Abstract
Objectives
Physical inactivity in end-stage renal disease (ESRD) patients is associated with increased mortality, and might be related to abnormalities in body composition (BC) and physical performance. It is uncertain to what extent starting dialysis influences the effects of ESRD on physical activity (PA). This study aimed to compare PA and physical performance between stage 5 chronic kidney disease (CKD-5) non-dialysis and dialysis patients, and healthy controls, to assess alterations in PA during the transition from CKD-5 non-dialysis to dialysis, and to relate PA to BC.
Methods
For the cross-sectional analyses 44 CKD-5 non-dialysis patients, 29 dialysis patients, and 20 healthy controls were included. PA was measured by the SenseWear™ pro3. Also, the walking speed and handgrip strength (HGS) were measured. BC was measured by the Body Composition Monitor©. Longitudinally, these parameters were assessed in 42 CKD-5 non-dialysis patients (who were also part of the cross-sectional analysis), before the start of dialysis and 6 months thereafter.
Results
PA was significantly lower in CKD-5 non-dialysis patients as compared to that in healthy controls but not as compared to that in dialysis patients. HGS was significantly lower in dialysis patients as compared to that in healthy controls. Walking speed was significantly lower in CKD-5 non-dialysis patients as compared to that in healthy controls but not as compared to that in dialysis patients. Six months after starting dialysis, activity related energy expenditure (AEE) and walking speed significantly increased.
Conclusions
PA is already lower in CKD-5 non-dialysis patients as compared to that in healthy controls and does not differ from that of dialysis patients. However, the transition phase from CKD-5 non-dialysis to dialysis is associated only with a modest improvement in AEE.
Keywords: Body composition, Dialysis, End-stage renal disease, Physical activity
Introduction
It is known that dialysis patients have decreased levels of physical activity (PA), matching a sedentary lifestyle, recently defined as number of steps <7,500/day [1]. Physical inactivity in dialysis patients is associated with an increased risk for hospitalization and mortality [2, 3], and with alterations in body composition (BC) and decreased muscle strength [4]. Also in earlier phases of chronic kidney disease (CKD) a reduced PA has been observed [5].
The transition phase from stage 5 CKD (CKD-5) non-dialysis to dialysis, as well as chronic dialysis treatment can have a major physiological and psychological impact on end-stage renal disease (ESRD) patients [6]. It is not well known whether PA is affected by chronic dialysis treatment or whether low PA is an inherent characteristic of ESRD. Whereas some literature suggests that PA is already decreased in the CKD-5 non-dialysis phase due to fatigue related to uremic disorders, the presence of comorbidity, and pre-existent lifestyle factors [7], others indicate that PA is adequately preserved in a cohort of CKD stages 4–5 patients [8]. Additionally, in theory, dialysis treatment may improve PA on non-dialysis days due to partial correction of the uremic state. Nevertheless, the treatment may induce fatigue and stimulate a sedentary lifestyle even on dialysis days [9].
PA may also be associated with BC changes. This may be of prognostic importance, as lower lean tissue index (LTI; lean tissue mass [LTM] corrected for height) is related to higher mortality, although the relation between fat tissue index (FTI; adipose tissue mass [ATM] corrected for height) and survival in dialysis patients is not straightforward [10]. However, to the best of our knowledge, only limited data are available to assess the association between PA and BC in a cohort including CKD-5 non-dialysis and dialysis patients [11, 12, 13].
This study first aimed to compare PA and physical performance cross-sectionally between CKD-5 non-dialysis and dialysis patients, and healthy controls. Second, we aimed to assess longitudinally alterations in PA and physical performance during the transition from CKD-5 non-dialysis to dialysis, and third, to assess the associations between PA and BC parameters in ESRD patients.
Materials and Methods
This study consisted of a cross-sectional and a longitudinal part. The cross-sectional analyses included 73 patients; 44 CKD-5 non-dialysis patients and 29 dialysis patients, and 20 healthy controls. Patients were recruited from dialysis centers in the Netherlands and Belgium: Maastricht, Eindhoven, Venlo, Sittard, Roermond, and Hasselt. CKD-5 non-dialysis patients were ESRD patients starting dialysis within one month. Dialysis patients had been treated with hemodialysis (HD) or peritoneal dialysis (PD) for at least 12 months.
The longitudinal analyses are part of an ongoing prospective study and included 42 of the CKD-5 non-dialysis patients (who were also part of the cross-sectional analysis) for whom 6 months follow-up data were available. Inclusion for the longitudinal analyses was solely based on the availability of the 6-month follow-up data. In addition, out of the 44 patients in the cross-sectional analyses, 2 patients received a kidney transplant before the 6-month follow-up measurement took place, and were excluded from the longitudinal analyses. Measurements were performed before the start of dialysis (maximum 4 weeks prior to the first dialysis session) and 5–6 months after starting dialysis by the same methods as used for the cross-sectional part.
Exclusion criteria for patients were: an acute start of dialysis treatment, active symptomatic coronary artery disease or cardiac failure New York Heart Association classification III or IV, active malignancies, active infections, and inability to provide informed consent. For bioimpedance measurements: no implantable cardioverter defibrillator (ICD) or pacemaker (interference with body composition monitor [BCM]). For walking test measurements: physical disability (patients had to be able to walk without help). There were no restrictions for other measurements in patients with an ICD or pacemaker or physical disability.
Healthy controls were non-diabetic, non-smokers, and not hypertensive, and recruited via advertisements at the university hospital.
