Abstract
Candidiasis is a multifaceted fungal disease including mucosal-cutaneous, visceral, and disseminated infections caused by yeast species of the genus Candida. Candida infections are among the most common human mycoses. Candida species are the third to fourth most common isolates from bloodstream infections in neutropenic or immunocompromised hospitalized patients. The mucosal-cutaneous forms—particularly vaginal infections—have a high prevalence. Vaginitis caused by Candida species is the second most common vaginal infection. Hence, candidiasis is a major subject for research, including experimental in vivo models to study pathogenesis, prevention, or therapy of the disease. The following review article will focus on various experimental in vivo models in different laboratory animals, such as mammals (mice, rats, rabbits), the fruit fly–Drosophila melanogaster, the larvae of the moth Galleria mellonella, or the free-living nematode Caenorhabditis elegans. The review will describe the induction of the different clinical forms of candidiasis in the various models and the validity of such models in mimicking the human clinical situations. The use of such models for the assessment of antifungal drugs, evaluation of potential vaccines to protect before candidiasis, exploration of Candida virulence factors, and comparison of pathogenicity of different Candida species will be included in the review. All of the above will be reported as based on published studies of numerous investigators as well as on the research of the author and his group.
Keywords: candidiasis, mammalian animal models, non-mammalian animal models
1. Introduction
Candidiasis is a multifaceted fungal disease including mucosal-cutaneous, visceral, and disseminated infections caused by yeast species of the genus Candida. Candida infections are among the most common human mycoses [1].
Candida species are the third to fourth most common isolates from bloodstream infections in neutropenic or immunocompromised hospitalized patients, primarily from intensive care units (ICUs) [2]. The Global Action Fund for Fungal Infections (Gaffi) [3] data indicate that ~300,000 cases of candidemia per year are predicted worldwide.
The mucosal-cutaneous forms—particularly vaginal infections—have a high prevalence. Vaginitis caused by Candida species is the second most common vaginal infection [4].
Gaffi data reveal that about 75 million women will suffer from recurrent vaginal infections. Oral thrush is an additional common mucosal Candida infection, afflicting over 9 million people [3].
Hence, candidiasis is a major subject for research, including experimental in vivo models to study pathogenesis, prevention, or therapy of the disease.
The following overview article aims to describe various experimental in vivo models in different laboratory animals, such as mammals (mice, rats, rabbits) [5], the fruit fly Drosophila melanogaster [6], the larvae of the moth Galleria mellonella [7], or the free-living nematode Caenorhabditis elegans [8]. The review will describe the induction of the different clinical forms of candidiasis in the various models and the validity of such models in mimicking the human clinical situations. The review will relate to models focusing on the evaluation of the efficacy of antifungal drugs in the treatment of the different clinical entities of candidiasis, or on evaluation of vaccines to assess protection before candidiasis. Exploration of Candida virulence factors or comparison of pathogenicity of different Candida species will be included in the review as well. All of the above will be reported based on published studies of numerous investigators, as well as on the research of the author and her group.
In order to mimic a human host, a mammalian animal would seem most rational; indeed: mice, rats, guinea-pigs and rabbits are the oldest known animal models. Candida experimental infections in these animals have been reported since the late 19th century [9].
2. The Mouse Model
Among the rodents, mice are the most widely-used animal models. A variety of clinical Candida entities have been studied in such models, including the mucosal oral or vaginal infections, the gastro-intestinal (GI), or deep-seated and systemic forms of candidiasis that have been induced experimentally in outbred and inbred mice strains. The most commonly used outbred mice strains are the ICR and Swiss white mice. Various inbred mice strains have been developed over the years for experimental work, of which the most commonly used strains in Candida studies are the BALB/C and BL 57 [10,11]. The author’s studies involved mostly the ICR strain for studies of systemic, vaginal, and oral Candida infections [12,13,14].
With the aim of mimicking the clinical situations in humans as closely as possible, experimental Candida infections can be induced not only in naïve mice, but also in mice rendered immunocompromised by pretreatment with various cytotoxic agents, such cyclophosphamide (CY) [12,15], 5-fluorouracyl (5FU) [16], or irradiation [16]. Other debilitating conditions can also be elicited in mice, such as experimental diabetes by pretreatment with streptozotocin [13] or continuous estrus stage by inoculation with estrogen [17].
Furthermore, the route of induction of infection may vary as well: systemic Candida infection can be induced by intravenous (IV) inoculation of the fungus, generally into the tail vein—a model widely used by most investigators [18,19], including the author [12,15]. Intraperitoneal (IP) injection is another route for the induction of systemic infection [20]. The GI route may also lead to a disseminated Candida infection [16].
