Abstract
Minimally invasive surgical mitral valve repair (MVRepair) has become routine for the treatment of mitral valve regurgitation, and indications have been expanded to include reoperations. Current European Society of Cardiology/European Association for Cardio-Thoracic Surgery guidelines for the management of valvular heart disease recommended standards in terms of mitral valve disease differentiation, timing of intervention and surgical techniques to improve patient care. Numerous minimally invasive techniques to lessen the invasiveness have been described, such as the minimal-access J-sternotomy (ministernotomy), the parasternal incision, the port-access technique and the right minithoracotomy. Despite the development of catheter-based techniques, surgical repair remains the gold standard today for nearly all patients with degenerative valvular diseases and the majority of patients with other types of valvular diseases. Techniques include resection of the prolapsed segment, neo-chordae implantation and ring annuloplasty. In this review, the current indications for mitral valve surgery are summarised and state-of-the-art MVRepair techniques are highlighted.
Keywords: Minimally Invasive Surgery (MIS), Minimally Invasive Mitral Valve Surgery, Minimally Invasive Mitral Valve Repair, Mitral Valve Surgery, Mitral Valve Repair, Endoscopic Mitral Valve Surgery, EndoAortic Crossclamping, Minithoracotomy, Periareolar Approach, 2017 ESC/EACTS Guidelines for the management of valvular heart disease, European Society of Cardiology (ESC), European Association for Cardio-Thoracic Surgery (EACTS)
Reconstructive valve surgery encompasses a comprehensive system of valve analysis and related techniques based on three basic principles as described by Alain Carpentier and colleagues: restoring or preserving the full mobility of the leaflets, creating a large surface of leaflet coaptation and remodelling the annulus to provide an optimal and stable orifice area.[1] Mitral valve regurgitation (MR) is one of the most common heart valve diseases represented in cardiac surgery.[2] The most common entities are primary and secondary MR due to degenerative changes and ischaemia.[3] Today, mitral valve repair (MVRepair) is the gold standard for the treatment of significant MR with results of high patient satisfaction, short hospital stay, low perioperative morbidity and mortality rates and excellent long-term outcomes.[4] The enhancements of minimally invasive surgical techniques has led to a decrease in surgical trauma and accelerated postoperative recovery, resulting in increased acceptance of these operating techniques.[4,5] Despite these satisfying results, there is ongoing discussion about the ideal timing for the intervention in asymptomatic patients.[6] Some groups prefer a watchful waiting strategy; others promote an early intervention, which is recommended in the recently updated 2017 European Society of Cardiology/European Association for Cardio-Thoracic Surgery (ESC/EACTS) guidelines for the management of valvular heart disease.[7]
History of the Minimally Invasive Surgical Access for Mitral Valve Repair
Less invasive approaches to cardiac surgical procedures have been developed in an effort to decrease rates of patient morbidity and enhance postoperative recovery in comparison with conventional methods.[8] The different approaches that have been used for minimally invasive MV surgery are parasternal incision, minimal-access J-sternotomy (ministernotomy) and right minithoracotomy.[8–14]
Cosgrove and Navia initially used a 10 cm right parasternal incision to expose both the aortic valve and MV.[8,12] This technique included resection of the third and fourth costal cartilages.[8] Although safe and effective operations were performed using the parasternal incision, significant disadvantages included occasional chest-wall instability, sacrifice of the right internal thoracic artery, occasional difficulty with aortic valve exposure and difficult conversion to median sternotomy.[8]
The ministernotomy approach, as described by Svensson,[15] has been used for aortic valve and MV procedures.[8,11,16,15] This incision provides good exposure of the MV, allows central cannulation, and when necessary, can easily be enlarged to full sternotomy without the need for a second skin incision.[8] Furthermore, Loulmet and colleagues found that patients who underwent ministernotomy suffered less pain than those who were treated with a thoracotomy.[8,11] However, this strongly depends on the length of incision and degree of spreading of the ribs.[8]