Exclusion criteria for healthy controls were hypertension during the screening; systolic blood pressure higher than 170 mm Hg, and/or diastolic blood pressure larger than 100 mm Hg, diabetes mellitus, and inability to provide informed consent.
All measurements took place on a non-dialysis day, or before a dialysis session for practical reasons, except for PA measurements, which were carried out in the home environment of the participants. Patients as well as healthy controls were asked to be in a fasting state during the measurements, except for the PA measurements.
PA Measurements
Participants were requested to wear a SenseWear™ pro 3 armband (Bodymedia®, Pittsburg, PA, USA) to measure PA parameters (total energy expenditure [TEE], activity related energy expenditure [AEE], number of steps) for 2 consecutive days (CKD-5 non-dialysis patients: 2.01 ± 0.41 days, mean on-body time: 94.9%; dialysis patients: 2.30 ± 0.73 days, mean on-body time: 96.7%; controls: 2.07 ± 0.54 days, mean on-body time: 96.1%), which is considered to be sufficient to obtain data with regard to daily PA [14, 15]. For the primary analyses, the mean of the total on-body time was calculated (expressed as TEE, AEE and number of steps per 24 h) to include both the dialysis and non-dialysis day. Furthermore, TEE and AEE were expressed per kilogram body weight. No differentiation was made between data collected on week or weekend days for all participants.
In an additional analysis, a 24-h measurement cycle was analyzed for the dialysis patient group to exclude the dialysis session. For the longitudinal analysis in the CKD-5 non-dialysis group, the available data were not sufficient to exclude dialysis session.
BC Measurements
BC was determined by bioimpedance spectroscopy with the Body Composition Monitor (BCM®, Fresenius Medical Care, Bad Homburg, Germany), which uses a 3-compartment model (ATM, LTM, and separate fluid overload [FO] compartment) described by Chamney et al. [16]. ATM and LTM were corrected for height to derive FTI and LTI. Measurements were taken as described in the manufacturer's manual [17]. Patients were in the supine position. In HD patients, measurements were collected before dialysis, in agreement with previous studies [18, 19, 20]. In PD patients, measurements were taken during a visit at the out-patient clinic. Measurements in PD patients were taken with a full abdomen for practical reasons and because sequestered fluid in the trunk has only a minor influence on whole-body bioimpedance measurements [21, 22, 23]. Body weight was corrected for PD fluid. Not all patients were in a fasting state as requested before measurements, due to diabetes (n = 7) or for practical reasons (n = 18).
Muscle Strength
Muscle strength was determined by measuring the handgrip strength (HGS) twice with a handheld dynamometer (Jamar®, Sammons Preston Inc., Bolingbrook, IL, USA) in a standing position with the arm in a flexed position of 90 degrees, contralateral of the shunt arm or in PD patients and healthy controls in the dominant hand.
Four Meter Walking Test
A 4-m walking test was conducted to determine walking speed (m/s). Several studies confirmed the validity and sensitivity of this test for determining walking speed [24, 25, 26, 27] and physical performance in ESRD patients [28].
Comorbidity Score
Comorbidity index was determined by the Davies comorbidity index scoring system [29], which is commonly used in ESRD patients [29, 30, 31]. Patients were divided into 3 risk groups; low, medium, and high risk of mortality.
Biochemical Parameters
Hemoglobin (HB) and dialysis adequacy (Kt/V) were determined during routine patient laboratory measurements.
Statistical Analysis
Data are expressed as mean ± SD or median (25th-75th percentile) unless indicated otherwise. For the cross-sectional analyses, differences in the categorical variables were assessed using chi-square tests. Differences in the continuous variables PA, physical performance, and BC between groups were assessed by independent-samples t tests or Mann-Whitney U tests, as appropriate. In additional analyses, we adjusted these between-group differences for differences in the distribution of age, gender, and diabetes status with the use of multivariable regression analyses. In these analyses, there was no evidence of multicollinearity (tolerance >0.20). For the longitudinal analyses, changes over time within the CKD-5 non-dialysis patient group were evaluated using the dependent-samples t test or Wilcoxon-matched pairs signed rank sum test, as appropriate. Differences in change between dialysis modalities were conducted with the independent sample t test or Wilcoxon-matched pairs signed rank sum test, as appropriate.
Statistical analyses were performed by IBM SPSS Statistics for Windows version 24 (IBM Corp., Armonk, NY, USA). p values ≤0.05 were considered to be statistically significant.
Results
Patient Characteristics
Patient characteristics for the cross-sectional part are summarized in Table 1a and for the longitudinal part in Table 1b.
Table 1.
a.