The mouse model was intensively explored in studies aiming to elucidate mechanisms of pathogenicity and fungal virulence attributes [21], or comparison of virulence of different Candida species or strains within a specific species [15]. Lionakis et al. [18] studied organ-specific immune responses in experimental invasive candidiasis.
Great importance is given to studies exploring the activity of new antifungal drugs in the mouse model [19,22], both in naïve and compromised mice, which represents activity in vivo and is thus an essential step in drug development. Such studies may also focus on the antifungal activity of the drug in terms of its pharmacokinetic characteristics, such as tissue distribution, excretion, or other pharmacokinetic parameters [23].
The mouse model is also suitable for exploring of the immune responses elicited by the fungus and evaluating possible induction of immunity to the infection. This is an essential step in the assessment of the possibility for development of a vaccine. The immunization step is then followed by a challenge of the immunized animals with live microorganisms, and the degree of protection to withstand the challenge is assessed in comparison to non-vaccinated control animals [20]. Such experiments were extensively reported in the literature with various Candida immunogens, such as killed Candida, attenuated live organisms, or various subcellular Candida fractions [24,25,26,27], including studies by the author’s group using Candida ribosomes as an immunogen [20].
Preventive measures to protect before Candida infection may include antifungal coverage or vaccination. Another approach to prevention may be based on interference in the infectious process by inhibition of specific steps in the process. An example of this approach is the use of inhibition in the step of adherence to host tissues—an initial step of the infectious process in the evolution of candidiasis. Examples of such studies can be demonstrated by competitive binding of adhesins or receptors to inhibit the adhesion process [28].
2.1. Induction of Infection
2.1.1. Systemic Infection
In the author’s group, most studies involved 4–6 week-old female ICR mice which were rendered immunocompromised by IP inoculation of 200 mg/kg cyclophosphamide (CY) [12,15]. Three days post-CY inoculation (peak of immunosuppression, as demonstrated by low numbers of white blood cells and a decrease in weight), the mice were inoculated IV into the tail vein with 104 Candida albicans yeasts/mouse.
This mode of infection induction leads to systemic candidiasis suitable for the evaluation of antifungal drugs, immune responses, or comparison of the pathogenicity of C. albicans strains.
Infection was monitored for a period of up to 30 days and assessed by survival rate (%), mean survival time (MST), and fungal burden, as determined by enumeration of Candida colony forming units (CFU) in the kidneys [12,15]. In some experiments, evaluation also included histopathological examination of kidney tissues. In specific experiments, other organs, such as lungs, liver, spleen, or brain were also examined [23].
Naïve mice were inoculated with a higher inoculum: 5 × 104 organisms/mouse.
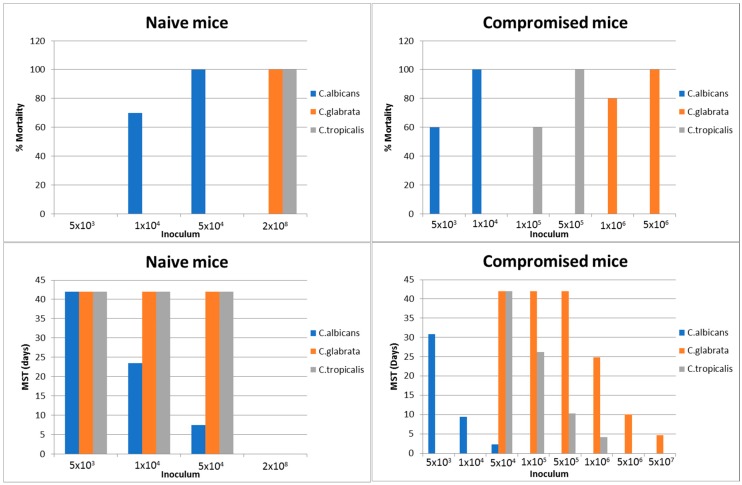
Infection by non-albicans Candida species (C. tropicalis, C. glabrata, C. krusei, etc.) required much higher inoculum to elicit infection (see Figure 1).
Figure 1.
Systemic candidiasis in naïve and compromised ICR mice. Adapted from Schadkchan and Segal [29,30]; Naïve and compromised mice were injected intravenously; Follow-up of 42 days; Data of % survival refer to inoculum which resulted in 100% mortality; MST = Mean survival time.
Intraperitoneal (IP) inoculation of C. albicans can also lead to systemic candidiasis. However, in this model, higher fungal inoculum is required [20].
