Abstract
Background:
Oxidative stress is one of the causative factors in the pathogenesis of neuro-degenerative diseases including mild cognitive impairment (MCI) and dementia. We previously reported that molecular hydrogen (H2) acts as a therapeutic and preventive antioxidant.
Objective:
We assess the effects of drinking H2-water (water infused with H2) on oxidative stress model mice and subjects with MCI.
Methods:
Transgenic mice expressing a dominant-negative form of aldehyde dehydrogenase 2 were used as a dementia model. The mice with enhanced oxidative stress were allowed to drink H2-water. For a ran-domized double-blind placebo-controlled clinical study, 73 subjects with MCI drank ~300 mL of H2-water (H2-group) or placebo water (control group) per day, and the Alzheimer's Disease Assessment Scale-cognitive subscale (ADAS-cog) scores were determined after 1 year.
Results:
In mice, drinking H2-water decreased oxidative stress markers and suppressed the decline of memory impairment and neurodegeneration. Moreover, the mean lifespan in the H2-water group was long-er than that of the control group. In MCI subjects, although there was no significant difference between the H2- and control groups in ADAS-cog score after 1 year, carriers of the apolipoprotein E4 (APOE4) geno-type in the H2-group were improved significantly on total ADAS-cog score and word recall task score (one of the sub-scores in the ADAS-cog score).
Conclusion:
H2-water may have a potential for suppressing dementia in an oxidative stress model and in the APOE4 carriers with MCI.
Keyword: ADAS-cog score, aldehyde dehydrogenase 2, ApoE4, hydrogen, hydrogen water, mild cognitive impairment, oxidative stress, randomized clinical study
1. INTRODUCTION
Oxidative stress is one of the causative factors in the pathogenesis of major neurodegenerative diseases including Alzheimer’s disease (AD), mild cognitive impairment (MCI), and Parkinson disease (PD) [1, 2]. Moreover, the genotype of apolipoprotein E4 (APOE4) is a genetic risk for AD, and the increased oxidative stress in the APOE4 carriers is considered as one of the modifiers for the risk [3].
To explore effective dietary antioxidants to mitigate age-dependent neurodegeneration, it may be useful to construct model mice in which AD phenotypes would progress in an age-dependent manner in response to oxidative stress. We constructed transgenic DAL101 mice expressing a polymorphism of the mitochondrial aldehyde dehydrogenase 2 gene (ALDH2*2) [4]. ALDH2*2 is responsible for a deficiency in ALDH2 activity and is specific to North-East Asians [5]. We reported previously that ALDH2 deficiency is a risk factor for late-onset AD in the Japanese population, [6] which was reproduced by Chinese and Korean studies in their respective populations [7, 8]. DAL101 mice exhibited a decreased ability to detoxify 4-hydroxy-2-nonenal (4-HNE) in cortical neurons, and consequently an age-dependent neurodegeneration, cognitive decline, and a shortened lifespan [4].
We proposed that molecular hydrogen (H2) has potential as a novel antioxidant, [9] and numerous studies have strongly suggested its potential for preventive and therapeutic applications [10-12]. In addition to extensive animal experiments, more than 25 clinical studies examining the efficacy of H2 have been reported, [11, 12] including double-blind clinical studies. Based on these studies, the field of hydrogen medicine is growing rapidly.
There are several methods to administer H2, including inhaling hydrogen gas (H2-gas), drinking H2-dissolved water (H2-water), and injecting H2-dissolved saline (hydrogen-rich saline) [13]. Drinking H2-water prevented the chronic stress-induced impairments in learning and memory by reducing oxidative stress in mice [14] and protects neural cells by stimulating the hormonal expression of ghrelin [15]. Additionally, injection of hydrogen-rich saline improved memory function in a rat model of amyloid-β-induced dementia by reducing oxidative stress [16]. Moreover, hydrogen inhalation during normoxic resuscitation improved neurological outcome in a rat model of cardiac arrest independently of targeted temperature management [17].
In this study, we examined whether drinking H2-water could suppress aging-dependent memory impairment induced by oxidative stress in DAL101 mice. Next, in a randomized double-blind placebo-controlled study, we investigated whether H2-water could delay the progression of MCI as assessed by the scores on the Alzheimer's Disease Assessment Scale-cognition sub-scale (ADAS-cog) [18, 19] from baseline at 1-year. We found a significant improvement in cognition at 1 year in carriers with the APOE4 genotype in the H2-group using sub- and total ADAS-cog scores.
2. MATERIALS AND METHODS
2.1. Ethical Approval and Consent to Participate
This animal study was approved by the Animal Care and Use Committee of Nippon Medical School. The methods were carried out in “accordance” with the relevant guidelines and regulations.
The clinical study protocol was approved by the ethics committees of University of Tsukuba, and registered in the university hospital medical information network (UMIN) as UMIN000002218 on July 17, 2009 at https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=history&action =list&type= summary&recptno= R000002-725&language=J.
Participants were enrolled from July 2009. All patients provided written informed consent prior to research investigations, which were conducted according to the Declaration of Helsinki and subsequent revisions.
2.2. Transgenic DAL101 Mice
Transgenic mice (DAL101) that express a transgene containing a mouse version of ALDH2*2 were constructed as described previously [4]. Since the number of mice used for each experiment was not consistent because of a breeding difficulty, the number of the mice used was specified. All mice were kept in a 12-hr light/dark cycle with ad libitum access to food and water. Examiners performed experiments in a blinded fashion. Since no significant decline was observed in cognitive impairment at the age of 18 months in wild-type mice with the same genetic background (C57BL/6), [4] the effects of H2-water were not assessed in this study.
2.3. Hydrogen Water
For animal experiments, saturated H2-water was prepared as described previously [14]. In brief, H2 was dissolved in water under high pressure (0.4 MPa) to a supersaturated level, and the saturated H2-water was stored under atmospheric pressure in an aluminum bag with no headspace. As a control, H2-water was completely degassed by gentle stirring for one day. Mice were given water freely using closed glass vessels equipped with an outlet line containing two ball bearings, which kept the water from being degassed. The vessel was freshly refilled with H2-water 6 days per week at 2:00 pm. The H2-concentration was still more than 0.3 mM on the next day.
For this clinical study, commercially available H2-water was a gift from Blue Mercury, Inc. (Tokyo, Japan). The H2-water (500 mL) was packed in an aluminum pouch with no headspace to maintain H2 concentration, and sterilized at 80°C for 30 min. The concentration of H2 was measured using a hydrogen sensor (Unisense, Aarhus N, Denmark), and used if the value was more than 0.6 mM. Placebo water packed in an identical package (500 mL) was also provided by Blue Mercury Inc. This company played no role in collection of data, management, analysis, or interpretation of the data. One package with 500 mL of placebo or H2-water per day was provided after showing previous empty packages, by which self-reported compliance rates in the intervention group were calculated as the volume of H2-water at 1-year.
2.4. Measurement of Oxidative Stress
As an oxidative stress marker, 8-OHdG [20] was measured using urine samples, which were collected between 9:00 and 10:00 am as described previously [21], by using a competitive enzyme-linked immunoassay (New 8-OHdG check; Japan Institute for the Control of Aging, Shizuoka, Japan). The values were normalized by urinary creatinine concentration, which was assayed using a standard kit (Wako, Kyoto, Japan). As an additional oxidative stress marker in the brain, accumulated MDA was determined using a Bioxytech MDA-586 Assay Kit (Percipio Biosciences, CA, USA). Malondialdehyde(MDA)levels were normalized against protein concentrations.
