Abstract
Background:
Balloon angioplasty revolutionised percutaneous treatment for coronary ar-tery disease four decades ago, but vessel-threatening dissections, elastic recoil and restenosis were major drawbacks to an otherwise successful long-lasting intervention. Subsequent advances with bare metal stents and then drug eluting stents followed, aiming to mitigate the risks of acute vessel closure and restenosis. However, stent implantation often necessitates dual antiplatelet therapy for a pro-longed period of time, which in itself can lead to adverse outcomes, especially in the frail elderly pop-ulation at higher risk of bleeding. More recently, bioabsorbable stents have been implemented in clini-cal practice enabling earlier intimal coverage of the stent and apposition.
However, another addition to the armamentarium of percutaneous coronary intervention is the use of drug-coated balloons without the need for deploying any coronary stents or scaffolds. Drug-coated balloons are semi-compliant balloons coated with an antiproliferative agent that is rapidly released on contact with the vessel intima exerting an anti-restenotic effect. The absence of a metallic scaffold means that the need for antiplatelet therapy can potentially be negated in the longer term if required. In this article, we will review the history of percutaneous coronary intervention and the available evi-dence for the appropriate use of drug-coated balloons especially in the elderly population.
Conclusion:
We will conclude this review by demonstrating the potential use of drug-coated balloon rather percutaneous stenting through case examples.
Keywords: Coronary artery disease, drug-coated balloons, elderly, vessel-threatening dissections, elastic recoil, restenosis
1. Introduction
Percutaneous coronary intervention began in the 1970s with balloon angioplasty [1]. Bare metal stents were soon developed in the mid 80’s to initially treat dissections and acute recoil, and subsequently also arterial restenosis but were associated with new complications such as stent restenosis and thrombosis [2]. Drug Eluting Stents (DES), introduced early in the millennium, have significantly reduced the rate of in-stent restenosis but the risk of stent thrombosis remains an important complication necessitating prolonged duration of dual antiplatelet therapy [3]. More recently, biodegradable scaffolds have been developed strategically to combine the temporary benefits of a stent with mechanical support and drug delivery with the added advantage of resorption, theoretically allowing a return to more normal vascular structure and function long-term [4]. The Drug-coated Balloon (DCB) is another addition to the therapeutic armamentarium available to the interventional cardiologist, combining the benefits of local drug delivery and a shorter duration of dual antiplatelet therapy without the complications of stent implantation, in cases where a stent might not be necessary or feasible [5]. This might prove invaluable in the modern era characterised by an ageing population with multiple comorbidities and high bleeding risk. The treatment of coronary artery disease is common in patients over the age of 75 years of age especially within the context of acute coronary syndromes. Careful consideration of the treatment strategy for acute coronary syndromes in this age group is particularly important in view of their increased complexity, complication rate and hospitalisation [6]. In this review, we will briefly discuss the history of percutaneous coronary intervention and then focus on the use of drug-coated balloons giving particular emphasis on their use in de-novo lesions and in the elderly.
2. Historical perspective
The first Balloon Angioplasty (BA) was performed by Dr Andreas Gruntzig in 1977 [7] when he dilated the left anterior descending artery on a conscious patient. In 1979, the results of the first few cases of BA were published and it was estimated that about 10-15% of candidates for bypass surgery were suitable for this new technique [1]. It soon became apparent that the technique was promising, but not without complications, including acute vessel closure and restenosis which affected up to a third of the patients in the first 6 months requiring repeat procedures or bypass surgery [2].
Bare Metal Stents (BMS) were soon developed in an attempt to prevent the acute vessel closure or recoil with initial favourable results being first published in 1987 [8]. In 1994, it was demonstrated by randomised control trials that elective stent implantation significantly reduced the rate of restenosis and need for repeat coronary intervention and thus drove the era of elective stent implantation [9, 10]. Of particular note however, there was no proven prognostic benefit in routine elective stent implantation for patients with stable angina. The use of BMS rapidly expanded over the next few years. It resolved problems such as acute vessel closure due to dissections and mitigated the issues of acute recoil and constrictive remodelling linked to BA but this was at the expense of increased risk of (sub)acute thrombosis and in-stent restenosis caused by in-stent neo-intimal hyperplasia and activation of vascular and smooth muscle cells [11].
In the early 2000s Drug Eluting Stents (DES) were developed to combine the benefit of a mechanical stent scaffold with the local delivery of an anti-proliferative drug to inhibit in-stent neo-intimal proliferation. They had demonstrably lower rates of in-stent restenosis compared to BMS and their use rapidly proliferated through the cardiology community [11]. Despite DES exhibiting a reduced rate of in-stent restenosis compared with BMS, the risk of stent thrombosis still remained, with the added requirement for an extended period of dual antiplatelet therapy for the first 12 months and antiplatelet monotherapy thereafter, which in turn increases the bleeding risk especially in the elderly [12, 13].
Thus coronary artery stents were developed to treat some of the early complications associated with BA and they fulfil this role both acutely (dissection, vessel recoil) or within the first few months post-implantation (restenosis). Whilst the main benefits of their use are seen in the first few months post-implantation the presence of the permanent metallic scaffolding and the need for longer dual antiplatelet therapy can be associated with an adverse bleeding profile and prognosis in the longer term. Therefore, biodegradable scaffolds were soon developed which would allow the vessel to return to a more physiological state after complete resorption with an anticipated benefit that this could allow earlier discontinuation of dual antiplatelet therapy when compared to DES; a benefit not fully supported by clinical studies [4, 14, 15].