Casselman and colleagues demonstrated that 93.5 % of patients who received a right minithoracotomy approach reported minimal to almost no procedure-related pain.[17] The surgeons only used a soft-tissue retractor, and, therefore, avoided any tension on the ribs. This approach offers excellent visualisation of the MV and gives a direct line of view of the left atriotomy and the MV, as the image is perpendicular to the visual plane.[8,18,19] Furthermore, the right minithoracotomy approach carries a cosmetic advantage over midline incisions, particularly in women.[8,20]
Subsequently, robotic MV surgery was fashioned on the already well-developed platform of minimally invasive surgical (MIS) MVRepair and added features such as true 3D high-magnification visualisation with a dual camera scope, multidirectional endoaortic cardiac instrument articulation, motion scaling, tremor filtration, and to a degree, a potentially even smaller incision, approaching a totally endoscopic procedure. [22] Operative, cardiopulmonary bypass (CPB), and cross-clamp times tend to be longer than nonrobotic minimally invasive approaches.[21,22] Excellent results from Dr Chitwood’s large East Carolina University series of 200 robotic MVRepairs have been reported.[22–24
Indications for Minimally Invasive Surgical Mitral Valve Repair
MR is the second-most frequent indication for valve surgery in Europe.[7,25] It is essential to distinguish primary from secondary MR, particularly regarding surgical and transcatheter interventional management.[7,26]
Primary Mitral Valve Regurgitation and Indications for Intervention
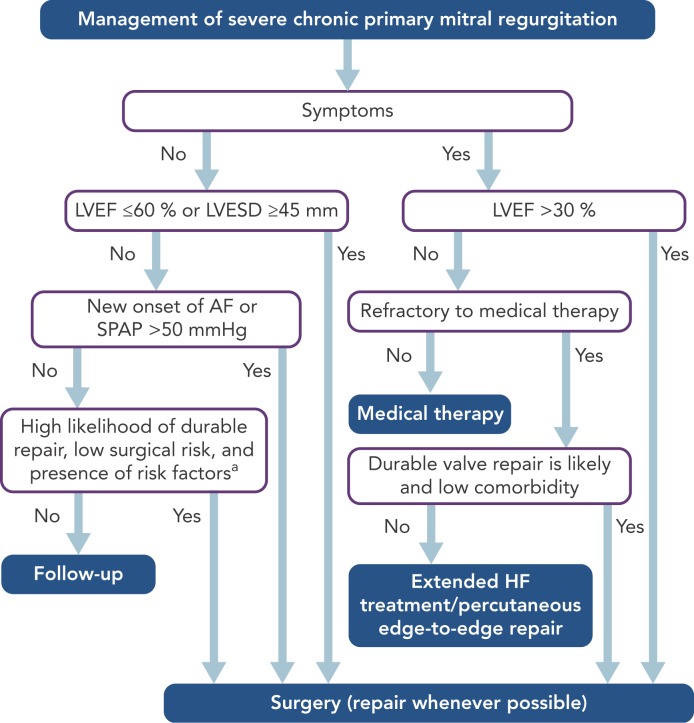
In primary MR, one or several components of the MV apparatus are directly affected. The most frequent aetiology is degenerative (prolapse, flail leaflet).[7] Endocarditis is one of the causes of primary MR, which is specifically discussed in ESC guidelines.[7,27] According to the 2017 ESC/EACTS guidelines for the management for valvular heart disease, indications for surgery in severe chronic primary MR are shown in Table 1 and Figure 1.
Table 1: Indications for Interventions for Severe Primary Mitral Valve Regurgitation.
| Recommendations | Class | Level |
|---|---|---|
| Mitral valve repair should be the preferred technique when the results are expected to be durable. | I | C |
| Surgery is indicated in symptomatic patients with LVEF >30%. | I | B |
| Surgery is indicated in asymptomatic patients with LV dysfunction (LVESD >45 mm* and/or LVEF <60%). | I | B |
| Surgery should be considered in asymptomatic patients with preserved LV function (LVESD <45 mm and LVEF >60%) and atrial fibrillation secondary to mitral regurgitation or pulmonary hyper-tension (systolic pulmonary pressure at rest >50 mmHg**). | IIa | B |
Surgery should be considered in asymptomatic
patients with preserved LVEF (>60%) and LVESD 40-44 mm* when a durable
repair is likely, surgical risk is low, the repair is performed in heart
valve centres, and at least one of the following findings is present:
|
IIa | C |
| Mitral valve repair should be considered in symptomatic patients with severe LV dysfunction (LVEF <30% and/or LVESD >55 mm) refractory to medical therapy when likelihood of successful repair is high and comorbidity low. | IIa | C |
| Mitral valve replacement may be considered in symptomatic patients with severe LV dysfunction (LVEF <30% and/or LVESD >55 mm) refractory to medical therapy when likelihood of successful repair is low and comorbidity low. | IIb | C |
| Percutaneous edge-to-edge procedure may be considered in patients with symptomatic severe primary mitral regurgitation who fulfil the echocardiographic criteria of eligibility and are judged inoperable or at high surgical risk by the Heart Team, avoiding futility. | IIb | C |
Source: courtesy of Professor Volkmar Falk; adapted from Baumgartner, et al., 2017. BSA = body surface area; LA = left atrial; LV = left ventricular; LVEF = left ventricular ejection fraction; LVESD = left ventricular end-systolic diameter; SPAP = systolic pulmonary artery pressure. *Cut-offs refer to average-size adults and may require adaptation in patients with unusually small or large stature. **If an elevated SPAP is the only indication for surgery, the value should be confirmed by invasive measurement.
Figure 1: Management of Severe Chronic Primary Mitral Valve Regurgitation.
Source: Courtesy of Professor Volkmar Falk; adapted from Baumgartner, et al., 2017. AF = atrial fibrillation; BSA = body surface area; CRT = cardiac resynchronisation therapy; HF = heart failure; LA = left atrial; LVEF = left ventricular ejection fraction; LVESD = left ventricular end-systolic diameter; SPAP = systolic pulmonary arterial pressure. aWhen there is a high likelihood of durable valve repair at a low risk, valve repair should be considered (IIa C) in patients with LVESD ≥40 mm and one of the following is present: flail leaflet or LA volume ≥60 ml/m2 BSA at sinus rhythm. bExtended HF management includes the following: CRT, ventricular assist devices, cardiac restraint devices and heart transplantation.
