Abstract
The cornea is one of the most densely innervated and sensitive tissues in the body. In addition to their important sensory functions, corneal nerves induce reflex tear production, blinking, and the release of trophic factors – all of which combined help to maintain the structural and functional integrity of the surface of the eye. Consequently, damage to corneal nerves as a result of disease, surgery, or trauma can lead to diminished corneal sensitivity, epithelial defects, and possible blindness. In this review, we describe commonly used tools that have provided considerable new information on corneal architecture and sensation in healthy and diseased corneas, with special emphasis on changes seen in herpes zoster ophthalmicus, corneal and other therapeutic ocular procedures, antiglaucoma medical therapy, aging, and diabetes. With its potential applications ranging from managing ocular-specific to systemic diseases, the study of corneal innervation has implications for future therapies extending beyond just the eye itself.
Keywords: corneal sensation, corneal innervation, ophthalmology, subbasal nerve plexus, ophthalmic disease
Introduction
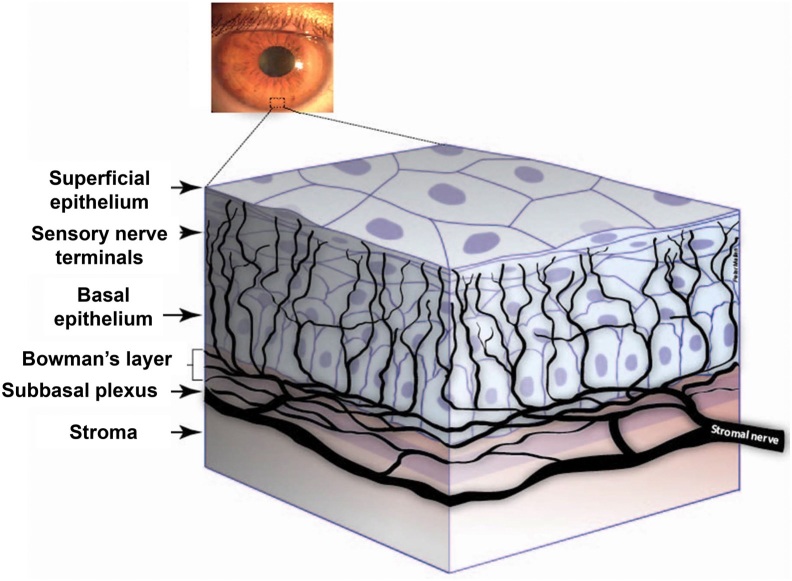
The cornea, the transparent tissue covering the front portion of the eye, is the most densely innervated tissue in the body [1]. It is comprised of five layers: the epithelium, Bowman's layer, the stroma, Descemet's membrane, and the endothelium. In 1957, Kitano demonstrated that innervation of the corneal epithelium first occurs at 5 months of gestation [2]. Corneal sensory nerves originate from the ophthalmic division of the trigeminal ganglion [3], traveling in the nasociliary nerve and its long ciliary nerve branches, and ultimately branching into nerve fibers that penetrate the cornea. These branches divide and run parallel to the superficial surface of the cornea between the basal epithelium and Bowman’s layer, forming the subbasal nerve plexus (SBNP) that supplies the overlying corneal epithelium (Figure 1) [4,5]. Extensive branching of the corneal nerve fibers produces large receptive fields for each sensory axon. This organization results in poor stimuli localization or acuity as a consequence of overlapping receptive fields, but produces an extraordinary level of sensitivity to external stimuli [6]. With a central corneal nerve density of approximately 7,000 nociceptors per square millimeter [4,7], the cornea is 300 to 600 times more sensitive than skin [8].
Figure 1.
Diagrammatic representation of human corneal nerves. Reprinted from [5], with permission from Elsevier®.
Corneal Nerve Function
Its abundant sensory nerve supply allows the cornea to transduce various thermal, mechanical, and chemical stimuli into the conscious perception of ocular dryness, discomfort, or pain [9]. Approximately 20 percent of corneal nociceptors are mechanoreceptors that convey acute sharp pain in response to mechanical contact with the corneal surface through thin myelinated Aδ type corneal nerves [10]. Approximately 70 percent are polymodal nociceptors, which through slow-conducting unmyelinated C type nerves, convey sharp and sustained pain in response to chemical stimuli like acetylcholine, prostaglandins, and bradykinin, as well as heat and mechanical irritants to the cornea [11]. The remaining 10 percent are Aδ and C fiber cold receptors, which fire in response to tear film evaporation and exposure of the cornea to cold solutions or air [12,13].
