studies and interrupted time-series studies if these contained at least three points of comparison pre-and post-intervention. Studies were excluded if they included residents of long-term health-care or nursing facilities; studied malaria, human immunodeficiency virus, mycobacterial or fungal infections, Helicobacter pylori eradication, or care pathways (such as malnutrition bundles); compared antibiotic regimens; were written in language other than English, Dutch, French, German, Portuguese or Spanish; or had no full-text article available.
Box 1. Search strategy for the review of antibiotic stewardship interventions in hospitals in low-and middle-income countries.
We searched the following databases from inception to 5 December 2017: Cochrane Central Register of Controlled Trials, EMBASE®, MEDLINE®, regional databases of the Global Index Medicus and the World Health Organization’s Virtual Health Library. The combination of the following and related terms was used: “low- and middle-income country”, “antibiotic”, “stewardship”, “inpatient” and terms related to study design such as “clinical trial”, “randomized controlled trial”, “interrupted time series”, “controlled before after”. Syntax and wording was adapted to the different libraries. Moreover, we searched reference lists of selected studies and of relevant reviews and consulted experts for additional literature. The full search strategy can be viewed online.13
Titles and abstracts were independently screened for eligibility by two authors. In case of disagreement, consensus was sought after reading the full-text article. The study selection was piloted by screening 630 abstracts and 44 full-text articles. These results were discussed among a panel of experts, after which the eligibility criteria were fine-tuned.
Two researchers extracted the data using an electronic form. The authors of original studies were not contacted in cases of incomplete or missing data. Data that were analysed inappropriately in the original studies were excluded. The quality of the studies was evaluated at the study level by two researchers independently. We used the 2017 quality criteria for randomized controlled trials and quasi-experimental studies of the Effective Practice and Organisation of Care Review Group.15 Reporting was done in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.16 For controlled trials, intention-to-treat analyses were reported unless indicated otherwise. If the original paper did not mention a relative risk (RR), we calculated a RR and 95% confidence interval (CI) if the necessary data were available. Due to the heterogeneity of the interventions and their reported outcomes, we present our findings using a narrative approach. Because of the large number of reported outcomes, we were unable to report all. We therefore selected the outcomes that were reported most frequently across the studies. We grouped studies by intervention type: structural, persuasive, enabling or intervention bundle.
Results
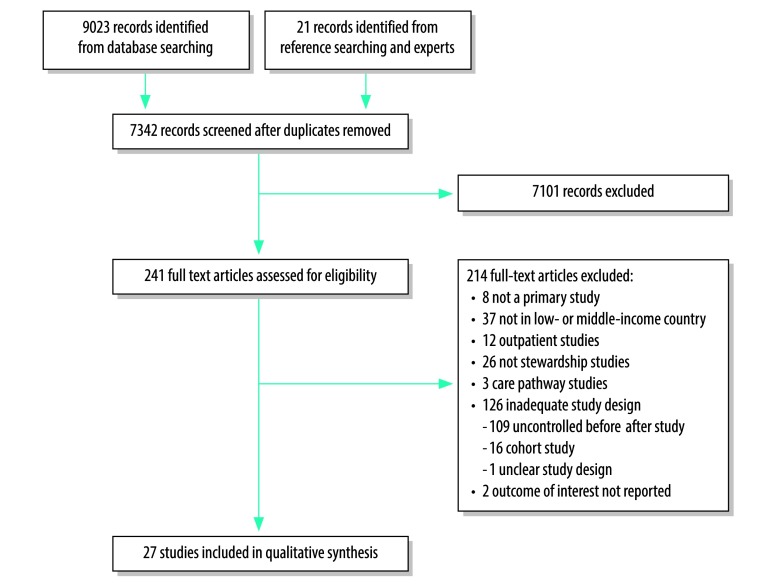
We screened 7342 abstracts, selected 241 full-text articles and included 27 studies:17–43 12 interrupted time-series, 9 randomized controlled trials, 3 cluster randomized controlled trials and 3 non-randomized controlled trials (Fig. 1). The studies were performed between 1996 and 2015 in 13 different countries. Two countries were considered low-income at the time of the study, one country transitioned from low to lower-middle income and the remaining were middle-income countries. Nine studies were conducted in multiple hospitals (range 2–65) but the majority was single-centre (18 studies). The interventions were implemented hospital-wide (10 studies) or on specific wards (17 studies) and targeted therapeutic prescriptions (20 studies), surgical prophylaxis (3 studies) or a combination of those (4 studies; Table 1).
Fig. 1.
Flowchart of the selection of studies included in the review of antibiotic stewardship interventions in hospitals in low-and middle-income countries
Table 1. Characteristics of studies included in the review of antibiotic stewardship interventions in hospitals in low-and middle-income countries.
| Authors, year | Study design | Country | Setting | Participants | Intervention type | Intervention details | Target illness |
|---|---|---|---|---|---|---|---|
| Weinberg et al., 200139 | Interrupted time-series | Colombia | 2 referral hospitals | Surgeons performing caesarean sections | Bundle | Guidelines on surgical antibiotic prophylaxis; structural changes (availability of prophylactic antibiotics on site); audit and feedback to physicians and nurses at hospital and individual level | Surgical site infections after caesarean section |
| Perez et al., 200340 | Interrupted time-series | Colombia | 2 university hospitals | Hospital A: all prescribers; hospital B: anaesthesiologists | Bundle | Prescription form with (un)restricted antibiotics; educational campaign; reminders in the workplace | NR |
| Gülmezoglu et al., 200727 | Cluster randomized controlled trial | Mexico and Thailand | 22 non-university maternity hospitals | Physicians, midwives, interns, students | Structural | Access to WHO’s online Reproductive Health Library44 and workshops on its use | Surgical site infections after caesarean section |
| Hadi et al., 200834 | Interrupted time-series | Indonesia | 1 teaching hospital | All prescribers of 5 internal medicine wards | Enabling | Antibiotic guidelines; education for prescribers | NR |
| Özkaya et al., 200926 | Non-randomized controlled trial | Turkey | 1 university hospital | Paediatric emergency department residents | Structural | Antibiotic initiation guided by influenza rapid test versus no laboratory investigation | Mild influenza-like illness |
| Rattanaumpawan et al., 201032 | Non-randomized controlled trial | Thailand | 1 public university hospital | All prescribers | Persuasive | Audit and feedback to prescribers by infectious diseases specialist | NR |
| Long et al., 201118 | Randomized controlled trial | China | 1 university hospital | Emergency department physicians | Structural | Antibiotic initiation and discontinuation guided by serum procalcitonin level versus routine carea | Community-acquired pneumonia |
| Maravić-Stojković et al., 201120 | Randomized controlled trial | Serbia | 1 tertiary hospital | Cardiac surgery and intensive care unit staff | Structural | Antibiotic initiation guided by serum procalcitonin level versus routine care (based on clinical signs, C-reactive protein levels and leukocyte count) | Infections after coronary artery bypass grafting or valve surgery |
| Shen et al., 201133 | Cluster randomized controlled trial | China | 1 tertiary hospital | All prescribers of 2 pulmonary wards | Persuasive | Audit and feedback to prescribers by clinical pharmacist | Respiratory tract infections |
| Opondo et al., 201137 | Cluster randomized controlled trial | Kenya | 8 district hospitals | Nurses, clinical and medical officers | Bundle | Guidelines for treatment of non-bloody diarrhoea; education for prescribers; audit and feedback to prescribers on hospital performance | Non-bloody diarrhoea |