Secondary Mitral Valve Regurgitation and Indications for Intervention
In secondary MR (previously also referred to as ‘functional MR’), the valve leaflets and chordae are structurally normal and MR results from an imbalance between closing and tethering forces on the valve secondary to alterations in left ventricular geometry.[7,28] It is most commonly seen in dilated or ischaemic cardiomyopathies. Annular dilatation in patients with chronic atrial fibrillation and left articular enlargement can also be an underlying mechanism.[7] The presence of chronic secondary MR is associated with impaired prognosis.[29] However, in contrast to primary MR, there is currently no evidence that a reduction of secondary MR improves survival. According to the 2017 ESC/EACTS guidelines for the management for valvular heart disease, the limited data regarding secondary MR had led to a lower level of evidence for treatment recommendations (see Table 2) and highlight the importance of decision making by the heart team (as heart failure and electrophysiology specialists should be involved).[7]
Table 2: Indications for Mitral Valve Intervention in Chronic Secondary Mitral Regurgitation.
| Recommendations | Class | Level |
|---|---|---|
| Surgery is indicated in patients with severe secondary mitral regurgitationundergoing CABG and LVEF >30%. | I | C |
| Surgery should be considered in symptomatic patients with severe secondary mitral regurgitation, LVEF <30% but with an option for revascularization, and evidence of myocardial viability. | IIa | C |
| When revascularization is not indicated, surgery may be considered in patients with severe secondary mitral regurgitation and LVEF >30%, who remain symptomatic despite optimal medical management (including CRT if indicated) and have a low surgical risk. | IIb | C |
| When revascularization is not indicated, surgery may be considered in patients with severe secondary mitral regurgitation and LVEF >30%, who remain symptomatic despite optimal medical management (including CRT if indicated) and have a low surgical risk. | IIb | C |
| In patients with severe secondary mitral regurgitation and LVEF <30% who remain symptomatic despite optimal medical management (including CRT if indicated) and who have no option for revascularization, the Heart Team may consider percutaneous edge-to-edge procedure or valve surgery after careful evaluation for ventricular assist device or heart transplant according to individual patient characteristics. | IIb | C |
Source: courtesy of Professor Volkmar Falk; adapted from Baumgartner, et al., 2017. CABG = coronary artery bypass grafting; CRT = cardiac resynchronisation therapy; LVEF = left ventricular ejection fraction.
Operative Techniques for Minimally Invasive Surgical Access for Mitral Valve Repair
For the purpose of widespread surgical applicability and equipment availability, this section focuses on the right lateral minithoracotomy nonrobotic, transthoracic cross-clamping and endoaortic cross-clamping techniques.
Repair Techniques
The surgical technique for repairing the MV can be selected according to the aetiology of the failing valve, and the abnormal segments of the valve.[30] Three principal goals of MVRepair were introduced by Carpentier: stabilisation of the annulus with the retention of an adequately sized mitral orifice, restoration of physiological leaflet motion and recreation of a sufficient line of coaptation.[1,6] The first technique to reach this was the ‘French correction’, introduced in 1983.[6,31]
Degenerative Mitral Valve Regurgitation
At the German Heart Center Berlin, almost all patients with degenerative MR undergo ring annuloplasty using a semi-rigid ring (Carpentier-Edwards Physio II Annuloplasty Ring, Edwards Lifesciences) and neochordae using the loop technique.[32] Ring annuloplasty is necessary to achieve a durable repair.[21,33]
Ischaemic Mitral Valve Regurgitation
Ischaemic MR repair includes annuloplasty with a closed, and often, undersized ring.
Mitral Valve Endocarditis
MR repair techniques include excision of all infected and inflamed tissue, and repair of the missing tissue with fresh pericardial patch or primary suturing. Some patients need implantation of artificial chordae and most undergo ring annuloplasty.
Rheumatic Mitral Valve Disease
Rheumatic valve repair techniques include commissural fusion release, detachment of papillary muscle fusion, resection of prolapsing segments and ring annuloplasty.
Part of the complexity of MV neochordal replacement with expanded polytetrafluoroethylene (ePTFE) sutures is determining the correct replacement chordal length and knotting the ePTFE suture without sliding the knot. von Oppell and Mohr described this technique of measuring the required chordal length and making a ‘premeasured’ Gore-Tex chordal loop that abolishes problems of inadvertently altering chordal length during fixation.[32]
Setup in the Operating Room for Minimally Invasive Surgical Access for Mitral Valve Repair Through a Right Lateral Minithoracotomy
The right lateral minithoracotomy in combination with adjunctive videoscopy and peripheral CPB has become the preferred approach at the German Heart Center Berlin. Images from the operative setups are shown in Figures 2 and 3.
Figure 2: The Minimally Invasive Periareolar ‘Nipple-cut’ Approach for Surgical Mitral Valve Repair.