In response to external threats and stimuli, such as dust, pathogens, or xenobiotics, corneal nerves not only induce tear production, but also stimulate the blinking reflex through an elaborate interplay between the corneal surface and lacrimal glands [14]. Maintaining a well-lubricated and smooth ocular surface with an intact epithelium helps to minimize visual distortion and dry eye symptoms, while protecting the ocular surface. In addition, corneal nerves release numerous trophic substances such as neuropeptides, neurotrophins, and growth factors that are crucial to regulating proliferation of corneal epithelium, epithelial integrity, and wound healing in the cornea [15,16]. Loss of sensory innervation of the cornea can result in a vision-threatening clinical condition known as neurotrophic keratopathy, which is characterized by reduced corneal sensation, tear film abnormalities and, in the most severe cases, persistent corneal epithelial defects, ulceration, and perforation of the stroma. Many other diseases are associated with decreased corneal sensitivity in humans, including herpetic keratitis, keratoconjunctivitis sicca, and keratoconus [4,17].
Various neurotransmitters, including substance P (SP), calcitonin gene-related peptide (CGRP), neuropeptide Y, vasoactive intestinal peptide, catecholamines, and acetylcholine are present in the cornea [15]. SP is released directly from corneal fibers following inflammation, and has received increasing attention given its effects on promoting epithelial proliferation and corneal wound healing in synergism with insulin-like growth factor-1 (IGF-1) and epidermal growth factor (EGF) [6,18]. On the basis of this research, investigators have formulated eye drops containing peptide sequences derived from SP and IGF-1 for the treatment of persistent corneal epithelial defects associated with neurotrophic keratopathy [19]. CGRP, among other neurotransmitters, is also involved in neurogenic inflammation and has been shown to enhance corneal epithelial healing in vitro [20].
Neurotrophic factors are another family of biomolecules thought to maintain homeostasis and repair of the cornea. They include nerve growth factor (NGF), neurotrophin 3 (NT-3), neurotrophin 4/5 (NT-4), brain-derived neurotrophic factor (BDNF), ciliary neurotropic factor (CNTF), and glial cell-derived neurotrophic factor (GDNF) as well as their corresponding tyrosine receptor kinase (TrK) receptors, many of which are expressed in the human cornea [21]. Mice with wounded corneas treated with CNTF-containing eye drops showed an increase in nerve fiber density 8 weeks after wounding [22]. NGF is expressed in the cornea during re-innervation after nerve surgical transection [21]. Both NGF and GDNF promote epithelial colony formation and proliferation [23]. This has translated into human case studies and clinical trials where NGF has been shown to improve non-healing epithelial defects such as corneal neurotrophic ulcers, with return of corneal sensation [24,25]. BDNF is capable of inducing both central and peripheral nerve growth and regeneration [26], and stimulating other neurotrophic factors such as NT-4/5 and Gap-43 to achieve neurite re-growth [27]. On the other hand, loss of neurotrophic signaling negatively impacts corneal nerve function. For example, inactivation of TrkA, results in loss of nociceptive neurons, stromal nerves, and corneal epithelium, as well as reduced response to noxious stimuli [28].
Thus, corneal sensation is critical to the structural and functional integrity of the ocular surface. Surgical procedures, trauma, and disease in the eye can all potentially damage corneal nerves and diminish sensation, resulting in transient or long-lasting ocular complications.
Assessing the Subbasal Corneal Nerve Plexus
Corneal nerves of the adult human eye have been extensively studied both ex vivo and in vivo, with the aim of capturing changes in morphology via imaging or qualitative assessment, in addition to quantitative assessment of corneal sensitivity and pain [29,30]. However, ex vivo studies by light and electron microscopy may generate unreliable results, since human corneal nerves are known to degenerate within the first 14 hours of death [31]. As an in vivo exam of the cornea, slit-lamp biomicroscopy is a vital and routinely used tool in the ophthalmologist’s office. Combined with use of various dyes, such as fluorescein and lissamine green, it can help reveal the general health of the ocular surface. However, a major restriction of the slit-lamp biomicroscopy is limited magnification, offering only up to 40x or 100x, and precluding visualization of the corneal cellular architecture and the SBNP.
