SUMMARY
Every animal species has a signature blood glucose level or glycemic set point. These set points are different and the normal glycemic levels (normoglycemia) of one species would be life threatening for other species. Mouse normoglycemia can be considered diabetic for humans. The biological determinants of the glycemic set point remain unclear. Here we show that the pancreatic islet imposes its glycemic set point on the organism, making it the bona fide glucostat in the body. Moreover, and in contrast to rodent islets, glucagon input from the alpha cell to the insulin secreting beta cell is necessary to fine-tune the distinctive human set point. These findings impact transplantation and regenerative approaches to treat diabetes because restoring normoglycemia may require more than replacing only the beta cells. Furthermore, therapeutic strategies using glucagon receptor antagonists as hypoglycemic agents need to be reassessed as they may reset the overall glucostat in the organism.
Keywords: Glucose homeostasis, insulin secretion, beta cell, alpha cell, glucagon, paracrine signaling, human pancreatic islet
Graphical abstract
By transplanting human, monkey and mice pancreatic islets into diabetic mice, XXX et al show that islet grafts transfer the glycemic set point of the donor species, independent of transplanted islet mass. Human islet grafts sense glucose levels and adjust their insulin secretion accordingly, with help from adjacent alpha cells.
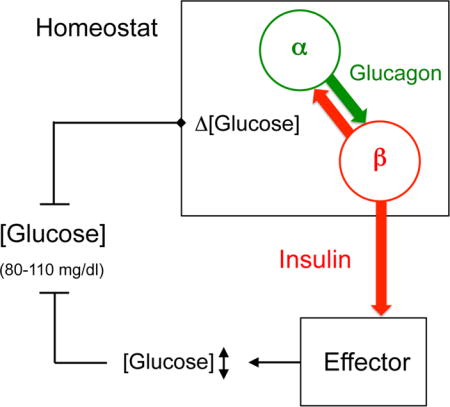
INTRODUCTION
Blood glucose levels are tightly regulated. In human beings, glucose homeostasis rapidly returns glycemia after feeding or during fasting to values around a set point of 90 mg/dL. Lower levels (hypoglycemia) or higher levels (hyperglycemia) are potential threats to our health. Animal species, however, can have strikingly different target glycemic levels [glycemic set points, Figure 1A; see also (Davalli et al., 1995; Graham et al., 2011; Meng et al., 2016)], possibly reflecting evolutionary adaptation (Schermerhorn, 2013). A given species maintains glycemia within a narrow range that can be completely out of the normoglycemic range of another species. Glucose homeostasis engages metabolic regulatory organs such as the liver, neural organs such as the hypothalamus, and endocrine organs such as the pancreatic islet, which all contribute to the regulation and utilization of blood glucose (Matschinsky et al., 2006; Schuit et al., 2001). Because the intricate interactions between these organs and because each one of them has its own glucose set point, it remains unclear if there is a leading organ or mechanism that maintains glycemia within the characteristic narrow range of the species. There is no systematic study addressing where the target value for normoglycemia is set, that is, where the glucostat resides in the body.
Figure 1. Pancreatic islet grafts transfer the glycemic set point of the islet donor species to recipient mice.
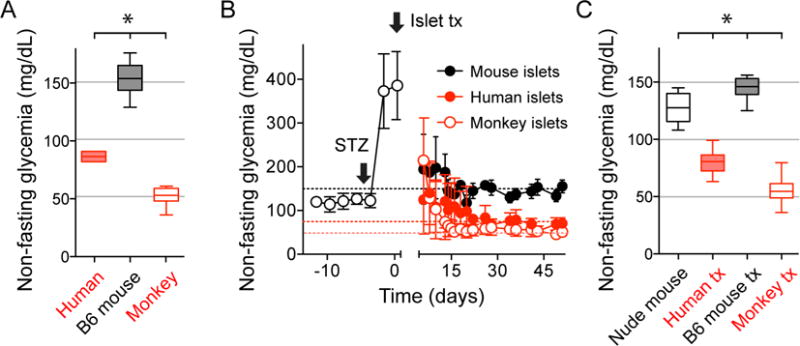
(A) Non-fasting glycemic levels of humans (n = 5), C57Bl6J mice (n = 20), and cynomolgus monkeys (n = 11) were significantly different.
(B) Nude mice rendered diabetic with streptozotocin (STZ) transplanted with islets from humans (n = 47 recipient mice), monkeys (n = 22), and C57Bl6J mice (n = 8) became normoglycemic with glycemic levels of the islet donor species. Curves are shown as average ± SD.
(C) Quantification of results shown in B. All glycemic values were significantly different from each other. Data are shown as box and whisker plots and compared with one-way ANOVA followed by Tukey’s multiple comparison tests. Asterisks denote significance (P < 0.05).
Results from islet xenotransplantation studies show that islet grafts transfer the glycemic levels typical of the islet donor species (Carroll et al., 1992; Georgiou and Mandel, 1987), making the islet a plausible candidate for being the overall glucostat in the organism. It has been proposed that glucose sensing and insulin secretion by the beta cell is the key regulatory element. In the human islet, however, beta cells are surrounded by glucagon secreting alpha cells and somatostatin secreting delta cells whose secretory products influence insulin secretion (Bosco et al., 2010; Brissova et al., 2005; Cabrera et al., 2006a; Caicedo, 2013). We therefore hypothesized that the glycemic set point results from the pancreatic islet working as an organ, where the hormonal output is governed by features and mechanisms intrinsic to the islet tissue.
To test our hypothesis, we transplanted pancreatic islets from different species into diabetic and non-diabetic mice and measured glycemia and glucose tolerance in the recipient mice. We found that the engrafted islets transferred the glycemic levels of the donor species. These results indicate that glucose sensing and insulin secretion from the islet was sufficient to establish target values for glycemia. To determine how alpha cell input influences insulin secretion and glycemia, we treated human islet grafts with a glucagon receptor antagonist that does not affect mouse glucagon receptors. This treatment decreased insulin secretion from human islet grafts and increased glycemia to levels that can be considered pre-diabetic. These findings demonstrate that beta cell secretion has to be amplified by input from adjacent alpha cells to establish the human glycemic set point.
RESULTS
The pancreatic islet imposes its glycemic set point on the organism
We used a xenotransplantation approach to determine whether the pancreatic islet serves as the bona fide glucostat in the body. Our strategy consisted of isolating the homeostatic contribution of the islet by transplanting islets from different species into the anterior chamber of the eye or under the kidney capsule of immunodeficient nude mice rendered diabetic with streptozotocin. As donors for the islets we used three species that differ widely in their normoglycemia, namely humans, cynomolgus monkeys, and C57Bl6 mice (Figure 1A). When islets from these species were transplanted into diabetic nude mice they restored normoglycemia to values that were indistinguishable from those of the respective donors [Figures 1B and C; human (86 ± 5.2 mg/dL) versus mice with human islets (80.2 ± 8.5 mg/dL); C57BL6 (153 ± 14 mg/dL) versus mice with C57BL6 islets (144.8 ± 10 mg/dL), monkeys (52 ± 7 mg/dL) versus mice with monkey islets (55 ± 9.6 mg/dL); mean ± SD]. Human and monkey islets imposed lower glycemic levels, whereas islets from C57BL6 mice engrafted under the kidney capsule forced higher glycemic levels upon the recipient nude mice. These results suggest that the islet alone can set the target glycemic values of the species.
Islets from species with lower glycemic set point dominate glycemia
How does transplanting islets affect the normoglycemia already established by islet grafts from a different species? We transplanted human islets under the kidney capsule of diabetic nude mice and, once normoglycemia was restored to human levels, we transplanted mouse islets into the eye (Figure 2A). Despite adding islet mass, this procedure did not change glycemia. When human islet grafts were removed, glycemic levels increased to reach the mouse donor’s normoglycemia (Figures 2A and B). These results showed that both human and mouse islets engrafted functionally. More importantly, they indicate that human islets were dominant. This can be explained by human islets having a glucose-dependency curve of insulin secretion that is shifted to lower glucose concentrations, with a glucose concentration threshold ~54-72 mg/dL for human islets versus ~90 mg/dL for mouse islets (Henquin et al., 2006). Hence, the most likely interpretation of our results is that insulin secretion from human islet grafts was stimulated at lower glucose levels, thus preventing glycemia from reaching levels that would activate beta cells in the mouse islet grafts. In this scenario, islets with the lower set point impose glycemia.
Figure 2. Human islet grafts impose their glycemic set point.