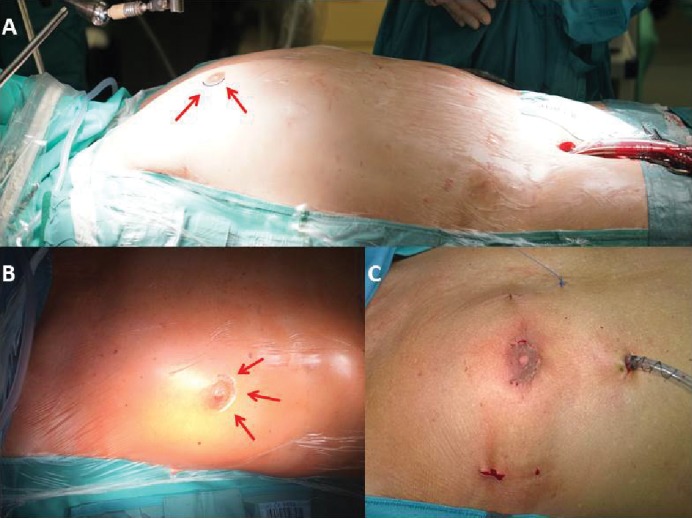
The minimally invasive periareolar nipple-cut approach for surgical mitral valve repair in male patients entails a 3cm small convex incision that straddles the right areolar border. A. Just before incision, the right periareolar border gets delined. B. Incised skin C. Aesthetically appealing postoperative result after the procedure is performed.
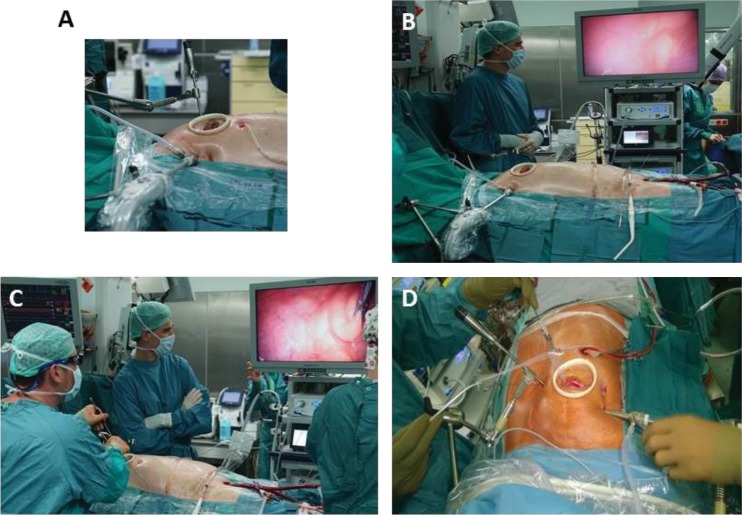
Figure 3: Complete Setup for Fully Endoscopic Highdefinition 3D Minimally Invasive Mitral Valve Surgery.
High-definition 3D minimally invasive mitral valve surgery as performed by Jörg Kempfert, MD and his team at the German Heart Center, Berlin. A. The minimally invasive periareolar approach for surgical mitral valve repair in male patients entails a 3 cm small convex incision that straddles the right areolar border. A soft-tissue retractor without additional rib spreading is used. B. A 3D screen is used. C. The surgeon operating on the mitral valve fully endoscopically with 3D glasses. Complete peripheral cardiopulmonary bypass with endoaortic cross-clamping using the endoaortic balloon is being performed. D. Ring annuloplasty.
Patient Positioning and Anaesthetics
The patient is intubated with a double lumen endotracheal tube and positioned supine with a small pillow under the right scapula to elevate the right hemithorax. If endoaortic balloon occlusion cross-clamping is planned, placement of bilateral radial arterial catheters will provide immediate warning of cephalad displacement of the endoballoon and subsequent innominate arterial obstruction.
Incisional Approach
A small 4 cm right lateral minithoracotomy, inframammary in men and in the submammary crease in women, is used to enter the thorax through the fourth intercostal space (ICS). A more lateral incision provides a more en face view of the MV, albeit at the expense of distance. A more medial approach provides closer access to all structures. A small thoracic and soft tissue retractor is used to spread the ribs. Alternative access as a variation of the standard right lateral minithoracotomy is the permutative periareolar ‘nipple-cut’ to surgically approach the intrathoracic organs and perform the MVRepair. This minimally invasive periareolar approach for surgical MVRepair in male patients entails a 3 cm small convex incision that straddles the right areolar border (Figure 2). Therefore, we use a soft-tissue retractor without additional rib spreading. Patient scar assessment scale scores suggest that this periareolar approach delivers patient-satisfying results regarding cosmetic and sensory function outcomes. The endoscopic periareolar approach is safe, efficient and cosmetically appealing, and compared with the conventional minithoracotomy for MIS MVRepair, the periareolar approach proves to be feasible and allows for surgery through an elegant incision without traumatic rib spreading. This technique is reproducible and even allows for complex MVRepair, additional tricuspid valve (TV) procedures and cryo-ablation (results of this study were presented during an oral presentation at ISMICS 2017 in Rome and EACTS 2017 in Vienna).
Intrathoracic Exposure
The right hemidiaphragm is retracted caudal and to the right with a suture placed in the tendonous dome and brought out by a suture hook through a stab incision in the right sixth or seventh space. The pericardium is opened 3-4 cm anterior and parallel to the phrenic nerve from the distal ascending aorta to the diaphragm.