2.5. Measurement of Memory Impairment: Object Recognition Task
Learning and memory abilities were examined using objection recognition task (ORT) [4]. A mouse was habituated in a cage for 4 h, and then two different-shaped objects were presented to the mouse for 10 min as training. The number of times of exploring and/or sniffing each object was counted for the first 5 min (Training test). The frequencies (%) in training test were considered as the backgrounds. To test memory retention after 1 day, one of the original objects was replaced with a novel one of a different shape and then times of exploration and/or sniffing was counted for the first 5 min (Retention test). When mice would lose learning and memory abilities, the frequencies of exploration and/or sniffing of each object should be equal (about 50%) in the training session, indicating that mice showed a similar interest in each object because of lack of memory for the objects. Learning and memory abilities were evaluated as the subtraction of the frequencies (%) in the retention test from each background (Training test).
2.6. Measurement of Memory Impairment: Passive Avoidance Task (PA)
The apparatus consisted of two compartments, one light and the other dark, separated by a vertical sliding door [22]. On day 1, we initially placed a mouse in the light compartment for 20 s. After the door was opened, the mouse could enter the dark compartment (mice instinctively prefer being in the dark). On day 2, the mouse was again placed in the light section to allow the mouse to move into the dark section. After the mouse entered the dark compartment, the door was closed. After 20 s, the mouse was given a 0.3 mA electric shock for 2 s. The mouse was allowed to recover for 10 s, and was then returned to the home cage. On day 3, 24h after the shock, the mouse was again placed in the light section with the door opened to allow the mouse to move into the dark section. We examined the latency time for stepping through the door. Learning and memory abilities were assessed as the subtraction of the latency times after the electric shock from each background (before).
2.7. Immunostaining of the Hippocampal CA1 Region
To examine neuronal loss and glial activation, the hippocampus region was stained with a pyramidal neuron-specific anti-NeuN antibody (clone A60; Merck Millipore, Darmstadt, Germany), an astrocyte-specific anti-glial fibrillary acidic protein (anti-GFAP) antibody (Thermo Scientific, MA, USA) or a microglia-specific anti-IbaI antibody (Wako). Mice were transcardially perfused to be fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) under anesthesia, and their brains were cryoprotected with 30% sucrose, and then frozen brain was sectioned at 8 μm thickness. After incubation with each primary antibody, sections were treated with secondary antibodies (Vector Laboratories, CA, USA) and their immunereactivity was visualized by the avidin-biotin complex method (Vector Laboratories).
2.8. Subjects of the Clinical Study
This study was a randomized, double-blind, placebo-controlled trial undertaken as a part of Tone project, an ongoing epidemiological study conducted in Tone Town, Ibaraki, Japan as described in detail previously [23, 24]. This town is located approximately 40 km northeast of central Tokyo and consists of 22 districts. The baseline survey of the Tone project included 1,032 participants in July 2009, and subjects of the present study were recruited from these participants.
Eligibility criteria are age 67 years or older, being able to give written informed consent for participation in the present study, with a diagnosis of MCI, being able to observe the following requirement: good compliance with water consumption; participation in the scheduled examinations for assessment; keeping a log-diary recording consumption of the water, with a modified Hachinski Ischemic score of 4 or less and a 15-item Geriatric Depression Scale score of 6 or less. In brief, 3 months before this clinical study, all participants underwent a group assessment which used a set of 5 tests that measured the following cognitive domains: attention; memory; visuospatial function; language; and reasoning as described previously [25]. Objective impairment in at least 1 cognitive domain based on the average of the scores on the neuropsychological measures within that domain and 1 SD cut-off using normative corrections for age, years of education, and sex.
Exclusion criteria were having “The Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV TR” criteria for dementing illnesses, a serious or unstable illnesses, a history within the past 5 years of serious infectious disease affecting the brain and/or malignant diseases, a history of alcohol or drug abuse or dependence (on DSM-IV TR) within the past 5 years, and receiving any types of anti-Alzheimer drugs and recent (within 4 weeks) initiation of medications that affect the central nervous system. When the score of Mini Mental State Examination (MMSE) [26] was less than 24, the subjects were excluded.
In this study, subjects were randomly assigned to either to an intervention group, who received H2-water every-day for 1 year, or a control group, who received placebo water. The allocation sequence was determined by computer-generated random numbers that were concealed from the investigators and subjects. Drs. Nakajima and Ikejima generated the random allocation sequence, enrolled participants, and assigned participants to interventions. Any participants and care providers were blindly masked.
In the original protocol, we planed to administer H2-water for 2 years and assess the secondary outcomes; however, we had to stop the project in 2011 by the Tsunami-disaster and could not obtained the 2-year data and secondary outcomes.
The APOE4 genotype was determined as described [25].
2.9. Statistical Considerations
All statistical analyses were performed by an academic biostatistician using SAS software version 9.2 (SAS Institute Inc, Cary, NC, USA). Results were considered significant at p < 0.05.
For the comparison of two groups in learning and memory abilities, and lifespans, unpaired two-tailed Student’s t-test was used for the comparison of H2-group with control group. For the other animal experiments, one-way analysis of variance (ANOVA) with Tukey-Kramer or Dunnett post hoc analysis was applied unless otherwise mentioned.
For the clinical trial, we planned to recruit a total of 120 patients, which would provide 90% power to detect an effect size of 0.6 using a two-sided test with a 5% significance level, but the actual sample size for the primary analysis was 73, leading to 70% power in the same setting. End-points were scores in the Japanese version of ADAS-cog at 1-year, and the changes were evaluated by Mann-Whitney's U test (non-parametric analysis) as well as Student’s t-test (parametric analysis).
3. RESULTS
3.1. Hydrogen-water Reduced Oxidative Stress in DAL Mice
Male DAL101 mice were given H2- or control water to drink ad libitum from the age of 1 month, and continued until the age of 18 months. The H2-water DAL101 group showed a significant decrease in the level of an oxidative stress marker, urinary 8-hydroxy-2’-deoxyguanosine (8-OHdG)[20] at the age of 14months (Suppl. Fig. S1A (223.8KB, pdf) ). Moreover, DAL101 mice increased oxidative stress in the brain as measured by the level of MDA as an alternative oxidative stress marker, and H2-water showed a significant recovery of this increased level of MDA in DAL101 mice (Suppl. Fig. S1B (223.8KB, pdf) ).
