Abstract
In epidemiological and nutrition research, it is very important to evaluate the stability of biomarkers as function of both storage time and temperature. In this study, the stability of folate and vitamin B12 in human serum samples has been tested after long-term storage at −80°C up to 13 years. Serum samples of 16 individuals were used in this study. The concentration of folate and vitamin B12 has been determined at t=0 and at 1, 8, and 13 years after storage at −80°C. The folate concentrations in serum samples remained stable at −80°C. The concentration of vitamin B12 was decreasing during the time of the study to about 50%. The correlation of the folate and also of the vitamin B12 concentrations in the stored samples compared with the starting values was still good. Therefore, although the concentration of vitamin B12 decreased upon storage, reliable comparative analyses can still be performed.
1. Introduction
Folate and vitamin B12 are both vitamins from the one-carbon metabolism and serve as compounds that repair DNA and re-methylate homocysteine [1–3].
The folate status is associated with several chronic diseases with a large impact on public health [4]. Folate deficiency is also associated with birth defects [5]. Both vitamins are related to both cardiovascular diseases [3, 6, 7, 9] and cognition [7–10]. Therefore, they are very important in nutrition studies to assess the dietary patterns. In addition, in epidemiological and clinical research, these vitamins are often used to make risk assessments in prospective studies. In these studies, often serum or plasma samples were used from biobanks which have been stored for several years and sometimes even for decades. Therefore, the assessment of the stability of these biomarkers during long-term storage is of great importance [11].
The measurements of folate and to a lower extend vitamin B12 are important biomarkers in clinical medicine and in epidemiologic studies. Both vitamins can be measured by a variety of methods, but the use of autoanalyzers have become common practice [12].
In the present study, folate and vitamin B12 have been measured with a commercially dedicated autoimmunoanalyzer. The stability of folate and vitamin B12 in serum samples was determined after a long-term storage of up to 13 years at −80°C.
2. Materials and Methods
2.1. Blood Withdrawal and Storage
For the stability study, serum samples of 16 human volunteers (blood donors) were used. Samples were obtained from the Central Blood Laboratory of the Red Cross (Amsterdam, the Netherlands) with written permission of the volunteers. After blood withdrawal, serum samples were prepared within two hours, divided in aliquots, and stored at −20°C and −70°C. In this study, only samples that have been stored at −70°C were used.
The initial concentrations of folate and vitamin B12 at t=0 have been determined within 4 hours after centrifugation. For long-term stability, samples have been stored for 1, 8, and 13 years, at −70°C for one year and at −80°C for the remaining period. Samples stored at −70°C were kept in a freezer equipped with temperature recorder and sound alarm. From 1 year onwards, the samples were stored at −80°C in a freezer equipped with an automatic temperature registration system with an alarm function. In Figure 1, the flow diagram of the present study is shown.
Figure 1.
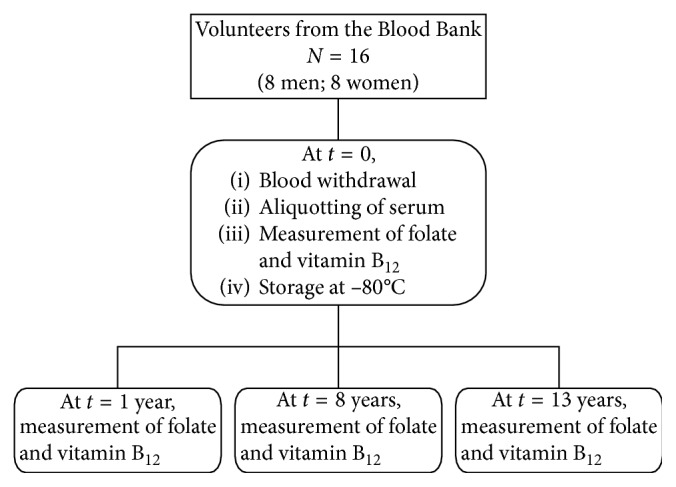
Flow diagram of the study.
2.2. Measurements of Biomarkers
The serum samples were kept on ice for less than 1 hr before the first measurements at t=0. The measurements were performed as a single measurement, except on day 0 (duplicate measurements). At time points 0 and 1 year, folate and vitamin B12 have been determined with an autoanalyzer (Hitachi 912, Roche Diagnostics, Almere, the Netherlands). A reference serum (AHSL-1) was included in each assay. During the first year of storage, the inter assay variation for folate was 1.9% (N=8). The inter assay variation for vitamin B12 was 3.4% (N=8).
