Abstract
Focal adhesion kinase (FAK) is a non-receptor protein tyrosine kinase localized at focal adhesions and is believed to mediate adhesion-stimulated effects. Although ablation of FAK impairs cell movement, it is not clear whether FAK might be involved in the guidance of cell migration, a role consistent with its putative regulatory function. We have transfected FAK-null fibroblasts with FAK gene under the control of the tetracycline repression system. Cells were cultured on flexible polyacrylamide substrates for the detection of traction forces and the application of mechanical stimulation. Compared with control cells expressing wild-type FAK, FAK-null cells showed a decrease in migration speed and directional persistence. In addition, whereas FAK-expressing cells responded to exerted forces by reorienting their movements and forming prominent focal adhesions, FAK-null cells failed to show such responses. Furthermore, FAK-null cells showed impaired responses to decreases in substrate flexibility, which causes control cells to generate weaker traction forces and migrate away from soft substrates. Cells expressing Y397F FAK, which cannot be phosphorylated at a key tyrosine site, showed similar defects in migration pattern and force-induced reorientation as did FAK-null cells. However, other aspects of F397-FAK cells, including the responses to substrate flexibility and the amplification of focal adhesions upon mechanical stimulation, were similar to that of control cells. Our results suggest that FAK plays an important role in the response of migrating cells to mechanical input. In addition, phosphorylation at Tyr-397 is required for some, but not all, of the functions of FAK in cell migration.
Keywords: autophosphorylation, cell migration, signal transduction
Focal adhesion kinase (FAK or pp125FAK) was first identified as a v-src substrate in chicken embryo fibroblasts (1). It was subsequently found to be a ubiquitous non-receptor protein tyrosine kinase (2), colocalizing with integrins at focal adhesions in adherent cells (3–5). The C-terminal domain contains multiple binding sites for focal adhesion proteins that associate with integrin clusters, such as paxillin, p130cas (6), and talin (7, 8). It is believed that integrin-dependent autophosphorylation of FAK recruits and activates Src family kinases, which in turn trigger downstream signaling events. A particularly important site of autophosphorylation, Tyr-397, was identified at the juncture between the N-terminal and the catalytic domain (9). Phosphorylation of this site promotes the binding of FAK with the SH2 domain of Src family kinase (9) and other proteins carrying this domain, such as phospholipase C (10).
The biological function of FAK is still a subject of much speculation. There is evidence that FAK is essential for integrin-stimulated cell migration (11–17), cell spreading (16), and proliferation (11, 18, 19). Overexpression of FAK increases the migration rate (20), whereas abolition of FAK expression impairs cell migration and leads to embryonic lethality (21). Tyr-397 autophosphorylation site is required for the maximal adhesion-induced FAK activation and for FAK-enhanced cell spreading and migration (16). So far, speculations have centered on the possible role of FAK in the detachment of cells from the substrate, as initially suggested by the apparent increase in the size of focal adhesions in FAK-null cells (21). However, because the turnover of focal adhesion is closely coupled to cell migration, it is possible that the increase in size or stability of the focal adhesion is associated with other defects in cell migration.
In this study, we pursued the possibility that FAK is involved in focal adhesion-mediated responses of cells to physical signals. There is increasing evidence that mechanical signals regulate not only cell migration, but also cell growth, apoptosis, and gene expression (22–26). Experiments with flexible substrates demonstrated that both mechanical forces and substrate rigidity could profoundly affect cell shape and migration rate (27). For example, when 3T3 cells are stretched with mechanical forces, protrusions that extended toward forces expand into dominant lamellipodia whereas other protrusions retract (28). In addition, cells plated on soft substrates showed both increased motility (27) and decreased growth (26). The localization of FAK at the juncture between integrins and the cytoskeleton makes it an attractive candidate for converting external mechanical stimulations into intracellular chemical events.
To address this possibility, we have used a tetracycline repression system to achieve inducible expression of wild-type FAK (WT-FAK) or Tyr-397 mutant FAK (F397-FAK) in fibroblasts derived from FAK-knockout mouse embryos (16). Cells under either gene inhibition or expression conditions were plated on flexible collagen-coated polyacrylamide substrates, to compare their traction forces and their responses to mechanical stimulations. Our results support the hypothesis that FAK is involved in mechanosensing and in coordinating motile activities for efficient directional migration. In addition, the autophosphorylation site at Tyr-397 appears to be involved in a subset of FAK functions.
Materials and Methods
Preparation of Polyacrylamide Substrates.
