Abstract
Sudden cardiac death is defined as an unexpected decease of cardiac origin. In individuals under 35 years old, most of these deaths are due to familial arrhythmogenic syndromes of genetic origin, also known as channelopathies. These familial cardiac syndromes commonly follow an autosomal dominant pattern of inheritance. Diagnosis, however, can be difficult, mainly due to incomplete penetrance and variable expressivity, which are hallmarks in these syndromes. The clinical manifestation of these diseases can range from asymptomatic to syncope but sudden death can sometimes be the first symptom of disease. Early identification of at-risk individuals is crucial to prevent a lethal episode. In this review, we will focus on the genetic basis of channelopathies and the effect of genetic and non-genetic modifiers on their phenotypes.
Keywords: sudden cardiac death, channelopathies, genetics, incomplete penetrance, variable expressivity
1. Introduction
Sudden death (SD) is defined as a death that occurs unexpectedly within 1 h of the onset of symptoms or occurs un-witnessed within 24 h of the deceased being seen alive and in a normal state of health before the body is found [1]. Nearly 80% of all SDs are of cardiac origin (sudden cardiac death, SCD). SCD is the leading cause of death in the Western world accounting for an estimated 15–20% of all deaths [2]. The median age ranges between 65 and 70 years with a male prevalence [3,4].
Coronary heart disease is the predominant cause of SCD among individuals over 55 years old; in young adults less than 35 years old, arrhythmogenic syndromes (cardiomyopathies and channelopathies) are the main cause of SCD [5]. Many of these cardiac causes of SCD among children and young adults have an underlying genetic basis, defined by monogenic genetic causes and are therefore inherited. The incidence of SCD in infants and youth less than 35 years old is approximately 2 to 8/100,000 person years, accounting for 2–10% of adult SCD [3,4].
In this review, we will focus on cardiac channelopathies, electrical disorders in structurally normal hearts caused by pathogenic alterations in genes encoding cardiac ion channels or their regulatory proteins.
2. Channelopathies
Inherited arrhythmogenic diseases are the main cause of SCD in young individuals. Unfortunately, SCD can be the first manifestation of one of these diseases; thus, identifying these disorders is crucial for family members. Consequently, current guidelines recommend that, for all victims having a structurally normal heart but no conclusive cause of death, a complete autopsy be performed, including molecular investigation to rule out a cardiac channelopathy [6].
2.1. Long QT Syndrome
Long QT syndrome (LQTS) is an inherited arrhythmic disorder characterized by a QT interval prolongation in the ECG; QTc is prolonged if >440 ms in men or >460 ms in women, in a structurally normal heart. This entity may be acquired (mainly due to pharmacological effects) or congenital. Electrical alterations may lead to palpitations, syncope and even SCD [7]. LQTS is the most prevalent inherited cardiac arrhythmia with an estimated prevalence of 1:2000–1:5000 and the leading cause for SD in young people [8].
LQTS is mainly transmitted in an autosomal dominant pattern (Romano–Ward syndrome); however, a few cases may follow an autosomal recessive pattern (Jervell and Lange–Nielsen syndrome) [9].
Current guidelines recommend genetic analysis of the three main prevalent LQTS-associated genes: KCNQ1 (encoding the Kv7.1, 30–35% of cases), KCNH2 (encoding the Kv11.1 potassium channel, 25–30% of cases) and SCN5A (encoding the Nav1.5 sodium channel, 5–10% of cases) [10]. Nevertheless, a few pathogenic variants have been identified in an additional 14 genes following an autosomal-dominant pattern of inheritance (AKAP9, ANK2, CACNA1C, CALM1, CALM2, CALM3, CAV3, KCNE2, KCNJ2, KCNJ5, RYR2, SCN1B, SCN4B and SNTA1), in another gene following an autosomal-recessive pattern (TRDN) and in one gene following both autosomal-dominant and recessive patterns of inheritance (KCNE1) [11].
Pathogenic genetic variants identified in potassium genes lead to a loss of function [5] whereas mutation in sodium and calcium channels lead to gain of function effect [12].
2.2. Brugada Syndrome
Brugada Syndrome (BrS) is a rare arrhythmogenic disease (estimated prevalence of 1–5/10,000 inhabitants) characterized by a ST-segment elevation in leads V1–V3 and cardiac conduction abnormalities in patients without structural heart alterations, leading to a high risk for ventricular arrhythmias and SCD [7].
BrS typically manifests in the third or fourth decade of life and mainly in men who are at rest. BrS shows a lower prevalence in Northern Europe (1.1 in 100,000 in Denmark) [13] and seems to be endemic in South-Eastern Asia, where the prevalence is 15–20 in 10,000 inhabitants [14].