3.2. Hydrogen Water Suppressed a Decline in Learning and Memory Impairment
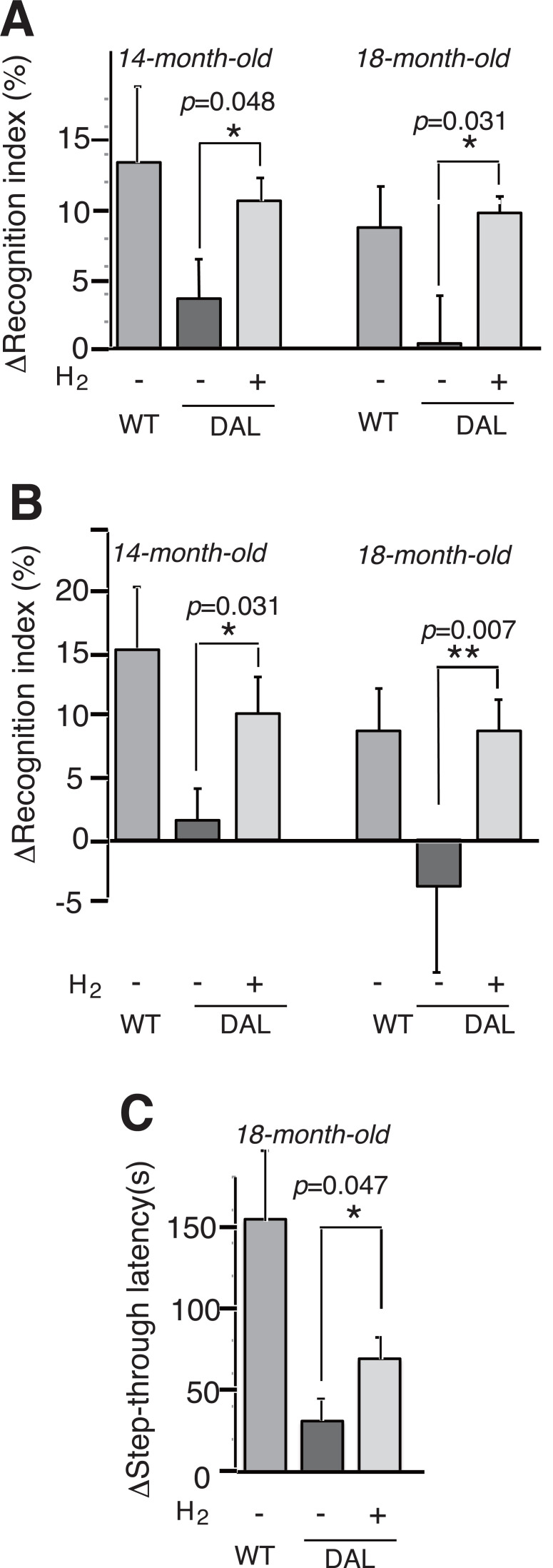
We examined learning and memory abilities using ORT [4]. As described in MATERIALS AND METHODS, learning and memory abilities were evaluated as the subtraction of the frequency (%) in Retention test from each background (Training test). Mice were provided with control or H2-water from the age of 1 month. At the age of 14 months, the H2-group significantly memorized the original objects and showed the preference for the novel object more than the control group (Fig. 1A 14-month-old).
Fig. (1).
Hydrogen water prevented cognitive decline. H2-water was provided from the age of 1 month (A, C), and from the age of 8 months (B). The mice were subjected to the first objection recognition task (ORT) at the age of 14 months (A, B, 14-month-old) and the second ORT at the age of 18 months (A, B, 18-month-old). The recognition indexes were obtained as the frequency (%) of exploring and/or sniffing the object that would be replaced or the novel one that had been replaced. ΔRecognition index (%) indicates the frequencies in Retention test of ORT after the subtraction of those in Training test (background). WT, wild-type; (DAL, H2-), DAL101 mice drinking degassed control water; (DAL, H2+), DAL101 mice drinking hydrogen water. Data are shown as the mean ± SEM. n = 9, *p < 0.05, **p < 0.01 by Student’s t-test. (C) The mice were subjected to a passive avoidance task. Step-through latencies before and after the electric shock are obtained and ΔStep-through latency (s) indicates the subtraction of Step-through latencies after from before the electric shock. WT, wild-type (n = 10); DAL, H2-, DAL101 mice receiving degassed control water (n = 8); and DAL, H2+, DAL101 mice receiving H2-water (n = 8). Data are shown as the mean ± SEM. *p < 0.05.
At the age of 18 months, the mice were subjected to the second ORT, which can be done by using different objects at the age of 18 months [14]. The aged DAL101 mice drinking H2-water still significantly memorized the original objects and preferred the novel one more than the control group (Fig. 1A 18-month-old).
Next, to test the drinking effects of H2-water from the later stage, we started giving H2-water to male DAL101 mice at the age of 8 months instead of 1 month, and subjected to ORT at the age of 14 months (Fig. 1B 14-month-old) and the second ORT at the age of 18 months (Fig. 1B 18-month-old). Even when the mice began to drink at the age of 8 months, H2-water significantly suppressed the decline in the learning and memory abilities at the age of 18 months as well as at the age of 14 months (Fig. 1B).
Moreover, we subjected the mice to PA [22] at the age 18 months as an alternative method. One day after a 0.3 mA electric shock for 2 s was given, wild-type C57BL/6 mice memorized the shock as evaluated by the subtraction of the latency time (s) to re-enter the dark compartment from each background (Fig. 1C). The H2-water group significantly suppressed the decline in learning and memory more than the control group (Fig. 1C).
Thus, drinking H2-water suppressed the learning and memory impairment in the oxidative stress mice.
3.3. Hydrogen-water Suppressed Neurodegeneration
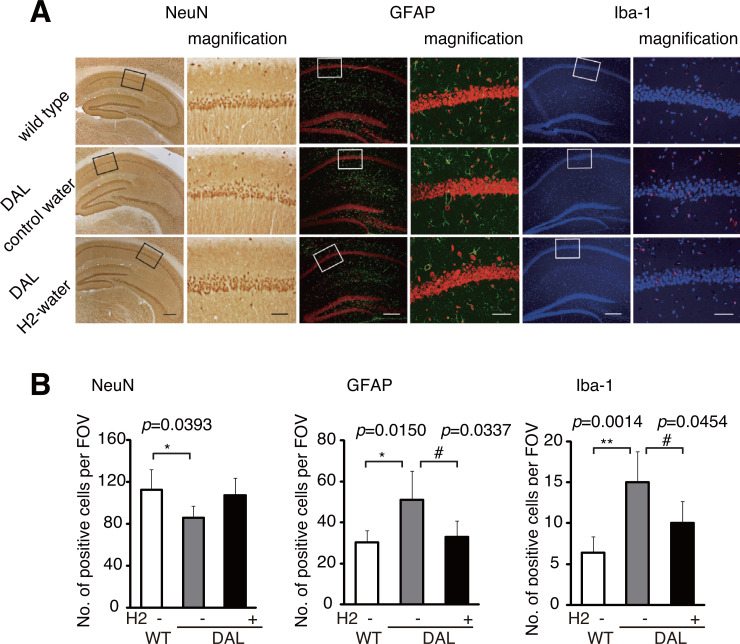
To examine whether H2-water could prevent neurodegeneration in aged DAL101 mice, we stained the hippocampus with a neuron-specific anti-NeuN antibody (Fig. 2A). Neurodegeneration was evaluated by glial activations using an anti-GFAP antibody and a microglia-specific anti-Iba-I antibody. Immune-positive cells per field of view (FOV) were counted in the CA1 region (Fig. 2B).
Fig. (2).
Hydrogen water suppressed neurodegeneration. (A) The hippocampal CA1 region was stained with antibodies against NeuN (a neuronal marker), GFAP (an astrocytic marker) or Iba-1 (a microglial marker) (Scale bars: 50 µm). Right panels show magnified images of the squares in the left panels (Scale bars: 10 µm). (B) Cells positive for anti-NeuN, anti-GFAP and anti-Iba-I antibodies per field of view (FOV) were counted in the CA1 region (n = 5). Data are shown as the mean ± SD. *p < 0.05, **p < 0.01 (wild-type vs DAL), #p < 0.05 (H2-water vs. control water in DAL).
The number of neurons was decreased in the control DAL101 group as the comparison with wild type group, and the H2-DAL101 group showed a trend in recovery of the decrease (Fig. 2A). As has been described previously, [4] the control DAL101 mice exhibited an increase in glial activation, and the H2-water group suppressed the enhanced glial activation in the CA1 region (Fig. 2, GFAP and Iba-I).