Polyacrylamide substrates coated with collagen I were prepared essentially as described previously (29, 30). The flexibility of the substrate was manipulated by maintaining the total acrylamide concentration while varying the bis-acrylamide concentration. Substrates with a transition in flexibility were prepared as described previously (28), with total acrylamide maintained at 5% and bis-acrylamide varying between 0.1 and 0.06%. Fluorescent beads (0.2 μm FluoSpheres, carboxylate-modified; Molecular Probes) were embedded in the soft part of the substrate. Other experiments were performed on substrates of 5% total acrylamide and 0.1% bis-acrylamide. Polyacrylamide substrates were soaked in DMEM for 30 min at 37°C before the cells were plated.
Cell Culture.
The generation of mouse embryonic fibroblasts expressing wild-type or F397-FAK under the control of tetracycline was described previously (16). All cells were maintained at 37°C and 5% CO2 in DMEM (Sigma) containing 4,500 mg/ml d-glucose, 584 mg/liter glutamine, 1 mM sodium pyruvate, supplemented with 100 μg/ml streptomycin, 100 units/ml penicillin, 0.25 μg/ml amphotericin (GIBCO/BRL), 1 mM nonessential amino acids (GIBCO/BRL), 10% FBS (Atlanta Biologicals, Norcross, GA), 1 μg/ml puromycin, and 1 μg/ml tetracycline (Calbiochem). Fresh tetracycline was added every other day to maintain the inhibition of FAK expression. Experiments with FAK-null cells were performed primarily with WT-FAK cells maintained in tetracycline; however, similar results were obtained with F397-FAK cells in the presence of tetracycline. To induce the expression of FAK, cells were replated in media lacking tetracycline and cultured for at least 48 h to reach maximal expression (16).
Microscopy and Measurements of Cell Motility.
Phase images or phase/fluorescence combination images were recorded with a Zeiss ×40 N.A. 0.65 Achromat phase objective lens on a Zeiss IM-35 microscope, by using a cooled charge-coupled device camera (TE/CCD-576EM; Princeton Instruments, Trenton, NJ).
To measure the rate and persistence of cell migration, cells were plated overnight on 35-mm coverglass chamber dishes at a low density, to minimize cell–cell interactions. Time-lapse phase images were collected at a 2-min interval over a period of 60 min. Centroids of the cell were then determined, and the speed (S) and directional persistence time (P) were obtained according to the persistent random walk equation as described by Dunn (31). Migration speed was calculated as 1/slope, and directional persistence was calculated as slope/6⋅y-intercept.
Micromanipulation of Substrates and Measurements of Traction Forces.
To apply mechanical forces to cells, the polyacrylamide substrate was pulled near the cell with a microneedle as described previously (28). Microneedles with a blunted tip was prepared with a microforge (Narishige, Greenvale, NY) and mounted on a micromanipulator (Leitz). The needle was gently lowered onto the substrate near a cell with a defined polarity and pushed toward or pulled away from a protrusion of the cell. The force was maintained through the period of observation.
Traction stress generated by the cell was determined as described previously (26, 32, 33), based on the displacement of fluorescent beads embedded in the polyacrylamide substrate, the cell boundary, the Young's modulus of the substrate, and the Poisson ratio. The total force output was computed by integrating the traction magnitude over the cell area.
Transfection with Greeen Fluorescent Protein (GFP)-Zyxin and Observation of Focal Adhesion Dynamics.
Human zyxin in a pEGFP-N1 vector was kindly supplied by Jurgen Wehland [Gesellschaft für Biotechnologische Forschung (GBF), Braunschweig, Germany]. Transient transfections were performed by using the Lipofectamine reagent (GIBCO/BRL) according to the manufacturer's instructions. The transfection time and the ratio of DNA to Lipofectamine were optimized to reach a transfection efficiency of about 20%.
Cells transiently expressing enhanced GFP (EGFP)-zyxin were plated on flexible substrates as described above. After 18 h, fluorescent EGFP-zyxin images were recorded with a Zeiss ×40 N.A. 0.75 Plan-Neofluar phase objective on a Zeiss Axiovert 10 microscope, by using a cooled charge-coupled device camera (MicroMax; Roper Scientific, Trenton, NJ).
Results
FAK-Null and F397-FAK-Expressing Cells Showed Decreased Migration Speed and Directional Persistence.