2.1.2. Gastrointestinal (GI) Infection
Candida albicans is a human commensal and part of the human gastrointestinal (GI) mycobiota, from where it can spread under specific conditions into other sites of the body and cause a variety of clinical entities, including systemic candidiasis [1]. Although C. albicans is not part of the mouse mycobiota (they harbor Candida pintolopesii), mice have been used for experimental GI candidiasis.
We investigated the interaction of C. albicans with the GI tissue in ICR mice treated with cytotoxic anti-cancer drugs methotrexate (MTX) or 5-fluorouracil (5FU) to mimic human situations [16].
We adapted an experimental model of fatal systemic candidiasis originating from the gastrointestinal (GI) tract of compromised mice [16]. Female ICR mice were compromised by a single anti-cancer treatment: irradiation (4 or 6 Greys i.e., 400–600 rads), methotrexate (MTX) (3 mg per mouse, intraperitoneally), or 5-fluorouracil (5FU) (200 mg kg−1, intravenously). Three days later, compromised and control mice were administered orally with C. albicans. Morbidity and mortality due to candidiasis were monitored for 30 days post-Candidal inoculation. Increased and longer GI colonization was noted among the MTX and 5FU treated or irradiated mice. The stomach was found to be the major part of the GI tract involved in fungal colonization. It is worth emphasizing that a significant number (53.8–83.3%) of the anti-cancer-treated mice developed systemic candidiasis originating from the GI tract, which was fatal in 30–80% of the infected animals. Candida could be found in the liver, spleen, and kidneys. In systemically-infected animals, Candidal antigen was demonstrated in the serum, and fungal abscesses containing C. albicans were observed in the liver, kidneys, and spleen.
2.1.3. Vaginal Infection
Experimental Candida vaginitis can be induced in mice and rats.
Very early observations [31] indicated that the optimal timing for the induction of vaginal infection is during the estrus stage of the mice. The estrus stage is characterized by the massive appearance of epithelial cells in the vaginal exudate. The estrus-cycle of mice is 3–4 days in duration. Mice infected during the estrus stage will develop the infection, which may disappear spontaneously afterward. Hence, most investigators—including the author—use models in which a constant estrus state is maintained by inoculating female mice with estradiol benzoate [17] 3–4 days prior to inoculation with Candida. Infection is induced by intravaginal inoculation of 107 C. albicans yeast cells, and can be maintained by repeated weekly inoculations of estradiol benzoate.
Diabetic women suffer from higher rates of Candida vaginitis [32]. In the frame of studies to inhibit adhesion of Candida to host tissues and thereby prevent infection, we explored this aspect in an experimental infection in diabetic mice. Mice were rendered diabetic by intraperitoneal (IP) injection of 160 mg/kg streptozotocin [13]. Diabetic state was apparent 2–7 days later. Mice were inoculated intra-vaginally with 107–1010 C. albicans and followed-up for 35 days. The diabetic mice developed a massive infection of long duration while in the non-diabetic control mice the infection was less massive and cleared earlier (see Table 1). Furthermore, the fungal burden in the vaginal wash—as measured by Candida CFU—was 10-fold lower in the non-diabetic mice than in the diabetic counterparts. Thus, this model mimics a real clinical situation and therefore seems to be valid.
Table 1.
Vaginal infection induced in diabetic and naïve mice.
| Days After Inoculation | Diabetic Mice with Estrus (% Infected) | Diabetic Mice Without Estrus (% Infected) | Naïve Mice |
|---|---|---|---|
| 1 | 100 | 92 | 70 |
| 3 | 100 | 92 | 20 |
| 5 | 83 | 58 | 10 |
| 7 | 83 | 55 | 0 |
| 14 | 67 | 55 | 0 |
| 21 | 50 | 55 | 0 |
| 28 | 50 | 55 | 0 |
| 35 | 33 | 55 | 0 |
Adapted from Segal and Yosef-Lev [13].
3. The Drosophila melanogaster Model
Non-mammalian animal models are of significance for biological and medical research as they are easier and simpler to handle, less costly, and save manpower. This rationale led to attempts seeking non-mammalian alternatives. Among several non-mammalian animal models in-use in biological and medical research, the oldest is Drosophila melanogaster.
Drosophila melanogaster is a small (~3 mm long) fruit fly and it has served as model—particularly in genetics and developmental biology—for almost a century. It is a small animal with a short life cycle of just two weeks, and is cheap and easy to keep large numbers. Mutant flies with defects in any of several thousand genes are available, and the entire genome has recently been sequenced [33].
Its importance and vast use in biological research was emphasized by the Nobel prize in physiology in 1995 given to Ed Lewis, Christiane Nusslein-Volhard, and Eric Wieschaus.