Nonetheless, all available stenting options involve a “foreign” implanted scaffold albeit for a short period of time, which necessitates a period of dual antiplatelet therapy commonly varying from one month to one year. Despite the associated increase in bleeding risk, the majority of patients tend to tolerate antiplatelet therapy well in trial data [16, 17]. However, for patients with hypertension, renal failure, with prior history of peptic ulcer or increased age who can have significant bleeding risk [18], even short term dual antiplatelet therapy may convey an extremely risky bleeding profile that outweighs the potential benefit of stent implantation. In addition, for certain patients it may become necessary to stop dual antiplatelet therapy following implantation of a DES unpredictably such as those diagnosed with neoplasia requiring biopsy or urgent surgery [19]. Therefore, although the percutaneous options of metallic scaffolding have evolved and improved through the years including transition from BMS to DES and biodegradable scaffolds, the real question is whether the same effect of opening the stenosed vessel without the need of any metallic/biodegradable scaffolding is achievable. Drug-coated Balloons (DCB) were developed and now approved in Europe in an attempt to mitigate some of the disadvantages of stent implantation such as bleeding risk from prolonged dual antiplatelet therapy and stent thrombosis, whilst in the USA they have only received Food and Drug Administration (FDA) approval for peripheral non-coronary interventions at present [20].
3. Drug-coated balloon
Using a DCB facilitates delivery of an anti-proliferative drug to the vessel wall without implantation of a stent [20]. It comprises a semi-compliant angioplasty balloon coated with the anti-proliferative drug and an excipient, an inert carrier molecule that facilitates drug transfer and absorption to the vessel wall [21]. Adequate lesion preparation is essential which creates microdissections in the vessel wall and allows better uptake of the drug [3]. The balloon is then inflated for 30-60 seconds and the drug absorbed into the vessel wall in a homogenous manner. Currently, the vast majority of DCBs available use the anti-proliferative drug paclitaxel although more recently others using sirolimus have also become available in Europe. It is the anti-proliferative agent that binds to the β subunit of tubulin, arresting the microtubule function and inhibiting cell division. Its lipophilicity and ability to concentrate to the arterial wall make it an optimal agent for DCB [22]. There is a relative paucity in the guidance available recommending the duration of antiplatelet therapy in patients with DCB especially in patients with stable disease undergoing coronary intervention. For acute coronary syndrome patients, the need for dual antiplatelet therapy is mandated as part of the management [23] for the acute event. However, in DCB-trials, dual antiplatelet use was variable ranging from 1-12 months. Reassuringly, in most of the trials the duration of dual antiplatelet therapy was short at 1-3 months without an increase in the rate of Major Adverse Cardiac Events (MACE) when compared to trials with longer duration of dual antiplatelet therapy [5]. Therefore, dual antiplatelet therapy for 1 month following DCB implantation for stable coronary artery disease has been recommended by an expert group [24]. We will next review the evidence of DCB in various scenarios and conclude by addressing the specific use of DCB in the elderly. In this article, the terms DCB and Drug-eluting Balloon (DEB) are used interchangeably.
4. Drug-coated balloons in in-stent restenosis
The PEPCAD II trial compared the SeQuent Please DCB with the Taxus DES for the treatment of in-stent restenosis (ISR). At 6 month follow-up, the DCB group had significantly less in-stent Late Lumen Loss (LLL) while at 12 month follow-up there was no significant difference in MACE making DEB treatment a safe and efficacious alternative option for ISR [25]. These results were confirmed by the ISAR-DESIRE 3 trial in 2013 and the PEPDAC China ISR trial in 2014. The ISAR-DESIRE 3 trial was a three-arm randomised controlled trial comparing DEB vs. DES (Paclitaxel) vs. BA. At 7 month follow-up DEB was non-inferior to DES in terms of diameter stenosis and both groups were superior to BA alone [26]. The PEPCAD China ISR trial compared the SeQuent Please DCB with Taxus DES for the treatment of ISR in DES. At 9 month follow-up, the DCB group was non-inferior to DES group in terms of LLL and at 12 month follow-up the two groups had similar clinical events [27]. Despite these encouraging results when DCB was compared with Paclitaxel-DES, the RIBS IV trial in 2015 showed that Everolimus-DES was superior to SeQuent Please DCB for the treatment of ISR of DES. At 1 year follow-up, the DES group had significantly larger minimal lumen diameter, larger net luminal gain, lower diameter stenosis and fewer adverse clinical outcomes mainly driven by less need for Target Vessel Revascularisation (TVR). The authors took great care in terms of pre-dilatation and avoiding geographical mismatch [28]. However, a rather generous <50% stenosis was considered as an acceptable result post PCI in the DCB arm, whereas in most centres <30% stenosis would be considered acceptable.
At present, DCB remain a treatment option of ISR according to the European Society of Cardiology guidelines on myocardial revascularisation [29] and also appear to be cost-effective compared to DES for restenosis treatment [30].
5. Drug-coated balloons in small vessel disease
In an increasingly diverse arena with multiple therapeutic options available, small calibre vessels were studied as a likely indication where drug-coated balloons may offer the most reasonable solution. Small calibre vessels, one of the strongest predictors of restenosis even in the era of DES, allow DCB to utilise their unique properties [31]. PEPCAD I was the first single-arm study that evaluated a DCB (Sequent Please) in small calibre vessels (vessel diameter of 2.25-2.8mm) in patients with an average age of 68 years. The DCB-only group achieved very good angiographic and clinical results which were maintained at 12 and 36 months follow-up. Patients who needed BMS-bailout had worse results in terms of Late Lumen Loss (LLL), restenosis and Target Lesion Revascularisation (TLR) and worse clinical outcomes at 12 and 36 month follow up. These were attributed possibly to geographical mismatch as most of the restenosis occurred at stent edges [32, 33].