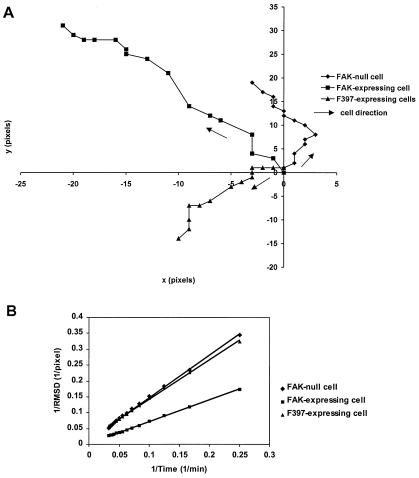
We first compared the migration of FAK-null, WT-FAK, or F397-FAK mouse embryonic fibroblasts on coverslips with time-lapse recording. To avoid possible clonal artifacts, the expression of WT-FAK or F397-FAK was placed under the regulation of tetracycline (16), and FAK-null cells were obtained by culturing cells in the presence of tetracycline. Typical migrational paths were shown in Fig. 1A. Quantitative analyses of the speed and persistence were carried out with double reciprocal plots of root-mean-square displacement against time, according to Dunn (31; Fig. 1B). Averaged results from 14 paths for FAK-null cells, 18 for WT-FAK-expressing cells, and 15 for F397-FAK-expressing cells indicated that FAK-null cells and cells expressing F397-FAK migrated with both a reduced speed and decreased directional persistence as compared with cells expressing WT-FAK (Table 1). These results suggest that defects in the migration of FAK-null cells and cells expressing F397-FAK, as reported previously (16, 21), were due to not only a more sluggish movement but also a more random migration pattern.
Figure 1.
Measurement of migration speed and directional persistence. Paths of the nuclear centroid of a representative FAK-null cell (♦), a cell expressing WT-FAK (■), and a cell expressing F397-FAK (▴) are shown in A. The position at t = 0 is placed at the origin, and the subsequent positions are plotted every 2 min. (B) Double reciprocal plot of root-mean-square displacement against time for a representative FAK-null cell (♦), a cell expressing WT-FAK (■), and a cell expressing F397-FAK (▴). Migration speed is calculated as 1/slope, and directional persistence is calculated as slope/6⋅y-intercept.
Table 1.
Comparison of migration speed and directional persistence of FAK-null and FAK-expressing cells
| Speed, μm/min | Persistence, min | |
|---|---|---|
| FAK-null | 0.364 ± 0.175 (n = 14) | 13.668 ± 8.145 (n = 14) |
| WT-FAK | 0.827 ± 0.345 (n = 18) | 20.039 ± 10.87 (n = 18) |
| F397-FAK | 0.585 ± 0.222 (n = 15) | 10.002 ± 4.959 (n = 15) |
Values are means ± SD. Migration speed and directional persistence of FAK-null and F397 cells were both significantly smaller than those of FAK-expressing cells (P = 0.0003 and 0.033 for speed, P = 0.0186 and 0.0087 for persistence, respectively).
FAK-Null and F397-FAK-Expressing Cells Failed To Reorient in Response to Mechanical Forces.
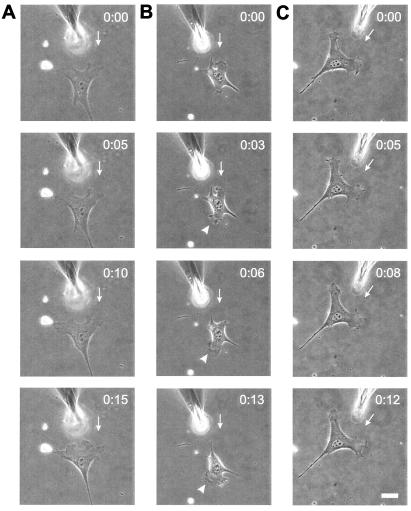
The decrease in directional persistence suggests that FAK-null cells may not be able to respond to physical or chemical guidance cues. We recently demonstrated that NIH 3T3 fibroblasts reorient in response to mechanical forces, by expand protrusions toward pulling forces and retracting those near pushing forces (28). These responses took place locally at the affected protrusion, within a period shorter than the persistence time of nuclear migration. To test whether FAK plays a role in this response, cells were cultured on flexible polyacrylamide substrates, and pushing forces were applied with a microneedle on the substrate in front of an approaching protrusion. None of the 18 FAK-null cells and 14 cells expressing F397-FAK tested showed a clear repulsive response (Fig. 2 A and C). In contrast, 62% (8/13) of cells expressing WT-FAK retracted from pushing forces within 5 min (Fig. 2B). Similar defects were observed when FAK-null or F397-FAK cells were stretched with pulling forces (not shown).