This syndrome is inherited following an autosomal pattern. Nearly 450 pathogenic variants in 25genes have been associated with BrS (ABCC9, CACNA1C, CACNA2D1, CACNB2b, FGF12, GPD1-L, HCN4, HEY2, KCND2, KCND3, KCNE3, KCNE5, KCNH2, KCNJ8, LRRC10, PKP2, RANGRF, SCN10A, SCN1B, SCN2B, SCN3B, SCN5A, SEMA3A, SLMAP and TRPM4). Variants in these genes can explain 30–35% of the cases, thus 65–70% of BrS cases remain genetically unsolved [11]. These genes encode for sodium, potassium and calcium ion channels or associated proteins. Despite recent genetic reports identifying patients with variants in these genes, conclusive association between most of these genes and BrS remains to be clarified. Current clinical guidelines only recommend genetic analysis of SCN5A (encoding the Nav1.5 sodium channel) since it is attributable in 25% of all BrS cases [6]. Loss-of function mutations in the SCN5A-encoded a-subunit of the cardiac sodium channel represent the most common genetic substrate for BrS cases. Although most of the genetic mutations in the genes involved in BrS result in a loss-of function effect, variants in KCND3, KCNE3 and KCNE5 genes cause a gain-of function effect in BrS patients.
2.3. Catecholaminergic Polymorphic Ventricular Tachycardia
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an inherited disease characterized by the presence of adrenergic-induced bidirectional or polymorphic ventricular tachycardia in individuals with a normal basal electrocardiogram and structurally normal heart. CPVT patients can present syncope and SCD triggered by physical or emotional stress at young ages and SCD can be the first manifestation in up to 30% of cases [15].
The prevalence of CPVT in the general population is estimated to be 1:10,000. Even though it is a rare entity, timely recognition is of vital importance because CPVT plays an important role in SCD in the young [16].
Sixty to sixty-five percent of CPVT patients carry a pathogenic variant in the RYR2 gene (encoding the ryanodine receptor 2), which is transmitted in an autosomal dominant pattern. Other genes have also been associated with CPVT (ANK2, CALM1, CALM2, CALM3, CASQ2, KCNJ2 and TRDN). CPVT is rarely diagnosed with a pathogenic variant in any of these other genes [10,17]. Pathogenic variants in the CASQ2 gene are transmitted following an autosomal recessive pattern but are also found in a small percentage of cases.
2.4. Short QT Syndrome
Short QT syndrome (SQTS) was first described in 2000 by Gussak et al. as a new clinical entity [18]. This syndrome is extremely rare with an estimated prevalence of less than 1 in 10,000 [19] and characterized by abnormally short QT intervals on the ECG. Currently, SQTS is defined as a QTc ≤ 330 ms or a QTc interval <360 ms and one or more of the following: history of cardiac arrest or syncope, family history of SCD at age 40 or younger, or a family history of SQTS [20].
The disease appears to be highly lethal in all age groups, including children in their first months of life and the probability of a first cardiac arrest by the age of 40 years is >40% [6].
Currently, six genes encoding potassium and calcium channels have been associated with SQTS, following an autosomal dominant pattern of inheritance: KCNH2, KCNQ1, KCNJ1, CACNA1C, CACNB2 and CACNA2D1. A comprehensive genetic analysis identifies the genetic origin of this entity in nearly 60% of cases. Clinical guidelines recommend performing a genetic analysis of the main genes: KCNH2 and KCNQ1 [6].
3. Incomplete Penetrance and Variable Expressivity
Generally, the inheritable cardiac arrhythmia syndromes follow an autosomal dominant pattern of inheritance but may exhibit incomplete penetrance, variable expressivity and phenotypic overlap.