| Bucher et al., 201225 | Randomized controlled trial | Peru | 1 public hospital | Paediatric emergency department physicians | Structural | Antibiotic initiation guided by faecal rotavirus rapid test in combination with a faecal leukocyte test versus faecal leukocyte test only | Acute diarrhoea |
| Magedanz et al., 201241 | Interrupted time-series | Brazil | 1 university hospital | All prescribers of the cardiology department | Bundle | Restriction of certain antibiotics; audit and feedback to prescribers by (i) infectious diseases specialist and (ii) pharmacist | NR |
| Qu et al., 201224 | Randomized controlled trial | China | 1 municipal hospital | Intensive care unit staff | Structural | Antibiotic initiation and discontinuation guided by serum procalcitonin level versus standard 14 days of antibiotics | Severe acute pancreatitis |
| Ding et al., 201317 | Randomized controlled trial | China | 1 tertiary hospital | Respiratory ward physicians | Structural | Antibiotic initiation and discontinuation guided by serum procalcitonin level versus routine care (based on clinical experience, sputum bacteriology results and leukocyte count) | Acute exacerbation of idiopathic pulmonary fibrosis |
| Aiken et al., 201336 | Interrupted time-series | Kenya | 1 public referral hospital | Nursing, medical and operating theatre staff | Bundle | Guidelines on surgical antibiotic prophylaxis; clinician education; patient education posters; audit and feedback to prescribers | Surgical site infections |
| Oliveira et al., 201323 | Randomized controlled trial | Brazil | 2 public university hospitals | Intensive care unit staff | Structural | Antibiotic discontinuation guided by serum procalcitonin level versus C-reactive protein test | Sepsis or septic shock |
| Tang et al., 201321 | Randomized controlled trial | China | 1 university hospital | Emergency department physicians | Structural | Antibiotic initiation guided by serum procalcitonin level versus routine carea | Acute asthma exacerbation |
| Chandy et al., 201435 | Interrupted time-series | India | 1 private tertiary hospital | All prescribers | Enabling | Implementation and dissemination of antibiotic prescribing guidelines | NR |
| Long et al., 201419 | Randomized controlled trial | China | 1 university hospital | Emergency department physicians | Structural | Antibiotic initiation guided by serum procalcitonin level versus routine carea | Acute asthma exacerbation |
| Najafi et al., 201522 | Randomized controlled trial | Islamic Republic of Iran | 1 university hospital | Intensive care unit staff | Structural | Antibiotic initiation guided by serum procalcitonin level versus routine carea | Severe inflammatory response syndrome |
| Bao et al., 201542 | Interrupted time-series | China | 65 public hospitals (30 tertiary; 35 secondary) | All prescribers | Bundle | Implementation of a nationally imposed multifaceted antibiotic stewardship programme | NR |
| Sun et al., 201543 | Interrupted time-series | China | 15 public tertiary hospitals | All prescribers | Bundle | Implementation of a nationally imposed multifaceted antibiotic stewardship programme | NR |
| Gong et al., 201638 | Interrupted time-series | China | 1 tertiary paediatric hospital | Paediatricians | Bundle | Antibiotic guidelines and prescribing restrictions; audit and feedback to prescribers by pharmacists and infection control physicians; financial penalties according to number of noncompliant prescriptions | NR |
| Brink et al., 201629 | Interrupted time-series | South Africa | 47 private hospitals | Physicians, other clinical staff and managers | Persuasive | Audit and feedback to prescribers by a pharmacist | NR |
| Li et al., 201730 | Non-randomized controlled trial | China | 6 university hospitals | Physicians of 8 intensive care units | Persuasive | Audit and feedback to prescribers by a pharmacist versus no intervention | NR |
| Tuon et al., 201728 | Interrupted time-series | Brazil | 1 university hospital | All prescribers | Structural | Mobile phone application providing antibiotic prescribing guidance | NR |
| Wattal et al., 201731 | Interrupted time-series | India | 1 tertiary hospital | Surgeons of 45 units | Persuasive | Audit and feedback to prescribers; focus group discussions per specialty | NR |
NR: not reported; WHO: World Health Organization.
a The content of routine care was not specified.
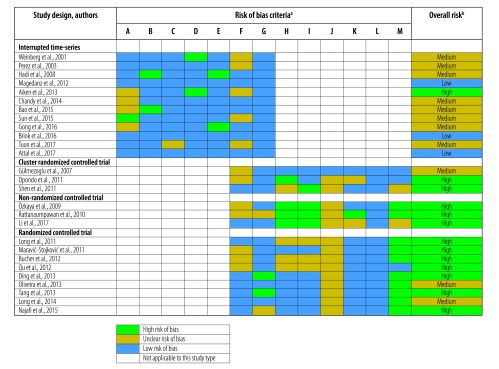
Risk of bias assessment
For the 12 interrupted time-series studies the risk of bias was low (3 studies), medium (8 studies) or high (1 study; Fig. 2). The main risks of bias were that the intervention was not independent of other changes (5 studies) and that incomplete data were not adequately addressed (5 studies). For the 15 (non)randomized trials the risk of bias was medium (3 studies) or high (12 studies). The main risks of bias included the absence of baseline outcome measurement (14 studies), lack of protection against contamination (prescribers could have been involved in treatment of both the intervention and control group; 11 studies), non-random or unclear randomization methods (8 studies) and incomplete data not being adequately addressed (7 studies).
Fig. 2.
Assessment of risk of bias in studies included in the review of antibiotic stewardship interventions in hospitals in low-and middle-income countries
a The criteria were: A: intervention independent of other changes; B: shape of intervention pre-specified; C: intervention unlikely to affect data collection; D: knowledge of allocated interventions adequately prevented during study; E: seasonality taken into account; F: incomplete outcome data adequately addressed; G: study free from selective outcome reporting; H: adequate allocation sequencing; I: adequate allocation concealment; J: baseline outcome measures similar; K: baseline characteristics similar; L: any blinding reported; M: study protected against contamination.
b The risk of bias was considered low if all criteria were scored as low, medium if one or two criteria were scored as medium or high, and high if more than two criteria were scored as medium or high.15
Structural interventions
Structural interventions were reported by 12 studies,17–28 eight of which were randomized controlled trials of the effect of using serum procalcitonin levels to guide antibiotic treatment (Table 2).17–24 Five of these studies reported antibiotic use as the outcome. All of them found a significant decrease in the percentage of patients receiving antibiotics in the procalcitonin group compared with routine care or C-reactive protein testing. RR ranged between 0.40 and 0.87.17–21 Five studies reported patient deaths as the outcome and found no significant effect of procalcitonin-guided antibiotic use on in-hospital or 30-day mortality.17,20,22–24
Table 2. Outcomes of interventions to improve appropriate prescribing and use of antibiotics in hospitals in low-and middle-income countries: controlled trials.