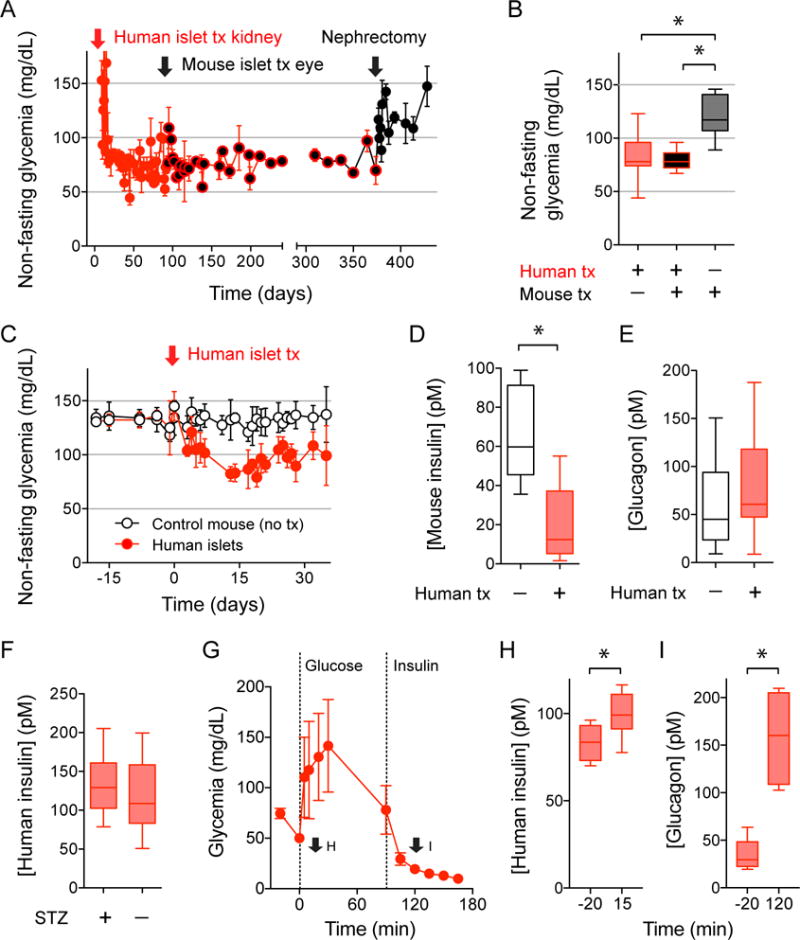
(A and B) Non-fasting glycemia of diabetic nude mice transplanted with human islets under the kidney capsule (red arrow) and then with islets from C57Bl6J mice into the eye (black arrow; n = 11 recipient mice). Human islets grafts were later removed by nephrectomy, which changed glycemic values to mouse levels (quantified in B). Data are shown as average ± SD (A) or box and whisker plots (B) and compared with one-way ANOVA followed by Tukey’s multiple comparison tests. Asterisks denote significance (P < 0.05).
(C-E) Non-fasting glycemia shows that non-diabetic nude mice transplanted with human islets (black symbols, n = 5) acquired the human glycemic set point (C). Endogenous release of mouse insulin was inhibited in the presence of human islet grafts (D), but plasma glucagon levels were not affected (E).
(F) Human insulin plasma levels in transplanted mice without endogenous islets (STZ-treated, STZ+) and with endogenous islets (STZ−; 15 measurements in 5 mice). Asterisks denote significance (P < 0.05, Student’s t-tests).
(G-I) Intraperitoneal glucose tolerance test (4g/kg glucose) followed by an insulin tolerance test (0.75 U/kg insulin) performed in diabetic nude mice transplanted with human islets (G; n = 6 mice) show adequate insulin and glucagon responses to the glucose challenge (H) and the induced hypoglycemia (I), respectively. Hormone plasma levels were measured at the time points indicated in G (arrows). Asterisks denote significance (P < 0.05, Student’s t-tests).
It is possible that the diabetic mouse model we used compromised glucose counterregulation, the protective response against hypoglycemia (Farhy et al., 2008; Shi et al., 1996). This could limit the recipient mouse ability to counteract the lower glycemia imposed by human islets. To address this issue, we transplanted human islets into intact, non-diabetic nude mice and found that this procedure still moved glycemic levels to the human set point (Figure 2C). In these mice, endogenous (mouse) beta cell insulin secretion was inhibited by ~85% (Figure 2D), while glucagon plasma levels were similar to those of control non-transplanted mice (Figure 2E). Plasma human insulin levels were the same whether or not transplanted mice had endogenous islets (Figure 2F), confirming that human islet grafts prevented glycemia from reaching levels that activate mouse beta cells. Alpha cells were not activated probably because the threshold for the glucagon counterregulatory response in mice is between 63 and 72 mg/dL (Malmgren and Ahrén, 2015), which is below the human glycemic set point (~80 mg/dL). Glucagon responses, however, could be elicited by hypoglycemia (< 50 mg/dl), and insulin responses by hyperglycemia (>120 mg/dl), demonstrating that hormone secretion from human islet grafts was not passive but appropriately regulated by changes in glycemia (Figures 2G-I and Figure S1). We therefore conclude that hormone secretion from human islet grafts was responsible for maintaining normoglycemia in the recipient mouse.
Islet mass is not a determinant of the glycemic set point
Functional engraftment of human and monkey islets required transplanting a larger islet mass. To determine the effects of islet graft mass on glycemia we transplanted different numbers of mouse and human islet equivalents into recipient mice (Figure 3). A titration of mouse islet graft mass in the eye showed that mice receiving smaller amounts of islets took longer to recover from diabetes (Figure 3B). However, mice in all groups returned to the typical glycemic level of the donor mouse (Figure 3C; see also Figure S2) despite having different islet graft volumes and graft insulin contents (Figures 3D, E, and J). Doubling the number of human islet grafts under the kidney capsule (Figure 3F) resulted in different graft insulin contents (Figure 3I), but did not affect the characteristic human glycemic set point in the recipient mouse (Figures 3H and J, Figure S2). Insulin plasma levels were also independent of islet graft mass (Figures 3E and I). These results rule out that islet mass affected the glycemic set point.
Figure 3. Glycemic levels depend on donor species but are independent of transplanted islet mass.
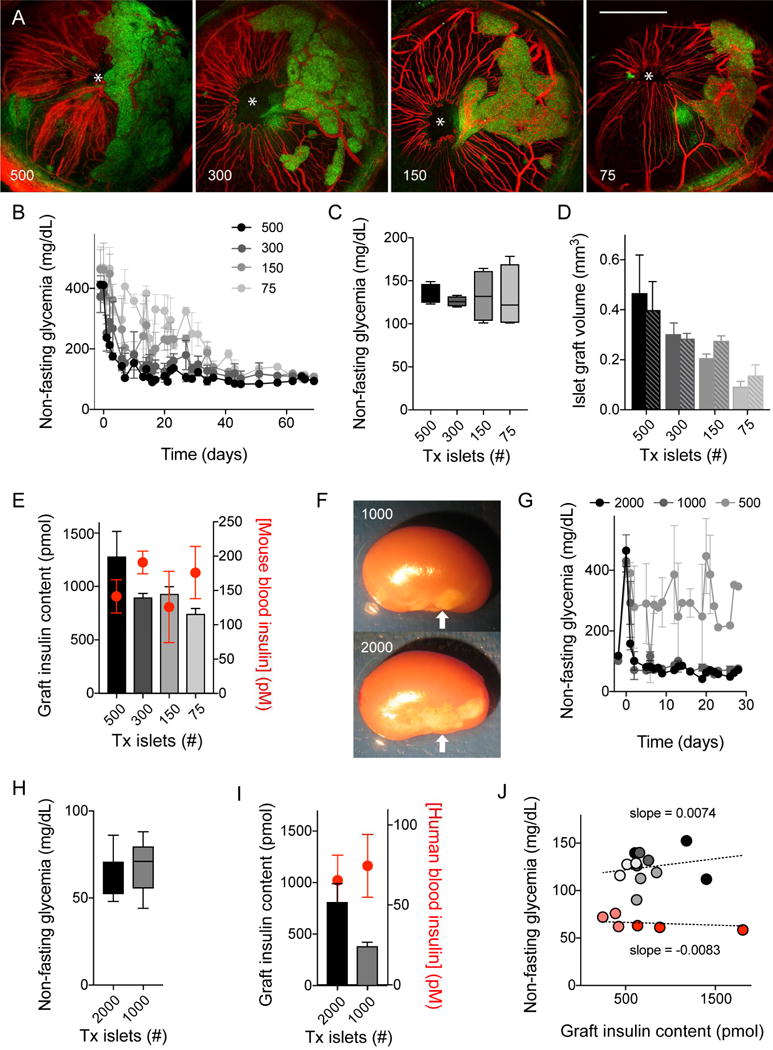
(A) Z-stacks of confocal images of the eyes of nude mice transplanted with 500, 300, 150, or 75 islet equivalents (islet backscatter shown in green and blood vessels in red. Asterisks indicates pupils; images acquired at day 70 after transplantation).