3.4. Hydrogen-water Extended the Average Lifespan of Mice
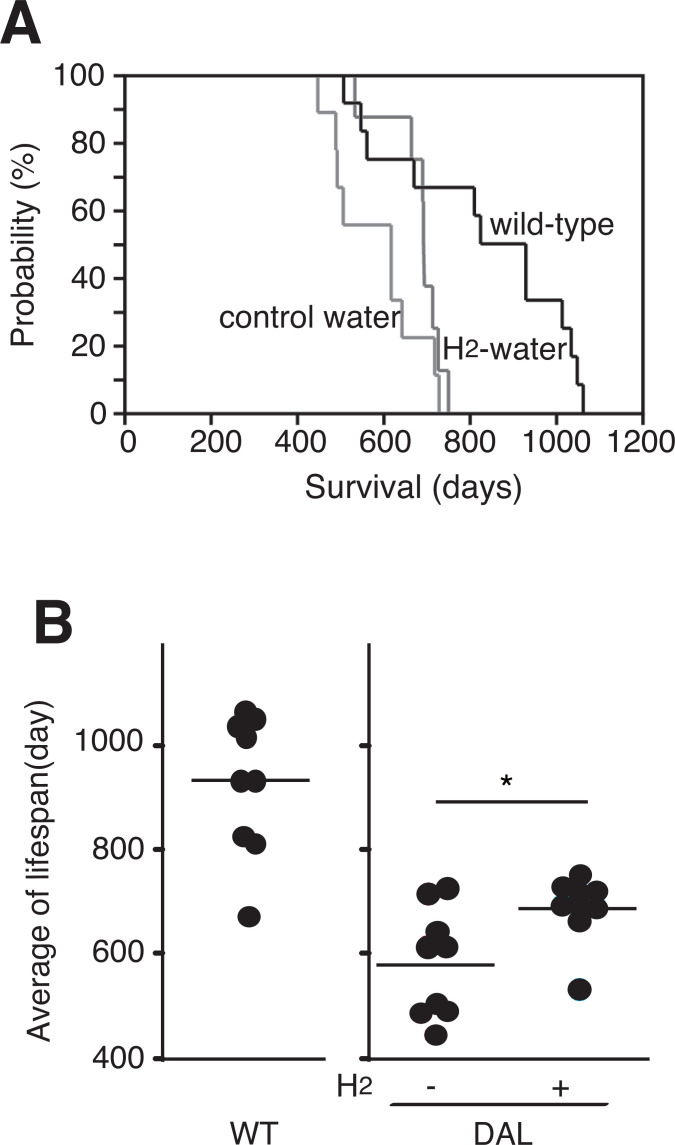
DAL101 mice showed a shorter lifespan, which has also been described previously [4]. To examine whether consumption of H2-water attenuated the shortened lifespan, female DAL101 mice started drinking control or H2-water at the age of 1 month. Although H2-water did not extend the maximum lifespan (Fig. 3A), H2-water significantly extended the mean of lifespan of DAL101 mice (Fig. 3B).
Fig. (3).
Extension of the average lifespan by continuous drinking H2-water. (A) Kaplan-Meier curve representing the survival of female C57BL/6 mice (wild-type), female DAL101 mice drinking control water (control water) and H2-water (H2-water). (B) Each dot indicates the lifespan of each mouse. The bars indicate the average lifespan of each group. *p < 0.05 (p = 0.036) by Student’s t-test.
3.5. A Randomized, Placebo Controlled Clinical Study
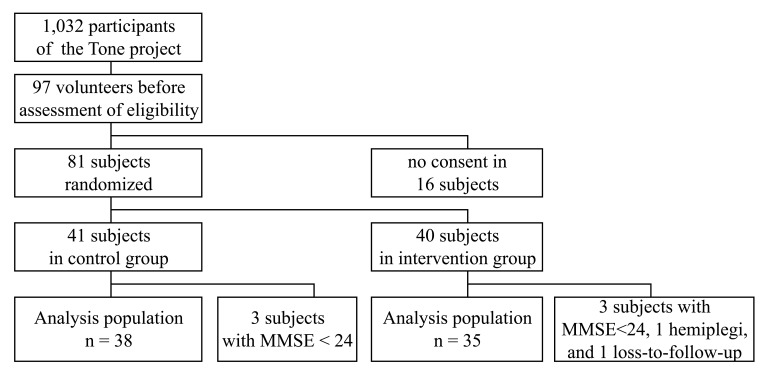
Fig. (4) shows the profile on the recruitment, randomization, and follow-up of this study. A total of 81 subjects of the 1,032 participants were randomized; however, 3 in the control group and 5 in the intervention group were diagnosed as ineligible after randomization and not included in this analysis. Baseline characteristics and lifestyle factors were balanced between the study groups (Table 1). Random assignment was stratified by age of ~74 years and MMSE score of ~28 points. The average compliance rate of drinking water was estimated as 64% in both groups at 1-year, meaning the subjects drank 320 mL/day on the average. The mean total ADAS-cog scores in the H2- and control groups were 8.04 and 7.89, respectively, with no significance.
Fig. (4).
Profile of the recruitment, randomization, and follow-up of this study. This study was a randomized, double-blind, placebo-controlled trial undertaken as a part of Tone project, an ongoing epidemiological study conducted in Tone Town, Ibaraki, Japan [23, 24].
Table 1.
Background characteristics of 73 subjects with mild cognitive impairment.
| Control (n=38) | Intervention (n=35) | |||
|---|---|---|---|---|
| Mean | SD or % | Mean | SD or % | |
| Woman * | 20 | (52.6%) | 19 | (54.3%) |
| Age (years) | 74.45 | 5.44 | 73.97 | 5.11 |
| Body mass index (kg/m2) | 23.55 | 2.59 | 23.19 | 4.08 |
| Systolic blood pressure (mmHg) | 131.26 | 12.35 | 135.14 | 13.31 |
| Diastolic blood pressure (mmHg) | 77.92 | 7.13 | 78.89 | 9.53 |
| Education (years) | 11.26 | 2.71 | 11.57 | 2.83 |
| Current alcohol drinker * | 19 | (50.0%) | 14 | (40.0%) |
| Current smoker * | 4 | (10.5%) | 5 | (14.3%) |
| Current exercise habit * | 27 | (71.1%) | 22 | (62.9%) |
| APOE4 carrier * | 6 | (15.7%) | 7 | (20.0%) |
| Family history * | 2 | (5.3%) | 2 | (5.7%) |
| Comorbidity * | ||||
| Hypertension | 15 | (39.5%) | 14 | (40.0%) |
| Diabetes mellitus | 4 | (10.5%) | 5 | (14.3%) |
| Dyslipidemia | 4 | (10.5%) | 4 | (11.4%) |
| Stroke | 2 | (5.3%) | 1 | (2.9%) |
| Depression | 1 | (2.6%) | 2 | (5.7%) |
| MMSE | 28.08 | 1.66 | 27.83 | 1.74 |
| ADAS-cog | 7.89 | 3.19 | 8.04 | 3.47 |
* indicates frequency (%).