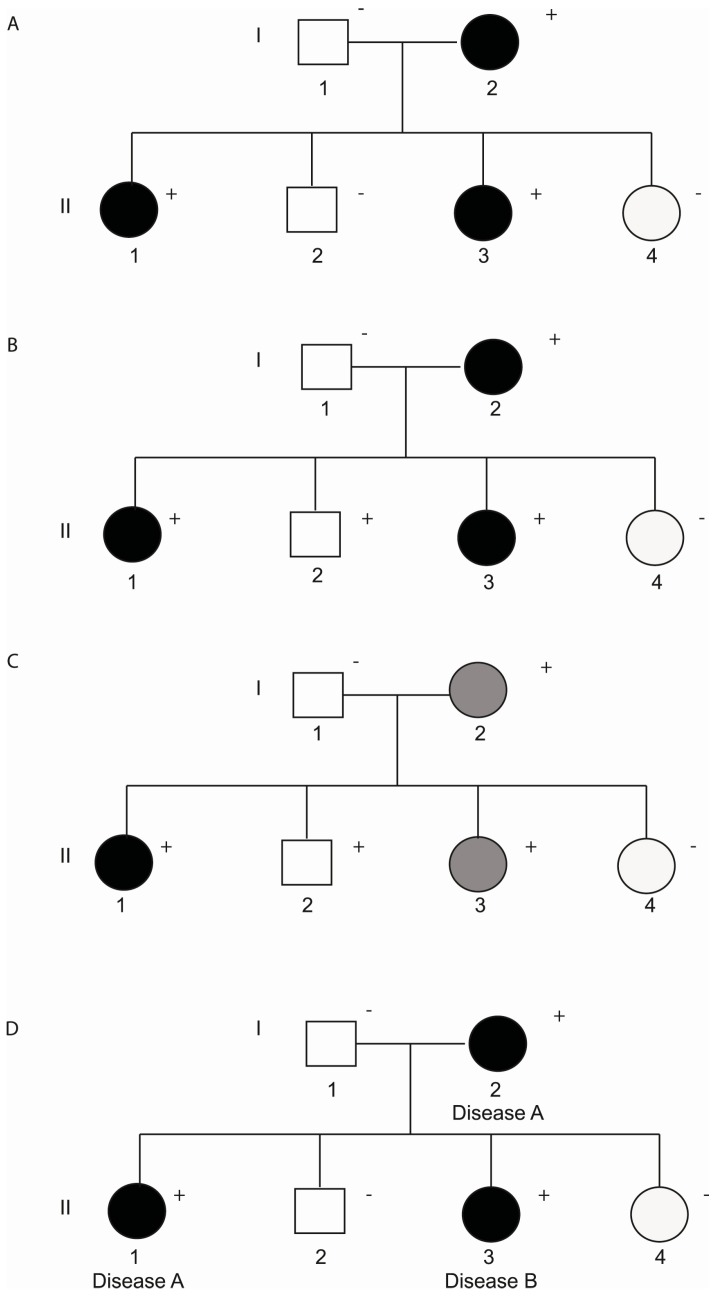
Penetrance is defined as the proportion of people with a specific genotype who manifest a particular clinical characteristic or phenotype. Variable expressivity refers to the series of signs and symptoms that can occur in different people with the same genetic condition. Both incomplete penetrance and variable expressivity are probably due to a combination of genetic, environmental and lifestyle factors. Only a few studies have focused on these modifiers, probably due to the high number of parameters that must be analysed. In addition to incomplete penetrance and variable expressivity, some cases with disease-causing mutations display phenotypic overlap, also known as pleiotropy. The pleiotropy occurs when mutations in a single gene may have a different effect and result in different heritable cardiac channelopathies in the same multigenerational pedigree (Figure 1).
Figure 1.
(A) Pedigree displaying complete penetrance; (B) Pedigree with incomplete penetrance because individual II:2 carries the mutation but is not affected; (C) Pedigree demonstrating incomplete penetrance and variable expressivity due to individual II:3 who carries the mutation but presents a less severe disease; (D) Pedigree showing phenotypic overlap because individual II:3 shows a different cardiac channelopathy. Clinically affected patients are shows in black, clinically unaffected patients are show in white and grey indicates a less severe phenotype. A plus sign indicates a carrier of the genetic variant. A minus sign indicates a non-carrier.
Mutations in specific genes have been associated with different hereditary cardiac arrhythmias. For instance, mutations in SCN5A have been related to LQTS, BrS, sick sinus syndrome and cardiac conduction disturbance, leading to overlapping electrocardiographic phenotypes. In 2016, Veltmann et al. demonstrated high penetrance for LQTS, BrS and cardiac conduction disease in a large family harbouring the SCN5A-E1784K mutation [21]. There are several other SCN5A mutations, including delK1500-SCN5A, delKPQ1505-1507 and 1795insD-SCN5A, associated with a spectrum of arrhythmia phenotypes [22].
Distinct overlapping features have been identified in BrS and arrhythmogenic right ventricular cardiomyopathy (ARVC) patients and this has been a subject of intense debate. Both syndromes could interact at a genetic level, considering that mutations in genes linked with ARVC could cause electrophysiological changes that facilitate the pathophysiological mechanism of BrS [23].
Another explanation for the phenotypic overlap could be a misdiagnosis of the heritable cardiac channelopathy phenotypes. The intravenous flecainide test allows unmasking BrS cases and oral flecainide is a proposed treatment for LQTS3 patients. In 2000, Priori et al. demonstrated that flecainide may induce an elevation of the ST segment in the ECG in patients with LQTS3, leading to a misdiagnosis of these cases [24].
The degree of penetrance among channelopathies is highly variable and also varies with the number of cases analysed among publications. In 2000, Priori et al. analysed 4 families affected by BrS based on their ECG analysis and identified a penetrance of 16% (range 12.5% to 50%) [25]. The penetrance of CPVT ranges from 63 to 78% [26,27] and for LQTS the mean penetrance is ~40% [22].