(B and C) Non-fasting glycemic values show that transplanting different numbers of islets from C75Bl6J mice into diabetic nude mice reversed diabetes and produced similar levels of glycemia (quantified in B, n = 3 mice per group). Note, however, that recipient mice with a smaller mass of transplanted islets needed longer to return to normoglycemia.
(D) Islet graft volumes of mice shown in A-C estimated by measuring islet backscatter (green) at days 35 (solid bars) and 70 (patterned bars) after transplantation. Mice receiving 500 islets had significantly more islet mass than those receiving 75 islets (P < 0.05, ANOVA followed by multiple comparison test). Over time, there was a small increase in islet volume in mice transplanted with fewer islets.
(E) Mouse graft insulin contents were different for the four groups of mice at day 70 (gray scale columns; 500 significantly different from 75; P < 0.05, ANOVA followed by multiple comparison test), but plasma insulin concentrations (red symbols) were similar.
(F) Photograph of nude mouse kidneys transplanted with 1000 or 2000 human islets. Arrows point at islet grafts, which were used for quantifications of mass in I and J.
(G and H) Transplantation of 1000 and 2000, but not 500, human islets reversed diabetes in recipient nude mice (n = 3 mice per group). Mice with successful islet engraftment showed human normoglycemic values that were independent of the number of transplanted islets (quantified in H).
(I) Human graft insulin contents were different for the two groups of mice at day 30 (P < 0.05, Student’s t-tests), but plasma insulin concentrations (red symbols) were similar. (J) In mice transplanted with different human (red symbols) and mouse (grey symbols) islet masses, graft insulin content did not correlate with target glycemia (slopes of regression lines not significantly different from 0), indicating that, once above the marginal mass required to achieve glucose homeostasis, islet mass does not impact the glycemic set point.
Artificial manipulation of nervous input changes the glycemic set point established by mouse but not by human islets transplanted into the mouse eye
Our results support the hypothesis that the islet serves as the overall glucostat in the organism. If so, manipulating the physiology of islet grafts should change the glycemic set point. We previously showed that intraocular mouse islet grafts are re-innervated according to their innervation pattern in the pancreas and that the autonomic nervous input to intraocular grafts can be manipulated via the pupillary light reflex (Rodriguez-Diaz et al., 2012). We found that artificially manipulating the nervous input to mouse islet grafts with light changed insulin secretion and normoglycemia in recipient mice (Rodriguez-Diaz et al., 2012), indicating that the glycemic set point can be adjusted by modulating islet function.
We transplanted human islets into the eye and found that the innervation patterns of intraocular islet grafts mimicked those of islets in the pancreas (Rodriguez-Diaz et al., 2011a), that is, human islet grafts were innervated almost exclusively by sympathetic axons mostly targeting blood vessels (Figures 4A and B). By contrast, intraocular mouse islet grafts were densely innervated by parasympathetic axons (Figure 4C), as they are in the pancreas (Rodriguez-Diaz et al., 2011a). Activating parasympathetic input by increasing the ambient illumination did not affect glycemia or glucose tolerance in mice with intraocular human islet grafts (Figure 4D and Figure S3) or in mice with mouse islet grafts under the kidney capsule (Figure 4D), but decreased glycemia in mice with mouse islet grafts in the eye (Figure 4D, see also Rodriguez-Diaz et al., 2012). These results indicate that nervous input to intraocular grafts can be manipulated with light. This manipulation, however, does not affect human islet grafts, which is in line with anatomical findings showing that the parasympathetic innervation of the human islet is sparse (Rodriguez-Diaz et al., 2011a). Our findings thus suggest that the human glycemic set point does not depend on nervous input to the islet.
Figure 4. Modulating nervous input to islet grafts affects glycemia in nude mice transplanted with mouse islets, but not in mice transplanted with human islets.
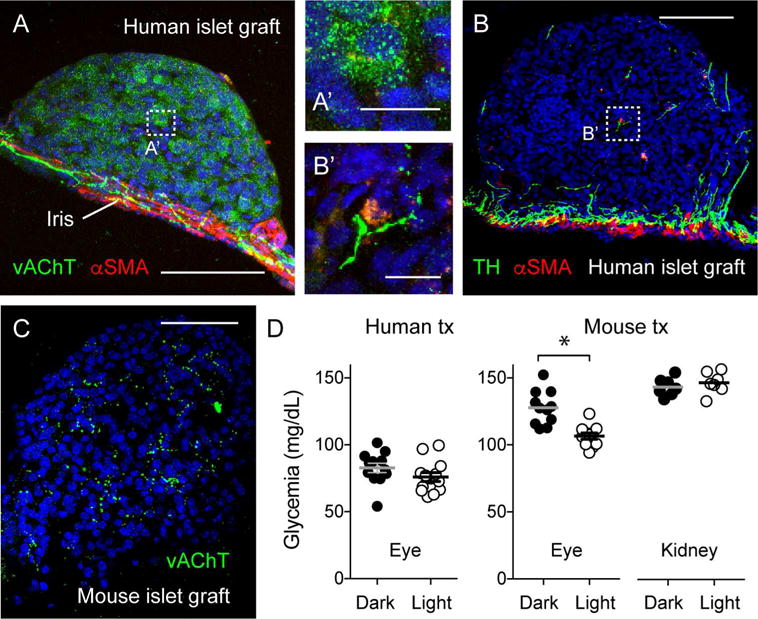
(A and B) Maximal projections of Z-stacks of confocal images of intraocular human islet grafts 90 days after transplantation showing immunostaining for the parasympathetic and sympathetic axon markers vAChT and TH, respectively. Notice that parasympathetic axons of the iris do not turn into the graft and that vAChT is present in endocrine cells. By contrast, some sympathetic axons reach into the islet parenchyma along blood vessels (labeled for αSMA). These staining patterns resemble those of islets in the native human pancreas (Rodriguez-Diaz et al, 2011a, 2011b). A’ and B’ are higher magnification images of regions denoted by boxes in A and B.
(C) In contrast to human islet grafts, C57Bl6J mouse islet grafts showed a high density of parasympathetic axons in the islet parenchyma (see also Rodriguez-Diaz et al, 2012). Scale bars, 50 μm (A-C) and 10 μm (A’ and B’).
(D) Non-fasting glycemic values show that modulating nervous input to human islet grafts via the pupillary light reflex with ambient illumination did not change glycemic levels. By contrast, increased nervous input reduced glycemic levels in mice with intraocular mouse islet grafts, but not in mice with mouse islets transplanted under the kidney capsule (P < 0.05, Student’s t-tests). Values obtained > 2 months after transplanting 1000 human islets into both eyes (n ≥ 12 mice per group) or 300 C7Bl6J mouse islet into the right eye or the kidney of diabetic nude mice (n = 4-5 mice per group).
Beta cell secretion has to be amplified by adjacent alpha cells to establish the human glycemic set point
What are the intrinsic properties of islets from different species that dictate different levels of glycemia? A salient feature of human islets is that they contain a larger proportion of glucagon-secreting alpha cells than mouse islets (Brissova et al., 2005; Cabrera et al., 2006b). Human alpha cells secrete glucagon and acetylcholine, which are strong potentiators of glucose-induced insulin secretion in vitro (Huypens et al., 2000; Rodriguez-Diaz et al., 2011b). We therefore hypothesized that alpha cell input, by increasing the efficacy of beta cell responses to glucose, affects glycemic levels. To test this hypothesis, our strategy was to transplant human islets into diabetic nude mice and, after restoring normoglycemia, inhibit human glucagon receptors with a specific antagonist (L-168,049) that does not affect mouse glucagon receptors (Cascieri et al., 1999; de Laszlo et al., 1999). To block cholinergic signaling we topically applied the muscarinic antagonist tropicamide to mouse eyes with human islet grafts, as previously described (Rodriguez-Diaz et al., 2012). Injection of L-168,049 (50 mg/kg, ip) did not induce changes in the glycemia of control nude mice, but increased glycemia in transplanted mice by ~50 mg/dL (73% change; Figures 5A and B). This treatment decreased plasma human insulin levels by 44% (Figure 5C).
Figure 5. Glycemic values set by insulin secretion from human islet grafts require glucagon input from neighboring alpha cells.