Figure 2.
Response of cells to locally applied pushing forces. A blunted microneedle was carefully inserted into the substrate near the front end of a FAK-null cell (A), a cell expressing WT-FAK (B), or a cell expressing F397-FAK (C). The needle was then pushed toward the cell. The FAK-null cell and the cell expressing F397-FAK maintained its migration direction and morphology over the period of observation, whereas the cell expressing WT-FAK moved away from the needle by retracting its original protrusion and expanding a protrusion at the opposite end. The direction of needle movement is indicated by an arrow, and the newly developed lamellipodium is indicated by an arrowhead. The starting time of needle manipulation is designated as time 0. Bar = 40 μm.
Pulling Forces Stimulated the Reorganization of Focal Adhesions in WT-FAK- and F397-FAK-Expressing, but Not FAK-Null, Cells.
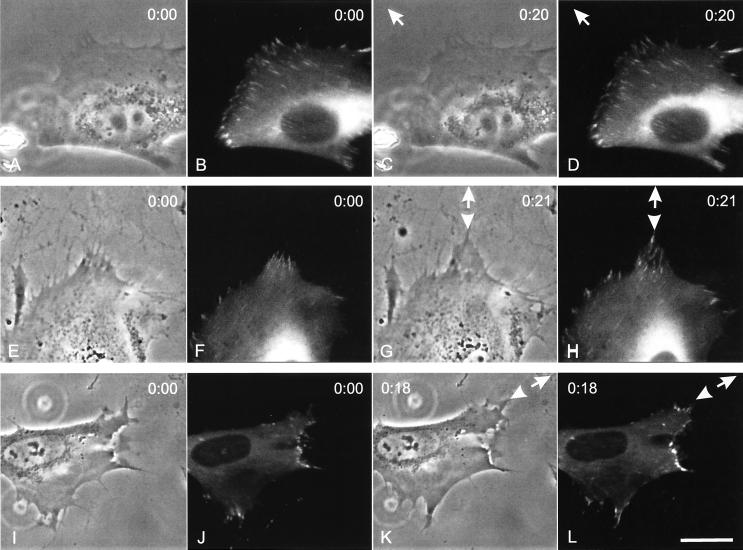
We next investigated whether the response to mechanical forces involved the reorganization of focal adhesions, which were shown to respond to mechanical signals in a recent report (34). The responses of focal adhesions to applied forces were examined by transfecting the WT-FAK or F397-FAK cell lines with EGFP-zyxin, a known component of the focal adhesion (35). Without mechanical forces, focal adhesions reorganized only slowly at the leading edge, such that no significant change in the overall distribution was observed in most cells over a period of 20 min. However, upon the application of pulling forces near a protrusion, prominent focal adhesions were observed within 20 min at the leading edge of cells expressing WT-FAK (Fig. 3 E–H; 11 of 20 cells), or F397-FAK (Fig. 3 I–L; 9 of 14 cells). In contrast, none of the FAK-null cells showed the response of focal adhesions to pulling forces (Fig. 3 A–D; total 15 cells).
Figure 3.
Induction of focal adhesions by pulling forces. A blunted microneedle was carefully inserted into the substrate near the front of a FAK-null cell (A–D), a cell expressing WT-FAK (E–H), or a cell expressing F397-FAK (I–L), which also transiently expressed EGFP-zyxin. The needle was then pulled away from the cell to exert stretching forces. Images of EGFP-zyxin were shown immediately after (B, F, and J), and ≈20 min after (D, H, and L) the application of forces. The FAK-null cell failed to show a response in focal adhesions, whereas the cell expressing WT-FAK and F397-FAK responded by forming longer and/or brighter focal adhesions in the region facing the microneedle (arrowheads). The direction of needle pulling is indicated by an arrow. A, C, E, G, I, and K show the corresponding phase images. Bar = 40 μm.
FAK-Null Cells Were Incapable of Durotaxis.
In addition to mechanical forces, NIH 3T3 cells use the flexibility of the substrate as a guidance cue, a phenomenon referred to as “durotaxis” (28). All of the three cell lines were able to migrate from soft substrates to stiff substrates (data not shown). In addition, both WT-FAK- and F397-FAK-expressing mouse embryonic fibroblasts avoid soft substrates: all of the 16 observed WT-FAK-expressing cells and 18 of 21 F397-FAK-expressing cells turned around when they arrived at the rigidity boundary from the stiff side, and none was able to migrate entirely onto the soft side (Fig. 4 B and C). In contrast, 8 of 10 FAK-null cells were able to migrate entirely from the stiff side onto the soft side (Fig. 4A). The poor directional persistence caused some FAK-null cells to cross the rigidity boundary repeatedly over a period of 12 h.