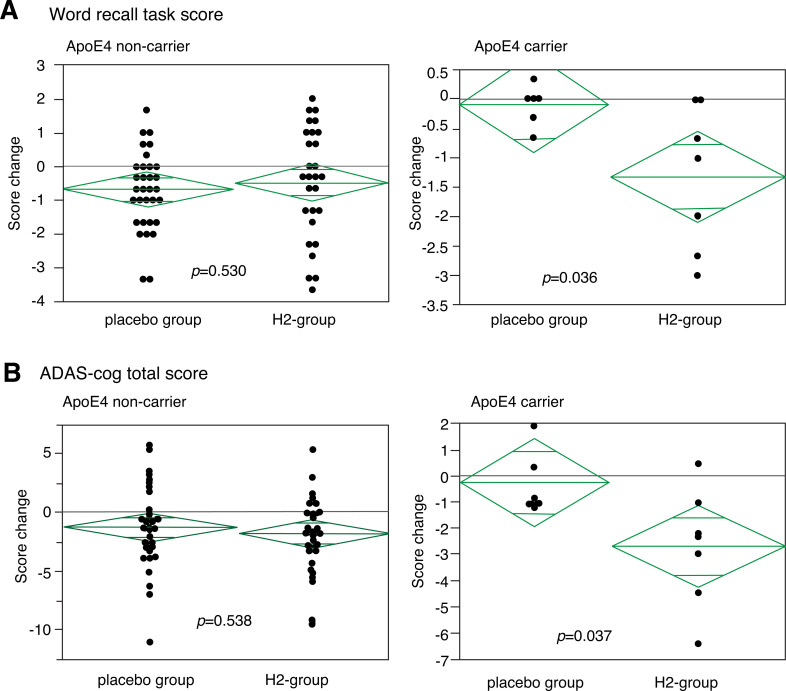
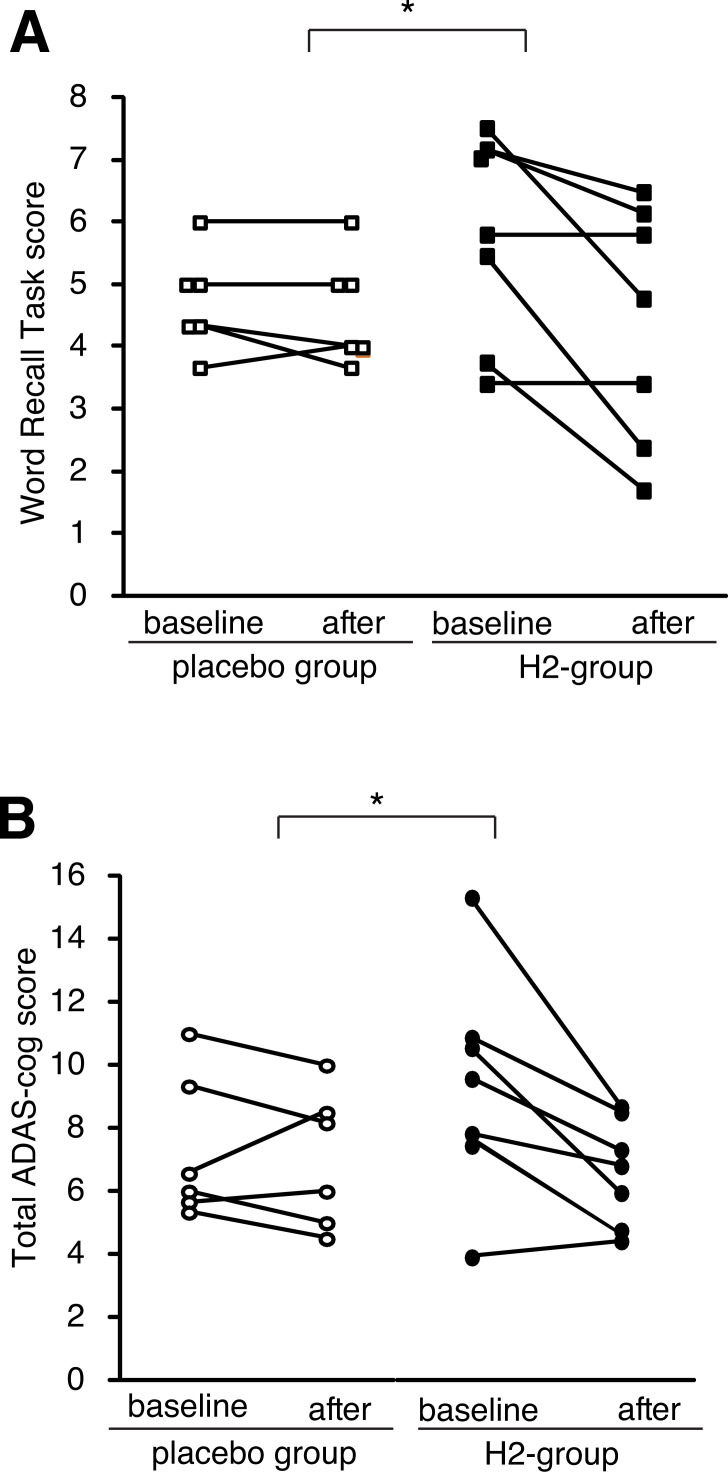
After 1 year, no observable harms or unintended effects in each group were found, and there was a trend to improve total ADA-cog score both in the H2- and control-groups (Suppl. Table S1 (223.8KB, pdf) ), probably because of interventions such as moderate exercise by the Tone project. Moreover, the subjects in the H2-group had more trends for the improvement than those in the control-groups although there was no significance (Suppl. Table S1 (223.8KB, pdf) ). However, when we pay attention to score-changes in carriers of the APOE4 genotype, the total ADAS-cogs and word recall task scores (one of the sub-scores) significantly improved as assessed by the distribution of the score change in each subject (Fig. 5). In the APOE4 carriers, the H2-group significantly improved, whereas the control group slightly worsened. Moreover, Fig. (6) shows the score change of each subject as an alternative presentation. Although the subjects in the control group did not improved, six and five out of 7 subjects improved on the total ADAS score and word recall task scores, respectively, in the H2-group of the APOE4 carriers.
Fig. (5).
Distribution of changes of sub- and total-ADAS-cog score. Distribution of change of word recall task score (A), a sub-score of ADAS-cog, and (B) total ADAS-cogs score in APOE4 non-carriers (left) and APOE4 carriers (right). Each dot indicates the change of individual subjects. The difference between the H2- and control groups was significant in APOE4 carriers by a non-parametric analysis as well as a parametric analysis. (A) p = 0.036 (by Student’s t-test) and p =0.047 (by Mann-Whitney's U test) and (B) p = 0.037 (by Student’s t-test) and p = 0.044 (by Mann-Whitney's U test) for (A) and (B), respectively. Middle bars in lozenges indicate median values.
Fig. (6).
Changes in a sub-sore and total ADAS-cog score of each subject in the APOE4 carriers. Each line indicates the 1-year change in the word recall task score (A) and total ADAS-cog score (B) of a subject in the APOE4 carriers. * indicates p < 0.05 as shown in the legend of Fig. 5.
DISCUSSION
Age-dependent neurodegenerative disorders are involved in oxidative stress. In this study, we showed that drinking
H2-water suppressed the biochemical, behavioral, and pathological decline in oxidative stress mice. The score of ADAS-cog [18] is the most widely used general cognitive measure in clinical trials of AD [27, 28]. The ADAS-cog score assesses multiple cognitive domains including memory, language, praxis, and orientation. Overall, the ADAS-cog has proven successful for its intended purpose. The present clinical study shows that drinking H2-water significantly improved the ADAS-cog score of APOE4 genotype-carriers.