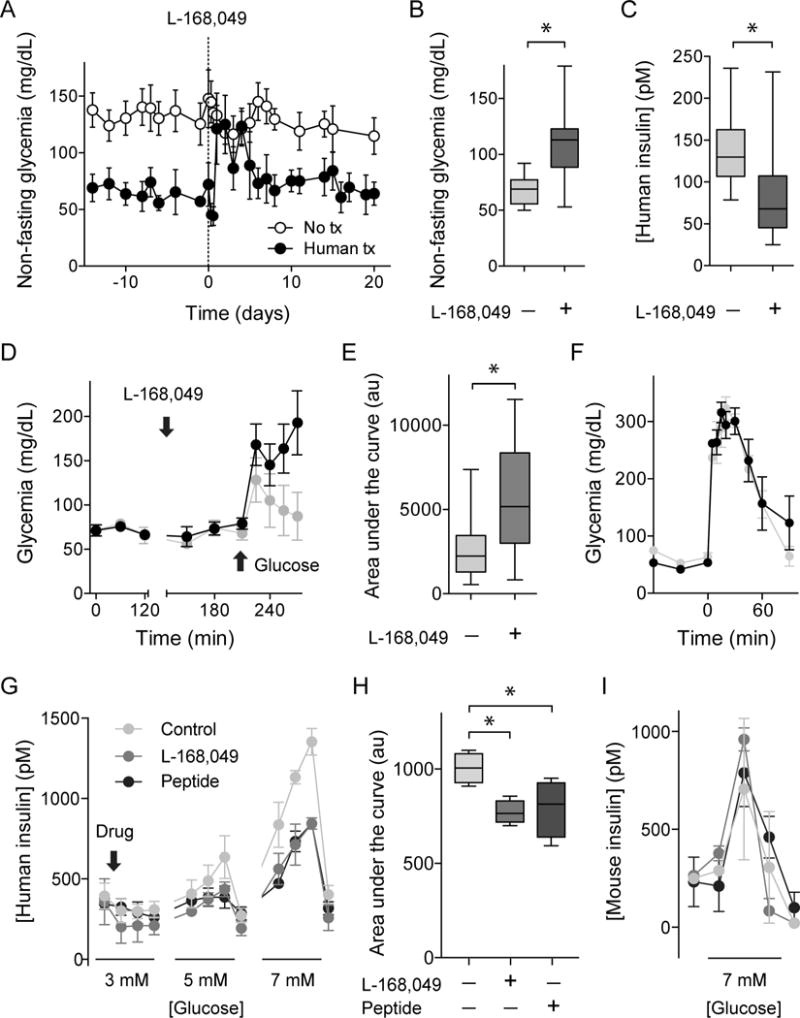
(A-C) Injection of the human-specific glucagon receptor antagonist L169,049 (50 mg/kg, ip, day 0) increased glycemia in nude mice with human islets transplanted into the eye (glycemic levels quantified and compared to levels before treatment in B; n = 6 mice). During treatment with L169,049, human insulin plasma levels were significantly reduced in recipient mice (C).
(D and E) Local application of L169,049 to the eye (4 mM) made transplanted mice glucose intolerant in intaperitoneal glucose tolerance tests (2 g/kg glucose, quantification as area under the curve of glucose excursion shown in E, n = 8 mice).
(F) Local application of the muscarinic antagonist tropicamide (0.5%, 17 mM) did not affect glucose tolerance (4 g/kg glucose) in mice transplanted with human islets (n = 3 mice). Data are shown as average ± SEM and compared with Student’s t-test. Asterisks denote significance (P < 0.05).
(G-I) Perifusion assays to measure insulin secretion showing that the glucagon receptor antagonists L-168,49 (50 nM) and des-His1-[Glu9]-Glucagon (1-29) amide (1μM) diminished glucose-stimulated (3, 5, and 7 mM) insulin secretion from human islets (G and H), but not from mouse islets (I). Antagonists were added at 3 mM glucose concentration (arrow) and maintained throughout the experiment. H shows a quantification (area under curve during stimulation with 5 and 7 mM glucose concentration) of experiments shown in G. Asterisks denote statistical significance (n = 4 islet human donors; P < 0.05; ANOVA followed by multiple comparisons).
We further tested islet graft function by using intraperitoneal glucose tolerance tests and found that inhibiting the human glucagon receptor made recipient mice glucose intolerant (Figures 5D and E). By contrast, tropicamide did not produce changes in glucose tolerance (Figure 5F). We previously showed that the glucagon-like peptide-1 (GLP-1) analog liraglutide, an incretin mimetic, accelerated engraftment and diabetes reversal but did not change the glycemic set point (Abdulreda et al., 2016). These results indicate that local glucagon input, but not cholinergic or incretin input, was needed to produce an adequate beta cell response to the glucose challenge.
To confirm that glucagon input influences insulin secretion from human beta cells, we performed in vitro perifusion studies of hormone secretion. The glucagon receptor antagonists L-168,49 (50 nM) and des-His1-[Glu9]-glucagon (1-29) amide (1 μM) decreased insulin secretion stimulated by step increases in glucose concentration by ~25% (Figures 5G and H). Inhibition of glucose stimulated insulin secretion from human islets measured in vitro with static incubation has been reported for des-His1-[Glu9]-glucagon (1-29) amide (Huypens et al., 2000). These antagonists did not affect insulin secretion from mouse islets (Figure 5I), indicating that the impact of glucagon on insulin secretion is minimal in this mouse strain under these conditions.
It is well established that the percentages of alpha cells vary from islet to islet and from person to person. Although we found that intraocular human islet grafts maintain a typical cytoarchitecture and cellular composition (Figure S4; Cabrera et al., 2006), variations in alpha cell numbers could change the level of glucagon input to beta cells and thus affect glycemia. To address this issue, as well as that of human islet variability in general, we examined the glycemic outcomes of individual islet preparations from > 30 human donors after transplantation into the eye or under the kidney capsule of recipient mice. While we observed variations in glycemia, all transplanted mice showed glycemic levels that were within the typical human range (70-110 mg/dl), and did not reach mouse glycemic levels (> 120 mg/dl, Figure S5). These results indicate that the islet mechanisms that set the glycemic levels are robust and override potential irregularities in alpha cell content, islet quality, transplanted islet numbers, or human donor characteristics. It this context, it should be noted that we found that the mouse strains C57Bl6J and 129X1/SvJ had strikingly different percentages of alpha cells in their islets (Figure S6). 129X1/SvJ mice had a larger percentage of alpha cells (29% versus 15%) and had lower glycemic levels that were transferred by islet transplantation into nude mice (Figure S6). These results suggest that the relative number of alpha cells, and hence the putative levels of intra-islet glucagon, could affect the glycemic set point in mice other than the alpha-cell-poor C57BL6 mouse.
DISCUSSION
Our results establish the pancreatic islet as the dominant player in determining the glycemic set point in the organism. Despite being exposed to non-physiological glycemia for months under the control of the “mismatched” islets, the recipient mice did not or could not deploy mechanisms to compensate for the chronically altered glycemic levels imposed by the engrafted islets. The main conclusion from our studies is that the transplanted islets sense glucose levels and adjust insulin secretion until the organism reaches the species’ glycemic set point. Our results further demonstrate that paracrine glucagon signaling in the islet is critical for the beta cell to secrete the appropriate insulin amounts that sustain the human glycemic set point. This is in line with studies showing that glucagon signaling through glucagon and GLP-1 receptors contributes substantially to the beta cell’s secretory responsiveness and competence by increasing cAMP levels (Bertuzzi et al., 1995; Huypens et al., 2000; Pipeleers et al., 1982; Samols et al., 1965).
Our findings are in sharp contrast to studies showing that the impact of alpha cells on beta cell function is negligible in rodents (King et al., 2007; Moens et al., 2002; Shiota et al., 2013; Thorel et al., 2011). The smaller proportion and spatial segregation of alpha cells in mouse and rat islets likely explains why rodent studies could not have predicted that the glycemic set point depends on islet glucagon signaling. Moreover, mouse alpha cells have an activation threshold [~70 mg/dL; (Malmgren and Ahrén, 2015)] that is much lower than the mouse glycemic set point (~140 mg/dL) and thus cannot contribute to set glycemic target values. By contrast, in the human islet under normoglycemic conditions (~90 mg/dl), alpha cell activation overlaps with beta cell activation. Indeed, when isolated human islets are exposed to step increases or decreases in glucose concentration, it is clear that both insulin and glucagon secretion are stimulated at 90 mg/dl (Figure 6A). This is contrary to the general notion that glucagon and insulin secretion are mutually exclusive. Although seemingly counterintuitive because these hormones have antagonistic effects on plasma glucose levels, these findings make sense if we consider glucagon a local paracrine signal that amplifies insulin secretion to stabilize glucose levels.
Figure 6. Alpha cells and beta cells of the human islet cooperate to maintain the glycemic set point.