Huge inter-individual variability cannot be exclusively explained by a single genetic variant, which leads us to consider possible non-genetic (demographic variables, such as sex or age and exogenous factors) and genetic (coding or non-coding variants) modifiers (Table 1).
Table 1.
Genetic and non-genetic factors associated with incomplete penetrance and variable expressivity.
| Non-Genetic Modifiers | |
|---|---|
| Gender | Worse prognosis in males |
| Age | Severe phenotypes in early manifestations, age-related penetrance to the ajmaline provocation test |
| Exogenous factors | Fever, excessive alcohol and large meals |
| Genetic Modifiers | |
| Coding variants—Rare Variants (MAF < 1%) | Additive effect of multiple independent mutations |
| CNVs | |
| New genes involved with the disease | |
| Coding variants—Common Variants (MAF > 1%) | Second hits (i.e., p.H558R_SCN5A; p.K897T_KCNH2; p.D85N_KCNE1 |
| Non-coding variants | 5′UTR and 3′UTR variants, microRNAs |
3.1. Non-Genetic Modifiers
Sex plays an important role in channelopathies. Males have a worse prognosis and greater risk of SCD than females. In the BrS-associated phenotype, intrinsic modifications in ionic currents and hormonal effects are thought to cause these variations [28]. Similar to this arrhythmogenic disease, SQTS, CPVT and LQTS display a sex-dependent penetrance [29,30].
Early manifestations of the symptoms may indicate an aggressive form of a disease, emphasizing the importance of changes in hormones, such testosterone [31]. On the other hand, there is some experimental evidence showing that sodium channels may decline with age leading to a more serious phenotype for a SCN5A pathogenic variant [32]. Mac Millant et al. identified the possibility of an age-related penetrance to the ajmaline provocation test and supports the need to retest with a negative ajmaline test in earlier childhood [33]. Conte et al. report for the first time the changes in the response to ajmaline over time and unmasked BrS in 23% of relatives with a previously negative drug test performed during childhood [34].
Several exogenous factors together with disease-causing variants can modulate ECG parameters altering the genotype-phenotype correlation. For example, the ECG pattern type I in BrS may be unmasked by fever, excessive alcohol intake and large meals. Drugs or electrolyte imbalances in LQTS may prolong or unmask the QT interval [6].
3.2. Genetic Modifiers
3.2.1. Coding Variants
• Rare variants
Genetic variants with minor allele frequency (MAF) <1% are considered rare and are often the main cause of heritable cardiac arrhythmias. Nevertheless, a wide percentage of families with a well-studied disease-causing mutation do not show full penetrance and complete expressivity. This fact may be due to the additive effect of multiple independent mutations, which can disturb and often increase the severity of the phenotype. More recently, it has become clear that LQTS patients carrying two or more pathogenic variants either in the same gene (compound heterozygotes) or in different genes (digenic heterozygosity) make up 4–9% of patients [35]. As a further example, a family affected by BrS was found to have a double pathogenic variant in the SCN5A gene associated with the severity of the disorder in the proband and his deceased sibling [36].
In 2011, the publication of exome data from the NHLBI GO Exome Sequencing project (ESP) increased access to the distribution of genetic variations in the general population. Two studies identified a high prevalence of genetic variants previously associated with LQTS (1:31) and BrS (1:23) in the new exome data [37,38]. These results suggest that some variants previously associated with a rare disease may not be disease-causing mutations.
Other types of variants, not identifiable by Sanger, can now be detected with high throughput technologies (next-generation sequencing, NGS). These include copies of large genomic regions, also known as copy number variants (CNVs). These genomic imbalances have been identified in some channelopathies, including LQTS [39] and BrS [40] but do not seem to be major contributors to these arrhythmogenic disorders (less than 5% of all diagnosed cases).
Lastly, pathogenic coding variants in genes not previously associated with the heritable cardiac arrhythmias may explain the lack of full penetrance. Whole exome sequencing in well studied families is emerging as the most feasible strategy to identify potential new arrhythmia-associated genes.
• Common variants
Variants with MAF > 1% are considered common variants or polymorphisms. A further explanation for the low penetrance in the heritable cardiac diseases is the coexistence of modified variants in the same ion channel genes, possibly common single polymorphisms, altering susceptibility to arrhythmias. These common variants could alter the severity of the disease caused by another disease-causing mutation or act independently to modulate susceptibility to ECG abnormalities in healthy individuals.