Figure 4.
Movements of cells on substrates with a rigidity gradient. Images were recorded with simultaneous phase and fluorescence illumination. Fluorescent beads embedded in the soft part facilitate the detection of the rigidity boundary. A FAK-null cell (A) approached the rigidity boundary from the hard side and moved into the soft side of the substrate. In contrast, cells expressing WT-FAK (B) or F397-FAK (C) reversed the direction of migration as part of the cell entered the soft substrate. (Bottom) Paths of these cells plotted every 10 min over a period of 3 h. Bar = 40 μm.
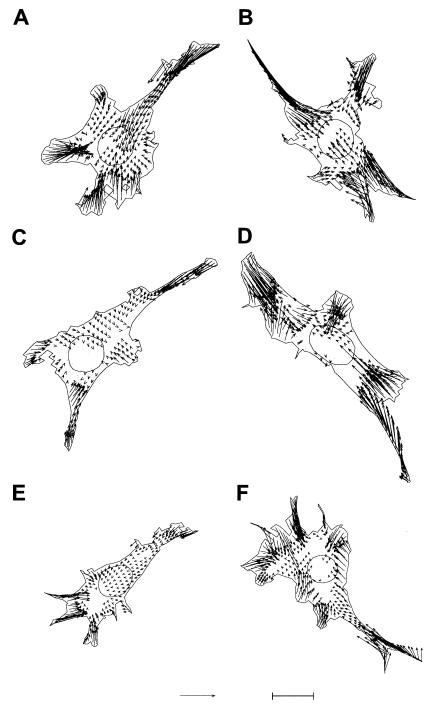
Durotaxis likely involves the modulation of traction forces by substrate rigidity, with stiffer substrate eliciting stronger traction forces (28). We therefore compared the magnitude and pattern of traction forces exerted by FAK-null or FAK-expressing cells on polyacrylamide substrates of different flexibility. On stiffer substrates (Young's modulus 280 kdyn/cm2), FAK-null, WT-FAK- and F397-FAK-expressing cells all exerted a similar pattern of traction stress, showing a general centripetal pattern with strong traction forces located at the frontal and lateral protrusions (Fig. 5). However, the total output of traction forces for WT-FAK- and F397-FAK-expressing cells decreased significantly on soft substrates, as was reported for NIH 3T3 cells (28), whereas traction forces of FAK-null cells were insensitive to such change in substrate flexibility (Table 2).
Figure 5.
Comparison of calculated traction forces of FAK-null cells (A and B), cells expressing WT-FAK (C and D), and cells expressing F397-FAK (E and F) on soft (A, C, and E; Young's modulus of 150 kdyn/cm2) or stiff (B, D, and F; 280 kdyn/cm2) substrates. These six cells selected for display generated a total force output close to the average value (Table 2). Statistically significant traction stresses within the cell boundary are shown as vectors. Bar in E = 100 kdyn/cm2. Bar in F = 20 μm.
Table 2.
Comparison of integrated traction forces of FAK-null and FAK-expressing cells on soft and hard substrates
| Y* = 280 kdyn/cm2 | Y = 150 kdyn/cm2 | |
|---|---|---|
| FAK-null | 0.555 ± 0.158 (n = 18) | 0.559 ± 0.197 (n = 18) |
| WT-FAK | 0.661 ± 0.218 (n = 18) | 0.514 ± 0.161 (n = 18) |
| F397-FAK | 0.710 ± 0.194 (n = 12) | 0.475 ± 0.193 (n = 12) |
The total force output was computed by integrating the magnitude of traction stress over the cell area. Values are means ± SD, in dynes. Traction forces of cells expressing WT-FAK or F397-FAK on stiff substrates were significantly larger than those on soft substrates (P = 0.03 and 0.01, respectively). No statistically significant difference was found for FAK-null cells on different substrates (P = 0.95).
Young's modulus.
Discussion
FAK is known to be involved in tyrosine phosphorylation during integrin-mediated signaling (11). However, its exact role in cell adhesion and migration is unclear. As indicated by the formation of apparently normal focal adhesions in FAK-null cells (21), FAK is not required for the assembly or maintenance of focal adhesions. Conversely, integrin-mediated activation of FAK is also independent of the formation of focal adhesions (36). Therefore FAK more likely plays a regulatory role in cell migration as initially suggested by results with Boyden chamber (16, 21), or wound healing (11, 37).