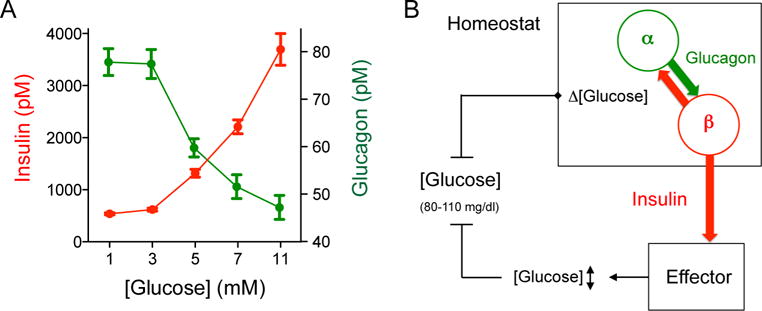
(A) Glucose concentration-response relationship for insulin and glucagon secretion from human islets. Values were obtained in dynamic perifusions and represent the secretory levels during step increases in glucose concentration (n = 4 human pancreas preparations). Notice that glucagon and insulin secretion overlap substantially around 5 mM glucose concentration (equivalent to 90 mg/dl). Glucagon secretion at 5 mM was significantly different from that at 11 mM glucose concentration (one-way ANOVA followed by Tukey’s multiple comparison tests).
(B) Cartoon depicting our view of the glucose homeostat. Fluctuations in glucose concentration (Δ [glucose]) around the glycemic set point are sensed by alpha and beta cells that continuously influence each other to fine-tune insulin secretion. Insulin serves as the control signal that regulates glucose uptake in effector organs (e.g. liver, muscles, and adipose tissue) to maintain normoglycemia. Without paracrine glucagon input to beta cells, the glucose homeostat fails to achieve target glycemic levels.
The fluid dynamics in the native pancreas may hinder glucagon to reach local concentrations that activate or prime beta cells. Indeed, an in vitro study using the perfused rat pancreas model showed that glucose-induced insulin secretion occurs independently of an amplifying signal from neighboring alpha cells (Moens et al., 2002). While determining local glucagon concentration in the islet in vivo is beyond what current methods can detect, there is nevertheless a strong case to be made that local glucagon amplifies beta cell activity in the living organism. We found that local glucagon affects insulin secretion using an in vivo model that reproduces blood flow, capillarity, and ultrastructural features of the islet vasculature in the pancreas (Speier et al., 2008a; Almaça et al, 2014). In the human islet, the percentage of alpha cells is higher, alpha cells and beta cells are aligned randomly along blood vessels, and most beta cells face alpha cells (> 70%, Cabrera et al., 2006), making it likely for beta cells to be directly exposed to glucagon secretion. In mice, glycemic levels are lower when the percentages of alpha cells are higher (Figure S6), suggesting that if glucagon input is increased it may lead to similar effects. The strong insulinotropic effects of glucagon (Samols et al., 1965; Huypens et al, 2000; present study), the increased insulin secretion from beta cells overexpressing glucagon receptors (Gelling et al., 2009), and the association of glucagon receptor mutations with reduced insulin secretion and type 2 diabetes (Hager et al, Nature Genetics 1995; Hansen et al., Diabetes, 1996), all lend further support to the notion that intra-islet glucagon influences insulin secretion.
Glucagon is a major hyperglycemic hormone in the organism that counters decreases in plasma glucose levels. Indeed, glucagon secretion provides the first line of defense in glucose counterregulation (Cryer et al., 2003). Glucagon, however, has also been known for decades as a strong amplifier of insulin secretion (Samols et al., 1965), whose effects are opposite to those of glucagon. One answer to this conundrum is to consider glucagon secretion as a mechanism that participates in two different regulatory circuits. In our view, glucagon secretion during normoglycemia reaches concentrations in the islet that amplify insulin secretion from neighboring beta cells (Figure 6B). As we show here, this local secretion is needed to maintain the human glycemic set point. Glucagon secretion under these circumstances is probably not strong enough to reach plasma levels that produce systemic responses. By contrast, when glycemia drops, glucagon secretion becomes strong enough to produce systemic, hyperglycemic effects, but cannot stimulate beta cells because glucose levels are no longer permissive for insulin secretion.
How can the role of glucagon secretion during normoglycemia be described in terms of homeostatic control? A regulatory system that maintains glucose homeostasis must include sensors, disturbance detectors, an integrator, and effectors. It is clear that both alpha and beta cells are specialized glucose detectors endowed with mechanisms to sense glucose. Any change (i.e. disturbance) in glucose concentration, the regulated variable, produces changes in alpha and beta cell physiology that can be considered disturbance signals (e.g. cell membrane depolarization or hyperpolarization, changes in intracellular Ca2+ concentration). These error signals ultimately converge on insulin granule exocytosis, which is the eventual integrator (controller) that uses the disturbance signals to send out the control signal insulin to the effector organs (liver, muscles, and fat). By increasing cAMP concentration in beta cells, glucagon secretion produces a disturbance signal that is one of the input signals for insulin exocytosis. When activated during glucose counterregulation, by contrast, alpha cells become integrators (controllers) themselves, and glucagon acts as a control signal that directly instructs effector organs to produce and release glucose.
The glycemic set point likely arises from the dynamic interactions between alpha and beta cells. Mathematical models of glucose homeostasis predict that interactions between alpha and beta cells are beneficial because they provide more stable, efficient and accurate control of glycemia (Jo et al., 2009; Koeslag et al., 2003). In these models, the interactions between alpha and beta cells need to be asymmetric to build a negative feedback loop for both cells. Indeed, glucagon and acetylcholine, both secreted from alpha cells in human islets, stimulate insulin secretion, whereas all known secretory products of beta cells inhibit glucagon secretion (Caicedo, 2013). This arrangement favors stability by attenuating exacerbated responses and works best with the prevailing small fluctuations in plasma glucose levels. As we show here, interrupting this feedback loop by inhibiting glucagon receptors on beta cells acutely destabilizes the glycemic set point, thus confirming the predictions made by the mathematical models.
Islet regulation of glucose homeostasis can be supplemented with feedforward mechanisms that temporarily override the glucostat. These anticipatory control mechanisms ensure that the islet is prepared for upcoming disturbances. Thus, incretin hormones produce anticipatory responses in the islet to food in the gut, and the autonomic nervous system produces anticipatory responses to food ingestion, to intense muscular activity, as well as to several other cues. Circulating signals derived from other organs may also affect islet function [e.g. bile acids from the liver, (Seyer et al., 2013)]. However, our transplantation results indicate that these additional regulators do not supersede the islet’s intrinsic ability to establish the glycemic set point. It can be argued that human and monkey islet grafts may not respond to mouse cues, but our results show that islets from C57Bl6 mice impose higher glycemic levels on recipient nude mice without being affected by circulating factors or other compensatory mechanisms.
Based on our results, we conclude that the human glucostat depends on the functional cooperation between alpha and beta cells, not solely on the beta cell. This has implications for therapies aimed at reconstituting the beta cell population to treat diabetes because the glycemic levels set by beta cells without glucagon input would likely be pre-diabetic. In addition, new approaches to inhibit the contribution of glucagon to hyperglycemia need to be reexamined because inhibiting glucagon receptors systemically may also eliminate this crucial local input to the beta cell.
Limitations of study
In our study, we used an experimental transplantation model in which rodents are used as recipients of islets. Although transplantation of human islets into mice nicely recapitulates in vivo human islet anatomy and physiology (Figures 2G-2I and 4, Figures S1 and S4), it is also clear that our model does not mimic all aspects that contribute to glucose homeostasis in the human being. Yet it is important to study the effects of human islets or of their best surrogate, non-human primate islets, in the living organism because primate islets differ substantially from those in rodents (Brissova et al, Cabrera et al, Dolensek et al, 2015). To implement a more comprehensive and clinically relevant model of glucose metabolism in primates, we have successfully transplanted monkey islets into monkey eyes (Diez et al, 2017). A further limitation of our study is that our conclusions are based mainly on pharmacological manipulation of glucagon receptors. Fortunately, the receptor antagonist we used has a higher affinity for the human glucagon receptor and thus we could avoid confounding effects on glucagon receptors in the mouse. Genetic manipulation of glucagon receptors in human islet grafts was not possible in our model.
STAR Methods Text
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Rayner Rodriguez-Diaz (r.diaz4@med.miami.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
All animal procedures were performed under protocols approved by the Institutional Animal Care Use Committee of the University of Miami. All animals were housed in virus antibody-free (VAF) rooms and kept in micro-isolated cages (5 mice per cage) with free access to autoclaved food and water.
Immune-compromised mice used as islet recipient
Female athymic nude mice (Hsd:Athymic Nude-nu; age: 8 weeks/weight: 22 g) were purchased from Envigo RMS, Inc. formerly Harlan. These mice were rendered diabetic (see below) and then transplanted with isolated human islets.
Mice used as islet donors
Male C57Bl6J (JAX stock #000664; age: 12 weeks/weight: ≥ 26 g) and 129X1/SvJ (JAX stock #000691; age: 12 weeks/weight: ≥ 26 g) mice were purchased from The Jackson Laboratory (JAX). The pancreas from these mice was processed for islet isolation as described elsewhere (Cabrera et al., 2006a).