The common polymorphisms or “second hits” are located on the opposite allele of the gene carrying the primary disease-causing mutation or in a completely separate channelopathy-susceptibility gene and they can either worsen or attenuate the effect of a primary disease-causing mutation [22]. Probably one of the most studied modifiers, p.H558R_SCN5A (MAF = 0.2217) seems to reduce the pathogenic effect in carriers of other disease-causing mutations, producing a less severe phenotype. In 2005, Crotti et al. demonstrated that the p.K897T_KCNH2 (MAF = 0.1871) polymorphism can act as a genetic modifier of LQTS clinical severity in the presence of other rare variants in the same gene. An additional recognized modifier of penetrance and expressivity, p.D85N_KCNE1 (MAF = 0.009158) causes loss-of-function effects on both IKr and IKs and behaves as a disease-causing variant [41]. Moreover, these common polymorphisms occur more frequently in patients with some cardiac channelopathies than in healthy individuals [41].
Genome-wide genetic association studies (GWAS) use a statistically powerful approach to detect genetic variants with small effect sizes. GWAS studies revealed a strong and replicable association between common variants in NOS1AP (not previously associated with cardiac electrophysiology), KCNQ1, KCNE1, KCNH2, SCN5A and KCNJ2 genes and QT interval duration in a control population. Another important GWAS study in BrS patients performed by Bezzina et al. in 2013 identified a significant association with the SCN10A locus (rs10428132) and HEY2 gene (rs9388451). The association signals at SCN5A-SCN10A demonstrated that genetic polymorphisms modulating cardiac conduction can also influence susceptibility to cardiac arrhythmia. These results indicate that common genetic variation can have a strong impact on the predisposition to rare diseases [42].
3.2.2. Non-Coding Variants
Only a small part of the genome encodes for proteins and at least some non-coding DNA has important biological functions. Non-coding regions located upstream (5′UTR) and downstream (3′UTR) of genes are transcribed but not translated into proteins. They have a regulatory role in gene expression, especially in the control of mRNA stability and translation. Therefore, variable transcription of different genes encoding ion cardiac channels is a possible mechanism for determining arrhythmia susceptibility.
In 2012, Amin et al. identified 3′UTR SNPs (rs2519184, rs8234 and rs10798) that potently modify the disease severity of LQT1. Patients with the derived SNP variants in their mutated KCNQ1 allele had shorter QTc and fewer symptoms, while patients with the derived SNP variants in their normal KCNQ1 allele had longer QTc and more symptoms [43]. Nevertheless, Crotti et al. (2016) analysed the effect of 3 mutations (rather than the 33 used by Amin et al.) in 747 subjects (versus the 168 used by Amin et al.) and demonstrated that 3′UTR SNPs are not acting as genetic modifiers in LQT1 patients [44].
MicroRNAs are small (about 22-nucleotide long) non-coding RNAs that regulate gene expression through translational repression or mRNA degradation, usually by binding to target sites located in the 3’UTRs of mRNAs. They are involved in the pathogenesis of many diseases, including cardiovascular and neurodegenerative diseases, immune disorders and cancer [45]; however, little is known about the role of miRNAs in regulating genes encoding ionic cardiac channels and may represent an important area for future analyses.
4. Conclusions
Cardiac channelopathies mainly follow an autosomal dominant pattern of inheritance but usually display incomplete penetrance, variable expressivity and phenotypic overlap. These phenotypic variations are due to genetic modifiers and environmental and lifestyle factors but most pathophysiological pathways implicated in these effects remain unknown. Basic analysis and further genotype-phenotype correlation studies in large families are crucial for understanding the cellular mechanism underlying these conditions as well as performing accurate SC-risk prediction for each individual.
Acknowledgments
This work was supported by Obra Social ‘La Caixa,’ Fondo de Investigacion Sanitaria (FIS, PI14/01773) from the Instituto de Salud Carlos III (ISCIII), “Fundacio Privada Daniel Bravo Andreu”, and Fundació La Marató de TV3. The CIBERCV is also an initiative of the ISCIII, Spanish Ministry of Economy and Competitiveness.