Patient demographics
| CKD-5 non-dialysis patients | Dialysis patients | Healthy controls | |
|---|---|---|---|
| Number of patients | 44 | 29 | 20 |
| Male, % | 75.0 | 69.0 | 65.0 |
| HD/PD | - | 21*/8 | - |
| Age, years | 61.3±12.0 | 58.2±14.7 | 59.7±14.1 |
| Height, cm | 173.8±9.3 | 171.6±9.6 | 174.8±11.4 |
| Weight, kg | 79.2±17.3 | 82.7±15.3 | 76.7±15.8 |
| BMI, kg/m2 | 26.0±4.1 | 28.1±4.4 | 24.9±3.4 |
| FO, L | 1.4±2.0 (n = 43) | 1.2±1.5 (n = 28) | 0.1±0.8 |
| Hemoglobin, mmol/L/g/dL | 6.7±0.9/10.7±1.5 (n = 39) | 6.9±0.7/11.2±1.2 (n = 28) | - |
| Residual urine output, mL/24 h | 2,100 (1,700–2,400) (n = 27) | 500 (0–1,445.0) | - |
| Dialysis vintage, years | - | 3.6±3.2 | - |
| Origin of end-stage renal disease, % | |||
| Diabetic nephropathy | 4.5 | 20.7 | - |
| Polycystic kidney disease | 27.3 | 17.2 | |
| Nephrosclerosis | 15.9 | 6.9 | |
| Hypertensive nephropathy | 9.1 | 10.3 | |
| Nephrotic syndrome | 11.4 | 3.4 | |
| Unknown cause | 11.4 | 13.8 | |
| Other | 20.5 | 27.6 | |
| Diabetes mellitus | 15.9 | 41.4 | - |
| Cardiovascular disease | 34.1 | 37.9 | - |
| Risk of mortality by Davies index | |||
| Low risk, % | 47.7 | 41.4 | - |
| Medium risk, % | 43.2 | 37.9 | |
| High risk, % | 9.1 | 20.7 | |
| Previous transplant, % | 22.7 | 31.0 | 0.0 |
| SBP, mm Hg | 145.9±21.4 | 152.4±26.5 | 138.0±13.4 |
| DBP, mm Hg | 83.3±13.0 | 80.8±12.4 | 82.3±6.9 |
Data are given as mean ± SD or median (25th–75th percentile).
HD, hemodialysis; PD, peritoneal dialysis; BMI, body mass index; FO, fluid overload; SBP, systolic blood pressure; DBP, diastolic blood pressure.
All HD patients have arteriovenous fistulas.
b.
Baseline patient demographics longitudinal analyses
| Patient demographics | |||
|---|---|---|---|
| Number of patients | 42 | ||
| Male, % | 73.8 | ||
| HD*/PD# | 21/21 | ||
| Age, years | 61.0±12.1 | ||
| Height, cm | 173.6±9.5 | ||
| Weight, kg | 78.7±17.5 | ||
| BMI, kg/m2 | 25.9±4.2 | ||
| FO (n = 41), L | 1.5±2.0 | ||
| Hemoglobin (n = 39), mmol/L/g/dL | 6.7±0.9/10.7±1.4 | ||
| Residual urine output (n = 25), mL/24 h | 1,980.2±461.6 | ||
| Origin of end-stage renal disease, % | |||
| Diabetic nephropathy | 4.8 | ||
| Polycystic kidney disease | 26.2 | ||
| Nephrosclerosis | 16.7 | ||
| Hypertensive nephropathy | 9.5 | ||
| Nephrotic syndrome | 11.9 | ||
| Unknown cause | 11.9 | ||
| Other | 19.0 | ||
| Diabetes mellitus | 14.3 | ||
| Cardiovascular disease | 31.0 | ||
| Risk of mortality by Davies index | |||
| Low risk, % | 50.0 | ||
| Medium risk, % | 42.9 | ||
| High risk, % | 7.1 | ||
| Previous transplant, % | 21.4 | ||
| SBP, mm Hg | 145.9±21.7 | ||
| DBP, mm Hg | 83.6±13.2 |
Data are given as mean ± SD.
HD, hemodialysis; PD, peritoneal dialysis; BMI, body mass index; FO, fluid overload; SBP, systolic blood pressure; DBP, diastolic blood pressure.
2HD patients have a central venous catheter.
1 PD patient switched from PD to HD via CVC 3 months after the start of dialysis.
PA Parameters
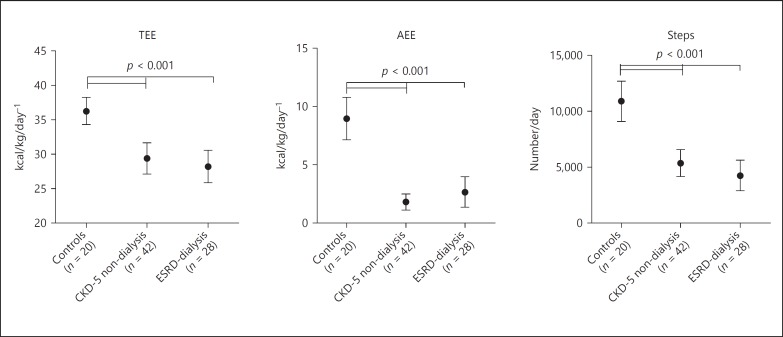
PA parameters, number of steps, and AEE were significantly lower in both CKD-5 non-dialysis and dialysis patients as compared to those in healthy controls (Table 2; Fig. 1). No significant differences were found between CKD-5 non-dialysis and dialysis patients (Table 2; Fig. 1). TEE was significantly lower in both CKD-5 non-dialysis patients and dialysis patients as compared to that in healthy controls (Table 2; Fig. 1). Additionally, after the exclusion of the dialysis day from the analyses, results were similar (data not shown). Further, after adjustment for age, gender, and diabetes prevalence outcomes were not materially changed (data not shown).
Table 2.