Chamilos et al. [6] developed a model in Drosophila melanogaster and studied fungal virulence attributes and drug discovery. Specifically, these investigators developed a model of candidiasis in Toll (Tl)–deficient Drosophila melanogaster. C. parapsilosis was less virulent than C. albicans in the Tl mutant flies, mimicking the human condition. Comparison of the findings of the attenuated cph1/cph1 and efg1/efg1 C. albicans mutant in the mouse model with those in the T1 mutant flies indicated similarity. Hence, these researchers concluded that the Drosophila melanogaster model is a promising model for large-scale studies of virulence mechanisms and antifungal drug activity in candidiasis. Glitenberg et al. [33] assessed the virulence of various clinical isolates of C. albicans in wild-type Drosophila melanogaster, and found that the virulence of the isolates correlated with that noted previously in the murine model. Hence, these investigators also concluded that the Drosophila model is a relevant model to study Candida infection.
A different group of investigators [34] used immune-deficient Drosophila melanogaster to explore the innate immune response to human fungal pathogens. They showed that specific C. albicans mutants differing in virulence in murine model exhibited a similar pattern of virulence in the Drosophila model. Moreover, in this model they could detect virulence characteristics not detected in an immunocompetent model.
It is of interest that in Drosophila, C. albicans can change its morphology from yeast to the pseudohyphal form, similar to the situation occurring during infection in mammalian hosts [35]. Furthermore, a very recent publication [36] asks an intriguing question—whether the fruit fly could be a potential vector of opportunistic pathogens, since they isolated 18 species of fungi, including Candida and Aspergillus species, from wild fruit flies.
4. The Caenorhabditis elegans Model
Caenorhabditis elegans is a small (about 1 mm in length) free-living transparent nematode found in temperate soil environments, which has been used as an animal model in developmental biology research since 1974 [8,37].
The advantages of this model system lie in its being a eukaryotic multicellular organism and its being transparent, enabling easy observation. In addition, C. elegans is cheap and easy to grow, can be frozen without losing viability, and hence can be stored easily for long periods of time. C. elegans does not poses an adaptive immune system but only an innate immune system by which to defend itself [38].
In relevance to fungi, C. elegans has been involved, among others, in the areas of antimicrobial drug discovery studies [39]; studies related to antifungal immune defenses [40] and antifungal efficacy against non-C. albicans species [41].
Pukkila-Worle et al. [40] showed that live C. albicans can establish an intestinal infection in C. elegans, while heat-killed organisms cannot. These authors also reported that by transcription profiling of C. elegans they demonstrated that exposure to C. albicans stimulated a host response in this nematode. The response is mediated through “pattern recognition”, recognizing pathogen-associated molecular patterns (PAMPs).
Of interest is the statement of Ewbank and Zagusti [39] that C. elegans cannot completely replace mammalian systems due to various differences, among which a major one is the inability of C. elegans to grow at the mammalian body temperature of 37 °C.
Scorzoni et al. [41] studied antifungal efficacy against C. krusei infection in two non-mammalian in vivo systems: C. elegans and Galleria mellonella. The authors found a correlation between the in vivo data and the in vitro susceptibility assays’ data, which lends validity to the in vivo models.
5. The Galleria mellonella Model
Another non-mammalian animal model introduced into biological-medical research in the late 1990s–early 2000s which is currently used by many investigators is the larvae of the moth Galleria mellonella [7]. In this case as well, the rationale is to save human resources and costs associated with use of mammalian animal models. The larvae are commercially available and easy to handle. Furthermore, due to the strong structural and functional similarities between the immune response of insects and the innate immune responses of mammals [42], insects can be used to study alterations in microbial virulence.
Galleria mellonella has been used as a model for the in vivo assessment of the pathogenicity of bacterial and fungal species [43,44,45,46].
The author’s group studied the comparative pathogenicity of C. albicans isolates from blood-stream Candida infection vs. isolates from vaginitis, both in Galleria mellonella and in a mouse model [15].
Table 2 shows the results of such tests. It can be noted that in the control strain CBS 562 and the blood isolate S14, the data of both models showed comparable results, with both strains showing the highest virulence. However, in other strains (M33 and M39), differences between the two models were noted. It should also be added that while both models enabled the assessment of mortality rate as a criterion of pathogenicity, the mouse model also enabled the assessment of morbidity, as determined by quantitative evaluation of mouse kidney colonization.
Table 2.