The PICCOLETO study compared the DIOR-PCB with Taxus DES in small vessels (<2.75mm). This study included elderly patients with the average age of the patients being 67 and 68 years, respectively in the two groups and followed up for 9 months. The study had to be stopped prematurely due to clearly superior clinical outcomes of the Taxus group mainly driven by TLRs [34]. However, it is difficult to draw definitive conclusions from this study as the first generation DCB used (DIOR placlitaxel-coated balloon) achieved low tissue drug dosage. In addition lesion preparation was inadequate with a pre-dilation rate of only 25% and low inflation pressures [3, 33].
The BELLO study was the first study that showed positive findings comparing the In.Pact Falcon DCB to Taxus DES in small vessels (vessel diameter <2.8mm). The average age was 64 and 66 years of age respectively. The primary outcome of in-stent (in-balloon) late loss was significantly less in the DCB group compared to the DES group. There were similar rates of revascularisation and restenosis. It has to be noted that the pre-dilation rate before DEB was 96.8% and that in cases of BMS-bailout avoidance of geographical mismatch was considered. However, in the subgroup of patients who needed BMS-bailout (20%) the LLL was significantly increased and similar to the DES group [3, 35].
6. Drug-eluting balloons in bifurcation lesions
Coronary bifurcation lesions account for approximately 20% of all percutaneous coronary interventions as they continue to provide a significant challenge, being associated with lower procedural success rates and higher rates of adverse cardiac events, even in experienced centres in the era of DES.
The DEBIUT was a three-arm trial designed to assess the efficacy of DEB pre-dilatation of the Side Branch (SB) in bifurcation lesions. Groups A and B were treated with a BMS in the Main Branch (MB) after pre-dilatation of both MB and SB with DIOR-I DEB and regular balloon respectively. Group C was treated with a paclitaxel DES at the MB after dilatation with a regular balloon of the MB and SB. Final kissing regular balloon dilatation was mandatory in all groups. The DES group was significantly better than both other groups while the DEB group did not show angiographic superiority compared to BMS group. The authors made the comment that tissue drug delivery with the DIOR-I is significantly less compared to Sequent Please even though the loading doses are comparable, and thus opened the gate for future studies in that area [36].
The BABILON trial assessed provisional BMS T-stenting technique with pre-dilatation of both SB and MB with SeQuent Please DEB versus Xience V DES T-stenting technique with pre-dilatation with regular balloons. Final kissing balloon dilatation was at operator’s discretion and there was a significant difference between the two groups with the BMS/DEB group having significantly less final kissing. The average age of patients was 64 years of age. The main advantage of DEB is negating the need for any stent placement and therefore this was not assessed in this study as the DEB group always had BMS associated it. As the BMS is known to be inferior to DES with regards to in-stent restenosis, it was therefore no surprise that the DES group had significantly less in-stent restenosis while the BMS/DEB group had significantly more TLR but the difference was due to main branch TLR. Importantly, there were no significant angiographic differences in the SB. Additionally, the two groups did not have any significant differences in MACE. The authors concluded that although an everolimus-DES provisional strategy appeared to be the preferred strategy the use of DEB in the SB appeared safe, and therefore further studies using DES in the MB and SB vs. DES in the MB and DEB in the SB were warranted [37].
Another important study in intervention of bifurcation lesions with T stenting was by Herrador and colleagues who compared 50 patients who had a DES (Taxus Liberte) in the MB and DEB in the SB (Sequent Please) against 50 patients with a DES in the MB and conventional ballooning in the SB with both groups having kissing balloon post dilation [38]. The average age in this study was 62 years old. There was no difference in clinical MACE between the two groups but there was a trend towards less SB restenosis in the DEB group (7% vs. 20%, p=0.08) suggesting that DEB is a safe and possibly more effective strategy.
The BIOLUX-1 study was the first multicentre study to assess the safety and efficacy of an everolimus-DES (Xience V/Xience Prime) in the MB with a Paclitaxel-DEB (Pantera LUX) in the SB for bifurcation lesions. The average age was 66 years old. The MB was pre-dilated first, the SB was treated with a DEB and then the MB was treated with a DES. Only one case received pre-dilatation of the SB with a regular balloon before the DEB and even though the protocol mandated final kissing balloon inflation, this only occurred in 37.2% of cases. Furthermore, a core angiographic laboratory assessment showed that only 11 out of the 35 patients had true bifurcation lesions. A separate assessment of the patients with true bifurcation lesions showed that the low LLL was maintained and the complications rate was low. Despite the limitations of this small pilot study, the authors concluded that the combination of DES in the MB with DEB in the SB is safe and effective [39].
The PEPCAD BIF trial evaluated the use of SeQuent Please DCB vs. BA in bifurcation lesions (with no proximal main branch disease though). The average age of the patients was 67 years of age. At 9-month follow up, the DCB group had less restenosis rate and LLL. The authors concluded that DCB treatment is a sound option for bifurcation lesions as long as there is only class A or B dissection and recoil <30% [40].
7. Drug-coated balloons in diabetes
It has long been demonstrated that patients with diabetes have increased risk of in-stent restenosis with vessel calibre, length of stent and lower BMI being the most important predictors of restenosis [41]. The PEPCAD IV trial compared the use of SeQuent Please DCB followed by cobalt-chromium stent vs. DES (Taxus Liberte) in patients with diabetes. The average age of patients was 62 and 58 years of age respectively. Even though it was recommended by the protocol, only 31% of patients in the DCB group underwent pre-dilatation. However, at 9-month follow up there was no significant difference in the clinical or angiographic outcomes. The LLL, TLR and MACE were similar [42]. Although this study showed some promising results for the DCB group, there was no arm including DCB without stenting which could have shown even better results.