Cannulation and CPB
The patient is connected to CPB by cannulation of the femoral artery and vein (single venous cannula for isolated MV procedures) through a 2 cm oblique incision in the groin. Transoesophageal echocardiography (TEE) is mandatory to confirm the optimum location of the tip of the venous cannula in the right atrium (Figure 3). Body temperature is maintained at around 34°C and vacuum-assisted venous drainage is used throughout the procedure. In general, a 14, 16 or 18 FR arterial cannula is sufficient depending on patient size and arterial diameter. Introduction of the cannula should be carried out with TEE confirmation of the endoaortic luminal position of the wire to minimise the risk of localised vessel dissection or injury. A 22 FR long venous cannula is sufficient and can be positioned at the inferior vena cava (IVC)/right articular junction by TEE guidance.
Aortic Clamping (see Figure 4)
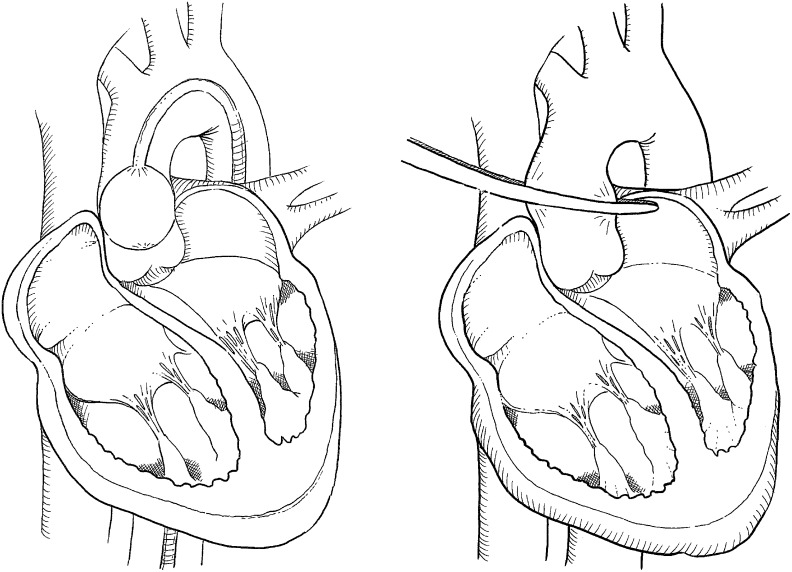
Figure 4: Schematic Drawings of Aortic Cross-clamping Techniques.
Left: Endoaotic cross-clamping using an endoaortic balloon. Right: Transthoracic cross-clamping using a transthoracic (Chitwood) clamp.
Transthoracic Cross-clamping
The transthoracic Chitwood clamp can be inserted via a 5 mm port through the third ICS at the right midaxillary line and positioned near the ascending aorta. Two litres of antegrade cardioplegia is delivered directly into the aortic root through a long needle and repeated after 90-120 minutes, if necessary.
Endoartic Cross-clamping
Using an aortic endoclamp placed through a side limb of the femoral arterial CPB cannula, aortic cross-clamping, antegrade cardioplegia administration and aortic root venting can be accomplished. The endoclamp is a multi-lumen catheter with an inflatable balloon at its distal end, which provides endo-aortic clamping. A central lumen can provide antegrade cardioplegia delivery or alternatively aortic root venting. A second tip lumen allows monitoring of aortic root pressure.
Video-assisted Endoscopic Monitoring
Once the thorax has been entered, a high-definition standard (0° for direct vision minimally invasive MV surgery or 30° for fully-endoscopic high-definition 3D MIS MV surgery [see Figure 3]) thoracoscope can be placed into the chest via a 10 mm port through the second or third interspace at the right anterior axillary line. The thoracoscope not only provides an additional view from which to perform subsequent work, but also brightly illuminates the entire chest. Throughout the procedure, the surgical field is flooded with carbon dioxide through the camera port.
MV Exposure and Surgical Mitral Valve Repair
The MV is accessed through a paraseptal incision and a left atrial retractor is used to expose the MV. MVRepair for degenerative MV disease is most commonly performed using the Gore-Tex neochordae using the loop technique.[32] Assessment of the optimal length and precise fixation of neochordae to the papillary muscles and the free edge of the mitral leaflets are the fundamental aspects of this technique. A semi-rigid annuloplasty ring is implanted to support the repair. MV competency is restored in patients with Barlow’s disease, using a myriad of different techniques from leaflet resection to neochordae to Alfieri’s edge-to-edge repair. Ischaemic MR is corrected using an undersized annuloplasty ring.
De-airing and Closure
After completing the mitral procedure, the right superior pulmonary vein vent should be placed across the competent MV to a depth such that venting holes are in both the ventricle and the atrium. The left atrium is then de-aired by filling it with saline during closure. A direct closure of a patient foramen ovale (PFO)/atrial septal defect (ASD) can be easily performed through the left atrial approach; however, patch closure of the ASD, TV repair or TV replacement have to be accessed through the right atrium after establishing total CPB by clamping the superior and inferior vena cavae. TV repair or TV replacement can also be performed after releasing the aortic clamp. Epicardial pacing wires should be placed while the heart is still decompressed on CPB. Following this, separation from CPB, decannulation, TEE examination of adequacy of mitral repair, and reversal of anticoagulation are all conducted as in a standard operation.