At time points 8 and 13 years, folate and vitamin B12 have been determined in serum with an immunoanalyzer (Access-2, Beckman-Coulter, Woerden, the Netherlands). A pretreatment converts the folate polyglutamic acid forms to the monoglutamic acid form predominant in serum. The measurements were performed as a single measurement, with dedicated kits from Beckman-Coulter. At time point 8 years, the intra-assay variation was determined with a reference serum (Biorad number: 40192). For folate, the intra-assay variation was 1.1% (N=2). The intra-assay variation for vitamin B12 was 4.3% (N=2). At time point 13 years, the intra-assay variation was determined with three reference sera (Biorad-1, -2, and AHLS-2). For folate, the mean intra-assay variation was 1.6% (N=4). The mean intra-assay variation for vitamin B12 was 2.7% (N=4).
The statistical differences (95% confidence interval) of the values from the initial value at t=0 were determined with a t-test for two-samples assuming equal variances.
3. Results
At the start of the study (t=0), the initial concentrations of folate and vitamin B12 of the 16 volunteers were determined. The mean concentration for folate at t=0 was 6.1 ±1.7 ng/mL, and the median value was 5.8 ng/mL, with a range of 3.9–10.5 ng/mL. The mean concentration for vitamin B12 at t=0 was 471 ± 75 pg/mL, and the median value was 464 pg/mL, with a range of 381–709 pg/mL.
3.1. Stability of Folate and Vitamin B12
The stability of both folate and vitamin B12 in human serum was followed during 13 years. In Figure 2, the mean values of the 16 volunteers are shown at each time point, expressed as percentage of the mean value of their concentrations at t=0 (before freezing of the samples).
Figure 2.
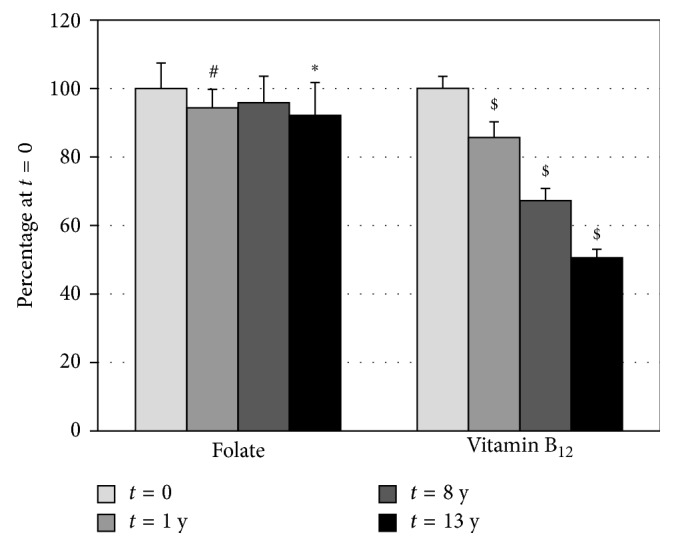
Stability of folate and vitamin B12 at 1, 8, and 13 years relative to t=0. The concentrations have been expressed as percentage of the mean value of their concentrations at t=0. Statistics: ∗ P < 0.05, # P < 0.05, and $ P < 0.001.
The levels for folate are rather constant during 13 years of storage at −80°C. Somewhat lower values, but statistically significant, were observed at time points 1 and 13 years of 94.4% and 92.0%, respectively.
For vitamin B12, an almost linear decrease was observed during the time period of storage. The mean level of vitamin B12 decreased from 100% to 85.6%, 62.2%, and 50.5% at time points 1, 8, and 13 years, respectively.
3.2. Correlations for Folate
In Figure 3, the correlation lines of the folate concentrations of the 16 volunteers before the storage of the sera and after storage at 1, 8, and 13 years are shown. The correlations of the time points with the starting values are very good. The correlation lines have the following equations: y = 0.870x + 1.02, y = 0.703x + 3.53, and y = 0.552x + 5.11 for 1, 8, and 13 years, respectively. The correlation coefficients (R 2) were 0.961, 0.867, and 0.889 for 1, 8, and 13 years, respectively. The statistics between 2 sets of data are P=0.0019 for 0 versus 1 year, P=0.155 for 0 versus 8 years, and P=0.031 for 0 versus 13 years as determined with a t-test for equal variances.
Figure 3.