Comparison of pathogenicity of Candida albicans strains in mouse and Galleria mellonella.
| Mouse Model | Galleria Model | |||
|---|---|---|---|---|
| Strain | MST (Days) | SD | MST (Days) | SD |
| S2 | 28.27 | 4.9 | 2.75 | 1.95 |
| S11 | 27.87 | 4.7 | 2.00 | 0.97 |
| S19 | 27.67 | 4.0 | 1.90 | 0.80 |
| S5 | 27.5 | 5.9 | 2.18 | 1.20 |
| S14 | 12.6 | 9.3 | 1.70 | 0.75 |
| CBS | 4.47 | 2.2 | 1.31 | 0.51 |
| M33 | 16.47 | 6.7 | 3.33 | 2.08 |
| M32 | 17.93 | 9.5 | 2.65 | 1.37 |
| M42 | 21.67 | 9.4 | 1.40 | 1.35 |
| M29 | 24.64 | 8.5 | 2.95 | 1.84 |
| M39 | 27.53 | 5.5 | 1.40 | 0.78 |
| M26 | 29.53 | 1.8 | 2.45 | 1.25 |
Adapted from Frenkel et al. [15]; Mice were inoculated with bloodstream (S) and vaginal (M) C. albicans strains and mean survival time was determined (MST); No significant difference was observed between the M and S strains in both models.
It is of interest that the literature demonstrates differences between researchers. While Amorim-Vaz et al. [46] observed a discrepancy in the results obtained in the two models, Hirakawa et al. [47], Brenan et al. [48], and Slater et al. [45] reported similar results in both models. Thus, in any in vivo assessment, research differences between models among researchers should be taken into consideration.
The Galleria model has also been used for testing antimicrobial efficacy [49] and the assessment of the pharmacokinetic characteristics of antimicrobial agents [50]. Mesa-Aranga et al. [51] tested the efficacy of antifungal drugs against Candida tropicalis, and Ames et al. [49] evaluated the activity of antifungals against Candida glabrata. Furthermore, the group of Astvad et al. [50] studied the pharmacokinetics of fluconazole in the larvae of Galleria mellonella in comparison to liquid chromatography.
Although some studies [52] indicated that transfer of the Galleria mellonella hemolymph may transfer immunity to Pseudomonas aeruginosa, no studies regarding anti-Candida or anti other-fungal vaccine involving vaccination and challenge with live Candida organisms has been described. Thus, Galleria mellonella is a system in which many of the biological aspects of microbial organisms can be investigated, however not to the same extent as in mammalian systems.
6. Summary
In summary, this article focused on experimental Candida infections in four different models: the mouse model as a representative of a mammalian model and three non-mammalian models: Drosophila melanogaster, Caenorhabditis elegans, and Galleria mellonella. All three non-mammalian models were generally compared to the traditional mammalian model. Many of the data correlated well with the mammalian model, but some were not compatible, suggesting that caution should be used in analysis and interpretation.
The major advantages of the non-mammalian models lie in the economy of human resources, affordable costs, and ease of handling. It should, however, be added in this context, that while the mammalian model enables research regarding pathogenicity, drug activity, and drug pharmacokinetics, as well as vaccination attempts involving immunization and challenge with the microbe to assess the elicited protection and immune responses, the non-mammalian models are not suitable for classical microbial vaccination and challenge studies, nor for organ colonization assessments. In addition, since some of the models (e.g., C. elegans) are not functional at the mammalian body temperature (37 °C) and lack an adaptive immune system, the validity of such models in reference to the human host is diminished.
Thus, the authors ultimately believe that the mammalian model more closely represents the human situation.
Acknowledgments
University funds.
Author Contributions
Esther Segal, the PI, contributed to the concept and writing of the article. Michael Frenkel performed experiments & contributed to analysis of data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Edwards J.E. Candida species. In: Bennett J.E., Dolin R., Blaser M.J., editors. Principles and Practice of Infectious Diseases. 8th ed. Elsevier; Amsterdam, The Netherlands: 2015. pp. 2879–2894. [Google Scholar]
- 2.Pfaller M.A., Diekema D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global Action Fund for Fungal Infections. Available online: https://www.gaffi.org.