Fibroblast migration involves complex interplay among the formation of cell-substrate adhesion, the exertion of propulsive forces, and the detachment of the adhesion sites (38). The rate and direction of migration is determined largely by differences in size, lifespan, and traction forces among multiple protrusions. Based on the apparently enlarged focal adhesions (21), and prolonged lifespan of focal adhesions during cell spreading (39), it was speculated that FAK may be required for the turnover of focal adhesions. Reduced turnover of focal adhesions may then impair cell detachment and explain the reduced speed of migration of FAK-null cells. In the present study, detailed analysis of the migration path suggested a second defect, that FAK-null cells are unable to maintain a steady course of migration. It is likely that multiple protrusions along opposite directions generate a tug-of-war, which leads to the instability of migration and further reduces the migratory speed. In addition, the randomized movements suggest that FAK may be involved in the guidance of cell migration in response to physical cues transmitted through the focal adhesions.
With their close proximity to the substrate, focal adhesions represent the primary structure for mediating mechanical interactions with the substrate, as shown recently with flexible substrates (40, 41). Through focal adhesions, the cell exerts forces on the substrate to propel its migration. In addition, migrating cells likely use the contact at focal adhesions as a means for probing the physical characteristics of the substrate and for detecting mechanical forces exerted through integrins. The present experiments with flexible polyacrylamide substrates provide direct evidence that FAK plays a key role in the response of fibroblasts to such mechanical signals. FAK-null cells showed no detectable response to pushing or pulling forces, with respect to both their migration and the assembly of focal adhesions. In contrast, control cells expanded their protrusions toward pulling forces and retracted away from pushing forces (Figs. 2 and 3). Furthermore, control cells responded to pulling forces by forming prominent focal adhesions, whereas FAK-nulls fail to show this response (Fig. 3). Therefore a likely scenario is that tension at focal adhesions regulates the assembly state of focal adhesions, with increasing tension stimulating the assembly and decreasing tension favoring the disassembly of focal adhesions. The stability of focal adhesions may in turn affect the expansion/retraction of local protrusions. Consistent with this notion, it was reported that forces exerted through integrin-ECM complexes can profoundly affect the organization of focal adhesion, as well as the stiffness and contractility of the cortex (34, 42, 43).
A related mechanism may account for the response of control cells to changing substrate flexibility. Here, instead of responding passively to applied forces, the cell most likely uses its traction forces to actively probe the substrate. Our measurements of traction forces demonstrated that, as for 3T3 cells (26, 28), FAK-expressing cells exerted stronger traction forces on stiffer substrates (Table 2), which may cause a positive feedback to amplify the initial mechanical response. In contrast, FAK-null cells were insensitive to substrate flexibility and generated similar total mechanical output on stiff or soft substrates.
Although biochemical studies indicated that autophosphorylation of Tyr-397 is critical for the interactions of FAK with Src and other proteins containing the SH-2 domain (9, 10), the role of Tyr-397 in the cellular function of FAK proved to be complicated. Mutation of Tyr-397 impairs the ability of FAK to promote cell migration and early spreading (16, 20); however, cells were eventually able to spread to a greater extent than did control cells (16). In addition, other phosphorylation sites, such as Tyr-576, Tyr-577, and Tyr-925, may play a complementary or alternative role in the regulation of FAK (16, 44). From the present results, it is clear that mutation at Tyr-397 did not abolish entirely the ability of FAK to regulate cell migration. Consistent with previous findings, cells expressing F397-FAK migrated with a reduced rate and persistence, similar to FAK-null cells. However, these cells were still able to amplify their focal adhesions in response to pulling forces, despite the lack of response in the direction of migration. In addition, cells expressing F397-FAK responded to substrate flexibility in a manner similar to control cells, showing both the weakening of traction forces on soft substrates and the ability to steer away from soft substrates. These observations suggest that unphosphorylated FAK, or FAK phosphorylated at alternative sites, was able to perform at least some of the functions, such as the regulation of traction forces. Furthermore, whereas WT-FAK and F397-FAK were both sufficient for the recruitment of proteins to focal adhesions upon mechanical stimulation, subsequent autophosphorylation of Tyr-397 in WT-FAK is required for stimulating the expansion of the protrusion and the reorientation of cell migration.