| Intervention type and study design | Study duration, weeks | No. of patients | Data summary | Outcome measure | Effect size | P |
|---|---|---|---|---|---|---|
| Structural intervention | ||||||
| Procalcitonin guidance | ||||||
| Randomized controlled trial18 | 201 | 172 | No. of patients receiving antibiotics: 72/86 in procalcitonin group; 79/86 in routine care group | RR of receiving antibiotic (95% CI) | 0.87 (0.79 to 0.96) | 0.01 |
| Randomized controlled trial20 | NR | 205 | No. of patients receiving antibiotics: 19/102 in procalcitonin group; 48/103 in routine care group | RR of receiving antibiotic (95% CI) | 0.40 (0.25 to 0.63) | 0.01 |
| No. of deaths: 3/102 in procalcitonin group; 3/103 in routine care group | RR of in-hospital death (95% CI) | 0.88 (0.33 to 2.35) | 0.80 | |||
| Randomized controlled trial17 | 154 | 78 | No. of patients receiving antibiotics: 26/39 in procalcitonin group; 35/39 in routine care group | RR of receiving antibiotic (95% CI) | 0.74 (0.58 to 0.95) | 0.01 |
| No. of deaths: 21/39 in procalcitonin group; 20/39 in routine care group | RR of death after 30 daysa (95% CI) | 1.11 (0.76 to 1.64) | 0.42 | |||
| Randomized controlled trial24 | 133 | 71 | No. of deaths: 7/35 in procalcitonin group; 8/36 in standard 14 days of antibiotics group | RR of in-hospital death (95% CI) | 0.90 (0.37 to 2.22) | 0.99 |
| Randomized controlled trial23 | 141 | 97 | No. of deaths: 21/50 in procalcitonin group; 21/47 in routine care group | RR of in-hospital death (95% CI) | 0.92 (0.59 to 1.44) | 0.84 |
| Randomized controlled trial21 | 283 | 265 | No. of patients receiving antibiotics: 59/132 in procalcitonin group; 95/133 in routine care group | RR of receiving antibiotic (95% CI) | 0.63 (0.50 to 0.78) | 0.01 |
| Randomized controlled trial19 | 133 | 180 | No. of patients receiving antibiotics: 44/90 in procalcitonin group; 79/90 in routine care group | RR of receiving antibiotic (95% CI) | 0.56 (0.44 to 0.70) | 0.01 |
| Randomized controlled trial22 | 52 | 60 | No. of deaths: 5/30 in procalcitonin group; 4/30 in routine care group | RR of in-hospital death (95% CI) | 1.25 (0.37 to 4.21) | 0.71 |
| Rapid diagnostic testing | ||||||
| Non-randomized controlled trial26 | 21 | 97 | No. of patients receiving antibiotics: 34/50 in influenza rapid diagnostic test group; 47/47 in routine care group | RR of receiving antibiotic (95% CI) | 0.68 (0.56 to 0.82) | 0.01 |
| Randomized controlled trial25 | 26 | 201 | No. of patients receiving antibiotics: 29/100 in faecal leukocyte + rotavirus rapid test group; 50/101 in faecal leukocyte test only group | RR of receiving antibiotic (95% CI) | 0.59 (0.41 to 0.84) | 0.03 |
| Library access plus workshops | ||||||
| Cluster randomized controlled trial27 | 43 to 52b | 1000 to 1022 per hospital | Mean % of operations with antibiotic prophylaxis: Mexico: 25.8 in intervention group; 6.5 in control group Thailand: 26.0 in intervention group; 14.7 in control group |
% of operations with antibiotic prophylaxis: difference in adjusted rate (95% CI) | Mexico: 19 (−8 to 46) | 0.12 |
| Thailand: 5 (−18 to 27) | 0.66 | |||||
| Persuasive intervention | ||||||
| Audit and feedback on individual patient cases | ||||||
| Non-randomized controlled trial32 | 17 | 948 | Mean no. of days of hospitalization: 30.4 in intervention group; 30.7 in control group | Mean difference in hospital length of stay (95% CI), days | −0.3 (−3.3 to −3.0) | 0.80 |
| Mean no. of days of treatment: 12.7 in intervention group; 16.4 in control group | Mean difference in treatment duration, days | −3.7 (−5.2 to −2.2) | 0.01 | |||
| Cluster randomized controlled tria33 | 43 | 436 | Mean no. of days of hospitalization: 14.2 in intervention group; 15.8 in control group | Mean difference in hospital length of stay (95% CI), days | −1.6 (−2.9 to −0.3) | 0.03 |
| Non-randomized controlled trial30 | 9 | 874 | Median no. of days of treatment: 4.0 in intervention group; 5.0 in control group | Difference in median no. of days of treatment | 1.0 | 0.03 |
| Intervention bundle | ||||||
| Treatment guidelines plus education plus audit and feedback | ||||||
| Cluster randomized controlled trial37 | 77 | 1160 | No. of patients receiving antibiotics for inappropriate indication: 313/594 in intervention group; 437/566 in control group | Absolute risk reduction for receiving antibiotic for inappropriate indication (95% CI) | 41 (−6 to 88) | 0.08 |
CI: confidence interval; DDD: defined daily doses; NR: not reported; RR: relative risk.
a Per protocol analysis.
b Different collection periods in different hospitals.
Note: Intention-to-treat analysis results are reported unless indicated otherwise. When significant P-values were not specified, we assumed P < 0.05 as significant.
A non-randomized controlled trial among 97 patients in a Turkish emergency department studied the effect of introducing a rapid diagnostic test for influenza-like disease.26 A lower percentage of tested patients were prescribed antibiotics compared with patients given clinical examination only (RR: 0.68; 95% CI: 0.56 to 0.82). In a randomized controlled trial among 201 patients in a Peruvian emergency department, use of a rapid test for rotavirus was associated with fewer patients receiving antibiotics (RR: 0.59; 95% CI: 0.41 to 0.84).25
In a cluster-randomized controlled trial in Mexico and Thailand health-care staff were given access to the WHO’s online Reproductive Health Library and workshops on its use.27 Thereafter, it was left open to the 22 participating hospitals whether certain activities, including antibiotic stewardship, were implemented. After 10‒12 months, no significant difference was found in the proportion of caesarean sections in which antibiotic prophylaxis was given, when comparing the 22 intervention hospitals to the 18 control hospitals (difference in adjusted rate in Mexico was 19.0%; 95% CI: −8.0 to 46.0% and in Thailand was 4.6%; 95% CI: −17.7 to 26.9%).