Parameters of physical activity, physical performance, and muscle strength
| Parameter | CKD-5 non-dialysis patients | Dialysis patients | Healthy controls | p value |
|---|---|---|---|---|
| TEE, kcal/kg/day−1 | 29.4 (25.4–33.3) (n = 42) | 27.1 (24.8–32.7) (n = 28) | 36.2 (32.9–39.7) (n = 20) | <0.001*/0.270†/<0.001# |
| AEE, kcal/kg/day−1 | 1.6 (0.8–3.1) (n = 42) | 1.9 (0.9–5.2) (n = 28) | 8.3 (6.2–12.4) (n = 20) | <0.001*/0.533†/<0.001# |
| Steps, number/day | 5,435.5 (3,212.8–7,384.3) (n = 42) | 3,994.5 (1,993.5–6,712.8) (n = 28) | 11,062.0 (7,687.0–13,839.0) (n = 20) | <0.001*/0.208†/<0.001# |
| HGS, kg | 29.2±9.8 (n = 44) | 27.2±12.2 (n = 28) | 32.0±11.0 (n = 20) | 0.320*/0.448†/0.174# |
| Walking speed, m/s | 1.4 (1.2–1.8) (n = 38) | 1.5 (1.2–1.9) (n = 27) | 1.8 (1.7–2.0) (n = 20) | 0.017*/0.699†/0.097# |
Data are given as mean ± SD or median (25th and 75th percentile).
TEE, total energy expenditure; AEE, activity related energy expenditure; HGS, handgrip strength.
p value for CKD-5 non-dialysis patients vs. healthy controls.
p value for CKD-5 non-dialysis patients vs. dialysis patients.
p value for dialysis patients vs. healthy controls.
Fig. 1.
Physical activity parameters between groups. CKD-5, stage 5 chronic kidney disease; ESRD, end-stage renal disease; TEE, total energy expenditure; AEE, activity related energy expenditure.
Muscle Strength and Walking Speed
No significant differences were found for HGS between CKD-5 non-dialysis patients and dialysis patients as well as for healthy controls (Table 2). However, after adjustment for differences in age, gender, and diabetes prevalence between groups, dialysis patients had significantly lower HGS as compared to that in healthy controls (−7.1 kg, 95% CI −12.1 to −2.1). Walking speed was significantly lower in CKD-5 non-dialysis patients as compared to that in healthy controls but not as compared to that in dialysis patients (Table 2). After adjustment for differences in age, gender, and diabetes prevalence between groups, findings were similar (data not shown).
Body Composition
LTI (kg/m2) was significantly lower in dialysis patients (13.0 ± 2.9 kg) as compared to that in CKD-5 non-dialysis patients (14.6 ± 2.5 kg, p = 0.020) and as compared to that in healthy controls (14.6 ± 1.9 kg, p = 0.039). FTI (kg/m2) was significantly higher in dialysis patients (14.3 ± 4.7 kg) as compared to that in CKD-5 non-dialysis patients (10.7 ± 4.1 kg, p = 0.002), and as compared to that in healthy controls (10.1 ± 3.0 kg, p = 0.001). For both LTI and FTI, no significant differences were found between CKD-5 non-dialysis patients when compared with the LTI and FTI of healthy controls. When adjusted for differences in age, gender, and diabetes prevalence between groups, LTI was significantly lower in dialysis patients as compared to that in CKD-5 non-dialysis patients (−1.6 kg/m2, 95% CI −2.7 to −0.5) and as compared to that in controls (−1.6 kg/m2, 95% CI −3.1 to −0.2). FTI was significantly higher in dialysis patients as compared to that in CKD-5 non-dialysis patients (+3.1 kg/m2, 95% CI 1.0–5.2) and as compared to that in healthy controls (+3.2 kg/m2, 95% CI 0.6–5.8) after adjustment for age, gender, and diabetes prevalence.
Comorbidity Score
Comorbidity scores are summarized in Table 1a. No significant differences were found between CKD-5 non-dialysis and dialysis patients in terms of the distribution for each category (low, medium, and high risk; p = 0.370).
Longitudinal Analyses
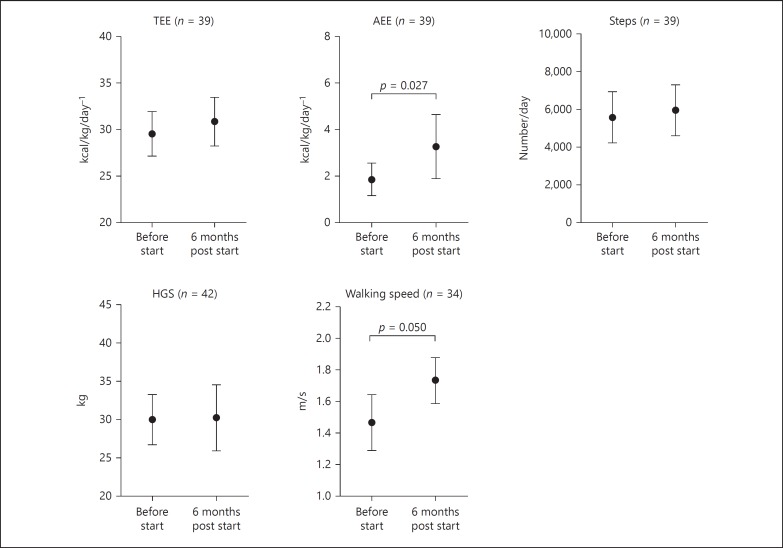
Changes over time in the first 6 months after starting dialysis were found for AEE (Table 3; Fig. 2). Walking speed significantly increased over time by median +0.2 (−0.1 to 0.4) m/s (p = 0.05; Table 3; Fig. 2). No changes were found for HGS during the 6-month follow-up period (Table 3; Fig. 2). Also, no significant changes were observed in BC and FO over the 6-month time period (Table 4). HB levels significantly increased in the first 6 months after starting dialysis +0.6 (−0.3 to 1.3) mmol/L (3.9 [−1.9 to 8.4] g/dL; p = 0.004; n = 39). Kt/V was 1.3 ± 0.2 for HD patients and 2.5 ± 1.0 for PD patients.