8. Drug-coated balloons in STEMI
With increasing experience of the DCB in coronary work, soon trials utilising them in acute myocardial infarction started to become available. The DEB-AMI trial was the first one to evaluate a DEB in the context of STEMI. It was a three-arm randomised control trial comparing BMS alone vs. BMS together with DEB vs. DES alone. It is important to note that the devices used were a modern cobalt chromium BMS, the DIOR DEB (first generation) and the TAXUS Liberte DES, and no randomised group utilised DEB monotherapy. The average age of patients was 60 years for the first two groups and 56 years for the third group. Even though it was mandated by the protocol, only 60% of the patients in the DEB group underwent pre-dilatation. The DIOR DEB group failed to show superiority compared to BMS while the DES group had significantly better angiographic and clinical results. The DEB group had fewer mal-apposed and uncovered struts compared to the DES group but more than BMS group as assessed by optical coherence tomography. Therefore even though there was no established clinical benefit with DEB compared to BMS, it was demonstrated that there was a drug effect. The authors felt that these data could be explained by reduced bioavailability of paclitaxel to the lesion either due to the excipient in the DEB or due to the fact that only 60% of patients underwent pre-dilatation. Indeed, subgroup analysis showed that the patients with pre-dilatation had significant less LLL compared to the patients without pre-dilatation [43]. It is important to realise that there was a subsequent 4th non-randomised arm of DEB-AMI trial showed that the DEB-only arm had similar outcomes compared to BMS or BMS with DEB but inferior to DES. The authors concluded the DEB-only could be considered as a treatment option during Primary Percutaneous Coronary Intervention (PPCI) in patients with contraindication to DES [44].
A more recent study from Amsterdam [45] also confirmed the safety and feasibility of a DCB in PPCI with the option of bailout stenting if required. A total of 100 patients presenting with a STEMI were included and 59 managed with DCB alone, while 41 patients required additional stenting indicating that DCB use as main treatment in this setting could be justified. Similar data from our unit with 253 patients treated with DCB in PPCI showed that stent bail out was only necessary for 9% of the patients, of which only 0.8% were for acute vessel closure. In addition at 12 month follow up TLR was 3.3% and mortality 6.3% supporting the existing data that a DCB initial approach in PPCI is possible and indicating that further research is warranted in this field [46].
9. Drug-coated balloons in elderly
Drug-coated balloons have been evaluated in various groups of patients. However, to the best of our knowledge, there is no randomised control trial specifically targeting the elderly population. In addition, as it can be noticed from the average age of the patients included in the studies mentioned above, the elderly have not been studied as a dedicated group; a subject that merits further discussion. According to the British Cardiovascular Intervention Society audit in 2015, 22551 coronary interventions took place in patients aged between 71-80 years old, 9422 PCIs in patients between 81-90 years old and 559 PCIs in patients >90 years old [47]. These data demonstrate that elderly patients undergo large numbers of percutaneous coronary interventions in routine clinical practice even through they only have a very small representation in clinical trials. With an increasingly ageing population, the prevalence of acute coronary syndromes in the elderly will continue to increase affecting both sexes [48]. This is an important group particularly as older patients are at a higher risk of bleeding even with single antiplatelet agents [49, 50]. In addition, elderly patients frequently have indications for anticoagulation, such as atrial fibrillation which becomes more frequent with advancing age. Therefore, an option that might allow a short-term use of dual antiplatelet therapy and then further allow complete discontinuation of the one remaining antiplatelet medication if signs of bleeding appear would be pioneering, as it can allow clinicians to protect patients during the immediate acute event and then tailor subsequent antiplatelet therapy in a personalised fashion. To date, there is only one prospective study in elderly with small vessel coronary artery disease acquired from the SeQuent Please Small Vessel ‘Paclitaxel-Coated Balloon Only’ Registry [51]. The older group (>75 years old) had significantly higher incidence of hypertension, renal insufficiency, atrial fibrillation and calcified lesions. There was no difference in MACE (4.2% vs. 6.1%) or TLR (3.9% vs. 3%) at 9 months between the younger and older group. According to the authors, this result appears similar to the everolimus-eluting stent TLR rate of 5.1% at 1 year from the SPIRIT Small Vessel Trial.
The appeal of DCB in the elderly population is mainly driven by the shortened period of dual antiplatelet therapy, therefore conveying a lesser bleeding risk, which is of particular importance in the presence of multiple comorbidities. A DCB approach can also enable discontinuation of all antiplatelets if clinically needed without predisposing patients to stent thrombosis as in the case with stents. Unfortunately, there is often a general perception that upper gastrointestinal bleeding is mainly non-disabling with low mortality rate, however whilst this might well be true for the majority of younger patients, the elderly patients do get more frequent and more significant, including lethal, bleeding. A recent prospective population-based cohort study in patients after a vascular event (Myocardial Infarction (MI), Transient Ischaemic Attach (TIA) and Stroke) treated with aspirin without proton pump inhibitor confirmed that elderly patients (>75 years old) have higher risk of major bleeding with significant risk of disabling or fatal upper gastrointestinal bleeding [50], indicating that a decision to prescribe even single long-term aspirin monotherapy in the elderly should be carefully considered. Therefore, treatment options such as DCB with their reduced duration for dual antiplatelet therapy might become very useful and relevant in the elderly patient with multiple comorbidities and high bleeding risk. However, at present there is little evidence to formulate a strong recommendation for DCB use as a first approach, with stenting as a secondary option if required, in the elderly for both elective and acute situations. New randomised studies comparing DCB with DES should be undertaken in the elderly population both in the acute and elective setting to establish the evidence on which to base further treatment options. Such studies should offer medium to long term follow up data especially as the benefit of DCB might be seen at later stages with reduced bleeding risk and mortality due to reduced antiplatelet use. On the balance of the current evidence we feel that an initial approach to use DCB in elderly patients with high risk of bleeding is justified, and if this approach fails then a metallic stent, either BMS or DES depending on the particular individual and clinical history, can be considered.