We have previously showed that DAL101 mice show age-dependent neurodegeneration and cognitive decline and the shorten lifespan [4]. DAL101 mice exhibit dementia phenotypes in an age-dependent manner in response to an increasing amount of oxidative stress [4]. Oxidative stress enhances lipid peroxidation, leading to the formation of highly reactive α, β-unsaturated aldehydes, such as MDA and 4-HNE [29]. The accumulation of 4-HNE-adducted proteins in pyramidal neurons has been observed in the brains of patients with AD and PD [30]. The decline of ALDH2*2 ability failed to detoxify cytotoxic aldehydes, and consequently increases in oxidative stress [31].
Moreover, double-transgenic mice were constructed by crossing DAL101 mice with Tg2576 mice, which express a mutant form of human amyloid precursor protein (APP). They showed accelerated amyloid deposition, tau phosphorylation, and gliosis, as well as impaired learning and memory abilities. The lifespan of APP/DAL mice was significantly shorter than that of APP and DAL101 mice [32]. Thus, these model animals may be helpful to explore antioxidants that could be able to prevent age-dependent dementia. Indeed, a diet containing Chlorella showed mitigated effects on cognitive decline in DAL101 [33].
One of the most potent risk factors for AD is carrier status of the APOE4 genotype, and the roles of APOE4 on the progression of AD have been extensively examined from various aspects [34, 35]. APOE4 also increase the number of atherogenic lipoproteins, and accelerate atherogenesis [36]. The increased oxidative stress in APOE4 carriers is considered as one of the modifiers for the risk [3]. A combination of antioxidants improved cognitive function of aged subjects after 3 years, especially in APOE4 carriers [23]. This previous clinical result agrees with the present study. H2 acts as an efficient antioxidant inside cells owing to its ability to rapidly diffuse across membranes [9]. Moreover, as a secondary anti-oxidative function, H2 seems to activate NF-E2-related factor 2 (Nrf2), [10] which reduces oxidative stress by expression a variety of antioxidant enzymes [37]. We reported that drinking H2-water prevented arteriosclerosis using APOE knockout mice, a model of the spontaneous development of atherosclerosis accompanying a decrease in oxidative stress [38]. Thus, it is possible that drinking H2-water improves vascular damage by decreasing oxidative stress as a direct or indirect antioxidant, leading to the improvement of a demintia model and MCI subjects. In this study, we focused on the genotype of APOE-isoforms; however, the polymorphism of the APOE gene in the promoter region influences the expression of the APOE gene [39]. Thus, it will be important to examine the effect of H2-water under this polymorphism.
For mitigating AD, significant attention has been given to regular, moderate exercise to help reduce the risk of dementia and prevent MCI from developing in aging patients [40 - 42]. Moderate exercise enhances energy metabolism and suppresses the expression of pro-inflammatory cytokines, [43] and protects vascular systems [40, 44, 45]. H2 exhibits multiple functions by a decrease in the levels of pro-inflammatory cytokines and an increase in energy metabolism in addition to anti-oxidative roles. To exert multiple functions, H2 regulates various signal transduction pathways and the expression of many genes [10]. For examples, H2 protects neural cells and stimulates energy metabolism by stimulating the hormonal expression of ghrelin [15] and fibroblast growth factor 21, [21] respectively. In contrast, H2 relieves inflammation by decreasing pro-inflammatory cytokines [46]. Thus, the combination of these functions of H2 on anti-inflammation and energy metabolism-stimulation might prevent the decline in brain function, [10] both of which are improved by regular and moderate exercise. Thus, it is possible that the multipe functions of H2, including energy metabolism-stimulation and anti-inflammation, may contribute to the improvement of the dementia model and the MCI subjects.
As an alternative aspect, H2 suppresses the nuclear factor of activated T cell (NFAT) transcription pathway to regulate various gene expression patterns [47]. NFAT signaling is altered in AD and plays an important role in driving amyloid β-mediated neurodegeneration [48]. Moreover, the NFAT transcriptional cascade contributes to amyloid β synaptotoxicity [49]. Additionally, an active involvement of the NFAT-mediated signaling pathway in α-syn-mediated degeneration of neurons in PD [50]. Indeed, patients with PD improved by drinking H2-water as revealed by a double-blind, placebo-controlled clinical study, [51] and a larger scale of a clinical trial is under investigation [52]. Thus, the beneficial effects of H2 on the neurodegenerative diseases may be explained by the suppression of NFAT transcriptional regulation.
CONCLUSION
The present study suggests a possibility for slowing the progress of dementia by drinking H2-water by means of animal experiments and a clinical intervention study for APOE4 carriers; however, a longer and larger scale of trials will be necessary to clarify the effect of H2-water on MCI.
ACKNOWLEDGEMENTS
We thank Blue Mercury, Inc. (Tokyo, Japan) for providing H2-water and placebo water, Ms. Hiroe Murakoshi for technical assistance and Ms. Suga Kato for secretarial work. Financial support for this study was provided by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (23300257, 24651055, and 26282198 to S.O.; 23500971 and 25350907 to K.N.). Financial support for this study was provided by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (23300257, 24651055, and 26282198 to S.O.; 23500971 and 25350907 to K.N.).
LIST OF ABBREVIATIONS
- APOE4
Apolipoprotein E4
- MCI
Mild cognitive Impairment
- ALDH2
Aldehyde Dehydrogenase 2
- ADAS-cog
Alzheimer's Disease Assessment Scale-cognitive subscale
- AD
Alzheimer’s Disease
- PD
Parkinson’s Disease
- DAL101
Dominant Negative Type 101 of the ALDH2 Mutant Polymorphism (ALDH2*2)
- 4-HNE
4-Hydroxy-2-nonenal
- 8-OHdG
8-Hydroxy-2’-deoxyguanosine
- MDA
Malondialdehyde
- ORT
Object Recognition Task
- PA
Passive Avoidance Task
- GFAP
Glial Fibrillary Acidic Protein
- PBS
Phosphate-buffered Saline
- ANOVA
One-way Analysis of Variance
- CI
Confidence Interval
- MMSE
Mini Mental State Examination
- FOV
Field of View
- APP
Amyloid Precursor Protein
- Nrf2
NF-E2-related Factor 2
- NFAT
Nuclear Factor of Activated T Cell
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s web site along with the published article.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The animal study was approved by the Animal Care and Use Committee of Nippon Medical School.
The human clinical study protocol was approved by the ethics committees of University of Tsukuba.
HUMAN AND ANIMAL RIGHTS
All animal research procedures followed were in accordance with the standards set forth in the eighth edition of Guide for the Care and Use of Laboratory Animals published by the National Academy of Sciences, The National Academies Press, Washington, D.C.).
All human material was obtained in accordance with the standards set forth in the Declaration of Helsinki principles of 1975, as revised in 2008 (http://www.wma.net/en/10ethics/10helsinki/<http://www.wma.net/en/10ethics/10helsinki/>).
Consent for Publication
All the patients provided written informed consent priority to research investigations.
CONFLICT OF INTEREST
We declare that there is no actual and potential conflict of interest on this study. Although SO was a scientific advisor of Blue Mercury, Inc. (Tokyo, Japan) from 2,005 to 2,008, there was no involvement during this study.