- 4.Sobel J.D. Vulvovaginal candidosis. Lancet. 2007;369:1961–1971. doi: 10.1016/S0140-6736(07)60917-9. [DOI] [PubMed] [Google Scholar]
- 5.Romani L. Current Protocols in Immunology. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 1999. Animal Models for Candidiasis. Unit 19.6. [DOI] [PubMed] [Google Scholar]
- 6.Chamilos G., Lionakis M.S., Lewis R.E., Lopez-Ribot J.L., Saville S.P., Albert N.D., Halder G., Kontoyiannis D.P. Drosophila melanogaster as a facile model for large-scale studies of virulence mechanisms and antifungal drug efficacy in Candida species. J. Infect. Dis. 2006;193:1014–1022. doi: 10.1086/500950. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs B.B., O’Brien E., El Khoury J.B., Mylonakis E. Methods for using Galleria mellonella as a model host to study fungal pathogenesis. Virulence. 2010;1:475–482. doi: 10.4161/viru.1.6.12985. [DOI] [PubMed] [Google Scholar]
- 8.Brenner S. In the Beginning Was the Worm. Genetics. 2009;182:413–415. doi: 10.1534/genetics.109.104976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joly V., Yeni P. Rodent models of Candida sepsis. In: Zak O., Sande M., editors. Handbook of Animal Models of Infection. Academic Press; Cambridge, MA, USA: 1999. pp. 650–657. [Google Scholar]
- 10.Inbred Strains of Mice: BALB. [(accessed on 1 December 2017)]; Available online: http://www.informatics.jax.org/inbred_strains/mouse/docs/BALB.shtml.
- 11.Aurora’s Guide to Mouse Colony Management at MIT. [(accessed on 1 December 2017)]; Available online: https://ki.mit.edu/files/ki/cfile/sbc/escell/mouseManagement.pdf.
- 12.Semis R., Mendlovic S., Polacheck I., Segal E. Activity of an Intralipid formulation of nystatin in murine systemic candidiasis. Int. J. Antimicrob. Agents. 2011;38:336–340. doi: 10.1016/j.ijantimicag.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 13.Segal E., Josef-Lev A. Induction of Candidal vaginitis in diabetic mice and attempts to prevent the infection. J. Med. Vet. Mycol. 1995;33:1–8. doi: 10.1080/02681219580000021. [DOI] [PubMed] [Google Scholar]
- 14.Segal E., Baranetz T., Sandovsky-Losica H., Gov Y., Teicher S., Dayan D. Experimental oral murine candidiasis and attempts of prevention. J. Med. Mycol. 1999;9:55–59. [Google Scholar]
- 15.Frenkel M., Mandelltat M., Alastruey-Izquierdo A., Mendlovic S., Semis R., Segal E. Pathogenicity of Candida albicans isolates from bloodstream and mucosal candidiasis assessed in mice and Galleria mellonella. J. Mycol. Med. 2016;26:1–8. doi: 10.1016/j.mycmed.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Sandovsky losica H., Barrnea L., Segal E. Fatal Systemic Candidiasis of Gastrointestinal Origin-an Experimental-Model in Mice Compromised by Anticancer Treatment. J. Med. Vet. Mycol. 1992;30:219–231. doi: 10.1080/02681219280000281. [DOI] [PubMed] [Google Scholar]
- 17.Segal E., Gottfried L., Lehrer N. Candidal Vaginitis in Hormone-Treated Mice—Prevention by a Chitin Extract. Mycopathologia. 1988;102:157–163. doi: 10.1007/BF00437398. [DOI] [PubMed] [Google Scholar]
- 18.Lionakis M.S., Lim J.K., Lee C.C., Murphy P.M. Organ-specific innate immune responses in a mouse model of invasive candidiasis. J. Innate Immunity. 2011;3:180–199. doi: 10.1159/000321157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohamed H.A., Radwan R.R., Raafat A.I., Ali A.E. Antifungal activity of oral (Tragacanth/acrylic acid) Amphotericin B carrier for systemic candidiasis: In vitro and in vivo study. Drug Deliv. Transl. Res. 2018;8:191–203. doi: 10.1007/s13346-017-0452-x. [DOI] [PubMed] [Google Scholar]
- 20.Levy R., Segal E., Eylan E. Protective Immunity against Murine Candidiasis Elicited by Candida-albicans Ribosomal Fractions. Infect. Immunity. 1981;31:874–878. doi: 10.1128/iai.31.3.874-878.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schulz B., Weber K., Schmidt A., Borg-von Zepelin M., Ruhnke M. Difference in virulence between fluconazole-susceptible and fluconazole-resistant Candida albicans in a mouse model. Mycoses. 2011;54:E522–E530. doi: 10.1111/j.1439-0507.2010.01970.x. [DOI] [PubMed] [Google Scholar]