One interrupted time-series study evaluated the implementation of an antibiotic treatment guide through a free-of-charge mobile application (Table 3). Twenty-four months after the intervention there were significant increases in the defined daily doses per 1000 bed-days of recommended antibiotics (amikacin and cefepime) and a significant decrease in non-recommended antibiotics (piperacillin; P = 0.02). Use of other non-recommended antibiotics (meropenem, ciprofloxacin and polymyxin) did not decrease significantly.28
Table 3. Outcomes of interventions to improve appropriate prescribing and use of antibiotics in hospitals in low-and middle-income countries: interrupted time-series studies.
| Intervention | Study segments (duration in weeks) | No. of data points per segment (no. of observations per data point) | Outcome measure | Effect sizea | P |
|---|---|---|---|---|---|
| Structural interventions | |||||
| Mobile phone application28 | S1: Pre-intervention (52) | 12 (NR) | DDD per 1000 bed-days | Baseline trend NR | N/A |
| S2: Post-intervention (52) | 12 (NR) | Trend increased for amikacinb | 0.02 | ||
| Trend increased for cefepimeb | 0.01 | ||||
| Trend decreased for piperacillinb | 0.02 | ||||
| Trend decreased for meropenemb | 0.44 | ||||
| Trend decreased for polymyxinb | 0.34 | ||||
| Trend decreased for ciprofloxacinb | 0.08 | ||||
| Persuasive interventions | |||||
| Audit and feedback on individual patient cases29 | S1: Pre-intervention (70) | 16 (NR) | DDD per 100 bed-days | Baseline level NR | N/A |
| Baseline trend +0.064/month | 0.62 | ||||
| S2: Implementation (104) | 24 (NR) | Level change NR | N/A | ||
| Trend change −0.56/month | 0.01 | ||||
| S3: Post-intervention (86) | 20 (NR) | Level change NR | N/A | ||
| Trend change −0.20/month | 0.05 | ||||
| Audit and feedback at department level31 | S1: Pre-intervention (52) | 12 (NR) | DDD per 100 bed-days | Baseline level: NR | N/A |
| Baseline trend: increasing in 1/35 wardsb | 0.05 | ||||
| S2: Post-intervention (13) | 3 (NR) | Level decreased in 3/35 wardsb | 0.05 | ||
| Enabling interventions | |||||
| Treatment guidelines34 | S1: Pre-intervention (16) | 9 (14) | DDD per 100 bed-days | Baseline level: NR | N/A |
| Baseline trend: −1.0 per 14 days | 0.53 | ||||
| S2: Guideline development (14) | 6 (14) | Level change: −31.9 | 0.03 | ||
| Trend change +2.1 per 14 days | 0.52 | ||||
| S3: Guideline declaration (8) | 4 (26) | Level change: −29.2 | 0.11 | ||
| Trend change: −9.5 per 14 days | 0.14 | ||||
| S4: Teaching sessions (8) | 4 (27) | Level change: +38.2 | 0.05 | ||
| Trend change: +10.0 per 14 days | 0.21 | ||||
| S5: Refresher course (8) | 5 (15) | Level change: −2.4 | 0.88 | ||
| Trend change: −9.8 per 14 days | 0.15 | ||||
| Treatment guidelines35 | S1: Pre-intervention (86) | 20 (NR) | DDD per 100 bed-days | Baseline level: 56.9 | N/A |
| Baseline trend: +0.95 per month | 0.01 | ||||
| S2: Guideline preparation and booklet dissemination (94) | 22 (NR) | Level change: NR | N/A | ||
| Trend change: +0.21 per month | 0.03 | ||||
| S3: No new intervention (104) | 24 (NR) | Level change: NR | N/A | ||
| Trend change: +0.31 per month | 0.01 | ||||
| S4: Guideline revision and booklet dissemination (104) | 24 (NR) | Level change: NR | N/A | ||
| Trend change: +0.05 per month | 0.64 | ||||
| S5: Guideline revision and booklet with electronic dissemination (86) | 20 (NR) | Level change: NR | N/A | ||
| Trend change: −0.37 per month | 0.01 | ||||
| Intervention bundles | |||||
| Treatment guidelines plus structural changes39 | Hospital A | ||||
| S1: Pre-intervention (13) | 3 (308) | % of operations with surgical site infection | Baseline level: 13.9 | N/A | |
| Baseline trend: NRc | NR | ||||
| S2: Guideline introduction with structural changes (30) | 7 (272) | Level change: −9.8 | 0.01 | ||
| Trend change: NRc | NR | ||||
| S3: Post-intervention (21) | 5 (217) | Level change: NRc | NR | ||
| Trend change: NRc | NR | ||||
| Hospital A | |||||
| S1: Pre-intervention (13) | 3 (308) | % of caesarean sections with administration of antibiotic prophylaxis | Baseline level: 47.5 | N/A | |
| Baseline trend: NRc | NR | ||||
| S2: Guideline introduction with structural changes (30) | 7 (272) | Level change: +31.6 | 0.01 | ||
| Trend change: NRc | NR | ||||
| S3: Post-intervention (21) | 5 (217) | Level change: −4.9 | 0.01 | ||
| Trend change: NRc | NR | ||||
| Hospital B: | |||||
| S1: Pre-intervention (13) | 3 (396) | % of caesarean sections with administration of antibiotic prophylaxis | Baseline level: 5.1 | N/A | |
| Baseline trend: NRc | NR | ||||
| S2: Guideline introduction with structural changes (39) | 9 (1026) | Level change: NRc | NR | ||
| Trend change: +5.4 per month | 0.01 | ||||
| S3: Post-intervention (52) | 12 (709) | Level change: +7.1 | 0.05 | ||
| Trend change: −4.1 | 0.01 | ||||
| Hospital A | |||||
| S1: Pre-intervention (13) | 3 (308) | % of caesarean sections with administration of antibiotic prophylaxis within 1 hour of delivery | Baseline level: 32.5 | N/A | |
| Baseline trend: NRc | NR | ||||
| S2: Guideline introduction with structural changes (30) | 7 (272) | Level change: 62.2 | 0.01 | ||
| Trend change: NRc | 0.01 | ||||
| S3: Post-intervention (21) | 5 (217) | Level change: NRc | NR | ||
| Trend change: NRc | NR | ||||
| Hospital B | |||||
| S1: Pre-intervention (13) | 3 (396) | % of caesarean sections with administration of antibiotic prophylaxis within 1 hour of delivery | Baseline level: 30.8 | N/A | |
| Baseline trend: +18.4 per month | 0.01 | ||||
| S2: Guideline introduction with structural changes (39) | 9 (1026) | Level change: NRc | NR | ||
| Trend change: −18.7 per month | 0.01 | ||||
| S3: Post-intervention (52) | 12 (709) | Level change: +15.2 | NR | ||
| Trend change: NRc | NR | ||||
| Prescription form plus education plus reminders40 | S1: Pre-intervention (103) | 103 (NR) | % of operations with incorrect timing of antibiotic prophylaxis | Baseline level: NR | N/A |
| Baseline trend: NR | N/A | ||||
| S2: Post-intervention (42) | 42 (NR) | Level change: −20 | 0.01 | ||
| Trend change: NR | NR | ||||
| Antibiotic restrictions plus audit and feedback41 | S1: Pre-intervention (129) | 30 (NR) | Antibiotic use, DDD per 100 bed-days | Baseline level: NR | N/A |
| Baseline trend +1.2 per month | 0.01 | ||||
| S2: Antibiotic restrictions plus audit and feedback by infectious diseases specialist (94) | 22 (NR) | Level change: −1.3 | 0.8 | ||
| Trend change: −2.7 per month | 0.01 | ||||
| S3: Antibiotic restrictions plus audit and feedback by pharmacist (86) | 20 (NR) | Level change: +4.7 | 0.4 | ||
| Trend change: +1.2 per month | 0.01 | ||||
| Treatment guidelines plus education plus audit and feedback36 | Timing study | ||||
| S1: Pre-intervention (26) | 26 (NR) | % of operations with incorrect timing of antibiotic prophylaxis | Baseline level: 99% | N/A | |
| Baseline trend: NR | N/A | ||||
| S2: Post-intervention (40) | 40 (NR) | Level decreasedb | 0.01 | ||
| Trend decreasedb | 0.01 | ||||
| Infection study | |||||
| S1: Pre-intervention (26) | 6 (223) | % of operations with surgical site infection | Baseline level: NR | N/A | |
| Baseline trend: −0.5 per month | 0.49 | ||||