Table 3.
Longitudinal analyses for parameters of physical activity, physical performance, and muscle strength
| Parameter | Visit | CKD-5 non-dialysis patients | p value |
|---|---|---|---|
| TEE (n = 39), kcal/kg/day−1 | Before start dialysis Six months after start dialysis |
29.6 (25.4–33.6) 30.7 (26.4–35.4) |
0.163 |
| AEE (n = 39), kcal/kg/day−1 | Before start Six months after start dialysis |
1.8 (0.7–3.1) 2.9 (1.1–5.8) |
0.027 |
| Steps (n = 39), number/day | Before start Six months after start dialysis |
5,747.0 (3,137.0–7,808.0) 5,486.0 (3,892.0–8,452.0) |
0.052 |
| HGS (n = 42), kg | Before start Six months after start dialysis |
29.3 (24.6–36.0) 29.0 (23.4–38.3) |
0.468 |
| Walking speed (n = 34), m/s | Before start Six months after start dialysis |
1.4 (1.2–1.8) 1.7 (1.5–2.0) |
0.050 |
Data are given as median (25th and 75th percentile).
TEE, total energy expenditure; AEE, activity related energy expenditure; HGS, handgrip strength.
Fig. 2.
Longitudinal changes in parameters of physical activity, physical performance, and muscle strength. TEE, total energy expenditure; AEE, activity related energy expenditure; HGS, handgrip strength.
Table 4.
Longitudinal analyses for body composition parameters
| Parameter | Visit | CKD-5 non-dialysis patients | p value |
|---|---|---|---|
| Body weight (n = 42), kg | Before start dialysis Six months after start dialysis |
80.9 (66.1–88.5) 79.6 (65.3–90.2) |
0.438 |
| BMI (n = 42), kg/m2 | Before start Six months after start dialysis |
25.7 (21.9–28.6) 25.9 (22.8–28.4) |
0.540 |
| LTI (n = 40), kg/m2 | Before start Six months after start dialysis |
14.5 (12.8–16.2) 14.1 (12.8–15.9) |
0.216 |
| FTI (n = 40), kg/m2 | Before start Six months after start dialysis |
10.4 (7.8–14.3) 10.0 (7.3–14.5) |
0.226 |
| FO (n = 41), L | Before start Six months after start dialysis |
1.1 (0.2–2.8) 1.3 (−0.1–2.6) |
0.577 |
Data are given in median (25th and 75th percentile).
BMI, body mass index; LTI, lean tissue index; FTI, fat tissue index; FO, fluid overload.
Additional analyses based on dialysis modality did not show significantly different outcomes for PA parameters, where 21 patients started HD and 21 patients started PD. The median change of TEE over 6 months was −0.2 (−1.6 to 2.1) kcal/kg/day−1 for PD patients and +2.3 (−1.2 to 7.1) kcal/kg/day−1 for HD patients (p = 0.086). For AEE, the median change over 6 months was +0.8 (−1.3 to 2.6) kcal/kg/day−1 for PD patients and +1.7 (−0.2 to 3.8) kcal/kg/day−1 for HD patients (p = 0.248). The median change in number of steps over 6 months of time was +1,809.0 (−1,053.0 to 3,458.0) steps/day for PD patients and +332.5 (−799.8 to 3,043.0) steps/day for HD patients (p = 0.714). For HGS, the median changes over 6 months were −1.5 (−4.0 to 2.0) kg in PD patients and +2.0 (−0.8 to 5.3) kg in HD patients (p = 0.027). No differences were found for walking speed between PD and HD patients over time, where the median change over time in walking speed was +0.1 (−0.21 to 0.34) m/s in PD patients and +0.2 (0.06–0.48) m/s in HD patients (p = 0.157).
Discussion
This study focused on the differences in PA between CKD-5 non-dialysis patients, dialysis patients and healthy controls, and on the alterations in PA from CKD-5 non-dialysis care to the first 6 months after starting dialysis. The cross-sectional analyses showed that PA parameters are already lower in the CKD-5 non-dialysis phase as compared to those in healthy controls, and comparable with those of dialysis patients, consistent with a sedentary lifestyle as previously defined by Avesani et al. [1]. The very low number of steps, as a hallmark of physical inactivity, in both CKD-5 non-dialysis and dialysis patients, is of serious concern, although not an entirely new finding in this field [1]. However, research on this topic has been controversial, since other studies show relatively preserved PA in patients with advanced CKD [8].
The results of the longitudinal study suggested that the start of dialysis appeared to have a positive effect only on PA parameter AEE, which significantly increased after the start of dialysis. However, whether this is related to a beneficial effect of starting dialysis per se or to the slight increase in HB levels following the start of dialysis is unknown.
Nonetheless, even after the start of dialysis, PA parameters remained lower as compared with those in healthy controls. These results reemphasize the need for additional interventions, such as exercise training, to increase PA parameters both before and after the start of dialysis, which has been shown to be beneficial in this patient group by several studies [32, 33], and furthermore to investigate whether or not such interventions are beneficial in the long term, given the fact that no differences in PA parameters were found between the CKD-5 non-dialysis group and the dialysis group. This underlines that the positive effect of starting dialysis is possibly cancelled out by a prolonged time on dialysis.