Author Contributions
Monica Coll and Oscar Campuzano wrote the paper. Ramon Brugada and Oscar Campuzano designed the study. Jesus Mates, Bernat del Olmo, Marta Puigmulé, Anna Fernandez-Falgueras, Anna Iglesias, Ferran Picó and Laura Lopez have revised the manuscript and improve it. All the authors revised and approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bayes de Luna A., Elosua R. Sudden death. Rev. Esp. Cardiol. 2012;65:1039–1052. doi: 10.1016/j.rec.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 2.Marsman R.F., Tan H.L., Bezzina C.R. Genetics of sudden cardiac death caused by ventricular arrhythmias. Nat. Rev. Cardiol. 2014;11:96–111. doi: 10.1038/nrcardio.2013.186. [DOI] [PubMed] [Google Scholar]
- 3.Martens E., Sinner M.F., Siebermair J., Raufhake C., Beckmann B.M., Veith S., Duvel D., Steinbeck G., Kaab S. Incidence of sudden cardiac death in Germany: Results from an emergency medical service registry in Lower Saxony. Europace. 2014;16:1752–1758. doi: 10.1093/europace/euu153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamin E.J., Blaha M.J., Chiuve S.E., Cushman M., Das S.R., Deo R., de Ferranti S.D., Floyd J., Fornage M., Gillespie C., et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campuzano O., Sarquella-Brugada G., Brugada R., Brugada J. Genetics of channelopathies associated with sudden cardiac death. Glob. Cardiol. Sci. Pract. 2015;2015:39. doi: 10.5339/gcsp.2015.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Priori S.G., Blomstrom-Lundqvist C., Mazzanti A., Blom N., Borggrefe M., Camm J., Elliott P.M., Fitzsimons D., Hatala R., Hindricks G., et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC) Eur. Heart J. 2015;36:2793–2867. doi: 10.1093/eurheartj/ehv316. [DOI] [PubMed] [Google Scholar]
- 7.Lieve K.V., Wilde A.A. Inherited ion channel diseases: A brief review. Europace. 2015 doi: 10.1093/europace/euv105. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz P.J., Stramba-Badiale M., Crotti L., Pedrazzini M., Besana A., Bosi G., Gabbarini F., Goulene K., Insolia R., Mannarino S., et al. Prevalence of the congenital long-QT syndrome. Circulation. 2009;120:1761–1767. doi: 10.1161/CIRCULATIONAHA.109.863209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ackerman M.J., Priori S.G., Willems S., Berul C., Brugada R., Calkins H., Camm A.J., Ellinor P.T., Gollob M., Hamilton R., et al. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA) Heart Rhythm. 2011;8:1308–1339. doi: 10.1016/j.hrthm.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Mizusawa Y. Recent advances in genetic testing and counseling for inherited arrhythmias. J. Arrhythm. 2016;32:389–397. doi: 10.1016/j.joa.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez-Falgueras A., Sarquella-Brugada G., Brugada J., Brugada R., Campuzano O. Cardiac Channelopathies and Sudden Death: Recent Clinical and Genetic Advances. Biology. 2017;6 doi: 10.3390/biology6010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ackerman M.J., Marcou C.A., Tester D.J. Personalized medicine: Genetic diagnosis for inherited cardiomyopathies/channelopathies. Rev. Esp. Cardiol. 2013;66:298–307. doi: 10.1016/j.recesp.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Holst A.G., Jensen H.K., Eschen O., Henriksen F.L., Kanters J., Bundgaard H., Svendsen J.H., Haunso S., Tfelt-Hansen J. Low disease prevalence and inappropriate implantable cardioverter defibrillator shock rate in Brugada syndrome: A nationwide study. Europace. 2012;14:1025–1029. doi: 10.1093/europace/eus002. [DOI] [PubMed] [Google Scholar]
- 14.Nademanee K. Sudden unexplained death syndrome in Southeast Asia. Am. J. Cardiol. 1997;79:10–11. doi: 10.1016/S0002-9149(97)00113-6. [DOI] [PubMed] [Google Scholar]
- 15.Wanguemert F., Bosch Calero C., Perez C., Campuzano O., Beltran-Alvarez P., Scornik F.S., Iglesias A., Berne P., Allegue C., Ruiz Hernandez P.M., et al. Clinical and molecular characterization of a cardiac ryanodine receptor founder mutation causing catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2015;12:1636–1643. doi: 10.1016/j.hrthm.2015.03.033. [DOI] [PubMed] [Google Scholar]
- 16.Lieve K.V., van der Werf C., Wilde A.A. Catecholaminergic Polymorphic Ventricular Tachycardia. Circ. J. 2016;80:1285–1291. doi: 10.1253/circj.CJ-16-0326. [DOI] [PubMed] [Google Scholar]