In the following section we will describe some cases managed by DCB alone, indicating their potential use in clinical practice.
10. Clinical cases where DCB was preferentially used
10.1. Case 1
10.1.1. DCB in a Patient at High Risk of Bleeding Requiring Anticoagulation for Atrial Fibrillation
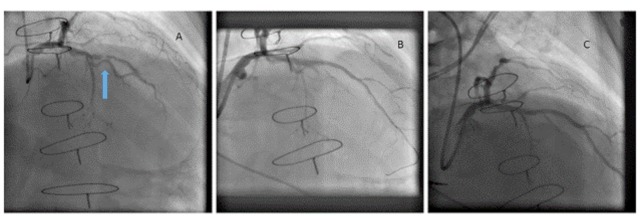
A 72 year old man with prior cardiac surgery (mitral valve repair), permanent atrial fibrillation and hypertension presented with increasing stable angina that interfered with his quality of life. Diagnostic angiography (Panel A) showed critical mid Left Anterior Descending (LAD) artery stenosis of a calcified vessel with TIMI 1 flow. Following discussion about the possible therapeutic options, rotablation was initially undertaken in the LAD and treated with a 2.5 x 20 mm SeQuent Please DCB with an excellent angiographic result (Panel B). He remained on a novel anticoagulant and antiplatelet monotherapy both in the acute and longer term period. At 6 months routine angiography revealed patent vessel (Panel C) (Fig. 1).
Fig. (1).
Coronary angiograpghy in a patient with stable angina showing significant calcific LAD disease (panel A, arrow) which was treated with DCB showing immediate (panel B) result and result at six months (panel C).
The patient remained completely asymptomatic when last seen 9 months following the intervention. This case highlights the benefit of DCB use in a patient with extensive cardiac history where repeat surgical revascularisation was not indicated and where a DES was likely to require dual antiplatelet therapy for 12 months in addition to oral anticoagulation, and then antiplatelet monotherapy long term alongside anticoagulation without the option of stopping the monotherapy if there was any significant concern about bleeding risk. However, with DCB the antiplatelet therapy could be discontinued if necessary.
10.2. Case 2
10.2.1. DCB Use in a Patient at High Bleeding Risk
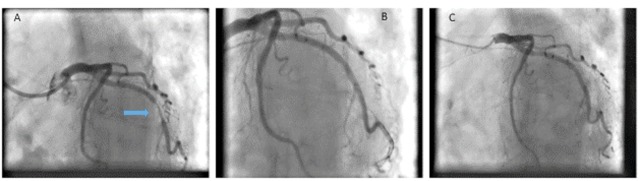
A 74 year old lady with stable angina underwent diagnostic angiography which showed significant circumflex disease (Panel A). She had a history of bleeding ulcers and the lesion was pre dilated with a semi-compliant balloon and final drug delivery was achieved with a 3.0 x 15 mm SeQuent Please DCB with a satisfactory result (Panel B). Dual anti platelets were continued for a month followed by monotherapy thereafter.
At 42 months repeat angiography was undertaken when she presented with atypical chest pain which showed an excellent long lasting result from the DCB (panel C) (Fig. 2). This case highlights the excellent long term outcome that can be achieved with DCB in a patient at high risk of bleeding.
Fig. (2).
A patient with severe circumflex lesion (panel A, arrow) was treated with DCB in view of bleeding history showing good result post DCB (panel B) and at 42 months (panel C).
10.3. Case 3
10.3.1. DCB Use in a Patient with an ST Elevation Myocardial Infarction
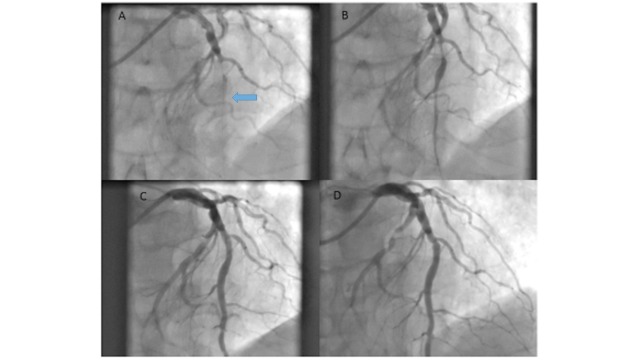
A 78 year old man presented with acute chest pain and electrocardiography confirmed an anterior STEMI. Emergency angiography revealed an occluded LAD which was treated successfully with thrombus aspiration and pre dilatation with a semi-compliant balloon followed by a final 4 x 20 mm SeQuent Please DCB. Panels A, B and C show pre, during and immediate post PCI angiography images respectively. Panel D shows follow up angiography at 3 months when he returned for staged intervention for by-stander circumflex disease. Dual antiplatelet therapy was continued for a year (for acute myocardial infarction) but it would have been possible to discontinue one of the antiplatelets at one month if the need arose (Fig. 3).