Our study showed that walking speed, as a parameter of physical performance, was already significantly decreased in the CKD-5 non-dialysis phase. Initiation of dialysis was associated with a significant increase in walking speed during the first 6 months after starting dialysis. Conversely, earlier research in elderly dialysis patients showed that the start of dialysis is related to an increased risk of disability, particularly to activities of daily living, next to an increased risk of mortality [34]. Nevertheless, that study population consisted mostly of frail elderly people in a nursing home environment, in which the start of dialysis could be an additional factor for further decline in physical functioning [35, 36], showing the importance of a patient's functional status prior to the start of dialysis. In contrast, we focused on a younger and less frail patient group (as was measured by determinants of frailty [37] such as body weight, HGS, PA, and walking speed), and studied parameters of PA and physical performance by field tests instead of self-reported tests. Additional analyses suggested that dialysis modality did not present different outcomes for PA parameters and physical performance. However, this may have been due to a lack of statistical power after stratification by dialysis modality.
HGS, as a parameter of muscle strength, tended to be under reference values as described by Webb et al. [38], in both CKD-5 non-dialysis patients as well as in dialysis patients, which is in line with earlier research in both older adults [39], as well as in ESRD patients starting dialysis [40]. Isoyama et al. [41] showed that muscle strength is a strong predictor for mortality in dialysis patients, even more strongly than muscle mass itself. In line with this study, our findings showed that starting dialysis does not seem to have a detrimental effect on HGS. However, no positive effect on HGS was observed during the first 6 months of dialysis treatment either.
Regarding BC, the cross-sectional analysis showed that LTI was significantly lower in dialysis patients when compared to that in CKD-5 non-dialysis patients and healthy controls. The decline in LTI in dialysis patients is in line with previous research [4, 42, 43]. In addition, after adjustment for age, gender, and diabetes, outcomes were similar.
In contrast, FTI was significantly higher in dialysis patients as compared to that in both CKD-5 non-dialysis patients and healthy controls. After adjustment for age, gender, and diabetes, the difference was similar in both groups, suggesting that only a part of high FTI levels was explained by the presence of diabetes (diabetes was more prevalent in the dialysis patient group as compared with the CKD-5 non-dialysis group). These results are in line with a recent cohort study in 8,227 patients, in which a mean increase in FTI of 0.95 kg/m2 and a mean decrease in LTI of 0.4 kg/m2 were observed within 2 years following the start of dialysis [44]. The higher FTI can possibly be explained by the effects of dialysis treatment, differences in food intake in dialysis patients, which were not captured in the present study, to a survivor bias, or to prolonged physical inactivity.
This study had some limitations; first, the study population was relatively small and consisted of both HD and PD patients. However, in the cross-sectional analysis, outcomes were not materially changed after the exclusion of PD patients. Also, the longitudinal study suggests no differences in changes in PA and physical performance between patients who started with HD as compared with those who started with PD, with the exception of HGS, which increased in HD patients and decreased in PD patients. A clear explanation for this finding is not directly at hand, and data on this topic are scarce, thereby creating a large knowledge gap in this field. Importantly, our results may have been due to the play of chance, given the low number of participants in each dialysis modality group. In addition, a study of Vogt et al. [45] did not find differences in HGS between HD and PD patients. Second, due to the fact that we have included relatively young and possibly less frail controls and patients, there might be a possibility of selection bias, as none of 42 CKD-5 non-dialysis patients in the longitudinally analyses died in the first 6 months of the transitional phase after starting dialysis. Third, PA was measured only for a relatively short period of time (48 h), which might have biased outcome parameters. However, data from previous studies suggest that 2 days of measurement are sufficient to obtain reliable data [14, 15]. Additionally, we chose to include an additional analysis of a non-dialysis day in order to exclude the effects of the time allocated to the dialysis treatment per se. Fourth, no data with regard to dietary intake were available. However, information with regard to nutritional status measured by the BCM [16] did not show significant alterations in the first 6 months after the start of dialysis. Finally, in the cross-sectional part, there was an imbalance in diabetics between the CKD-5 non-dialysis and dialysis patients. However, results did not materially change after adjustment for diabetes.
To conclude, PA parameters are already lower in the CKD-5 non-dialysis phase, consistent with a sedentary lifestyle and were not different from dialysis patients. The transition from CKD-5 non-dialysis to dialysis was associated with only a modest improvement in AEE in the first 6 months after the start of dialysis. These results underscore the importance of PA programs, which should also include the CKD-5 non-dialysis phase.
Statement of Ethics
Written informed consent was obtained from each patient prior to participation. The study was approved by the Ethical Committee (NL33129.068.10, NL35039.068.10) and the Hospital Board of the Maastricht University Medical Center+.
Disclosure Statement
Funding: J.P.K., F.M.S., N.J.H.B., and R.J.H.M. are supported by an unrestricted grant from Fresenius Medical Care Europe. P.W. and B.C. are employees of Fresenius Medical Care Europe.