With a growing interest in noninvasive techniques to study corneal tissue physiology with greater resolution, the invention and application of in vivo confocal microscopy (IVCM) enables ophthalmologists to image the cornea at the cellular level. The first scanning confocal microscope was developed by Minsky in 1988, which was followed by the invention of the tandem-, slit-, and more recently the laser-scanning confocal microscope, which allows for magnification up to 800x [32,33]. As a rapid and noninvasive technique, IVCM has many advantages and can provide images nearly comparable with in vitro histochemical methods [34]. IVCM has proven useful in the assessment of corneal cellular and nerve morphology in normal health, post-operative conditions, and a variety of diseases, including dry eye disease, contact lens wear, neurotrophic keratopathy, post-refractive surgery, among others [35-38]. Alterations in corneal nerve morphology in the SBNP, including nerve sprouting and thickening, reduced nerve fiber density, increased tortuosity, branching, reflectivity, neuromas, and beading, have been observed in patients with corneal pathology as compared to controls [39,40].
IVCM itself is not without limitations, however. Depending on the area and volume of the cornea sampled, and therefore the area of SBNP, findings cannot necessarily be extrapolated to the entire cornea [41]. The lack of consensus regarding quantifying or even defining SBNP density, tortuosity, beading, or branches can make it difficult to compare the results of different studies [42]. Manual analysis of these parameters is laborious, slow, and partial to human variability and bias. While research groups have developed automated analysis of IVCM images, to date, there is no commercially available software to standardize analysis [43,44].
Consequently, direct and quantitative measurement of corneal sensation in response to different stimuli has emerged as an increasingly popular method for assessing corneal nerve function. The Cochet-Bonnet esthesiometer, where a mechanical stimulus is applied to the corneal surface using nylon filaments of variable diameter and length [45], is widely used due to its portability and ease of use. The stimulus is applied successively with step-wise shortening of the filament until the patient reports a sensation. The longer the length of the filament that elicits sensation, the more sensitive the cornea. However, the Cochet-Bonnet requires contact with the eye and has a limited range of testing values, as it permits assessment only of corneal mechanoreceptors. The Belmonte’s gas esthesiometer addresses some of these limitations. Investigators can adjust the flow, composition, and temperature of a fine stream of gas to more precisely apply and assess three different modalities of stimuli to the ocular surface: mechanical sensitivity to air flow, chemical sensitivity to carbon dioxide, and thermal sensitivity to different temperatures [46]. Therefore, whereas Cochet-Bonnet esthesiometers are thought to quantify only Aδ fiber function, the Belmonte esthesiometer is potentially informative of the state and function of both Aδ and C fibers.
Alterations of Corneal Innervation and Sensation
Herpes Zoster Ophthalmicus
Herpes zoster ophthalmicus (HZO) or ocular zoster is a painful and potentially devastating condition that occurs when latent varicella-zoster virus is reactivated in V1, the ophthalmic division of the trigeminal nerve. Ocular zoster can affect any part of the eye from the conjunctiva to the optic nerve, and is associated with a range of ocular and neurological complications including conjunctivitis, keratitis, uveitis, central retinal artery occlusion, nerve palsies, or neurotrophic keratopathy with ulceration and corneal perforation [47,48]. Permanent sequelae may include ocular inflammation and scarring, chronic severe pain and/or vision loss [49].
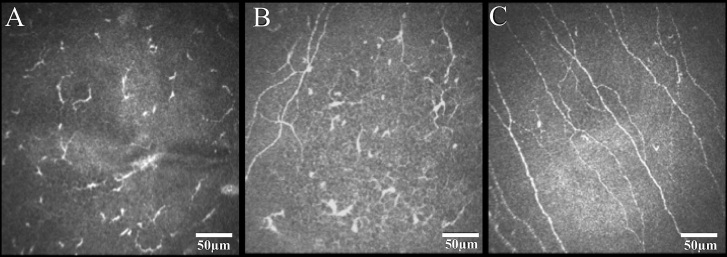
Several studies have examined the role of the structural destruction of corneal nerves in zoster infection, correlated with functional losses in sensation [50]. A scarcity of subbasal corneal nerves or significant decrease in SBNP density was shown in the eyes with unilateral HZO [36]. Surprisingly, patients with unilateral HZO exhibited a significant loss of the corneal nerve plexus even in the contralateral clinically unaffected eye as compared with normal subjects (Figure 2) [51]. Pathological alterations to the SBNP were strongly correlated with decreases in corneal sensation.