| S2: Post-intervention (39) | 9 (223) | Level change: NR | 0.05 | ||
| Trend change: −0.7 per month | 0.03 | ||||
| Multifaceted antibiotic stewardship programme42 | Outcome A | ||||
| S1: Pre-intervention (52) | 12 (NR) | % of patients receiving antibiotic | Baseline level: NR | N/A | |
| Baseline trend +0.3 per month | > 0.05 | ||||
| S2: Implementation (52) | 12 (NR) | Level change: −2.3 | > 0.05 | ||
| Trend change: −2.3 per month | 0.01 | ||||
| S3: Post-intervention (104) | 24 (NR) | Level change: −2.7 | 0.05 | ||
| Trend change: +1.9 per month | 0.01 | ||||
| Outcome B | |||||
| S1: Pre-intervention (52) | 12 (NR) | Antibiotic use, DDD per 100 bed-days | Baseline level: NR | N/A | |
| Baseline trend: −0.4 per month | 0.2 | ||||
| S2: Implementation (52) | 12 (NR) | Level change: +2.8 | > 0.05 | ||
| Trend change: −2.2 per month | 0.01 | ||||
| S3: Post-intervention (104) | 24 (NR) | Level change: −7.1 | 0.01 | ||
| Trend change: +2.4 per month | 0.01 | ||||
| Multifaceted antibiotic stewardship programme43 | S1: Pre-intervention (334) | 26 (58) | % of patients receiving antibiotic | Baseline level: 74.7 | N/A |
| Baseline trend: −0.3 per quarter | 0.01 | ||||
| S2: Post-intervention (78) | 6 (750) | Level change: −7.3 | 0.04 | ||
| Trend change: −1.5 per quarter | 0.07 | ||||
| Treatment guidelines plus antibiotic restrictions plus audit and feedback38 | S1: Pre-intervention (17) | 4 (375 985) | % of patients receiving antibiotic | Baseline level: 59.0 | N/A |
| Baseline trend: −3.0 per month | 0.01 | ||||
| S2: Guidelines and restrictions (21) | 5 (424 702) | Level change: +3.0 | 0.2 | ||
| Trend change: −0.4 per month | 0.6 | ||||
| S3: Financially punished audit and feedback (60) | 14 (446 727) | Level change: −9.0 | 0.01 | ||
| Trend change: +3.0 per month | 0.01 |
DDD: defined daily doses; N/A: not applicable; NR: not reported; RR: relative risk; S: segment.
a In interrupted times-series studies the linear curve which summarizes the outcome data in each study segment can be defined by its level (y-intercept) and trend (slope). Level change reflects the difference of the level of the current segment compared with the level of the previous segment. Trend change reflects the difference of the trend of the current segment compared to the trend of the previous segment.
b The authors reported no values for level or trend changes.
c The authors reported that there were no significant changes but with no values for levels or trend changes.
Persuasive interventions
Four studies evaluated the effect of audit and feedback to prescribers on individual patient cases by pharmacists (3 studies) or infectious diseases specialists (1 study):29,30,32,33 A non-randomized controlled trial including 577 patients in eight intensive care units reported a decrease of duration of antibiotic treatment of −1.0 day (P = 0.03) (Table 2).30 Another non-randomized controlled trial of 948 patients in a public university hospital reported a decrease of duration on antibiotic treatment of −3.7 days (P < 0.01) and a decrease in mean length of hospital stay of −1.6 days (P = 0.03).32 A cluster randomized trial found no significant difference in mean length of hospital stay among 436 patients (0.3 days; P = 0.8).33 An interrupted time-series study in 47 private hospitals in South Africa found a decreasing trend of antibiotic use during the implementation phase of the intervention (−0.56 defined daily doses per 100 bed-days per month; P < 0.01; Table 3).29 The trend was sustained in the 20 months post-implementation (−0.20 defined daily doses per 100 bed-days per month; P < 0.05).
An interrupted time-series study evaluated the effect of audit and feedback at the departmental level in 35 surgical wards. Three months after the intervention a significant decrease in defined daily doses per 100 bed-days was reported in 3 out of 35 wards (immediate decreases of −66.5%, −46.1% and −26.4% respectively; P < 0.05).31
Enabling interventions
Two interrupted time-series studied the effect of enabling interventions on antibiotic prescribing (Table 3).34,35 A study in an Indonesian hospital subsequently studied the development of treatment guidelines which were officially presented, followed by education and then refresher education. The authors reported a significant decrease of −31.9 defined daily doses per 100 bed-days (P = 0.03) after guideline development and a significant increase of +38.2 defined daily doses per 100 bed-days (P < 0.05) after education. The net effect of the intervention remains unclear.34 Another study in an Indian hospital evaluated the effect of an antibiotic policy guideline which was first developed and introduced, then revised and made available as booklet and lastly revised and made available through the intranet. The authors initially reported a baseline rising trend in antibiotic use of +0.95 defined daily doses per 100 bed-days per month (P < 0.01) which levelled off after the first two interventions and declined by −0.37 defined daily doses per 100 bed-days per month (P < 0.01) after the last intervention.35
Intervention bundles
Eight studies evaluated bundles combining different interventions.36–43 A cluster randomized controlled trial in eight Kenyan hospitals compared a bundle containing guidelines, education and face-to-face feedback to prescribers with a similar, but less intensive bundle (fewer hours of training, written feedback; Table 2).37 Comparing prescriptions for 594 children in intervention hospitals and 566 children in control hospitals showed that the intensive bundle was associated with a non-significant absolute risk reduction in inappropriate use of antibiotics for non-bloody diarrhoea of 41% (95% CI: −6 to 88%).
The other seven studies all used an interrupted time-series design (Table 3). One study in two Colombian hospitals implemented antibiotic prophylaxis guidelines for caesarean sections, immediate availability of antibiotics in the operating theatre and feedback to surgeons about surgical site infections.39 The study reported a significant increase in the percentage of caesarean section births in which prophylaxis was administered (immediate increase by +31.6% in hospital A; P < 0.01 and gradual increase by +5.4% per month in hospital B; P < 0.01), an increase in antibiotic administration within 1 hour of delivery (immediate increase by 62.2% in hospital A only; P < 0.01) and a significant decrease in the monthly rate of surgical site infections with 9.8% (P < 0.01) in hospital A.
In another study in a Kenyan hospital, surgical antibiotic prophylaxis guidelines were implemented, combined with training, personal feedback to prescribers and patient information posters.36 The proportion of operations with incorrect timing of antibiotic prophylaxis significantly decreased (no values reported) and the percentage of surgical site infections decreased after the intervention by −0.7% per month (P = 0.03).
Another Colombian study introduced an antibiotic prescription form containing a list of restricted antibiotics with information on dosing intervals and an educational campaign.40 The study found a decrease of 20% (P < 0.01) in the proportion of operations with incorrect timing of surgical prophylaxis.