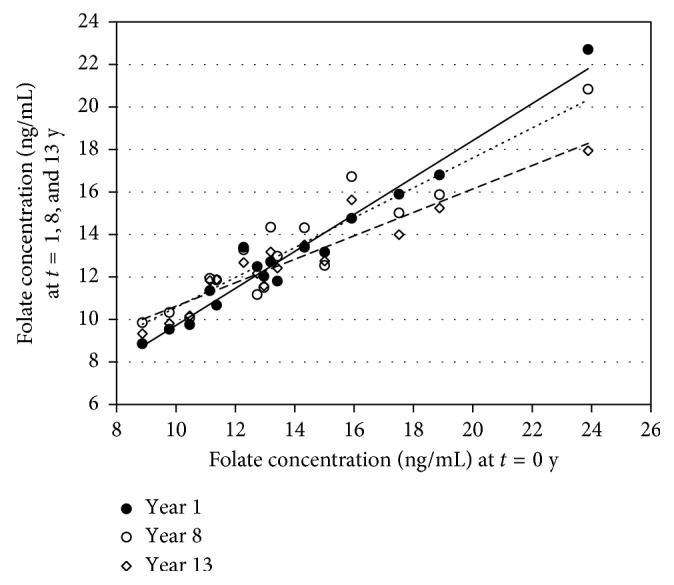
Correlation between the mean folate concentrations at t=0 and after storage for 1, 8, and 13 years at −70°C. The correlation lines have the following equations: y = 0.870x + 1.02
 , y = 0.703x + 3.53
, y = 0.703x + 3.53
 , and y = 0.552x + 5.11
, and y = 0.552x + 5.11
 for 1, 8, and 13 years, respectively.
for 1, 8, and 13 years, respectively.
3.3. Correlations for Vitamin B12
In Figure 4, the correlation lines of the vitamin B12 concentrations of the 16 volunteers before the storage of the sera and after storage at 1, 8, and 13 years are shown. Although the concentration of vitamin B12 decreased substantially with the duration of storage, as shown in Figure 1, the correlations of the time points with the starting values are still rather good. The correlation lines have the following equations: y = 0.829x + 2.80, y = 0.600x + 33.7, and y = 0.401x + 48.5 for 1, 8, and 13 years, respectively. The correlation coefficients (R 2) were 0.894, 0.890, and 0.865 for 1, 8, and 13 years, respectively. The statistics between the 2 sets of data are P=1.7∗E − 8 for 0 versus 1 year, P=1.2∗E − 11 for 0 versus 8 years, and P=3.0∗E − 12 for 0 versus 13 years as determined with a t-test for equal variances.
Figure 4.
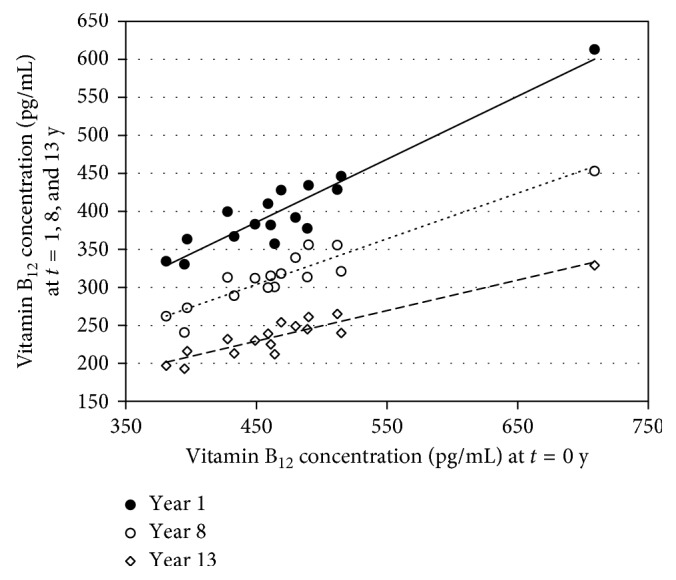
Correlation between the mean vitamin B12 concentrations at t=0 and after storage for 1, 8, and 13 years at −70°C. The correlation lines have the following equations: y = 0.829x + 2.80
 , y = 0.600x + 33.7
, y = 0.600x + 33.7
 , and y = 0.401x + 48.5
, and y = 0.401x + 48.5
 for 1, 8, and 13 years, respectively.
for 1, 8, and 13 years, respectively.