Monkey islet preparations
Cynomolgus monkeys (Macaca fascicularis, age: > 4 years) were obtained from Charles River BRF, Inc. (Houston, TX) and were negative for TB, Herpes B, SRV, SIV and STLV-1. Pair-housed monkeys were supplied with water ad libitum and fed twice daily. The University of Miami complies with the Animal Welfare Act of 1966 (PL89-544) as amended by the Welfare Act of 1970 (PL91-279), adheres to the principals stated in the guide for the care and use of laboratory animals – NIH publication #85-23 (revised) and is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Islet isolation, culture and quality control assessment were performed as previously described (Kenyon et al., 1999).
Human islet preparations
Human pancreatic islets were obtained through the cGMP/cGTP Cell Processing Facility (Diabetes Research Institute, Miami), City of Hope Integrated Islet Distribution Program (IIDP) and Prodo Laboratories, Inc. The experiments were conducted with islets preparations that were obtained from non-diabetic, cadaveric donors (age: 16 – 69 years, 39 % females). Human islets were incubated at 22°C in serum-free Miami media supplemented with glutathione (1 mg/100 ml) (Bottino et al., 1997).
METHOD DETAILS
Diabetes induction in recipient mice
Acute diabetes induction in the mice was achieved via single intravenous injection of streptozotocin (STZ; 150–220 mg/kg). When needed, up to two additional doses of STZ were administered at least 3 days apart to produce frank diabetes (three consecutive readings of nonfasting glycemia > 300 mg/dL).
Islet transplantation
Islet transplantation into the anterior chamber of the eye of diabetic nude mice and under the kidney capsule was performed as previously described (Abdulreda et al., 2016; Ichii et al., 2005; Speier et al., 2008a; Speier et al., 2008b). A total of 1,000 human islets (500 IEQs in each eye or 1,000 in one kidney), 1,000 monkey islets (kidney), or 300 mouse islets (eye or kidney) were transplanted into confirmed hyperglycemic nude mouse recipients. In addition, we transplanted different numbers of mouse islets (75, 150, 300, and 500) and human islets (1,000 and 2,000) to assess effects of islet mass on glycemia in the recipient mouse.
To measure total insulin content, mouse and human islet grafts were dissected out of the eyes and kidneys, respectively. Islet grafts were homogenized in 1 ml acid-ethanol (95% ethanol and 10.2 N HCl at a ratio of 50:1) by an Ultrasonic Homogenizer (Biologic) for 2 min applying 20 pulses. After an overnight incubation at 4°C, the extracts were centrifuged at 650g for 30 min at 4°C. Human insulin and mouse insulin concentrations were measured with ELISAs (Mercodia).
Glucagon-receptor antagonist experiment
Recipient treatment with the glucagon receptor antagonist L-168,049 (50 mg/kg/day, i.p., or 4 mM in 0.1% DMSO, topically applied to the eye) or with the muscarinic antagonist tropicamide (1% ophthalmic solution topically applied to the eye; Akorn Inc.) was started after full engraftment of human islets (> 1 month after transplantation). These doses did not affect glycemia in non-transplanted control mice (Figure 2).
The glucagon receptor antagonist L-168,049 was purchased from Tocris (Cat. Nr. 2311). L-168,049 is a potent and selective, non-competitive antagonist of the human glucagon receptor. It binds with high affinity to human glucagon receptors (IC50 = 3.7 nM) and has a moderate affinity to murine glucagon receptors (IC50 = 63 nM). Even at relatively high doses (50 mg/kg, per os, which leads to systemic concentrations of 15 μM; (de Laszlo et al., 1999)), L-168,049 does not inhibit glucagon-stimulated increases in glycemia in mice (Cascieri et al., 1999), indicating that its in vivo activity at the murine receptor is limited. The in vivo effects of L-168,049 could therefore be attributed to its action on glucagon receptors in human islet grafts.
In vivo imaging of islet graft volume
Islet graft volume was measured using laser backscatter of the islets transplanted in the eye as previously described in detail (Abdulreda et al, 2011). In brief, 3D confocal images (z-stacks) spanning the full thickness of the whole islet graft were acquired using 5X air objective in a Leica SP5 fluorescence confocal microscope system using a 633 nm laser (in backscatter/reflection mode) to visualize the islets and a 561nm laser (in fluorescence mode) to visualize blood vessels by TRITC-labeled dextran (2,000 kD M.W.; Invitrogen) injected intravenously (0.1 ml of 10 mg/mL solution). Volumetric measurements were obtained in the images (without any processing) in Volocity software (Perkin Elmer; USA) using built-in algorithms to detect the islets (in the backscatter channel) and the blood vessels (in the fluorescence channel). Islet graft volume was calculated by subtracting the blood vessel volume measured within the islets from the total measured volume of the corresponding islets for each mouse.
Mice follow up and blood sampling
Animals were weighed two to three times per week, and blood glucose was measured using portable glucometers (OneTouchUltra2). Blood samples (~100 mL) for hormone measurements during glucose challenge were collected from the tail vein into tubes containing K2 EDTA and immediately supplemented with 5 ml aprotinin (10,000 KIU/ml). Plasma levels of human- or mouse- specific insulin and cross-reactive glucagon were measured with ELISAs (Mercodia; see key resources table).
To exclude any residual function of the native pancreas (i.e., inadequate diabetes induction or islet regeneration), which may represent a confounding bias when assessing human islet function in vivo, the graft-bearing eyes (enucleation) and kidneys (nephrectomy) were surgically removed under general anesthesia (isoflurane 2% mix with oxygen, inhalation to effect). The eyes were carefully resected, and the orbit was packed with sterile gauze saturated with neomycin ointment to prevent bleeding. For nephrectomy, we made a small incision in the left flank region, extruded the kidney, and tied the vascular pedicle with nonresorbable stitch. The urether was dissected and the kidney removed. After confirming that proper hemostasis has been achieved, the muscle and skin were sutured. Animals were monitored post-operatively to confirm prompt return to hyperglycemia (non-fasting glycemic values > 250mg/dl). Animals showing sustained euglycemia after removal of the graft-bearing eyes or kidney were excluded from the analyses.
Intraperitoneal glucose- and insulin-tolerance tests
Intraperitoneal glucose-tolerance tests (IPGTTs) were performed after overnight fasting. Mice were injected with 200–300 ml glucose solution (2 or 4 g/kg body weight) and blood glucose (IPGTT) was monitored at predetermined time points after the injection. The higher-concentration glucose bolus was used to amplify the glucose excursion during challenges with glucose or insulin because the typical 2 g/kg bolus induced small changes in glycemia in nude mice transplanted with human islets. In our hands, mice transplanted with human islets typically returned to fasting glycemia levels within 60 min during IPGTTs. To induce hypoglycemia, we injected transplanted mice with insulin (i.p., 0.75 U/kg).
Dynamic measurements of hormone secretion
A high-capacity, automated perifusion system was used to dynamically measure hormone secretion from pancreatic islets (Biorep Perifusion V2.0.0, Miami, FL). A low pulsatility peristaltic pump pushed HEPES-buffered solution (mM: 125 NaCl, 5.9 KCl, 2.56 CaCl2, 1 MgCl2, 25 HEPES, and 0.1% BSA; pH 7.4; and a perifusion rate of 100 μL/min) through a column containing 100 pancreatic islets immobilized in Bio-Gel P-4 Gel (BioRad, Hercules, CA). Glucose concentration was increased stepwise from 1, 3, 5, 7, to 11 mM (10 minutes each) with or without the glucagon receptor antagonists L-168,49 (50 nM) or des-His1-[Glu9]-Glucagon (1-29) amide (1μM). The perifusate was collected in an automatic fraction collector designed for a 96 well plate format. The columns containing the islets and the perifusion solutions were kept at 37°C, and the perifusate in the collecting plate was kept at < 4°C. Perifusates were collected every minute. Human insulin and mouse insulin release was determined in the perifusate with ELISAs (Mercodia).