In a Chinese study, guidelines and antibiotic restrictions were introduced, followed by individual prescriber audit and feedback, with financial penalties and revocation of prescribing privileges in case of non-compliance.38 Before the intervention the proportion of patients on antibiotic treatment was decreasing significantly by −3% per month from a baseline level of 59% (P = 0.01). After the first intervention, no significant changes were reported. After the second intervention, a sudden drop of −9% (P = 0.01) was observed, followed by a steady increase of +3% per month (P = 0.01) in the next 14 months. The net effect of the intervention bundle remains unclear.
A study in a Brazilian cardiology hospital first introduced restriction of certain antibiotics with individual audit and feedback to prescribers by an infectious diseases specialist and subsequently more comprehensive audit and feedback by a pharmacist. Before the intervention, the total antibiotic consumption significantly increased during 30 months (+1.2 defined daily doses per 100 bed-days per month; P < 0.01). This trend decreased after the first intervention (−2.7 per month; P < 0.01) and increased after the second (+1.2 per month; P < 0.01). The net effect of the intervention bundle remains unclear.41
Two Chinese studies looked at the implementation of a multifaceted national antibiotic stewardship programme, containing structural changes, antibiotic restriction, education, guidelines, and audit and feedback, in 65 and 15 secondary and tertiary public hospitals respectively.42,43 Participation was compulsory and financial punishment for hospitals and disciplinary actions for individual prescribers could be imposed. Both studies reported a significant decrease in antibiotic use after the intervention. One study reported a decreasing trend of −2.2 defined daily doses per 100 bed-days per month (P < 0.01).42 The other study reported a decrease in the proportion of patients receiving antibiotics (−7.3%; P = 0.04).43
Discussion
In this systematic review the majority of the included studies reported a positive effect of antibiotic stewardship interventions for hospitalized patients. This is in line with previously published systematic reviews on stewardship interventions in hospitals, which did not focus specifically on low- and middle-income countries.8–10 However, we cannot make general recommendations to guide the selection of antibiotic stewardship interventions due to limitations of the included studies, including the low quality of methods, variations and shortcomings in outcome reporting, under-representation of certain settings, heterogeneity of the interventions and variations in implementation strategy.
When screening titles and abstracts, we found 153 articles that reported on stewardship activities in a hospital setting, but 126 of those were excluded because of the study design (mainly bias-prone uncontrolled before‒after studies). So, although antibiotic stewardship is taking place and is being studied in low- and middle-income countries, most studies fall short methodologically. The studies we did include were also generally of low quality. For those with a randomized study design, a major risk of bias was contamination, meaning that prescribers could be involved in treatment of both the intervention and control groups. Because it may not be feasible to randomize individual prescribers, wards or hospitals to overcome this bias, interrupted time-series design has been recommended as an alternative. In interrupted time-series, data are collected continuously, and trends and outcome levels are compared before and after the intervention. To minimize bias and confounding, interrupted-time-series should meet certain requirements: a minimum of 12 data points before and after intervention, 100 observations per data point and the use of analytic techniques or models.45 These requirements were seldom met by the included studies. Poor quality of methods is a consistent theme among reviews of antibiotic stewardship in countries of all income levels and this issue needs to be addressed to strengthen the evidence base.8,9,46
Many of the included studies focused on a quantitative reduction in antibiotic prescribing. However, stewardship is not merely concerned with a reduction in antibiotic use, but in finding the balance between the potency of antibiotics and their potentially hazardous effects. The goal is to improve patient outcomes, decrease antibiotic resistance and increase cost‒effectiveness of care. Therefore, it is recommended that clinical outcomes (including adverse events), microbiological and cost‒effectiveness outcomes are reported in all stewardship studies.8,47 Most of the studies included in this review failed to do so. There is an ongoing debate about which parameters should be reported to accurately reflect the above-mentioned outcomes.48,49 This generally leads to a wide variety of reported parameters, as we observed in our review. This lack of uniformity limits comparison and aggregation of data. Also, for low- and middle-income settings, the measurement of certain clinical or microbiological outcomes, for example infection with Clostridium difficile, may be challenging if not impossible. Defining feasible outcome measures that can be uniformly applied in low- and middle-income countries should be prioritized. In the meantime, parameters that are easy to assess, such as mortality or hospital length of stay, should be reported by every stewardship study.
The majority of studies were performed in tertiary care centres in urban areas in middle-income countries, which limits the generalizability of the results. Large differences exist in terms of resources, organization, prescription practices and financing between countries and between facilities within countries.11 The intervention most frequently studied in our review was the implementation of procalcitonin testing. Although this intervention showed promising results, it may not be feasible to implement in many health-care settings in low- and middle-income countries. In addition, good quality evidence from non-tertiary or rural hospitals in low-income countries is lacking. Studies focusing on these settings should therefore be prioritized.
The effectiveness of the interventions varied across the studies, even those that implemented similar interventions. This is likely due to differences in the intervention or the implementation strategy, which may have been adapted to fit local circumstances. A detailed description of the intervention and the implementation strategy is therefore mandatory to interpret the study findings. Stewardship interventions in hospitals usually aim to change individual prescriber’s behaviour. This behaviour is influenced by social norms, attitudes and beliefs.50 These are therefore important determinants of the effectiveness of the intervention and should be an integral part of studies of stewardship interventions. For this reason, collaboration with behavioural scientists has been recommended.46 None of the included studies reported behaviour determinants.
Our review has several limitations. We defined a broad search strategy, allowing different settings, participants, interventions and outcomes to be included. This strategy provides a good overview of what evidence is available, but limits the generalizability of the findings. Moreover, to ensure the validity of the results, studies had to fulfil high methodological standards to be included. This led to discarding numerous lower quality studies. Also, we did not include studies that only reported cost (effectiveness) as an outcome, as these require a different analysis model. Lastly, due to publication bias (not reporting negative results) and language restrictions we may have missed certain studies.
We conclude that, based on the currently available evidence, general recommendations regarding the effectiveness of antibiotic stewardship interventions in low- and middle-income countries cannot be made. As many hospitals in low- and middle-income countries are setting up antibiotic stewardship programmes, what should be the way forward? On the basis of our findings, we suggest the following actions should be prioritized to strengthen the evidence base: (i) provision of methodological and statistical support for commonly used, complex study designs such as interrupted-time-series; (ii) seeking consensus on relevant and feasible outcome measurements for low- and middle-income countries; (iii) performing methodologically solid studies in settings such as non-tertiary, rural and public hospitals in low-income countries; and (iv) accurate descriptions of interventions, implementation strategies and inclusion of behavioural aspects. While awaiting the effect of these actions, the current lack of evidence should not prevent health-care workers from engaging in stewardship. Evidence and examples both from high- and low-and middle-income countries can inspire and provide guidance in the meantime.8–11
Acknowledgments
The authors thank Tine Verdonck, Johan van Griensven, Kristien Wouters and Jan Jacobs.
Funding:
Janneke Cox received unrestricted funding from the Baillet-Latour fund.
Competing interests:
None declared.