- 17.Roux-Buisson N., Cacheux M., Fourest-Lieuvin A., Fauconnier J., Brocard J., Denjoy I., Durand P., Guicheney P., Kyndt F., Leenhardt A., et al. Absence of triadin, a protein of the calcium release complex, is responsible for cardiac arrhythmia with sudden death in human. Hum. Mol. Genet. 2012;21:2759–2767. doi: 10.1093/hmg/dds104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gussak I., Brugada P., Brugada J., Wright R.S., Kopecky S.L., Chaitman B.R., Bjerregaard P. Idiopathic short QT interval: A new clinical syndrome? Cardiology. 2000;94:99–102. doi: 10.1159/000047299. [DOI] [PubMed] [Google Scholar]
- 19.Mazzanti A., O’Rourke S., Ng K., Miceli C., Borio G., Curcio A., Esposito F., Napolitano C., Priori S.G. The usual suspects in sudden cardiac death of the young: A focus on inherited arrhythmogenic diseases. Expert Rev. Cardiovasc. Ther. 2014;12:499–519. doi: 10.1586/14779072.2014.894884. [DOI] [PubMed] [Google Scholar]
- 20.Priori S.G., Wilde A.A., Horie M., Cho Y., Behr E.R., Berul C., Blom N., Brugada J., Chiang C.E., Huikuri H., et al. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Europace. 2013;15:1389–1406. doi: 10.1093/europace/eut272. [DOI] [PubMed] [Google Scholar]
- 21.Veltmann C., Barajas-Martinez H., Wolpert C., Borggrefe M., Schimpf R., Pfeiffer R., Caceres G., Burashnikov E., Antzelevitch C., Hu D. Further Insights in the Most Common SCN5A Mutation Causing Overlapping Phenotype of Long QT Syndrome, Brugada Syndrome, and Conduction Defect. J. Am. Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giudicessi J.R., Ackerman M.J. Determinants of incomplete penetrance and variable expressivity in heritable cardiac arrhythmia syndromes. Transl. Res. 2013;161:1–14. doi: 10.1016/j.trsl.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoogendijk M.G. Diagnostic dilemmas: Overlapping features of brugada syndrome and arrhythmogenic right ventricular cardiomyopathy. Front. Physiol. 2012;3:144. doi: 10.3389/fphys.2012.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Priori S.G., Napolitano C., Schwartz P.J., Bloise R., Crotti L., Ronchetti E. The elusive link between LQT3 and Brugada syndrome: The role of flecainide challenge. Circulation. 2000;102:945–947. doi: 10.1161/01.CIR.102.9.945. [DOI] [PubMed] [Google Scholar]
- 25.Priori S.G., Napolitano C., Gasparini M., Pappone C., Della Bella P., Brignole M., Giordano U., Giovannini T., Menozzi C., Bloise R., et al. Clinical and genetic heterogeneity of right bundle branch block and ST-segment elevation syndrome: A prospective evaluation of 52 families. Circulation. 2000;102:2509–2515. doi: 10.1161/01.CIR.102.20.2509. [DOI] [PubMed] [Google Scholar]
- 26.Postma A.V., Denjoy I., Kamblock J., Alders M., Lupoglazoff J.M., Vaksmann G., Dubosq-Bidot L., Sebillon P., Mannens M.M., Guicheney P., et al. Catecholaminergic polymorphic ventricular tachycardia: RYR2 mutations, bradycardia, and follow up of the patients. J. Med. Genet. 2005;42:863–870. doi: 10.1136/jmg.2004.028993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van der Werf C., Nederend I., Hofman N., van Geloven N., Ebink C., Frohn-Mulder I.M., Alings A.M., Bosker H.A., Bracke F.A., van den Heuvel F., et al. Familial evaluation in catecholaminergic polymorphic ventricular tachycardia: Disease penetrance and expression in cardiac ryanodine receptor mutation-carrying relatives. Circ. Arrhythm. Electrophysiol. 2012;5:748–756. doi: 10.1161/CIRCEP.112.970517. [DOI] [PubMed] [Google Scholar]
- 28.Benito B., Sarkozy A., Mont L., Henkens S., Berruezo A., Tamborero D., Arzamendi D., Berne P., Brugada R., Brugada P., et al. Gender differences in clinical manifestations of Brugada syndrome. J. Am. Coll. Cardiol. 2008;52:1567–1573. doi: 10.1016/j.jacc.2008.07.052. [DOI] [PubMed] [Google Scholar]
- 29.Mazzanti A., Kanthan A., Monteforte N., Memmi M., Bloise R., Novelli V., Miceli C., O’Rourke S., Borio G., Zienciuk-Krajka A., et al. Novel insight into the natural history of short QT syndrome. J. Am. Coll. Cardiol. 2014;63:1300–1308. doi: 10.1016/j.jacc.2013.09.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Priori S.G., Napolitano C., Memmi M., Colombi B., Drago F., Gasparini M., DeSimone L., Coltorti F., Bloise R., Keegan R., et al. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106:69–74. doi: 10.1161/01.CIR.0000020013.73106.D8. [DOI] [PubMed] [Google Scholar]
- 31.Probst V., Denjoy I., Meregalli P.G., Amirault J.C., Sacher F., Mansourati J., Babuty D., Villain E., Victor J., Schott J.J., et al. Clinical aspects and prognosis of Brugada syndrome in children. Circulation. 2007;115:2042–2048. doi: 10.1161/CIRCULATIONAHA.106.664219. [DOI] [PubMed] [Google Scholar]