Results
Results of Minimally Invasive Surgical Mitral Valve Repair in Patients with Severe Primary Mitral Valve Regurgitation
Institutions such as the German Heart Center Berlin, the Heart Center Leipzig, Germany and the OLV Clinic Aalst, Belgium contribute immensely to the development, progress and quality of minimally invasive MV surgery. Therefore, these high-volume programmes have high and durable repair rates with minimal mortality.
Casselman and collaegues described the results of 187 patients who had undergone MIS MVRepair in a 4-year study.[17] The operative technique was successfully performed in all but two patients. Therefore, success rate was almost 100 % and it proved immediate technical feasibility of the minimally invasive procedure. Freedom from MV reoperation was 99.5 % ± 0.5 % at 30 days, 97.1 % ± 1.4 % at 1 year and 93.3 % ± 2.6 % at 4 years. Seeburger and colleagues described the results of 1,339 patients who had undergone MIS MVRepair in an 8-year study.[4,35] Success rate was almost 100 %. The 5-year Kaplan-Meier estimation for freedom from MV reoperation was 96.3 %. The 30-day mortality rate was 2.4 % and the 5-year survival rate was 82.6 %. Davierwala and colleagues described the results of 3,438 patients who had undergone minimally invasive MV surgery during 1999-2010, of which 2,829 were MVRepairs.[21] For MVRepair, the survival rates were 87.0 ± 0.7 % and 74.2 ± 1.4 % at 5 and 10 years, respectively. The rates of freedom from reoperation were 96.6 ± 0.4 % and 92.9 ± 0.9 % at 5 and 10 years, respectively.
Results of Minimally Invasive Surgical Mitral Valve Repair in Patients with Chronic Secondary Mitral Valve Regurgitation
Sündermann and colleagues reported that the outcome of patients undergoing MVRepair for secondary MR is dependent to the underlying cause of cardiomyopathy and the concomitant procedure.[6] Gummert and colleagues described a 30-day mortality rate of 6.1 % and a 5-year survival rate of 66 % in patients with dilated and ischaemic cardiomyopathy.[36] TV repair and atrial fibrillation ablation and atrial size reduction were the only accepted concomitant procedure.[6,36] Bax and colleagues reported their results for 51 patients undergoing coronary revascularisation and parallel restrictive MVRepair in ischaemic cardiomyopathy.[37] Their early mortality rate was 5.6 % and the 2-year survival was 84 %. One patient had to undergo reoperation for recurrent MR. The 2-year echocardiographic follow-up showed no or mild MR in all patients as well as a decrease in left ventricular end systolic and end diastolic dimensions. Additionally, differences in outcome in regards of the use of a complete or a partial annuloplasty ring have been observed by Kwon and colleagues.[38] In a retrospective study of 479 patients who had undergone MVRepair due to secondary MR, they found a greater freedom from recurrent MR in the 209 patients in whom a complete ring was used compared with 270 patients treated with a partial ring. A difference in survival rates during the follow-up could not be detected.
Perspective
According to guidelines, surgical repair should be performed whenever feasible and early intervention is warranted when durability is predicted.[39] However, careful patient selection (and operator experience) plays a key role in achieving ideal initial and sustained outcomes. Therefore, as postulated by Maisano and colleagues,[39] endovascular transcatheter therapy for MR (e.g. the MitraClip procedure) currently should be limited to patients who otherwise would not be eligible for surgery. In patients with low-risk degenerative MR, surgical repair will remain the standard of care for many years, with transcatheter MV repair (TMVRep) and transcatheter MV implantation (TMVI) playing a role in high-risk or inoperable patients who are not amenable for MIS MVRepair or eventually for TMVRep.[39] In patients with chronic secondary MR, the role of surgery is less well established in patients who are not candidates for coronary artery bypass graft, and most patients are treated medically. TMVRep may be a safe, palliative approach for such patients, and several large-scale randomised ongoing trials investigate the effectiveness of the MitraClip in this scenario. In the future, careful patient selection will play a fundamental role in identifying specific patients most likely to benefit from MV surgery versus TMVI versus TMVRep.[39]
Conclusions
The right anterolateral minithoracotomy in the fourth intercostal space is currently the most commonly applied approach. For these procedures, videoscopic assistance and the use of telemanipulative robots (e.g. da Vinci system) are adjunctive techniques for further decreasing trauma of the surgical access. In experienced hands, the minimally invasive approach has shown excellent results with regard to operative complications and the durability of surgical MVRepair. Furthermore, today MVRepair is the gold standard for treatment of significant MR with results of high patient satisfaction, short hospital stay, low perioperative morbidity and mortality rates and excellent long-term outcomes.
Acknowledgements
The authors would like to thank Professor Volkmar Falk for sharing [Figure 1 and Tables 1 and 2 with us. We also would like to thank our graphic team at the German Heart Center Berlin (Helge Haselbach and Christian Meier) for drawing the schematic figures and taking the professional photographs of the operative setups.