References
- 1.Holmes AH, Moore LS, Sundsfjord A, Steinbakk M, Regmi S, Karkey A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. 2016. January 9;387(10014):176–87. 10.1016/S0140-6736(15)00473-0 [DOI] [PubMed] [Google Scholar]
- 2.Antimicrobial resistance: global report on surveillance. Geneva: World Health Organization; 2014. [Google Scholar]
- 3.Leopold SJ, van Leth F, Tarekegn H, Schultsz C. Antimicrobial drug resistance among clinically relevant bacterial isolates in sub-Saharan Africa: a systematic review. J Antimicrob Chemother. 2014. September;69(9):2337–53. 10.1093/jac/dku176 [DOI] [PubMed] [Google Scholar]
- 4.Lestari ES, Severin JA, Verbrugh HA. Antimicrobial resistance among pathogenic bacteria in Southeast Asia. Southeast Asian J Trop Med Public Health. 2012. March;43(2):385–422. [PubMed] [Google Scholar]
- 5.Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, et al. Antibiotic resistance-the need for global solutions. Lancet Infect Dis. 2013. December;13(12):1057–98. 10.1016/S1473-3099(13)70318-9 [DOI] [PubMed] [Google Scholar]
- 6.The evolving threat of antimicrobial resistance: options for action. Geneva: World Health Organization; 2012. [Google Scholar]
- 7.Global action plan on antimicrobial resistance. Geneva: World Health Organization; 2015. [DOI] [PubMed] [Google Scholar]
- 8.Davey P, Marwick CA, Scott CL, Charani E, McNeil K, Brown E, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2017. February 9;2:CD003543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuts EC, Hulscher MEJL, Mouton JW, Verduin CM, Stuart JWTC, Overdiek HWPM, et al. Current evidence on hospital antimicrobial stewardship objectives: a systematic review and meta-analysis. Lancet Infect Dis. 2016. July;16(7):847–56. 10.1016/S1473-3099(16)00065-7 [DOI] [PubMed] [Google Scholar]
- 10.Honda H, Ohmagari N, Tokuda Y, Mattar C, Warren DK. Antimicrobial stewardship in inpatient settings in the Asia Pacific Region: a systematic review and meta-analysis. Clin Infect Dis. 2017. May 15;64 suppl_2:S119–26. 10.1093/cid/cix017 [DOI] [PubMed] [Google Scholar]
- 11.Cox JA, Vlieghe E, Mendelson M, Wertheim H, Ndegwa L, Villegas MV, et al. Antibiotic stewardship in low- and middle-income countries: the same but different? Clin Microbiol Infect. 2017. November;23(11):812–8. 10.1016/j.cmi.2017.07.010 [DOI] [PubMed] [Google Scholar]
- 12.Howard P, Pulcini C, Levy Hara G, West RM, Gould IM, Harbarth S, et al. ; ESCMID Study Group for Antimicrobial Policies (ESGAP); ISC Group on Antimicrobial Stewardship. An international cross-sectional survey of antimicrobial stewardship programmes in hospitals. J Antimicrob Chemother. 2015. April;70(4):1245–55. [DOI] [PubMed] [Google Scholar]
- 13.Van Dijck C, Cox JA, Vlieghe E. The impact of antibiotic stewardship interventions in hospitalized patients in low- and middle- income countries: a systematic literature review. PROSPERO International prospective register of systematic reviews. [internet]. York: University of York; 2016. Available from: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42016042019 [cited 2018 Jan 3]. [Google Scholar]
- 14.World Bank country and lending groups [internet]. Washington: World Bank; 2016. Available from: http://data.worldbank.org/about/country-and-lending-groups [cited 2016 Jan 24].
- 15.Suggested risk of bias criteria for EPOC reviews [internet]. London: Cochrane; 2017. Available from: http://epoc.cochrane.org/epoc-specific-resources-review-authors [cited 2017 Dec 17].
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009. July 21;6(7):e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding J, Chen Z, Feng K. Procalcitonin-guided antibiotic use in acute exacerbations of idiopathic pulmonary fibrosis. Int J Med Sci. 2013. May 20;10(7):903–7. 10.7150/ijms.4972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long W, Deng X, Zhang Y, Lu G, Xie J, Tang J. Procalcitonin guidance for reduction of antibiotic use in low-risk outpatients with community-acquired pneumonia. Respirology. 2011. July;16(5):819–24. 10.1111/j.1440-1843.2011.01978.x [DOI] [PubMed] [Google Scholar]
- 19.Long W, Li LJ, Huang GZ, Zhang XM, Zhang YC, Tang JG, et al. Procalcitonin guidance for reduction of antibiotic use in patients hospitalized with severe acute exacerbations of asthma: a randomized controlled study with 12-month follow-up. Crit Care. 2014. September 5;18(5):471. 10.1186/s13054-014-0471-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maravić-Stojković V, Lausević-Vuk L, Jović M, Ranković A, Borzanović M, Marinković J. Procalcitonin-based therapeutic strategy to reduce antibiotic use in patients after cardiac surgery: a randomized controlled trial. Srp Arh Celok Lek. 2011. Nov-Dec;139(11-12):736–42. 10.2298/SARH1112736M [DOI] [PubMed] [Google Scholar]
- 21.Tang J, Long W, Yan L, Zhang Y, Xie J, Lu G, et al. Procalcitonin guided antibiotic therapy of acute exacerbations of asthma: a randomized controlled trial. BMC Infect Dis. 2013. December 17;13(1):596. 10.1186/1471-2334-13-596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Najafi A, Khodadadian A, Sanatkar M, Shariat Moharari R, Etezadi F, Ahmadi A, et al. The comparison of procalcitonin guidance administer antibiotics with empiric antibiotic therapy in critically ill patients admitted in intensive care unit. Acta Med Iran. 2015;53(9):562–7. [PubMed] [Google Scholar]
- 23.Oliveira CF, Botoni FA, Oliveira CR, Silva CB, Pereira HA, Serufo JC, et al. Procalcitonin versus C-reactive protein for guiding antibiotic therapy in sepsis: a randomized trial. Crit Care Med. 2013. October;41(10):2336–43. 10.1097/CCM.0b013e31828e969f [DOI] [PubMed] [Google Scholar]
- 24.Qu R, Ji Y, Ling Y, Ye CY, Yang SM, Liu YY, et al. Procalcitonin is a good tool to guide duration of antibiotic therapy in patients with severe acute pancreatitis. A randomized prospective single-center controlled trial. Saudi Med J. 2012. April;33(4):382–7. [PubMed] [Google Scholar]
- 25.Bucher A, Rivara G, Briceño D, Huicho L. [Use of a rapid rotavirus test in prescription of antibiotics in acute diarrhea in pediatrics: an observational, randomized, controlled study]. Rev Gastroenterol Peru. 2012. Jan-Mar;32(1):11–5. Spanish. [PubMed] [Google Scholar]
- 26.Özkaya E, Cambaz N, Coşkun Y, Mete F, Geyik M, Samanci N. The effect of rapid diagnostic testing for influenza on the reduction of antibiotic use in paediatric emergency department. Acta Paediatr. 2009. October;98(10):1589–92. 10.1111/j.1651-2227.2009.01384.x [DOI] [PubMed] [Google Scholar]
- 27.Gülmezoglu AM, Langer A, Piaggio G, Lumbiganon P, Villar J, Grimshaw J. Cluster randomised trial of an active, multifaceted educational intervention based on the WHO Reproductive Health Library to improve obstetric practices. BJOG. 2007. January;114(1):16–23. 10.1111/j.1471-0528.2006.01091.x [DOI] [PubMed] [Google Scholar]