With its localization at the interface between the integrin receptor for ECM binding and the actin cytoskeleton for force generation, FAK easily fulfill a role in converting external mechanical input into chemical signals. Our previous studies indicated that the extent of protein tyrosine phosphorylation at focal adhesions is regulated by physical properties of the substrate, and that one of the main phosphoproteins is likely to be FAK (27). FAK is also required for stress-dependent morphological response of endothelial cells (45). One possibility is that FAK may respond to mechanical stress by changing its conformation and exposing its phosphorylation site, including not only Tyr-397 but also other activation sites previously shown to be critical for its function. Equally important is the mechanism linking FAK with the actin cytoskeleton. A recent study has shown a constitutive activation of Rho in FAK-null cells, implicating the small GTPase Rho in FAK-mediated signaling (39). With its multiple effectors affecting the actin cytoskeleton, Rho may serve as a crucial link between FAK phosphorylation and cortical contractility. The present study illustrates the value of combining biophysical, cellular, and gene manipulation approaches in future explorations into the complex mechanism of cell migration.
Acknowledgments
We are grateful to Dr. Jurgen Wehland [Gesellschaft für Biotechnologische Forschung (GBF), Braunschwick, Germany] and Victor Small (Austrian Academy of Sciences, Salzburg, Austria) for providing EGFP-zyxin plasmids. This work was supported by grants from the National Aeronautics and Space Administration (NAG2-1197) and by National Institutes of Health Grants GM-32476 to Y.-l.W., GM-61806 to M.D., and GM-49882 to S.K.H.
Abbreviations
- FAK
focal adhesion kinase
- WT-FAK
wild-type FAK
- F397-FAK
Tyr-397 mutant FAK
- GFP
green fluorescent protein
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Kanner S B, Reynolds A B, Vines R R, Parsons J T. Proc Natl Acad Sci USA. 1990;87:3328–3332. doi: 10.1073/pnas.87.9.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz M A, Schaller M D, Ginsberg M H. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 3.Hanks S K, Calalb M B, Harper M C, Patel S K. Proc Natl Acad Sci USA. 1992;89:8487–8491. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlaepfer D D, Hanks S K, Hunter T, vanderGeer P. Nature (London) 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 5.Hynes R O. Trends Cell Biol. 1999;9:M33–M37. [PubMed] [Google Scholar]
- 6.Guan J-L. Int J Biochem Cell Biol. 1997;29:1085–1096. doi: 10.1016/s1357-2725(97)00051-4. [DOI] [PubMed] [Google Scholar]
- 7.Chen H-C, Appeddu P A, Parsons J T, Hildebrand J D, Schaller M D, Guan J-L. J Biol Chem. 1995;270:16995–16999. doi: 10.1074/jbc.270.28.16995. [DOI] [PubMed] [Google Scholar]
- 8.Zheng C, Xing Z, Bian Z C, Guo C, Warner A, Guan J L. J Biol Chem. 1998;273:2384–2389. doi: 10.1074/jbc.273.4.2384. [DOI] [PubMed] [Google Scholar]
- 9.Schaller M D, Hildebrand J D, Shannon J D, Fox J W, Vines R R, Parsons J T. Mol Cell Biol. 1994;14:1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X, Chattopadhyay A, Ji Q S, Owen J D, Ruest P J, Carpenter G, Hanks S K. Proc Natl Acad Sci USA. 1999;96:9021–9026. doi: 10.1073/pnas.96.16.9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilmore A P, Romer L H. Mol Biol Cell. 1996;7:209–1224. doi: 10.1091/mbc.7.8.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cary L A, Han D C, Polte T R, Hanks S K, Guan J-L. J Cell Biol. 1998;140:211–221. doi: 10.1083/jcb.140.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sieg D J, Ilic D, Jones K C, Damsky C H, Hunter T, Schlaepfer D D. EMBO J. 1998;17:5933–5947. doi: 10.1093/emboj/17.20.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu J, Tamura M, Pankov R, Danen E H J, Takino T, Matsumoto K, Yamada K M. J Cell Biol. 1999;146:389–403. doi: 10.1083/jcb.146.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sieg D J, Hauck C R, Schlaepfer D D. J Cell Sci. 1999;112:2677–2691. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- 16.Owen D J, Ruest P J, Fry D W, Hanks S K. Mol Cell Biol. 1999;19:4806–4818. doi: 10.1128/mcb.19.7.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sieg D J, Hauck C R, Ilic D, Klingbeil C K, Schaefer E, Damsky C H, Schlaepfer D D. Nat Cell Biol. 2000;2:249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- 18.Zhao J-H, Reiske H, Guan J-L. J Cell Biol. 1998;143:1997–2008. doi: 10.1083/jcb.143.7.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao J, Zheng C, Guan J-L. J Cell Sci. 2000;113:3063–3072. doi: 10.1242/jcs.113.17.3063. [DOI] [PubMed] [Google Scholar]