Fig. (3).
shows an occluded LAD (panel A, arrow) which was treated with DCB with good result (panel B during procedure, panel C post-procedure) which was maintained 3 months later when the patient returned for a staged procedure to his by-stander circumflex disease.
This case highlights that a DCB can provide a solid strategy even in patients presenting with AMI to reduce the period needed for dual antiplatelet therapy if required (for example bleeding risk or scheduled surgery) and the angiographic outcome can be excellent.
CONCLUSION
There has been an amazing advancement in the field of percutaneous coronary intervention since its introduction in the 1970s. Using evidence-based therapy, we have moved from balloon angioplasty, to bare metal stents, and then to drug eluting stents and biodegradable scaffolds. However, in the modern era of the more complex and potentially frail patients, it is important for the interventional cardiologist to have a variety of treatment options available in their armamentarium. Drug-coated balloons are this latest development trying to find its place and role in an ever developing field. It is evident from the studies reviewed that there is not a uniform class effect among the drug-coated balloons as all individual components of the device can affect its performance. The results so far are encouraging such that the indications for drug-coated balloon use will continue to grow beyond in-stent restenosis and small vessel disease even though at present studies are in general small with some conflicting results. The elderly is a large group of patients encountered in daily clinical practice, yet unfortunately remain underrepresented in trials. They comprise a complex group of patients with multiple comorbidities and increased risk of bleeding risk who are also at a higher risk of acute coronary syndromes. Drug-coated balloons could provide a useful tool to allow the cardiologist to achieve very good clinical results in dilating culprit vessels without the need of longer term dual antiplatelet therapy, however further studies are urgently required in this group of patients to allow appropriate classification and solid guideline recommendation for their use.
ACKNOWLEDGEMENTS
Declared none.
List of abbreviations
- AMI
Acute Myocardial Infarction
- BA
Balloon Angioplasty
- BMS
Bare Metal Stent
- DCB
Drug-coated Balloon
- DEB
Drug Eluting Balloon
- DES
Drug Eluting Stent
- FDA
Food and Drug Administration
- ISR
In-stent Restenosis
- MACE
Major Adverse Cardiac Events
- MB
Main Branch
- MI
Myocardial Infarction
- LLL
Late Lumen Loss
- PCI
Percutaneous Coronary Intervention
- PPCI
Primary Percutaneous Coronary Intervention
- SB
Side Branch
- STEMI
ST Elevation Myocardial Infarction
- TIA
Transient Ischaemic Attack
- TLR
Target Lesion Revascularisation
- TVR
Target Vessel Revascularisation
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Gruntzig A., Senning A., Siegenthaler W. Nonoperative dilatation of coronary-artery stenosis: Percutaneous transluminal coronary angioplasty. N. Engl. J. Med. 1979;301:61–68. doi: 10.1056/NEJM197907123010201. [DOI] [PubMed] [Google Scholar]
- 2.Gruntzig A., King S., Scblumpf M., Siegenthaler W. Long-term follow-up after percutaneous transluminal coronary angioplasty. N. Engl. J. Med. 1987;316:1127–1132. doi: 10.1056/NEJM198704303161805. [DOI] [PubMed] [Google Scholar]
- 3.Richelsen R.K.B., Overvad T.F., Jensen S.E. Drug-eluting balloons in the treatment of coronary de novo lesions: A comprehensive review. Cardiol Ther Springer Healthcare. 2016;5:133–160. doi: 10.1007/s40119-016-0064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramadugu P., Latha Alikatte K. A review on biodegradable and bioabsorbable stents for coronary artery disease. J. Bioequivalence Bioavailab. 2016;08:64–67. [Google Scholar]
- 5.Jackson D., Tong D., Layland J. A review of the coronary applications of the drug coated balloon. Int J Cardiol Elsevier Ireland Ltd. 2017;226:77–86. doi: 10.1016/j.ijcard.2016.09.045. [DOI] [PubMed] [Google Scholar]
- 6.Gnanenthiran S.R., Kritharides L., D’Souza M., Lowe H.C., Brieger D.B. Revascularisation compared with initial medical therapy for non-ST-elevation acute coronary syndromes in the elderly: A meta-analysis. Heart. 2017;103(24):1962–1969. doi: 10.1136/heartjnl-2017-311233. [DOI] [PubMed] [Google Scholar]
- 7.Barton M., Gruntzig J., Husmann M., Rosch J. Balloon angioplasty - The legacy of Andreas Gruntzig, M.D. (1939-1985). Front. Cardiovasc. Med. 2014;1:1–25. doi: 10.3389/fcvm.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sigwart U., Puel J., Mirkvitch V., Joffre F., Kappenberger L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N. Engl. J. Med. 1987;316:701–706. doi: 10.1056/NEJM198703193161201. [DOI] [PubMed] [Google Scholar]
- 9.Serruys P., Jaegere P., Kiemeneij F., et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. N. Engl. J. Med. 1994;331:489–495. doi: 10.1056/NEJM199408253310801. [DOI] [PubMed] [Google Scholar]