Immunohistochemistry
Mouse pancreatic tissues from C57Bl6J and 129X1/SvJ mice and eyes containing islet grafts were fixed overnight in 4% PFA, cryoprotected in a sucrose gradient (10, 20 and 30% w/w sucrose), and frozen in Tissue-Tek Optimal Cutting Temperature (OCT) compound before cryosectioning (−20°C). After a rinse with PBS-Triton X-100 (0.3%), pancreatic or eye sections (40 m) were incubated in blocking solution (PBS-Triton X-100 0.3% and Universal Blocker Reagent; Biogenex, San Ramon, CA). Thereafter, sections were incubated for 48 h (20° C) with primary antibodies diluted in blocking solution. We immunostained for the endocrine markers insulin (1:2000, Accurate, cat. nr. ICCB39-1), glucagon (1:2000, Sigma, cat nr. G2654-5), and somatostatin (1;1000, Chemicon, cat nr. MAB354), for the vascular marker alpha smooth muscle actin (αSMA,1:250, Sigma, cat. nr. A5228), and for a sympathetic (tyrosine hydroxylase, 1:500, Millipore, cat. nr. AB152) and a parasympathetic fiber marker (vesicular acetylcholine transporter 1:1000, Synaptic Systems, cat. nr. 139103). Immunostaining was visualized by using Alexa Fluor conjugated secondary antibodies (1:500 in PBS; 16 h at 20°C; Invitrogen, Carlsbad, CA). Cell nuclei were stained with DAPI. Slides were mounted with Vectashield mounting medium (Vector Laboratories). Confocal images of immunostained sections were acquired on an inverted laser-scanning confocal microscope (Leica TCS SP5) with LAS AF software using a 63X oil immersion objective (NA 1.4) (Leica Microsystems).
QUANTIFICATION AND STATISTICAL ANALYSIS
For statistical comparisons, we used GraphPad Prism 5.0 and performed Student’s t test or one-way analysis of variance (ANOVA) followed by a Tukey’s Multiple Comparison Test. We considered statistical significance when P values were lower than 0.05. All data were assessed to ensure normal distribution and equal variance between groups. We present data in the manuscript as average ± SD (Figures 1 and 2) or ± SEM (Figures 3-5) or as box and whisker plots (minimum, first quartile, third quartile, maximum). The statistical details of experiments can be found in the figure legends of the manuscript.
Supplementary Material
Highlights.
Species-specific glycemic set points are transferred with islet transplantation
The pancreatic islet thus carries the information to impose the glycemic set point
The glycemic set point does not depend on islet graft mass
Control of glycemia by insulin secretion relies on paracrine input from alpha cells
Acknowledgments
This work was supported by funds from the Diabetes Research Institute Foundation (DRIF), grants from the NIH (F32DK083226 (M.H.A.), K01DK097194 (M.H.A.), R01DK084321 (A.C.), R56DK084321 (A.C.), R01DK111538 (A.C.), R01DK113093 (A.C.), R21ES025673 (A.C.), American Diabetes Association Innovative grant 1-17-ICTS-052 (A.C.), R21DK114418 (R.R.-D.) the Swedish Diabetes Association Fund, the Swedish Research Council, Novo Nordisk Foundation, the Family Erling-Persson Foundation, Strategic Research Program in Diabetes at Karolinska Institutet, the ERC-2013-AdG 338936-BetaImage, the Family Knut and Alice Wallenberg Foundation, Skandia Insurance Company Ltd, Diabetes and Wellness Foundation, the Bert von Kantzow Foundation, and the Stichting af Jochnick Foundation. We acknowledge the organ donors and their families for enabling our research with human pancreatic islets.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
It is not known if there is a leading organ that maintains blood glucose levels within the narrow physiological range. Rodriguez-Diaz and colleagues show that the pancreatic islet serves as the systemic glucostat and that paracrine glucagon input from alpha cells is essential for setting the glycemic set point. Therapeutic strategies using glucagon receptor antagonists to lower glycemia should thus be reassessed.
AUTHOR CONTRIBUTIONS
R.R.-D., D.M., M.H.A., N.K., C.R., A.P., A.C., and P.-O.B designed the study. R.R.-D., J.W., D.M., B.L, I.B.L., M.H.A., D.B., and A.C. designed and conducted experiments, analyzed and interpreted data. R.R.-D., A.C., and P.-O.B. initiated the study, interpreted data, and wrote and edited the manuscript. All authors reviewed the final draft of the manuscript.
COMPETING FINANCIAL INTERESTS
P.-O.B. is cofounder and CEO of Biocrine, an unlisted biotech company that is using the anterior chamber of the eye technique as a research tool. B.L., I.L., and M.H.A. are consultants for the same company.
A.P. is currently employed at the National Institutes of Health (NIH). The opinions expressed in this article are the author’s own and do not necessarily reflect the views of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
References
- Abdulreda MH, Rodriguez-Diaz R, Caicedo A, Berggren PO. Liraglutide Compromises Pancreatic β Cell Function in a Humanized Mouse Model. Cell Metab. 2016;23:541–546. doi: 10.1016/j.cmet.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almaça J, Molina J, Arrojo e Drigo R, Abdulreda MH, Jeon WB, Berggren PO, Caicedo A, Nam HG. Young capillary vessels rejuvenate aged pancreatic islets. Proc Natl Acad Sci USA. 2014;111:17612–17617. doi: 10.1073/pnas.1414053111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertuzzi F, Berra C, Socci C, Davalli AM, Calori G, Freschi M, Piemonti L, De Nittis P, Pozza G, Pontiroli AE. Glucagon improves insulin secretion from pig islets in vitro. J Endocrinol. 1995;147:87–93. doi: 10.1677/joe.0.1470087. [DOI] [PubMed] [Google Scholar]
- Bosco D, Armanet M, Morel P, Niclauss N, Sgroi A, Muller Y, Giovannoni L, Parnaud G, Berney T. Unique arrangement of alpha- and beta-cells in human islets of Langerhans. Diabetes. 2010;59:1202–1210. doi: 10.2337/db09-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottino R, Inverardi L, Valente U, Ricordi C. Serum-free medium and pyruvate improve survival and glucose responsiveness of islet beta cells in culture. Transplant Proc. 1997;29:1978. doi: 10.1016/s0041-1345(97)00191-7. [DOI] [PubMed] [Google Scholar]
- Brissova M, Fowler M, Nicholson W, Chu A, Hirshberg B, Harlan D, Powers A. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem. 2005;53:1087–1097. doi: 10.1369/jhc.5C6684.2005. [DOI] [PubMed] [Google Scholar]
- Cabrera O, Berman D, Kenyon N, Ricordi C, Berggrern P, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo A. Paracrine and autocrine interactions in the human islet: more than meets the eye. Semin Cell Dev Biol. 2013;24:11–21. doi: 10.1016/j.semcdb.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll P, Zeng Y, Alejandro R, Starzl T, Ricordi C. Glucose homeostasis is regulated by donor islets in xenografts. Transplant Proc. 1992;24:2980–2981. [PMC free article] [PubMed] [Google Scholar]
- Cascieri MA, Koch GE, Ber E, Sadowski SJ, Louizides D, de Laszlo SE, Hacker C, Hagmann WK, MacCoss M, Chicchi GG, et al. Characterization of a novel, non-peptidyl antagonist of the human glucagon receptor. J Biol Chem. 1999;274:8694–8697. doi: 10.1074/jbc.274.13.8694. [DOI] [PubMed] [Google Scholar]
- Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26:1902–1912. doi: 10.2337/diacare.26.6.1902. [DOI] [PubMed] [Google Scholar]
- Davalli AM, Ogawa Y, Scaglia L, Wu YJ, Hollister J, Bonner-Weir S, Weir GC. Function, mass, and replication of porcine and rat islets transplanted into diabetic nude mice. Diabetes. 1995;44:104–111. doi: 10.2337/diab.44.1.104. [DOI] [PubMed] [Google Scholar]
- de Laszlo SE, Hacker C, Li B, Kim D, MacCoss M, Mantlo N, Pivnichny JV, Colwell L, Koch GE, Cascieri MA, et al. Potent, orally absorbed glucagon receptor antagonists. Bioorg Med Chem Lett. 1999;9:641–646. doi: 10.1016/s0960-894x(99)00081-5. [DOI] [PubMed] [Google Scholar]