4. Discussion
Several short-term stability studies on folate and vitamin B12 were performed in the past [13–17]. But only one long-term stability study on folate was found in EDTA plasma for 48 months at −20°C [18]. After 6 months of storage the mean value of folate decreased to 25% of its original value, and this level remained constant until 48 months. For vitamin B12, only one long-term stability study was found [14] and showed a good stability during 48 months of storage in EDTA plasma at −20°C, which is a different finding with our present results.
The present study shows in which folate and vitamin B12 were measured after 1, 8, and 13 years of storage at −80°C that that the measurements of folate still can be done without any major change in the concentration. For vitamin B12, there is a consistent decrease to about 50% after 13 years of storage at −80°C. It must be noted that from 8 years on, the autoanalyzer and reagents were changed so that a possible level change could be expected, although a comparative study was performed that showed the same results for both methods. In a previous report after 1 year of storage [19], the decrease of vitamin B12 was already visible, but then it was not feasible to make a strong conclusion based on only one time point. Now after 13 years, it is clear that this decrease was consistent.
For epidemiological studies, however, this decrease in concentration of vitamin B12 has only little effect on the rank order and correlations with the starting concentrations. Rather good correlation coefficients (R 2) were found between 0.87 and 0.89 after storage. For folate, the concentrations of the stored samples remain close to the starting concentrations with good correlations with the starting concentrations; for example, the correlation coefficient (R 2) at 13 years was 0.89.
There has been one study on the long-term stability of B vitamins [20] in which storage at −25°C during 4, 6, 17, and 29 years was reported. Folate was measured as p-aminobenzoylglutamate declined at a slow rate of 0.98%/year and about 80% of the folate was recovered after 29 years of storage as determined with LC-MS/MS. For folate, p-aminobenzoylglutamate appeared to be the method of choice for the determination of folate status in stored serum samples. In our method, a pretreatment also converts the folate polyglutamic acid forms to the monoglutamic acid form which is predominant in serum.
In the same study, vitamin B12 status did not show any decline, as measured with a microbiological assay [20]. This is in contrast with our results. Maybe the method of choice has an influence of the outcome of the study.
In conclusion, this study shows that long-term storage of serum samples at −80°C, intended for future measurements of folate and vitamin B12, can still be used for data analyses, although the concentration of vitamin B12 decreases steadily with longer storage time.
Acknowledgments
Part of the study was conducted with the financial support from the Board of Directors of the National Institute of Public Health and the Environment (Bilthoven, the Netherlands), within the strategic project on antioxidant supplements for aging.
Ethical Approval
Samples were obtained from blood donors of the Central Blood Laboratory of the Red Cross (Amsterdam, the Netherlands) with written permission of each of the volunteers. In this study, principles of the Declaration of Helsinki were followed.
Disclosure
All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Conflicts of Interest
No potential conflicts of interest were reported by the authors.
References
- 1.Stover P. J. Physiology of folate and vitamin B12 in health and disease. Nutrition Reviews. 2004;62:S3–S12. doi: 10.1111/j.1753-4887.2004.tb00070.x. [DOI] [PubMed] [Google Scholar]
- 2.Chanarin I. Megaloblastic anaemia, cobalamin, and folate. Journal of Clinical Pathology. 1987;40(9):978–984. doi: 10.1136/jcp.40.9.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhonukshe-Rutten R. A. M., de Vries J. H. M., de Bree A., van der Put N., van Staveren W. A., de Groot L. C. P. G. M. Dietary intake and status of folate and vitamin B12 and their association with homocysteine and cardiovascular disease in European populations. European Journal of Clinical Nutrition. 2009;63(1):18–30. doi: 10.1038/sj.ejcn.1602897. [DOI] [PubMed] [Google Scholar]