- 20.Cary L A, Chang J F, Guan J-L. J Cell Sci. 1996;109:1787–1794. doi: 10.1242/jcs.109.7.1787. [DOI] [PubMed] [Google Scholar]
- 21.Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T, Aizawa S. Nature (London) 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 22.Mochitate K, Pawelek P, Grinnell F. Exp Cell Res. 1991;193:198–207. doi: 10.1016/0014-4827(91)90556-a. [DOI] [PubMed] [Google Scholar]
- 23.Banes A J, Tsuzaki M, Yamamoto J, Fischer T, Brigman B, Brown T, Miller L. Biochem Cell Biol. 1995;73:349–365. doi: 10.1139/o95-043. [DOI] [PubMed] [Google Scholar]
- 24.Davies P F. Physiol Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grinnell F, Zhou M, Carlson M A, Abrams J M. Exp Cell Res. 1999;248:608–619. doi: 10.1006/excr.1999.4440. [DOI] [PubMed] [Google Scholar]
- 26.Wang H-B, Dembo M, Wang Y-L. Am J Physiol Cell Physiol. 2000;279:C1345–C1350. doi: 10.1152/ajpcell.2000.279.5.C1345. [DOI] [PubMed] [Google Scholar]
- 27.Pelham R J, Jr, Wang Y-L. Proc Natl Acad Sci USA. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lo C-M, Wang H-B, Dembo M, Wang Y-L. Biophys J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y-L, Pelham R J., Jr Methods Enzymol. 1998;298:489–496. doi: 10.1016/s0076-6879(98)98041-7. [DOI] [PubMed] [Google Scholar]
- 30.Pelham R J, Jr, Wang Y-L. Mol Biol Cell. 1999;10:935–945. doi: 10.1091/mbc.10.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunn G A. Agents Actions. 1983;12,Suppl.:14–33. doi: 10.1007/978-3-0348-9352-7_1. [DOI] [PubMed] [Google Scholar]
- 32.Dembo M, Wang Y-L. Biophys J. 1999;76:2307–2316. doi: 10.1016/S0006-3495(99)77386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Munevar S, Wang Y-L, Dembo M. Biophys J. 2001;80:1744–1757. doi: 10.1016/s0006-3495(01)76145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riveline D, Zamir E, Balaban N Q, Schwarz U S, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky A D. J Cell Biol. 2001;153:1175–1185. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beckerle M C. BioEssays. 1997;19:949–957. doi: 10.1002/bies.950191104. [DOI] [PubMed] [Google Scholar]
- 36.Lyman S, Gilmore A, Burridge K, Gidwitz S, White G C., 2nd J Biol Chem. 1997;272:22538–22547. doi: 10.1074/jbc.272.36.22538. [DOI] [PubMed] [Google Scholar]
- 37.Ilic D, Kanazawa S, Furuta Y, Yamamoto T, Aizawa S. Exp Cell Res. 1996;222:298–303. doi: 10.1006/excr.1996.0038. [DOI] [PubMed] [Google Scholar]
- 38.Sheetz M P, Felsenfeld D P, Galbraith C G. Trends Cell Biol. 1998;8:51–54. doi: 10.1016/s0962-8924(98)80005-6. [DOI] [PubMed] [Google Scholar]
- 39.Ren X-D, Kiosses W B, Sieg D J, Otey C A, Schlaepfer D D, Schwartz MA. J Cell Sci. 2000;113:3673–3678. doi: 10.1242/jcs.113.20.3673. [DOI] [PubMed] [Google Scholar]
- 40.Beningo K, Dembo M, Kaverina I, Small J V, Wang Y-L. J Cell Biol. 2001;153:881–887. doi: 10.1083/jcb.153.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balaban N Q, Schwarz U S, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, Geiger B. Nat Cell Biol. 2001;3:466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 42.Wang N, Butler J P, Ingber D E. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 43.Choquet D, Felsenfeld D P, Sheetz M P. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- 44.Schlaepfer D D, Hunter T. Mol Biol Cell. 1996;16:5623–5633. doi: 10.1128/mcb.16.10.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naruse K, Yamada T, Sai X-R, Hamaguchi M, Sokabe M. Oncogene. 1998;17:455–463. doi: 10.1038/sj.onc.1201950. [DOI] [PubMed] [Google Scholar]