- 28.Tuon FF, Gasparetto J, Wollmann LC, Moraes TPD. Mobile health application to assist doctors in antibiotic prescription – an approach for antibiotic stewardship. Braz J Infect Dis. 2017. Nov-Dec;21(6):660–4. 10.1016/j.bjid.2017.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brink AJ, Messina AP, Feldman C, Richards GA, Becker PJ, Goff DA, et al. ; Netcare Antimicrobial Stewardship Study Alliance. Antimicrobial stewardship across 47 South African hospitals: an implementation study. Lancet Infect Dis. 2016. September;16(9):1017–25. 10.1016/S1473-3099(16)30012-3 [DOI] [PubMed] [Google Scholar]
- 30.Li Z, Cheng B, Zhang K, Xie G, Wang Y, Hou J, et al. Pharmacist-driven antimicrobial stewardship in intensive care units in East China: a multicenter prospective cohort study. Am J Infect Control. 2017. September 1;45(9):983–9. 10.1016/j.ajic.2017.02.021 [DOI] [PubMed] [Google Scholar]
- 31.Wattal C, Khanna S, Goel N, Oberoi JK, Rao BK. Antimicrobial prescribing patterns of surgical speciality in a tertiary care hospital in India: role of persuasive intervention for changing antibiotic prescription behaviour. Indian J Med Microbiol. 2017. Jul-Sep;35(3):369–75. [DOI] [PubMed] [Google Scholar]
- 32.Rattanaumpawan P, Sutha P, Thamlikitkul V. Effectiveness of drug use evaluation and antibiotic authorization on patients’ clinical outcomes, antibiotic consumption, and antibiotic expenditures. Am J Infect Control. 2010. February;38(1):38–43. 10.1016/j.ajic.2009.04.288 [DOI] [PubMed] [Google Scholar]
- 33.Shen J, Sun Q, Zhou X, Wei Y, Qi Y, Zhu J, et al. Pharmacist interventions on antibiotic use in inpatients with respiratory tract infections in a Chinese hospital. Int J Clin Pharm. 2011. December;33(6):929–33. 10.1007/s11096-011-9577-z [DOI] [PubMed] [Google Scholar]
- 34.Hadi U, Keuter M, van Asten H, van den Broek P; Study Group ‘Antimicrobial resistance in Indonesia: Prevalence and Prevention’ (AMRIN). Optimizing antibiotic usage in adults admitted with fever by a multifaceted intervention in an Indonesian governmental hospital. Trop Med Int Health. 2008. July;13(7):888–99. 10.1111/j.1365-3156.2008.02080.x [DOI] [PubMed] [Google Scholar]
- 35.Chandy SJ, Naik GS, Charles R, Jeyaseelan V, Naumova EN, Thomas K, et al. The impact of policy guidelines on hospital antibiotic use over a decade: a segmented time series analysis. PLoS One. 2014. March 19;9(3):e92206. 10.1371/journal.pone.0092206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aiken AM, Wanyoro AK, Mwangi J, Juma F, Mugoya IK, Scott JA. Changing use of surgical antibiotic prophylaxis in Thika Hospital, Kenya: a quality improvement intervention with an interrupted time series design. PLoS One. 2013. November 11;8(11):e78942. 10.1371/journal.pone.0078942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Opondo C, Ayieko P, Ntoburi S, Wagai J, Opiyo N, Irimu G, et al. Effect of a multi-faceted quality improvement intervention on inappropriate antibiotic use in children with non-bloody diarrhoea admitted to district hospitals in Kenya. BMC Pediatr. 2011. November 25;11(1):109. 10.1186/1471-2431-11-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gong S, Qiu X, Song Y, Sun X, He Y, Chen Y, et al. Effect of financially punished audit and feedback in a pediatric setting in China, within an antimicrobial stewardship program, and as part of an international accreditation process. Front Public Health. 2016. May 18;4:99. 10.3389/fpubh.2016.00099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinberg M, Fuentes JM, Ruiz AI, Lozano FW, Angel E, Gaitan H, et al. Reducing infections among women undergoing cesarean section in Colombia by means of continuous quality improvement methods. Arch Intern Med. 2001. October 22;161(19):2357–65. 10.1001/archinte.161.19.2357 [DOI] [PubMed] [Google Scholar]
- 40.Pérez A, Dennis RJ, Rodríguez B, Castro AY, Delgado V, Lozano JM, et al. An interrupted time series analysis of parenteral antibiotic use in Colombia. J Clin Epidemiol. 2003. October;56(10):1013–20. 10.1016/S0895-4356(03)00163-X [DOI] [PubMed] [Google Scholar]
- 41.Magedanz L, Silliprandi EM, dos Santos RP. Impact of the pharmacist on a multidisciplinary team in an antimicrobial stewardship program: a quasi-experimental study. Int J Clin Pharm. 2012. April;34(2):290–4. 10.1007/s11096-012-9621-7 [DOI] [PubMed] [Google Scholar]
- 42.Bao L, Peng R, Wang Y, Ma R, Ren X, Meng W, et al. Significant reduction of antibiotic consumption and patients’ costs after an action plan in China, 2010–2014. PLoS One. 2015. March 13;10(3):e0118868. 10.1371/journal.pone.0118868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun J, Shen X, Li M, He L, Guo S, Skoog G, et al. Changes in patterns of antibiotic use in Chinese public hospitals (2005–2012) and a benchmark comparison with Sweden in 2012. J Glob Antimicrob Resist. 2015. June;3(2):95–102. 10.1016/j.jgar.2015.03.001 [DOI] [PubMed] [Google Scholar]
- 44.The WHO Reproductive Health Library [internet]. Geneva: World Health Organization; 2017. Available from: https://extranet.who.int/rhl [cited 2017 Dec 17].
- 45.de Kraker MEA, Abbas M, Huttner B, Harbarth S. Good epidemiological practice: a narrative review of appropriate scientific methods to evaluate the impact of antimicrobial stewardship interventions. Clin Microbiol Infect. 2017. November;23(11):819–25. 10.1016/j.cmi.2017.05.019 [DOI] [PubMed] [Google Scholar]
- 46.Hulscher MEJL, Prins JM. Antibiotic stewardship: does it work in hospital practice? A review of the evidence base. Clin Microbiol Infect. 2017. November;23(11):799–805. 10.1016/j.cmi.2017.07.017 [DOI] [PubMed] [Google Scholar]
- 47.Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016. May 15;62(10):e51–77. 10.1093/cid/ciw118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morris AM. Antimicrobial stewardship programs: appropriate measures and metrics to study their impact. Curr Treat Options Infect Dis. 2014;6(2):101–12. 10.1007/s40506-014-0015-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moehring RW, Anderson DJ, Cochran RL, Hicks LA, Srinivasan A, Dodds Ashley ES; Structured Taskforce of Experts Working at Reliable Standards for Stewardship (STEWARDS) Panel. Expert consensus on metrics to assess the impact of patient-level antimicrobial stewardship interventions in acute-care settings. Clin Infect Dis. 2017. February 1;64(3):377–83. 10.1093/cid/ciw787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Charani E, Edwards R, Sevdalis N, Alexandrou B, Sibley E, Mullett D, et al. Behavior change strategies to influence antimicrobial prescribing in acute care: a systematic review. Clin Infect Dis. 2011. October;53(7):651–62. 10.1093/cid/cir445 [DOI] [PubMed] [Google Scholar]