- 10.Fischman D., Leon M., Baim D., Schatz R., et al. A randomised comparison of coronary stent placement and balloon angioplasty in the treatment of coronary artery disease. N. Engl. J. Med. 1994;331:496–501. doi: 10.1056/NEJM199408253310802. [DOI] [PubMed] [Google Scholar]
- 11.Serruys P.W., Kutryk M.J.B., Ong A.T.L. Coronary-artery stents. N. Engl. J. Med. 2006;354:483–495. doi: 10.1056/NEJMra051091. [DOI] [PubMed] [Google Scholar]
- 12.Stefanini G.G., Holmes D.R. Drug-eluting coronary-artery stents. N. Engl. J. Med. 2013;368:254–265. doi: 10.1056/NEJMra1210816. [DOI] [PubMed] [Google Scholar]
- 13.Li L., Geraghty O.C., Mehta Z., Rothwell P.M., Oxford Vascular Study Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: A population-based cohort study. Lancet. 2017;390(10093):490–499. doi: 10.1016/S0140-6736(17)30770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buccheri D., Caramanno G., Geraci S., Cortese B. Should we reconsider dual antiplatelet therapy duration following bioresorbable scaffold angioplasty? J. Thorac. Dis. 2017;9:417–418. doi: 10.21037/jtd.2017.03.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Souza S., Ferrante G., Tyczynski P., Di Mario C. Biodegradable stents - A new era? Eur. Cardiol. 2008;4:82–84. [Google Scholar]
- 16.Yusuf S., Mehra S., Chrolavicius S., et al. Effects of Clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N. Engl. J. Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 17.Mauri L., Kereiakes D.J., Yeh R.W., et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N. Engl. J. Med. 2014;371:2155–2166. doi: 10.1056/NEJMoa1409312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alfredsson J., Neely B., Neely M.L., et al. Predicting the risk of bleeding during dual antiplatelet therapy after acute coronary syndromes. Heart. 2017;103:1168–1176. doi: 10.1136/heartjnl-2016-310090. [DOI] [PubMed] [Google Scholar]
- 19.Song J.W., Soh S., Shim J.K. Dual antiplatelet therapy and non-cardiac surgery: Evolving issues and anesthetic implications. Korean J. Anesthesiol. 2017;70:13–21. doi: 10.4097/kjae.2017.70.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agostoni P., Sangiorgi G.M., Biondi-Zoccai G.G. Treatment of restenosis with a paclitaxel-coated balloon catheter. N. Engl. J. Med. 2007;356:1071–1072. [PubMed] [Google Scholar]
- 21.Byrne R., Joner M., Alfonso F., Kastrati A. Drug-coated balloon therapy in coronary and peripheral artery disease. Nat. Rev. Cardiol. 2014;11(1):13–23. doi: 10.1038/nrcardio.2013.165. [DOI] [PubMed] [Google Scholar]
- 22.Ng V.G., Mena C., Pietras C., Lansky A.J. Local delivery of paclitaxel in the treatment of peripheral arterial disease. Eur. J. Clin. Invest. 2015;45:333–345. doi: 10.1111/eci.12407. [DOI] [PubMed] [Google Scholar]
- 23.Roffi M., Patrono C., Collet J-P., et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2016;37:267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 24.Kleber F., Rittger H., Bonaventura K., et al. Drug-coated balloons for treatment of coronary artery disease: updated recommendations from consensus group. Clin. Res. Cardiol. 2013;102:785–797. doi: 10.1007/s00392-013-0609-7. [DOI] [PubMed] [Google Scholar]
- 25.Unverdorben M., Vallbracht C., Cremers B., et al. Paclitaxel-coated balloon catheter versus paclitaxel-Coated stent for the treatment of coronary in-stent restenosis. Circulation. 2009;119:2986–2994. doi: 10.1161/CIRCULATIONAHA.108.839282. [DOI] [PubMed] [Google Scholar]
- 26.Byrne R., Neumann F., Mehilli J., et al. Paclitaxel-eluting balloons, paclitaxel-eluting stents, and balloon angioplasty in patients with restenosis after implantation of a drug-eluting stent (ISAR-DESIRE 3): A randomised, open-label trial. Lancet. 2013;381:461–467. doi: 10.1016/S0140-6736(12)61964-3. [DOI] [PubMed] [Google Scholar]
- 27.Xu B., Gao R., Wang J., et al. A prospective, multicenter, randomized trial of paclitaxel-coated balloon versus paclitaxel-eluting stent for the treatment of drug-eluting stent in-stent restenosis: Results from the PEPCAD China ISR trial. JACC Cardiovasc. Interv. 2014;7:204–211. doi: 10.1016/j.jcin.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Alfonso F., Pérez-Vizcayno M.J., Cárdenas A., et al. A prospective randomized trial of drug-eluting balloons versus everolimus-eluting stents in patients with in-stent restenosis of drug-eluting stents. J. Am. Coll. Cardiol. 2015;66:23–33. doi: 10.1016/j.jacc.2015.04.063. [DOI] [PubMed] [Google Scholar]
- 29.Cardiovascular P., Eapci I., France J.C., et al. 2014 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2014;35:2541–2619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 30.Eccleshall S., Waliszewski M. The NICE recommendation for drug-coated balloons and its global impact. Ther. Adv. Cardiovasc. Dis. 2015;9:87–94. doi: 10.1177/1753944715574655. [DOI] [PubMed] [Google Scholar]
- 31.Biondi-Zocca G., Moretti C., Abbate A., Sheiban I. Percutaneous coronary intervention for small vessel coronary artery disease. Cardiovasc. Revasc. Med. 2010;11:189–198. doi: 10.1016/j.carrev.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Unverdorben M., Kleber F.X., Heuer H., et al. Treatment of small coronary arteries with a drug-eluting balloon catheter. The 3-year results of the PEPCAD I study. EuroInterventions. 2013;9(5):620–628. doi: 10.4244/EIJV9I5A99. [DOI] [PubMed] [Google Scholar]