References
- 1.Avesani CM, Trolonge S, Deleaval P, Baria F, Mafra D, Faxen-Irving G, et al. Physical activity and energy expenditure in haemodialysis patients: an international survey. Nephrol Dial Transplant. 2012;27:2430–2434. doi: 10.1093/ndt/gfr692. [DOI] [PubMed] [Google Scholar]
- 2.Stack AG, Molony DA, Rives T, Tyson J, Murthy BV. Association of physical activity with mortality in the US dialysis population. Am J Kidney Dis. 2005;45:690–701. doi: 10.1053/j.ajkd.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Tentori F, Elder SJ, Thumma J, Pisoni RL, Bommer J, Fissell RB, et al. Physical exercise among participants in the Dialysis Outcomes and Practice Patterns Study (DOPPS): correlates and associated outcomes. Nephrol Dial Transplant. 2010;25:3050–3062. doi: 10.1093/ndt/gfq138. [DOI] [PubMed] [Google Scholar]
- 4.van den Ham EC, Kooman JP, Schols AM, Nieman FH, Does JD, Franssen FM, et al. Similarities in skeletal muscle strength and exercise capacity between renal transplant and hemodialysis patients. Am J Transplant. 2005;5:1957–1965. doi: 10.1111/j.1600-6143.2005.00944.x. [DOI] [PubMed] [Google Scholar]
- 5.Johansen KL, Painter P. Exercise in individuals with CKD. Am J Kidney Dis. 2012;59:126–134. doi: 10.1053/j.ajkd.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broers NJ, Cuijpers AC, van der Sande FM, Leunissen KM, Kooman JP. The first year on haemodialysis: a critical transition. Clin Kidney J. 2015;8:271–277. doi: 10.1093/ckj/sfv021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gould DW, Graham-Brown MP, Watson EL, Viana JL, Smith AC. Physiological benefits of exercise in pre-dialysis chronic kidney disease. Nephrology (Carlton) 2014;19:519–527. doi: 10.1111/nep.12285. [DOI] [PubMed] [Google Scholar]
- 8.Wlodarek D, Glabska D, Rojek-Trebicka J. Physical activity of predialysis patients with chronic kidney disease measured using SenseWear Armban. J Sports Med Phys Fitness. 2011;51:639–646. [PubMed] [Google Scholar]
- 9.Fouque D, Pelletier S, Mafra D, Chauveau P. Nutrition and chronic kidney disease. Kidney Int. 2011;80:348–357. doi: 10.1038/ki.2011.118. [DOI] [PubMed] [Google Scholar]
- 10.Marcelli D, Usvyat LA, Kotanko P, Bayh I, Canaud B, Etter M, et al. Body composition and survival in dialysis patients: results from an international cohort study. Clin J Am Soc Nephrol. 2015;10:1192–1200. doi: 10.2215/CJN.08550814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cupisti A, D'Alessandro C, Fumagalli G, Vigo V, Meola M, Cianchi C, et al. Nutrition and physical activity in CKD patients. Kidney Blood Press Res. 2014;39:107–113. doi: 10.1159/000355784. [DOI] [PubMed] [Google Scholar]
- 12.Rhee CM, Kalantar-Zadeh K. Resistance exercise: an effective strategy to reverse muscle wasting in hemodialysis patients? J Cachexia Sarcopenia Muscle. 2014;5:177–180. doi: 10.1007/s13539-014-0160-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuo YT, Chiu KM, Tsang YM, Chiu CM, Chien MY. Influence of chronic kidney disease on physical function and quality of life in patients after coronary artery bypass grafting. Cardiorenal Med. 2015;5:237–245. doi: 10.1159/000433447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dontje ML, van der Wal MH, Stolk RP, Brügemann J, Jaarsma T, Wijtvliet PE, van der Schans CP, de Greef MH. Daily physical activity in stable heart failure patients. J Cardiovasc Nurs. 2014;29:218–226. doi: 10.1097/JCN.0b013e318283ba14. [DOI] [PubMed] [Google Scholar]
- 15.Almeida GJ, Wasko MC, Jeong K, Moore CG, Piva SR. Physical activity measured by the sensewear armband in women with rheumatoid arthritis. Phys Ther. 2011;91:1367–1376. doi: 10.2522/ptj.20100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chamney PW, Wabel P, Moissl UM, Muller MJ, Bosy-Westphal A, Korth O, et al. A whole-body model to distinguish excess fluid from the hydration of major body tissues. Am J Clin Nutr. 2007;85:80–89. doi: 10.1093/ajcn/85.1.80. [DOI] [PubMed] [Google Scholar]
- 17.Fresenius Medical Care . Bad Homburg, Germany: Fresenius Medical Care GmbH; 2007. Body Composition Monitor Manual Operation Procedure. [Google Scholar]
- 18.Wizemann V, Wabel P, Chamney P, Zaluska W, Moissl U, Rode C, et al. The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transplant. 2009;24:1574–1579. doi: 10.1093/ndt/gfn707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wabel P, Rode C, Moissl U, Chamney PW, Wizemann V. Accuracy of bioimpedance spectroscopy (BIS) to detect fluid status changes in hemodialysis patients (abstract) Nephrol Dial Transplant. 2007;22((suppl 6)):VI129. [Google Scholar]
- 20.Broers NJ, Martens RJ, Cornelis T, Diederen NM, Wabel P, van der Sande FM, et al. Body composition in dialysis patients: a functional assessment of bioimpedance using different prediction models. J Ren Nutr. 2015;25:121–128. doi: 10.1053/j.jrn.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Van Biesen W, Williams JD, Covic AC, Fan S, Claes K, Lichodziejewska-Niemierko M, et al. Fluid status in peritoneal dialysis patients: the European Body Composition Monitoring (EuroBCM) study cohort. PLoS One. 2011;6:e17148. doi: 10.1371/journal.pone.0017148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davison SN, Jhangri GS, Jindal K, Pannu N. Comparison of volume overload with cycler-assisted versus continuous ambulatory peritoneal dialysis. Clin J Am Soc Nephrol. 2009;4:1044–1050. doi: 10.2215/CJN.00020109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper BA, Aslani A, Ryan M, Zhu FY, Ibels LS, Allen BJ, et al. Comparing different methods of assessing body composition in end-stage renal failure. Kidney Int. 2000;58:408–416. doi: 10.1046/j.1523-1755.2000.00180.x. [DOI] [PubMed] [Google Scholar]