- 22.Sanchis M., Capilla J., Castanheira M., Martin-Vicente A., Sutton D.A., Fothergill A.W., Wiederhold N.P., Guarro J. Voriconazole minimum inhibitory concentrations are predictive of treatment outcome in experimental murine infections by Candida glabrata. Int. J. Antimicrob. Agents. 2016;47:286–288. doi: 10.1016/j.ijantimicag.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 23.Semis R., Nili S.S., Munitz A., Zaslavsky Z., Polacheck I., Segal E. Pharmacokinetics, tissue distribution and immunomodulatory effect of intralipid formulation of nystatin in mice. J. Antimicrob. Chemother. 2012;67:1716–1721. doi: 10.1093/jac/dks117. [DOI] [PubMed] [Google Scholar]
- 24.Segal E., Elad D. Fungal vaccines and immunotherapy. J. Mycol. Med. 2006;16:134–151. doi: 10.1016/j.mycmed.2006.06.004. [DOI] [Google Scholar]
- 25.Segal E. Testing Antifungal Vaccines in an Animal Model of Invasive Candidiasis and in Human Mucosal Candidiasis. Methods Mol. Biol. 2017;1625:343–353. doi: 10.1007/978-1-4939-7104-6_23. [DOI] [PubMed] [Google Scholar]
- 26.Ahmad E., Zia Q., Fatima M.T., Owais M., Saleemuddin M. Vaccine potential of plasma bead-based dual antigen delivery system against experimental murine candidiasis. Int. J. Biol. Macromol. 2015;81:100–111. doi: 10.1016/j.ijbiomac.2015.07.047. [DOI] [PubMed] [Google Scholar]
- 27.Saville S.P., Lazzell A.L., Chaturvedi A.K., Monteagudo C., Lopez-Ribot J.L. Efficacy of a Genetically Engineered Candida albicans tet-NRG1 Strain as an Experimental Live Attenuated Vaccine against Hematogenously Disseminated Candidiasis. Clin. Vaccine Immunol. 2009;16:430–432. doi: 10.1128/CVI.00480-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Segal E. Inhibition of Candida adhesion to prevent candidiasis. In: Kahane I., Ofek I., editors. Toward Anti Adhesion Therapy for Microbial Diseases of Advances in Experimental Medicine and Biology. Volume 408. Plenum Press; New York, NY, USA: London, UK: 1996. pp. 197–206. [DOI] [PubMed] [Google Scholar]
- 29.Shadkchan Y., Segal E. Antifungal activity of amphotericin B-lipid admixtures in experimental systemic candidosis in naive mice. J. Antimicrob. Chemother. 1999;44:787–790. doi: 10.1093/jac/44.6.787. [DOI] [PubMed] [Google Scholar]
- 30.Shadkchan Y., Segal E. Treatment of experimental candidosis with amphotericin B-Intralipid admixtures in immunocompromised mice. J. Antimicrob. Chemother. 2001;48:245–251. doi: 10.1093/jac/48.2.245. [DOI] [PubMed] [Google Scholar]
- 31.Taschdjian C.L., Reiss F., Kozin P.J. Experimental vaginal candidiasis in mice; its implications for superficial candidiasis in humans. J. Investig. Dermatol. 1960;34:89–94. doi: 10.1038/jid.1960.13. [DOI] [PubMed] [Google Scholar]
- 32.Peer A.K., Hoosen A.A., Seedat M.A., van den Ende J., Omar M.A. Vaginal Yeast Infections in Diabetic Women. S. Afr. Med. J. 1993;83:727–729. [PubMed] [Google Scholar]
- 33.Glittenberg M.T., Silas S., MacCallum D.M., Gow N.A., Ligoxygakis P. Wild-type Drosophila melanogaster as an alternative model system for investigating the pathogenicity of Candida albicans. Dis. Model. Mech. 2011;4:504–514. doi: 10.1242/dmm.006619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alarco A.M., Marcil A., Chen J., Suter B., Thomas D., Whiteway M. Immune-deficient Drosophila melanogaster: A model for the innate immune response to human fungal pathogens. J. Immunol. 2004;172:5622–5628. doi: 10.4049/jimmunol.172.9.5622. [DOI] [PubMed] [Google Scholar]
- 35.San-Blas G., Travassos L.R., Fries B.C., Goldman D.L., Casadevall A., Carmona A.K., Barros T.F., Puccia R., Hostetter M.K., Shanks S.G., et al. Fungal morphogenesis and virulence. Med. Mycol. 2000;38:79–86. doi: 10.1080/mmy.38.s1.79.86. [DOI] [PubMed] [Google Scholar]