References
- 1.Carpentier AF, Adams DH, Filsoufi F. Philadelphia: Elsevier; 2010. Carpentier’s Reconstructive Valve Surgery: From Valve Analysis to Valve Reconstruction. [Google Scholar]
- 2.Adams DH, Rosenhek R, Falk V. Degenerative mitral valve regurgitation: best practice revolution. Eur Heart J. 2010;31:1958–66. doi: 10.1093/eurheartj/ehq222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sündermann SH, Seeburger J, Scherman J et al. Innovations in minimally invasive mitral valve pair. Surg Technol Int. 2012;22:207–12. [PubMed] [Google Scholar]
- 4.Welp H, Martens S. Minimally invasive mitral valve repair. Curr Opin Anaesthesiol. 2014;27:65–71. doi: 10.1097/ACO.0000000000000038. [DOI] [PubMed] [Google Scholar]
- 5.Vanermen H, Farhat F, Wellens F et al. Minimally lnvasive video-assisted mitral valve surgery: from Port-Access Towards a totally endoscopic procedure. J Card Surg. 2000;15:51–60. doi: 10.1111/j.1540-8191.2000.tb00444.x. [DOI] [PubMed] [Google Scholar]
- 6.Sündermann SH, Falk V, Jacobs S. Mitral valve reconstruction - timing, surgical techniques and results. Swiss Med Wkly. 2012;142:w13715. doi: 10.4414/smw.2012.13715. [DOI] [PubMed] [Google Scholar]
- 7.Baumgartner H, Falk V, Bax JJ et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–91. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 8.Detter C, Boehm DH, Reichenspurner H. Minimally invasive valve surgery: different techniques and approaches. Expert Rev Cardiovasc Ther. 2004;2:239–51. doi: 10.1586/14779072.2.2.239. [DOI] [PubMed] [Google Scholar]
- 9.Arom KV, Emery RW. Minimally invasive mitral operations. Ann Thorac Surg. 1997;63:1219–20. [PubMed] [Google Scholar]
- 10.Cohn LH, Adams DH, Couper GS et al. Minimally invasive cardiac valve surgery improves patient satisfaction while reducing costs of cardiac valve replacement and repair. Ann Surg. 1997;226:421–8. doi: 10.1097/00000658-199710000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loulmet DF, Carpentier A, Cho PW et al. Less invasive techniques for mitral valve surgery. J Thorac Cardiovasc Surg. 1998;115:772–9. doi: 10.1016/S0022-5223(98)70354-X. [DOI] [PubMed] [Google Scholar]
- 12.Navia JL, Cosgrove DM., 3rd. Minimally invasive mitral valve operations. Ann Thorac Surg. 1996;62:1542–44. doi: 10.1016/0003-4975(96)00779-5. [DOI] [PubMed] [Google Scholar]
- 13.Chitwood WR, Jr, Wixon CL, Elbeery JR et al. Video-assisted minimally invasive mitral valve surgery. J Thorac Cardiovasc Surg. 1997;114:773–82. doi: 10.1016/S0022-5223(97)70081-3. [DOI] [PubMed] [Google Scholar]
- 14.Chitwood WR, Jr, Elbeery JR, Moran J. Minimally invasive mitral valve repair using transthoracic aortic occlusion. Ann Thorac Surg. 1997;63:1477–9. doi: 10.1016/s0003-4975(97)00242-7. [DOI] [PubMed] [Google Scholar]
- 15.Svensson LG. Minimal-access “j” or “j” sternotomy for valvular, aortic, and coronary operations or reoperations. Ann Thorac Surg. 1997;64:1501–3. doi: 10.1016/S0003-4975(97)00927-2. [DOI] [PubMed] [Google Scholar]
- 16.Gillinov AM, Cosgrove DM. Minimally invasive mitral valve surgery: mini-sternotomy with extended transseptal approach. Semin Thorac Cardiovasc Surg. 1999;11:206–11. doi: 10.1016/s1043-0679(99)70061-4. [DOI] [PubMed] [Google Scholar]
- 17.Casselman FP, Van Slycke S, Dom H et al. Endoscopic mitral valve repair: feasible, reproducible and durable. J Thorac Cardiovasc Surg. 2003;125:273–82. doi: 10.1067/mtc.2003.19. [DOI] [PubMed] [Google Scholar]
- 18.Onnasch JF, Schneider F, Falk V et al. Five years of less invasive mitral valve surgery: from experimental to routine approach. Heart Surg Forum. 2002;5:132–5. [PubMed] [Google Scholar]
- 19.Onnasch JF, Schneider F, Falk V et al. Minimally invasive approach for redo mitral valve surgery: a true benefit for the patient. J Card Surg. 2002;17:14–9. doi: 10.1111/j.1540-8191.2001.tb01214.x. [DOI] [PubMed] [Google Scholar]
- 20.Reichenspurner H, Boehm DH, Gulbins H et al. Three-dimensional video and robot-assisted port-access mitral valve operation. Ann Thorac Surg. 2000;69:1176–81. doi: 10.1016/s0003-4975(99)01561-1. [DOI] [PubMed] [Google Scholar]