- 24.Ostir GV, Volpato S, Fried LP, Chaves P, Guralnik JM. Reliability and sensitivity to change assessed for a summary measure of lower body function: results from the Womenʼs Health and Aging Study. J Clin Epidemiol. 2002;55:916–921. doi: 10.1016/s0895-4356(02)00436-5. [DOI] [PubMed] [Google Scholar]
- 25.Penninx BW, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A Biol Sci Med Sci. 2000;55:M691–M697. doi: 10.1093/gerona/55.11.m691. [DOI] [PubMed] [Google Scholar]
- 26.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartmann EL, Kitzman D, Rocco M, Leng X, Klepin H, Gordon M, et al. Physical function in older candidates for renal transplantation: an impaired population. Clin J Am Soc Nephrol. 2009;4:588–594. doi: 10.2215/CJN.03860808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davies SJ, Russell L, Bryan J, Phillips L, Russell GI. Comorbidity, urea kinetics, and appetite in continuous ambulatory peritoneal dialysis patients: their interrelationship and prediction of survival. Am J Kidney Dis. 1995;26:353–361. doi: 10.1016/0272-6386(95)90657-6. [DOI] [PubMed] [Google Scholar]
- 30.Davies SJ, Phillips L, Naish PF, Russell GI. Quantifying comorbidity in peritoneal dialysis patients and its relationship to other predictors of survival. Nephrol Dial Transplant. 2002;17:1085–1092. doi: 10.1093/ndt/17.6.1085. [DOI] [PubMed] [Google Scholar]
- 31.Van Manen JG, Korevaar JC, Dekker FW, Boeschoten EW, Bossuyt PM, Krediet RT. Adjustment for comorbidity in studies on health status in ESRD patients: which comorbidity index to use? J Am Soc Nephrol. 2003;14:478–485. doi: 10.1097/01.asn.0000043902.30577.c9. [DOI] [PubMed] [Google Scholar]
- 32.van den Ham EC, Kooman JP, Schols AM, Nieman FH, Does JD, Akkermans MA, et al. The functional, metabolic, and anabolic responses to exercise training in renal transplant and hemodialysis patients. Transplantation. 2007;83:1059–1068. doi: 10.1097/01.tp.0000259552.55689.fd. [DOI] [PubMed] [Google Scholar]
- 33.Bae YH, Lee SM, Jo JI. Aerobic training during hemodialysis improves body composition, muscle function, physical performance, and quality of life in chronic kidney disease patients. J Phys Ther Sci. 2015;27:1445–1449. doi: 10.1589/jpts.27.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361:1539–1547. doi: 10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kotanko P, Kooman J, van der Sande F, Kappel F, Usvyat L. Accelerated or out of control: the final months on dialysis. J Ren Nutr. 2014;24:357–363. doi: 10.1053/j.jrn.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Murray SA, Boyd K, Sheikh A. Palliative care in chronic illness. BMJ. 2005;330:611–612. doi: 10.1136/bmj.330.7492.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 38.Webb AR, Newman LA, Taylor M, Keogh JB. Hand grip dynamometry as a predictor of postoperative complications reappraisal using age standardized grip strengths. JPEN. 1989;13:30–33. doi: 10.1177/014860718901300130. [DOI] [PubMed] [Google Scholar]
- 39.Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, et al. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci. 2001;56:B209–B217. doi: 10.1093/gerona/56.5.b209. [DOI] [PubMed] [Google Scholar]
- 40.Heimburger O, Qureshi AR, Blaner WS, Berglund L, Stenvinkel P. Hand-grip muscle strength, lean body mass, and plasma proteins as markers of nutritional status in patients with chronic renal failure close to start of dialysis therapy. Am J Kidney Dis. 2000;36:1213–1225. doi: 10.1053/ajkd.2000.19837. [DOI] [PubMed] [Google Scholar]
- 41.Isoyama N, Qureshi AR, Avesani CM, Lindholm B, Barany P, Heimburger O, et al. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin J Am Soc Nephrol. 2014;9:1720–1728. doi: 10.2215/CJN.10261013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foley RN, Wang C, Ishani A, Collins AJ, Murray AM. Kidney function and sarcopenia in the United States general population: NHANES III. Am J Nephrol. 2007;27:279–286. doi: 10.1159/000101827. [DOI] [PubMed] [Google Scholar]
- 43.Mak RH, Cheung W. Cachexia in chronic kidney disease: role of inflammation and neuropeptide signaling. Curr Opin Nephrol Hypertens. 2007;16:27–31. doi: 10.1097/MNH.0b013e3280117ce7. [DOI] [PubMed] [Google Scholar]
- 44.Marcelli D, Brand K, Ponce P, Milkowski A, Marelli C, Ok E, et al. Longitudinal changes in body composition in patients after initiation of hemodialysis therapy: results from an international cohort. J Ren Nutr. 2016;26:72–80. doi: 10.1053/j.jrn.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Vogt BP, Borges MC, Goés CR, Caramori JC. Handgrip strength is an independent predictor of all-cause mortality in maintenance dialysis patients. Clin Nutr. 2016;35:1429–1433. doi: 10.1016/j.clnu.2016.03.020. [DOI] [PubMed] [Google Scholar]