REFERENCES
- 1.Lin M.T., Beal M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 2.Mecocci P., Polidori M.C. Antioxidant clinical trials in mild cognitive impairment and Alzheimer’s disease. Biochim. Biophys. Acta. 2012;1822:631–638. doi: 10.1016/j.bbadis.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Jofre-Monseny L., Minihane A.M., Rimbach G. Impact of apoE genotype on oxidative stress, inflammation and disease risk. Mol. Nutr. Food Res. 2008;52:131–145. doi: 10.1002/mnfr.200700322. [DOI] [PubMed] [Google Scholar]
- 4.Ohsawa I., Nishimaki K., Murakami Y., Suzuki Y., Ishikawa M., Ohta S. Age-dependent neurodegeneration accompanying memory loss in transgenic mice defective in mitochondrial aldehyde dehydrogenase 2 activity. J. Neurosci. 2008;28:6239–6249. doi: 10.1523/JNEUROSCI.4956-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen C.H., Ferreira J.C., Gross E.R., Mochly-Rosen D. Targeting aldehyde dehydrogenase 2: new therapeutic opportunities. Physiol. Rev. 2014;94:1–34. doi: 10.1152/physrev.00017.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamino K., Nagasaka K., Imagawa M., Yamamoto H., Yoneda H., Ueki A., et al. Deficiency in mitochondrial aldehyde dehydrogenase increases the risk for late-onset Alzheimer’s disease in the Japanese population. Biochem. Biophys. Res. Commun. 2000;273:192–196. doi: 10.1006/bbrc.2000.2923. [DOI] [PubMed] [Google Scholar]
- 7.Jo S.A., Kim E.K., Park M.H., Han C., Park H.Y., Jang Y., et al. A Glu487Lys polymorphism in the gene for mitochondrial aldehyde dehydrogenase 2 is associated with myocardial infarction in elderly Korean men. Clin. Chim. Acta. 2007;382:43–47. doi: 10.1016/j.cca.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Wang B., Wang J., Zhou S., Tan S., He X., Yang Z., et al. The association of mitochondrial aldehyde dehydrogenase gene (ALDH2) polymorphism with susceptibility to late-onset Alzheimer’s disease in Chinese. J. Neurol. Sci. 2008;268:172–175. doi: 10.1016/j.jns.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Ohsawa I., Ishikawa M., Takahashi K., Watanabe M., Nishimaki K., Yamagata K., et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 10.Ohta S. Molecular hydrogen as a preventive and therapeutic medical gas: initiation, development and potential of hydrogen medicine. Pharmacol. Ther. 2014;144:1–11. doi: 10.1016/j.pharmthera.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Ichihara M., Sobue S., Ito M., Ito M., Hirayama M., Ohno K. Beneficial biological effects and the underlying mechanisms of molecular hydrogen - comprehensive review of 321 original articles. Med. Gas Res. 2015;5:12. doi: 10.1186/s13618-015-0035-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iketani M., Ohsawa I. Molecular Hydrogen as a Neuroprotective Agent. Curr. Neuropharmacol. 2017;15:324–331. doi: 10.2174/1570159X14666160607205417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohta S. Molecular hydrogen as a novel antioxidant: overview of the advantages of hydrogen for medical applications. Methods Enzymol. 2015;555:289–317. doi: 10.1016/bs.mie.2014.11.038. [DOI] [PubMed] [Google Scholar]
- 14.Nagata K, Nakashima-Kamimura N, Mikami T, Ohsawa I, Ohta S. Consumption of molecular hydrogen prevents the stress-induced impairments in hippocampus-dependent learning tasks during chronic physical restraint in mice. 2009. [DOI] [PubMed]
- 15.Matsumoto A., Yamafuji M., Tachibana T., Nakabeppu Y., Noda M., Nakaya H. Oral ‘hydrogen water’ induces neuroprotective ghrelin secretion in mice. Sci. Rep. 2013;3:3273. doi: 10.1038/srep03273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J., Wang C., Zhang J.H., Cai J.M., Cao Y.P., Sun X.J. Hydrogen-rich saline improves memory function in a rat model of amyloid-beta-induced Alzheimer’s disease by reduction of oxidative stress. Brain Res. 2010;1328:152–161. doi: 10.1016/j.brainres.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 17.Hayashida K., Sano M., Kamimura N., Yokota T., Suzuki M., Ohta S., et al. Hydrogen inhalation during normoxic resuscitation improves neurological outcome in a rat model of cardiac arrest, independent of targeted temperature management. Circulation. 2014;130:2173–2180. doi: 10.1161/CIRCULATIONAHA.114.011848. [DOI] [PubMed] [Google Scholar]
- 18.Rosen W.G., Mohs R.C., Davis K.L. A new rating scale for Alzheimer’s disease. Am. J. Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 19.Connor D.J., Sabbagh M.N. Administration and scoring variance on the ADAS-Cog. J. Alzheimers Dis. 2008;15:461–464. doi: 10.3233/jad-2008-15312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Zwart L.L., Meerman J.H., Commandeur J.N., Vermeulen N.P. Biomarkers of free radical damage applications in experimental animals and in humans. Free Radic. Biol. Med. 1999;26:202–226. doi: 10.1016/s0891-5849(98)00196-8. [DOI] [PubMed] [Google Scholar]
- 21.Kamimura N., Nishimaki K., Ohsawa I., Ohta S. Molecular hydrogen improves obesity and diabetes by inducing hepatic FGF21 and stimulating energy metabolism in db/db mice. Obesity (Silver Spring) 2011;19:1396–1403. doi: 10.1038/oby.2011.6. [DOI] [PubMed] [Google Scholar]
- 22.O’Riordan K.J., Huang I.C., Pizzi M., Spano P., Boroni F., Egli R., et al. Regulation of nuclear factor kappaB in the hippocampus by group I metabotropic glutamate receptors. J. Neurosci. 2006;26:4870–4879. doi: 10.1523/JNEUROSCI.4527-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bun S., Ikejima C., Kida J., Yoshimura A., Lebowitz A.J., Kakuma T., et al. A combination of supplements may reduce the risk of Alzheimer’s disease in elderly Japanese with normal cognition. J. Alzheimers Dis. 2015;45:15–25. doi: 10.3233/JAD-142232. [DOI] [PubMed] [Google Scholar]
- 24.Miyamoto M., Kodama C., Kinoshita T., Yamashita F., Hidaka S., Mizukami K., et al. Dementia and mild cognitive impairment among non-responders to a community survey. J. Clin. Neurosci. 2009;16:270–276. doi: 10.1016/j.jocn.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki M., Kodama C., Hidaka S., Yamashita F., Kinoshita T., Nemoto K., et al. Prevalence of four subtypes of mild cognitive impairment and APOE in a Japanese community. Int. J. Geriatr. Psychiatry. 2009;24:1119–1126. doi: 10.1002/gps.2234. [DOI] [PubMed] [Google Scholar]
- 26.Arevalo-Rodriguez I., Smailagic N., Roque I.F.M., Ciapponi A., Sanchez-Perez E., Giannakou A., et al. Mini-Mental State Examination (MMSE) for the detection of Alzheimer’s disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst. Rev. 2015;3:CD010783. doi: 10.1002/14651858.CD010783.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ihl R., Ferris S., Robert P., Winblad B., Gauthier S., Tennigkeit F. Detecting treatment effects with combinations of the ADAS-cog items in patients with mild and moderate Alzheimer’s disease. Int. J. Geriatr. Psychiatry. 2012;27:15–21. doi: 10.1002/gps.2679. [DOI] [PubMed] [Google Scholar]
- 28.Karin A., Hannesdottir K., Jaeger J., Annas P., Segerdahl M., Karlsson P., et al. Psychometric evaluation of ADAS-Cog and NTB for measuring drug response. Acta Neurol. Scand. 2014;129:114–122. doi: 10.1111/ane.12153. [DOI] [PubMed] [Google Scholar]