- 33.Loh JP, Waksman R. Paclitaxel drug-coated balloons: A review of current status and emerging applications in native coronary artery de novo lesions. 2012. [DOI] [PubMed]
- 34.Cortese B., Micheli A., Picchi A., et al. Paclitaxel-coated balloon versus drug-eluting stent during PCI of small coronary vessels, a prospective randomised clinical trial. The PICCOLETO Study. Heart. 2010;96:1291–1296. doi: 10.1136/hrt.2010.195057. [DOI] [PubMed] [Google Scholar]
- 35.Latib A., Colombo A., Castriota F., et al. A randomized multicenter study comparing a paclitaxel drug-eluting balloon with a paclitaxel-eluting stent in small coronary vessels: The bello (balloon elution and late loss optimization) study. J Am Coll Cardiol Elsevier Inc. 2012;60:2473–2480. doi: 10.1016/j.jacc.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 36.Belkacemi A., Agostoni P., Voskuil M., Stella P.R. Coronary bifurcation lesions treated with the drug-eluting balloon: A preliminary insight from the DEBIUT study. EuroIntervention. 2011;7:66–69. doi: 10.4244/EIJV7SKA12. [DOI] [PubMed] [Google Scholar]
- 37.Mínguez J.R.L., Asensio J.M.N., Vecino L.J.D., et al. A prospective randomised study of the paclitaxel-coated balloon catheter in bifurcated coronary lesions (babilon trial): 24-month clinical and angiographic results. EuroIntervention. 2014;10:50–57. doi: 10.4244/EIJV10I1A10. [DOI] [PubMed] [Google Scholar]
- 38.Herrador J.A., Fernandez J.C., Guzman M., Aragon V. Drug-eluting vs. conventional balloon for side branch dilation in coronary bifurcations treated by provisional T stenting. J. Interv. Cardiol. 2013;26:454–462. doi: 10.1111/joic.12061. [DOI] [PubMed] [Google Scholar]
- 39.Worthley S., Hendriks R., Worthley M., et al. Paclitaxel-eluting balloon and everolimus-eluting stent for provisional stenting of coronary bifurcations: 12-month results of the multicenter BIOLUX-I study. Cardiovasc Revascularization Med. 2015;16:413–417. doi: 10.1016/j.carrev.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 40.Kleber F.X., Rittger H., Ludwig J., et al. Drug eluting balloons as stand alone procedure for coronary bifurcational lesions: Results of the randomized multicenter PEPCAD-BIF trial. Clin. Res. Cardiol. 2016;105:613–621. doi: 10.1007/s00392-015-0957-6. [DOI] [PubMed] [Google Scholar]
- 41.West N.E.J., Ruygrok P.N., Disco C.M.C., et al. Clinical and angiographic predictors of restenosis after stent deployment in diabetic patients. Circulation. 2004;109:867–873. doi: 10.1161/01.CIR.0000116750.63158.94. [DOI] [PubMed] [Google Scholar]
- 42.Ali R., Degenhardt R., Zambahari R. Paclitaxel-eluting balloon angioplasty and cobalt-chromium stents versus conventional angioplasty and paclitaxel-eluting stents in the treatment of native coronary artery stenoses in patients with diabetes mellitus. EuroIntervention. 2011;7(Suppl. K):K83–K92. doi: 10.4244/EIJV7SKA15. [DOI] [PubMed] [Google Scholar]
- 43.Belkacemi A., Agostoni P., Nathoe H.M., et al. First results of the DEB-AMI (Drug Eluting Balloon in Acute ST-segment elevation myocardial infarction) Trial: A multicenter randomized comparison of drug-eluting balloon plus bare-metal stent versus bare-metal stent versus drug-eluting stent in primary p. J. Am. Coll. Cardiol. 2012;59:2327–2337. doi: 10.1016/j.jacc.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 44.Nijhoff F., Agostoni P., Belkacemi A., et al. Primary percutaneous coronary intervention by drug-eluting balloon angioplasty: The non-randomised fourth arm of the drug-eluting balloon in ST-segment elevation myocardial infarction (DEB-AMI) trial. EuroIntervention. 2015;86(Suppl. 1):S34–S44. doi: 10.1002/ccd.26060. [DOI] [PubMed] [Google Scholar]
- 45.Vos N.S., Dirksen M.T., Vink M.A., et al. Safety and feasibility of a PAclitaxel-eluting balloon angioplasty in Primary Percutaneous coronary intervention in Amsterdam (PAPPA): One-year clinical outcome of a pilot study. EuroIntervention. 2014;10:584–590. doi: 10.4244/EIJV10I5A101. [DOI] [PubMed] [Google Scholar]
- 46.Wickramarachchi U., Corballis N., Maart C., et al. 2017 https://abstractbook.pcronline. com/export/pdf/id/364
- 47. https://www.bcis.org.uk
- 48.Ruff C.T., Braunwald E. The evolving epidemiology of acute coronary syndromes. Nat. Rev. Cardiol. 2011;8:140–147. doi: 10.1038/nrcardio.2010.199. [DOI] [PubMed] [Google Scholar]
- 49.Pisters R., Lane D., Nieuwlaat R. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation. Euro Heart Survey Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 50.Li L., Geraghty O.C., Mehta Z., Rothwell P.M. Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: a population-based cohort study. Lancet. 2017;6736:1–10. doi: 10.1016/S0140-6736(17)30770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sinaga D.A., Ho H.H., Zeymer U., et al. Drug coated balloon angioplasty in elderly patients with small vessel coronary disease. Ther. Adv. Cardiovasc. Dis. 2015;9:389–396. doi: 10.1177/1753944715598714. [DOI] [PubMed] [Google Scholar]