- 21.Davierwala PM, Seeburger J, Pfannmueller B et al. Minimally invasive mitral valve surgery : “The Leipzig experience”. Ann Cardiothorac Surg. 2013;2:744–50. doi: 10.3978/j.issn.2225-319X.2013.10.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woo YJ, Seeburger J, Mohr FW. Minimally Invasive Valve Surgery. Semin Thorac Cardiovasc Surg. 2007;19:289–98. doi: 10.1053/j.semtcvs.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Woo YJ, Rodriguez E, Atluri P, Chitwood WR. Minimally Invasive, robotic, and off-pump mitral valve surgery. Semin Thorac Cardiovasc Surg. 2006;18:139–47. doi: 10.1053/j.semtcvs.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez E, Kypson AP, Moten SC et al. Robotic mitral surgery at East Carolina University: a 6 year experience. Int J Med Robot. 2006;2:211–5. doi: 10.1002/rcs.80. [DOI] [PubMed] [Google Scholar]
- 25.Lung B, Baron G, Butchart EG et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J. 2003;24:1231–43. doi: 10.1016/s0195-668x(03)00201-x. [DOI] [PubMed] [Google Scholar]
- 26.De Bonis M, Al-attar N, Antunes M et al. Surgical and interventional management of mitral valve regurgitation : a position statement from the European Society of Cardiology Working Groups on Cardiovascular Surgery and Valvular Heart Disease. Eur Heart J. 2016;37:133–9. doi: 10.1093/eurheartj/ehv322. [DOI] [PubMed] [Google Scholar]
- 27.Habib G, Lancellotti P, Antunes MJ et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36:3075–128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 28.Levine RA, Schwammenthal E. Ischemic mitral regurgitation on the threshold of a solution: from paradoxes to unifying concepts. Circulation. 2005;112:745–58. doi: 10.1161/CIRCULATIONAHA.104.486720. [DOI] [PubMed] [Google Scholar]
- 29.Grigioni F, Enriquez-sarano M, Zehr KJ et al. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103:1759–64. doi: 10.1161/01.cir.103.13.1759. [DOI] [PubMed] [Google Scholar]
- 30.Spiegelstein D, Ghosh P, Sternik L et al. Current strategies of mitral valve repair. Isr Med Assoc J. 2007;9:303–9. [PubMed] [Google Scholar]
- 31.Schubert SA, Mehaffey JH, Charles EJ, Kron IL. Mitral Valve Repair: The French Correction Versus the American Correction. Surg Clin North Am. 2017;97:867–88. doi: 10.1016/j.suc.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Oppell UO, Mohr FW. Chordal replacement for both minimally invasive and conventional mitral valve surgery using premeasured Gore-Tex loops. Ann Thorac Surg. 2000;70:2166–8. doi: 10.1016/s0003-4975(00)02047-6. [DOI] [PubMed] [Google Scholar]
- 33.Gillinov AM, Tantiwongkosri K, Blackstone EH et al. Is prosthetic anuloplasty necessary for durable mitral valve repair? Ann Thorac Surg. 2009;88:76–82. doi: 10.1016/j.athoracsur.2009.03.089. [DOI] [PubMed] [Google Scholar]
- 34.Saunders PC, Grossi EA, Sharony R et al. Minimally invasive technology for mitral valve surgery via left thoracotomy: experience with forty cases. J Thorac Cardiovasc Surg. 2004;127:1026–31. doi: 10.1016/j.jtcvs.2003.08.053. [DOI] [PubMed] [Google Scholar]
- 35.Seeburger J, Borger MA, Falk V et al. Minimal invasive mitral valve repair for mitral regurgitation: results of 1339 consecutive patients. Eur J Cardiothorac Surg. 2008;34:760–5. doi: 10.1016/j.ejcts.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 36.Gummert JF, Rahmel A, Bucerius J et al. Mitral valve repair in patients with end stage cardiomyopathy: who benefits? Eur J Cardiothorac Surg. 2003;23:1017–22. doi: 10.1016/s1010-7940(03)00148-9. [DOI] [PubMed] [Google Scholar]
- 37.Bax JJ, Braun J, Somer ST et al. Restrictive annuloplasty and coronary revascularization in ischemic mitral regurgitation results in reverse left ventricular remodelling. Circulation. 2004;110:II-103–8. doi: 10.1161/01.CIR.0000138196.06772.4e. [DOI] [PubMed] [Google Scholar]
- 38.Kwon MH, Lee LS, Cevasco M et al. Recurrence of mitral regurgitation after partial versus complete mitral valve ring annuloplasty for functional mitral regurgitation. J Thorac Cardiovasc Surg. 2013;146:616–22. doi: 10.1016/j.jtcvs.2012.07.049. [DOI] [PubMed] [Google Scholar]
- 39.Maisano F, Alfieri O, Banai S et al. The future of transcatheter mitral valve interventions: competitive or complementary role of repair vs. replacement? Eur Heart J. 2015;36:1651–9. doi: 10.1093/eurheartj/ehv123. [DOI] [PubMed] [Google Scholar]