- Diez JA, Arrojo E Drigo R, Zheng X, Stelmashenko OV, Chua M, Rodriguez-Diaz R, Fukuda M, Kohler M, Leibiger I, Tum SBB, Ali Y, Augustine GJ, Barathi VA, Berggren PO. Pancreatic islet blood flow dynamics in primates. Cell Rep. 2017;8:1490–1501. doi: 10.1016/j.celrep.2017.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolensek J, Rupnik MS, Stozer A. Structural similarities and differences between the human and the mouse pancreas. Islets. 2015;7:e1024405. doi: 10.1080/19382014.2015.1024405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhy LS, Du Z, Zeng Q, Veldhuis PP, Johnson ML, Brayman KL, McCall AL. Amplification of pulsatile glucagon counterregulation by switch-off of alpha-cell-suppressing signals in streptozotocin-treated rats. Am J Physiol Endocrinol Metab. 2008;295:E575–585. doi: 10.1152/ajpendo.90372.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelling RW, Vuguin PM, Du XQ, Cui L, Rømer J, Pederson RA, Leiser M, Sørensen H, Holst JJ, Fledelius C, Johansen PB, Fleischer N, McIntosh CH, Nishimura E, Charron MJ. Pancreatic beta-cell overexpression of the glucagon receptor gene results in enhanced beta-cell function and mass. Am J Physiol Endocrinol Metab. 2009;297:E695–707. doi: 10.1152/ajpendo.00082.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou HM, Mandel TE. Transplanted fetal pancreas allografts regulate blood glucose to donor-strain levels. Transplant Proc. 1987;19:2922–2925. [PubMed] [Google Scholar]
- Graham ML, Bellin MD, Papas KK, Hering BJ, Schuurman HJ. Species incompatibilities in the pig-to-macaque islet xenotransplant model affect transplant outcome: a comparison with allotransplantation. Xenotransplantation. 2011;18:328–342. doi: 10.1111/j.1399-3089.2011.00676.x. [DOI] [PubMed] [Google Scholar]
- Hager J, Hansen L, Vaisse C, Vionnet N, Philippi A, Poller W, Velho G, Carcassi C, Contu L, Julier C, Cambien F, Passa P, Lathrop M, Kindsvogel W, Demenais F, Nishimura E, Froguel P. A missense mutation in the glucagon receptor gene is associated with non-insulin-dependent diabetes mellitus. Nat Genet. 1995;9:299–304. doi: 10.1038/ng0395-299. [DOI] [PubMed] [Google Scholar]
- Hansen LH, Abrahamsen N, Hager J, Jelinek L, Kindsvogel W, Froguel P, Nishimura E. The Gly40Ser mutation in the human glucagon receptor gene associated with NIDDM results in a receptor with reduced sensitivity to glucagon. Diabetes. 1996;45:725–730. doi: 10.2337/diab.45.6.725. [DOI] [PubMed] [Google Scholar]
- Henquin J, Dufrane D, Nenquin M. Nutrient control of insulin secretion in isolated normal human islets. Diabetes. 2006;55:3470–3477. doi: 10.2337/db06-0868. [DOI] [PubMed] [Google Scholar]
- Huypens P, Ling Z, Pipeleers D, Schuit F. Glucagon receptors on human islet cells contribute to glucose competence of insulin release. Diabetologia. 2000;43:1012–1019. doi: 10.1007/s001250051484. [DOI] [PubMed] [Google Scholar]
- Ichii H, Inverardi L, Pileggi A, Molano R, Cabrera O, Caicedo A, Messinger S, Kuroda Y, Berggren PO, Ricordi C. A novel method for the assessment of cellular composition and beta-cell viability in human islet preparations. Am J Transplant. 2005;5:1635–1645. doi: 10.1111/j.1600-6143.2005.00913.x. [DOI] [PubMed] [Google Scholar]
- Jo J, Choi MY, Koh DS. Beneficial effects of intercellular interactions between pancreatic islet cells in blood glucose regulation. J Theor Biol. 2009;257:312–319. doi: 10.1016/j.jtbi.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Kenyon NS, Chatzipetrou M, Masetti M, Ranuncoli A, Oliveira M, Wagner JL, et al. Long-term survival and function of intrahepatic islet allografts in rhesus monkeys treated with humanized anti-CD154. Proc Natl Acad Sci U S A. 1999;96:8132–8137. doi: 10.1073/pnas.96.14.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AJ, Fernandes JR, Hollister-Lock J, Nienaber CE, Bonner-Weir S, Weir GC. Normal relationship of beta- and non-beta-cells not needed for successful islet transplantation. Diabetes. 2007;56:2312–2318. doi: 10.2337/db07-0191. [DOI] [PubMed] [Google Scholar]
- Koeslag JH, Saunders PT, Terblanche E. A reappraisal of the blood glucose homeostat which comprehensively explains the type 2 diabetes mellitus-syndrome X complex. J Physiol. 2003;549:333–346. doi: 10.1113/jphysiol.2002.037895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmgren S, Ahrén B. Deciphering the Hypoglycemic Glucagon Response: Development of a Graded Hyperinsulinemic Hypoglycemic Clamp Technique in Female Mice. Endocrinology. 2015;156:3866–3871. doi: 10.1210/EN.2015-1314. [DOI] [PubMed] [Google Scholar]
- Matschinsky FM, Magnuson MA, Zelent D, Jetton TL, Doliba N, Han Y, Taub R, Grimsby J. The network of glucokinase-expressing cells in glucose homeostasis and the potential of glucokinase activators for diabetes therapy. Diabetes. 2006;55:1–12. [PubMed] [Google Scholar]
- Meng F, Zhu L, Huang W, Irwin DM, Zhang S. Bats: Body mass index, forearm mass index, blood glucose levels and SLC2A2 genes for diabetes. Sci Rep. 2016;6:29960. doi: 10.1038/srep29960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens K, Berger V, Ahn JM, Van Schravendijk C, Hruby VJ, Pipeleers D, Schuit F. Assessment of the role of interstitial glucagon in the acute glucose secretory responsiveness of in situ pancreatic beta-cells. Diabetes. 2002;51:669–675. doi: 10.2337/diabetes.51.3.669. [DOI] [PubMed] [Google Scholar]
- Pipeleers D, in’t Veld PI, Maes E, Van De Winkel M. Glucose-induced insulin release depends on functional cooperation between islet cells. Proc Natl Acad Sci U S A. 1982;79:7322–7325. doi: 10.1073/pnas.79.23.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Diaz R, Abdulreda MH, Formoso AL, Gans I, Ricordi C, Berggren PO, Caicedo A. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab. 2011a;14:45–54. doi: 10.1016/j.cmet.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Diaz R, Dando R, Jacques-Silva MC, Fachado A, Molina J, Abdulreda MH, Ricordi C, Roper SD, Berggren PO, Caicedo A. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat Med. 2011b;17:888–892. doi: 10.1038/nm.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Diaz R, Speier S, Molano RD, Formoso A, Gans I, Abdulreda MH, Cabrera O, Molina J, Fachado A, Ricordi C, et al. Noninvasive in vivo model demonstrating the effects of autonomic innervation on pancreatic islet function. Proc Natl Acad Sci U S A. 2012;109:21456–21461. doi: 10.1073/pnas.1211659110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samols E, Marri G, Marks V. Promotion of insulin secretion by glucagon. Lancet. 1965;2:415–416. doi: 10.1016/s0140-6736(65)90761-0. [DOI] [PubMed] [Google Scholar]
- Schermerhorn T. Normal glucose metabolism in carnivores overlaps with diabetes pathology in non-carnivores. Front Endocrinol (Lausanne) 2013;4:188. doi: 10.3389/fendo.2013.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuit FC, Huypens P, Heimberg H, Pipeleers DG. Glucose sensing in pancreatic beta-cells: a model for the study of other glucose-regulated cells in gut, pancreas, and hypothalamus. Diabetes. 2001;50:1–11. doi: 10.2337/diabetes.50.1.1. [DOI] [PubMed] [Google Scholar]
- Seyer P, Vallois D, Poitry-Yamate C, Schütz F, Metref S, Tarussio D, Maechler P, Staels B, Lanz B, Grueter R, et al. Hepatic glucose sensing is required to preserve β cell glucose competence. J Clin Invest. 2013;123:1662–1676. doi: 10.1172/JCI65538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi ZQ, Rastogi KS, Lekas M, Efendic S, Drucker DJ, Vranic M. Glucagon response to hypoglycemia is improved by insulin-independent restoration of normoglycemia in diabetic rats. Endocrinology. 1996;137:3193–3199. doi: 10.1210/endo.137.8.8754739. [DOI] [PubMed] [Google Scholar]
- Shiota C, Prasadan K, Guo P, El-Gohary Y, Wiersch J, Xiao X, Esni F, Gittes GK. α-Cells are dispensable in postnatal morphogenesis and maturation of mouse pancreatic islets. Am J Physiol Endocrinol Metab. 2013;305:E1030–1040. doi: 10.1152/ajpendo.00022.2013. [DOI] [PubMed] [Google Scholar]
- Speier S, Nyqvist D, Cabrera O, Yu J, Molano R, Pileggi A, Moede T, Kohler M, Wilbertz J, Leibiger B, et al. Noninvasive in vivo imaging of pancreatic islet cell biology. Nature Medicine. 2008a;14:574–578. doi: 10.1038/nm1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speier S, Nyqvist D, Köhler M, Caicedo A, Leibiger IB, Berggren PO. Noninvasive high-resolution in vivo imaging of cell biology in the anterior chamber of the mouse eye. Nat Protoc. 2008b;3:1278–1286. doi: 10.1038/nprot.2008.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorel F, Damond N, Chera S, Wiederkehr A, Thorens B, Meda P, Wollheim CB, Herrera PL. Normal glucagon signaling and β-cell function after near-total α-cell ablation in adult mice. Diabetes. 2011;60:2872–2882. doi: 10.2337/db11-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