- 32.Huang X., Du Y., Yang P., Lin S., Xi Y., Yang Z., Ma A. Age-dependent alterations of voltage-gated Na(+) channel isoforms in rat sinoatrial node. Mech. Ageing Dev. 2015;152:80–90. doi: 10.1016/j.mad.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 33.McMillan M.R., Day T.G., Bartsota M., Mead-Regan S., Bryant R., Mangat J., Abrams D., Lowe M., Kaski J.P. Feasibility and outcomes of ajmaline provocation testing for Brugada syndrome in children in a specialist paediatric inherited cardiovascular diseases centre. Open Heart. 2014;1:e000023. doi: 10.1136/openhrt-2013-000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conte G., de Asmundis C., Ciconte G., Julia J., Sieira J., Chierchia G.B., Brugada P. Follow-up from childhood to adulthood of individuals with family history of Brugada syndrome and normal electrocardiograms. JAMA. 2014;312:2039–2041. doi: 10.1001/jama.2014.13752. [DOI] [PubMed] [Google Scholar]
- 35.Napolitano C., Novelli V., Francis M.D., Priori S.G. Genetic modulators of the phenotype in the long QT syndrome: State of the art and clinical impact. Curr. Opin. Genet. Dev. 2015;33:17–24. doi: 10.1016/j.gde.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Bezzina C.R., Rook M.B., Groenewegen W.A., Herfst L.J., van der Wal A.C., Lam J., Jongsma H.J., Wilde A.A., Mannens M.M. Compound heterozygosity for mutations (W156X and R225W) in SCN5A associated with severe cardiac conduction disturbances and degenerative changes in the conduction system. Circ. Res. 2003;92:159–168. doi: 10.1161/01.RES.0000052672.97759.36. [DOI] [PubMed] [Google Scholar]
- 37.Risgaard B., Jabbari R., Refsgaard L., Holst A.G., Haunso S., Sadjadieh A., Winkel B.G., Olesen M.S., Tfelt-Hansen J. High prevalence of genetic variants previously associated with Brugada syndrome in new exome data. Clin. Genet. 2013;84:489–495. doi: 10.1111/cge.12126. [DOI] [PubMed] [Google Scholar]
- 38.Refsgaard L., Holst A.G., Sadjadieh G., Haunso S., Nielsen J.B., Olesen M.S. High prevalence of genetic variants previously associated with LQT syndrome in new exome data. Eur. J. Hum. Genet. 2012;20:905–908. doi: 10.1038/ejhg.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campuzano O., Sarquella-Brugada G., Mademont-Soler I., Allegue C., Cesar S., Ferrer-Costa C., Coll M., Mates J., Iglesias A., Brugada J., et al. Identification of Genetic Alterations, as Causative Genetic Defects in Long QT Syndrome, Using Next Generation Sequencing Technology. PLoS ONE. 2014;9:e114894. doi: 10.1371/journal.pone.0114894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mademont-Soler I., Pinsach-Abuin M.L., Riuro H., Mates J., Perez-Serra A., Coll M., Porres J.M., Del Olmo B., Iglesias A., Selga E., et al. Large Genomic Imbalances in Brugada Syndrome. PLoS ONE. 2016;11:e0163514. doi: 10.1371/journal.pone.0163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nishio Y., Makiyama T., Itoh H., Sakaguchi T., Ohno S., Gong Y.Z., Yamamoto S., Ozawa T., Ding W.G., Toyoda F., et al. D85N, a KCNE1 polymorphism, is a disease-causing gene variant in long QT syndrome. J. Am. Coll. Cardiol. 2009;54:812–819. doi: 10.1016/j.jacc.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Bezzina C.R., Barc J., Mizusawa Y., Remme C.A., Gourraud J.B., Simonet F., Verkerk A.O., Schwartz P.J., Crotti L., Dagradi F., et al. Common variants at SCN5A-SCN10A and HEY2 are associated with Brugada syndrome, a rare disease with high risk of sudden cardiac death. Nat. Genet. 2013 doi: 10.1038/ng1113-1409b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amin A.S., Giudicessi J.R., Tijsen A.J., Spanjaart A.M., Reckman Y.J., Klemens C.A., Tanck M.W., Kapplinger J.D., Hofman N., Sinner M.F., et al. Variants in the 3’ untranslated region of the KCNQ1-encoded Kv7.1 potassium channel modify disease severity in patients with type 1 long QT syndrome in an allele-specific manner. Eur. Heart J. 2012;33:714–723. doi: 10.1093/eurheartj/ehr473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crotti L., Lahtinen A.M., Spazzolini C., Mastantuono E., Monti M.C., Morassutto C., Parati G., Heradien M., Goosen A., Lichtner P., et al. Genetic Modifiers for the Long-QT Syndrome: How Important Is the Role of Variants in the 3’ Untranslated Region of KCNQ1? Circ. Cardiovasc. Genet. 2016;9:330–339. doi: 10.1161/CIRCGENETICS.116.001635. [DOI] [PubMed] [Google Scholar]
- 45.Bronze-da-Rocha E. MicroRNAs expression profiles in cardiovascular diseases. BioMed Res. Int. 2014;2014:985408. doi: 10.1155/2014/985408. [DOI] [PMC free article] [PubMed] [Google Scholar]