- 4.Fairfield K. M., Fletcher R. H. Vitamins for chronic disease prevention in adults, scientific review. Journal of the American Medical Association. 2002;287(23):3116–3126. doi: 10.1001/jama.287.23.3116. [DOI] [PubMed] [Google Scholar]
- 5.Blom H. J., Shaw G. M., den Heijer M., Finnell R. H. Neural tube defects and folate, case far from closed. Nature Reviews Neuroscience. 2005;7(9):724–731. doi: 10.1038/nrn1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke. Journal of the American Medical Association. 2002;288(16):2015–2022. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 7.Balk E. M., Raman G., Tatsioni A., Chung M., Lau J., Rosenberg I. H. Vitamin B6, B12, and folic acid supplementation and cognitive function, a systematic review of randomized trials. Archives of Internal Medicine. 2007;167(1):21–30. doi: 10.1001/archinte.167.1.21. [DOI] [PubMed] [Google Scholar]
- 8.Clarke R., Sherliker P., Hin H., et al. Folate and vitamin B12 status in relation to cognitive impairment and anaemia in the setting of voluntary fortification in the UK. British Journal of Nutrition. 2008;100(5):1054–1059. doi: 10.1017/s0007114508958001. [DOI] [PubMed] [Google Scholar]
- 9.Clarke R., Lewington S., Sherliker P., Armitage J. Effects of B-vitamins on plasma homocysteine concentrations and on risk of cardiovascular disease and dementia. Current Opinion in Clinical Nutrition and Metabolic Care. 2007;10(1):32–39. doi: 10.1097/mco.0b013e328011aa71. [DOI] [PubMed] [Google Scholar]
- 10.Ravaglia G., Forti P., Maioli F., et al. Homocysteine and folate as risk factors for dementia and Alzheimer disease. American Journal of Clinical Nutrition. 2005;82(3):636–643. doi: 10.1093/ajcn.82.3.636. [DOI] [PubMed] [Google Scholar]
- 11.Lippi G., Chance J. J., Church S., et al. Preanalytical quality improvement, from dream to reality. Clinical Chemistry and Laboratory Medicine. 2011;49(7):1113–1126. doi: 10.1515/cclm.2011.600. [DOI] [PubMed] [Google Scholar]
- 12.Quinlivan E. P., Hanson A. D., Gregory J. F. The analysis of folate and its metabolic precursors in biological samples. Analytical Biochemistry. 2006;348(2):163–184. doi: 10.1016/j.ab.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 13.van Eijsden M., Wal M. F., Hornstra G., Bonsel G. J. Can whole-blood samples be stored over 24 hours without compromising stability of C-reactive protein, retinol, ferritin, folic acid, and fatty acids in epidemiologic research? Clinical Chemistry. 2005;51(1):230–232. doi: 10.1373/clinchem.2004.042234. [DOI] [PubMed] [Google Scholar]
- 14.Zhang D. J., Elswick R. K., Miller W. G., Bailey J. L. Effect of serum-clot contact time on clinical chemistry laboratory results. Clinical Chemistry. 1998;44(6):1325–1333. [PubMed] [Google Scholar]
- 15.Komaromy-Hiller G., Nuttall K. L., Ashwood E. R. Effect of storage on serum vitamin B12 and folate stability. Annals of Clinical & Laboratory Science. 1997;27(4):249–253. [PubMed] [Google Scholar]
- 16.Clement N. F., Kendall B. S. Effect of light on vitamin B12 and folate. Laboratory Medicine. 2009;40(11):657–659. doi: 10.1309/lmwd82yz7qzpsnqp. [DOI] [Google Scholar]
- 17.Drammeh B. S., Schleicher R. L., Pfeiffer C. M., Jain R. B., Zhang M., Nguyen P. H. Effects of delayed sample processing and freezing on serum concentrations of selected nutritional indicators. Clinical Chemistry. 2008;54(11):1883–1891. doi: 10.1373/clinchem.2008.108761. [DOI] [PubMed] [Google Scholar]
- 18.Ocké M. C., Schrijver J., Obermann-De Boer G. L., Bloemberg B. P. M., Haenen G. R. M. M., Kromhout D. Stability of blood, provitamins during four years of storage at −20°C, consequences for epidemiologic research. Journal of Clinical Epidemiology. 1995;48(8):1077–1085. doi: 10.1016/0895-4356(94)00232-f. [DOI] [PubMed] [Google Scholar]
- 19.Jansen E. H. J. M., Beekhof P. K., Cremers J. W. J. M., Schenk E. Long-term, instability of folate and vitamin B12 in human serum. Clinical Chemistry and Laboratory Medicine. 2012;50(10):1761–1763. doi: 10.1515/cclm-2012-0108. [DOI] [PubMed] [Google Scholar]
- 20.Hannisdal R., Gislefoss R. E., Grimsrud T. K., Hustad S., Mørkrid L., Ueland P. M. Analytical recovery of folate and its degradation products in human serum stored at –25°C for up to 29 years. Journal of Nutrition. 2010;140(3):522–526. doi: 10.3945/jn.109.116418. [DOI] [PubMed] [Google Scholar]