- 36.Ramirez-Camejo L.A., Maldonado-Morales G., Bayman P. Differential Microbial Diversity in Drosophila melanogaster: Are Fruit Flies Potential Vectors of Opportunistic Pathogens? Int. J. Microbiol. 2017;2017:8526385. doi: 10.1155/2017/8526385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sulston J.E., Brenner S. The DNA of Caenorhabditis elegans. Genetics. 1974;77:95–104. doi: 10.1093/genetics/77.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ewbank J.J., Pujol N. Local and long-range activation of innate immunity by infection and damage in C. elegans. Curr. Opin. Immunol. 2016;38:1–7. doi: 10.1016/j.coi.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Ewbank J.J., Zugasti O. C. elegans: Model host and tool for antimicrobial drug discovery. Dis. Model. Mech. 2011;4:300–304. doi: 10.1242/dmm.006684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pukkila-Worley R., Feinbaum R.L., McEwan D.L., Conery A.L., Ausubel F.M. The evolutionarily conserved mediator subunit MDT-15/MED15 links protective innate immune responses and xenobiotic detoxification. PLoS Pathog. 2014;10:e1004143. doi: 10.1371/journal.ppat.1004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scorzoni L., de Lucas M.P., Mesa-Arango A.C., Fusco-Almeida A.M., Lozano E., Cuenca-Estrella M., Mendes-Giannini M.J., Zaragoza O. Antifungal efficacy during Candida krusei infection in non-conventional models correlates with the yeast in vitro susceptibility profile. PLoS ONE. 2013;8:e60047. doi: 10.1371/journal.pone.0060047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moghaddam M.R.B., Tonk M., Schreiber C., Salzig D., Czermak P., Vilcinskas A., Rahnamaeian M. The potential of the Galleria mellonella innate immune system is maximized by the co-presentation of diverse antimicrobial peptides. Biol. Chem. 2016;397:939–945. doi: 10.1515/hsz-2016-0157. [DOI] [PubMed] [Google Scholar]
- 43.Ramarao N., Nielsen-Leroux C., Lereclus D. The Insect Galleria mellonella as a Powerful Infection Model to Investigate Bacterial Pathogenesis. J. Vis. Exp. 2012:e4392. doi: 10.3791/4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coleman J.J., Muhammed M., Kasperkovitz P.V., Vyas J.M., Mylonakis E. Fusarium pathogenesis investigated using Galleria mellonella as a heterologous host. Fungal Biol. 2011;115:1279–1289. doi: 10.1016/j.funbio.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slater J.L., Gregson L., Denning D.W., Warn P.A. Pathogenicity of Aspergillus fumigatus mutants assessed in Galleria mellonella matches that in mice. Med. Mycol. 2011;49:S107–S113. doi: 10.3109/13693786.2010.523852. [DOI] [PubMed] [Google Scholar]
- 46.Amorim-Vaz S., Delarze E., Ischer F., Sanglard D., Coste A.T. Examining the virulence of Candida albicans transcription factor mutants using Galleria mellonella and mouse infection models. Front. Microbiol. 2015;6:367. doi: 10.3389/fmicb.2015.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirakawa M.P., Martinez D.A., Sakthikumar S., Anderson M.Z., Berlin A., Gujja S., Zeng Q.D., Zisson E., Wang J.M., Greenberg J.M., et al. Genetic and phenotypic intra-species variation in Candida albicans. Genome Res. 2015;25:413–425. doi: 10.1101/gr.174623.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brennan M., Thomas D.Y., Whiteway M., Kavanagh K. Correlation between virulence of Candida albicans mutants in mice and Galleria mellonella larvae. FEMS Immunol. Med. Microbiol. 2002;34:153–157. doi: 10.1111/j.1574-695X.2002.tb00617.x. [DOI] [PubMed] [Google Scholar]
- 49.Ames L., Duxbury S., Pawlowska B., Ho H.L., Haynes K., Bates S. Galleria mellonella as a host model to study Candida glabrata virulence and antifungal efficacy. Virulence. 2017;8:1909–1917. doi: 10.1080/21505594.2017.1347744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Astvad K.M.T., Meletiadis J., Whalley S., Arendrup M.C. Fluconazole Pharmacokinetics in Galleria mellonella Larvae and Performance Evaluation of a Bioassay Compared to Liquid Chromatography-Tandem Mass Spectrometry for Hemolymph Specimens. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.00895-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mesa-Arango A.C., Forastiero A., Bernal-Martinez L., Cuenca-Estrella M., Mellado E., Zaragoza O. The non-mammalian host Galleria mellonella can be used to study the virulence of the fungal pathogen Candida tropicalis and the efficacy of antifungal drugs during infection by this pathogenic yeast. Med. Mycol. 2013;51:461–472. doi: 10.3109/13693786.2012.737031. [DOI] [PubMed] [Google Scholar]
- 52.De Verno P.J., Aston W.P., Chadwick J.S. Transfer of Immunity against Pseudomonas-aeruginosa P11-1 in Galleria-mellonella Larvae. Dev. Comp. Immunol. 1983;7:423–434. doi: 10.1016/0145-305X(83)90027-7. [DOI] [PubMed] [Google Scholar]