- 29.Schneider C., Tallman K.A., Porter N.A., Brash A.R. Two distinct pathways of formation of 4-hydroxynonenal. Mechanisms of nonenzymatic transformation of the 9- and 13-hydroperoxides of linoleic acid to 4-hydroxyalkenals. J. Biol. Chem. 2001;276:20831–20838. doi: 10.1074/jbc.M101821200. [DOI] [PubMed] [Google Scholar]
- 30.Csala M., Kardon T., Legeza B., Lizak B., Mandl J., Margittai E., et al. On the role of 4-hydroxynonenal in health and disease. Biochim. Biophys. Acta. 2015;1852:826–838. doi: 10.1016/j.bbadis.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 31.Endo J., Sano M., Katayama T., Hishiki T., Shinmura K., Morizane S., et al. Metabolic remodeling induced by mitochondrial aldehyde stress stimulates tolerance to oxidative stress in the heart. Circ. Res. 2009;105:1118–1127. doi: 10.1161/CIRCRESAHA.109.206607. [DOI] [PubMed] [Google Scholar]
- 32.Kanamaru T., Kamimura N., Yokota T., Iuchi K., Nishimaki K., Takami S., et al. Oxidative stress accelerates amyloid deposition and memory impairment in a double-transgenic mouse model of Alzheimer’s disease. Neurosci. Lett. 2015;587:126–131. doi: 10.1016/j.neulet.2014.12.033. [DOI] [PubMed] [Google Scholar]
- 33.Nakashima Y., Ohsawa I., Konishi F., Hasegawa T., Kumamoto S., Suzuki Y., et al. Preventive effects of Chlorella on cognitive decline in age-dependent dementia model mice. Neurosci. Lett. 2009;464:193–198. doi: 10.1016/j.neulet.2009.08.044. [DOI] [PubMed] [Google Scholar]
- 34.De Marco M., Vallelunga A., Meneghello F., Varma S., Frangi A.F., Venneri A. ApoE epsilon4 allele related alterations in hippocampal connectivity in early Alzheimer’s disease support memory performance. Curr. Alzheimer Res. 2017;14:766–777. doi: 10.2174/1567205014666170206113528. [DOI] [PubMed] [Google Scholar]
- 35.Shackleton B., Crawford F., Bachmeier C. Apolipoprotein E-mediated modulation of ADAM10 in Alzheimer’s disease. Curr. Alzheimer Res. 2017;14:578–585. doi: 10.2174/1567205014666170203093219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanson A.J., Craft S., Banks W.A. The APOE genotype: modification of therapeutic responses in Alzheimer’s disease. Curr. Pharm. Des. 2015;21:114–120. doi: 10.2174/1381612820666141020164222. [DOI] [PubMed] [Google Scholar]
- 37.Johnson D.A., Johnson J.A. Nrf2-a therapeutic target for the treatment of neurodegenerative diseases. Free Radic. Biol. Med. 2015;88:253–267. doi: 10.1016/j.freeradbiomed.2015.07.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohsawa I., Nishimaki K., Yamagata K., Ishikawa M., Ohta S. Consumption of hydrogen water prevents atherosclerosis in apolipoprotein E knockout mice. Biochem. Biophys. Res. Commun. 2008;377:1195–1198. doi: 10.1016/j.bbrc.2008.10.156. [DOI] [PubMed] [Google Scholar]
- 39.Maloney B., Ge Y.W., Petersen R.C., Hardy J., Rogers J.T., Perez-Tur J., et al. Functional characterization of three single-nucleotide polymorphisms present in the human APOE promoter sequence: Differential effects in neuronal cells and on DNA-protein interactions. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2010;153B:185–201. doi: 10.1002/ajmg.b.30973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uemura K., Doi T., Shimada H., Makizako H., Yoshida D., Tsutsumimoto K., et al. Effects of exercise intervention on vascular risk factors in older adults with mild cognitive impairment: a randomized controlled trial. Dement. Geriatr. Cogn. Disord. Extra. 2012;2:445–455. doi: 10.1159/000343486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gates N., Fiatarone Singh M.A., Sachdev P.S., Valenzuela M. The effect of exercise training on cognitive function in older adults with mild cognitive impairment: a meta-analysis of randomized controlled trials. Am. J. Geriatr. Psychiatry. 2013;21:1086–1097. doi: 10.1016/j.jagp.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki T., Shimada H., Makizako H., Doi T., Yoshida D., Ito K., et al. A randomized controlled trial of multicomponent exercise in older adults with mild cognitive impairment. PLoS One. 2013;8:e61483. doi: 10.1371/journal.pone.0061483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smart N.A., Steele M. The effect of physical training on systemic proinflammatory cytokine expression in heart failure patients: a systematic review. Congest. Heart Fail. 2011;17:110–114. doi: 10.1111/j.1751-7133.2011.00217.x. [DOI] [PubMed] [Google Scholar]
- 44.Cooper C., Li R., Lyketsos C., Livingston G. Treatment for mild cognitive impairment: systematic review. Br. J. Psychiatry. 2013;203:255–264. doi: 10.1192/bjp.bp.113.127811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lavie C.J., Arena R., Swift D.L., Johannsen N.M., Sui X., Lee D.C., et al. Exercise and the cardiovascular system: clinical science and cardiovascular outcomes. Circ. Res. 2015;117:207–219. doi: 10.1161/CIRCRESAHA.117.305205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buchholz B.M., Kaczorowski D.J., Sugimoto R., Yang R., Wang Y., Billiar T.R., et al. Hydrogen inhalation ameliorates oxidative stress in transplantation induced intestinal graft injury. Am. J. Transplant. 2008;8:2015–2024. doi: 10.1111/j.1600-6143.2008.02359.x. [DOI] [PubMed] [Google Scholar]
- 47.Iuchi K., Imoto A., Kamimura N., Nishimaki K., Ichimiya H., Yokota T., et al. Molecular hydrogen regulates gene expression by modifying the free radical chain reaction-dependent generation of oxidized phospholipid mediators. Sci. Rep. 2016;6:18971. doi: 10.1038/srep18971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdul H.M., Sama M.A., Furman J.L., Mathis D.M., Beckett T.L., Weidner A.M., et al. Cognitive decline in Alzheimer’s disease is associated with selective changes in calcineurin/NFAT signaling. J. Neurosci. 2009;29:12957–12969. doi: 10.1523/JNEUROSCI.1064-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hudry E., Wu H.Y., Arbel-Ornath M., Hashimoto T., Matsouaka R., Fan Z., et al. Inhibition of the NFAT pathway alleviates amyloid beta neurotoxicity in a mouse model of Alzheimer’s disease. J. Neurosci. 2012;32:3176–3192. doi: 10.1523/JNEUROSCI.6439-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo J., Sun L., Lin X., Liu G., Yu J., Parisiadou L., et al. A calcineurin- and NFAT-dependent pathway is involved in alpha-synuclein-induced degeneration of midbrain dopaminergic neurons. Hum. Mol. Genet. 2014;23:6567–6574. doi: 10.1093/hmg/ddu377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoritaka A., Takanashi M., Hirayama M., Nakahara T., Ohta S., Hattori N. Pilot study of H(2) therapy in Parkinson’s disease: a randomized double-blind placebo-controlled trial. Mov. Disord. 2013;28:836–839. doi: 10.1002/mds.25375. [DOI] [PubMed] [Google Scholar]
- 52.Yoritaka A., Abe T., Ohtsuka C., Maeda T., Hirayama M., Watanabe H., et al. A randomized double-blind multi-center trial of hydrogen water for Parkinson’s disease: protocol and baseline characteristics. BMC Neurol. 2016;16:66. doi: 10.1186/s12883-016-0589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s web site along with the published article.