Figure 2.
In vivo confocal microscopy images of the subbasal corneal nerve plexus in eyes with herpes zoster ophthalmicus (HZO) and controls. Both the eyes affected by HZO (A) and the contralateral clinically unaffected eyes (B) demonstrated a significant reduction in subbasal nerve plexus including number of nerves, branches, and total nerve length when compared to normal controls (C). Reprinted from [51], with permission from Elsevier®.
A loss of corneal sensation may progress to non-healing epithelial defects or acute corneal lysis and perforation; about 25 percent of all HZO patients will develop these clinical signs of neurotrophic keratopathy because of permanent corneal anesthesia [52]. Therefore, there is increasing interest both in IVCM as a clinical tool for monitoring corneal status, as well as in the possibility of restoring corneal innervation and sensation in severe or persistent HZO cases. Cruzat et al. described spontaneous corneal nerve regeneration of central subbasal nerves with partial recovery of corneal sensation in a single case with severe neurotrophic zoster keratopathy after 5.3 years of follow-up [53], confirmed by IVCM, corneal esthesiometry, and ex vivo immunohistochemistry. This study, among others that have also successfully treated neurotrophic keratopathy of different etiologies [54,55], suggest that it may be possible for some patients with HZO neurokeratopathy to regain corneal innervation and function with either extended observation or therapy.
Corneal Transplantations and Laser Refractive Surgeries
Corneal transplantation represents the oldest, most common, and most successful form of tissue transplantation worldwide [56]. Common procedures today range from full-thickness penetrating keratoplasty (PKP), a technique requiring a full-thickness 360-degrees corneal incision, to lamellar keratoplasty techniques that replace only the diseased layer of the cornea. After PKP, regeneration of the transected SBNP fiber bundles occurs at a far slower rate than after cataract or refractive surgery [57]. The nerve fiber density and branching in the SBNP can still be abnormal 40 years after PKP [58]. Several studies have observed corneal sensation to be markedly reduced, if not absent, for decades after transplantation [59-61]. Impaired sensory innervation after PKP is thought to contribute to the relatively high frequency of epithelial complications observed after the procedure [62]. The effect of different lamellar surgical techniques on corneal nerves is variable, ranging from endothelial keratoplasty (EK) in which corneal sensitivity is relatively preserved [63], to deep anterior lamellar keratectomy (DALK) in which there is a progressive recovery in corneal sensitivity, with no statistically significant difference from that of post-PKP recovery [64].
Laser-assisted in situ keratomileusis (LASIK) and photorefractive keratectomy (PRK) are the most common corneal refractive procedures. Both involve tissue removal by excimer photoablation, the depth of which can influence the extent of corneal hypoesthesia after corneal refractive surgery [65]. Corneal subbasal nerves become depleted after PRK and LASIK and slowly recover over several years [66]; the SBNP density is barely detectable for up to 6 months post-LASIK, and remains less than half of the preoperative values 1 year after [67,68]. However, corneal sensitivity progressively approaches preoperative LASIK values by 6 to 12 months when measured with Cochet-Bonnet esthesiometry, and by 2 years when measured by gas esthesiometry [69,70]. The alteration of corneal nerves after LASIK is hypothesized to be the most likely cause of subjective dry eye symptoms after refractive surgery [71].
Non-corneal Ocular Procedures
Corneal changes have been extensively described not only following corneal, but also other ocular procedures. Using the Cochet-Bonnetesthesiometer and IVCM, Bitirgen et al. were able to show that repeated intravitreal injections of anti-VEGF appear to have no harmful effects on central corneal sensation and innervation [72]. However, uneventful cataract surgery performed with phacoemulsification and intraocular lens implantation is associated with marked loss of SBNP density and corneal sensitivity, with a return to normal values within 4 to 8 months postoperatively [73-75].
SBNP density and corneal sensitivity has been found to be reduced after laser panretinal photocoagulation (PRP) in treating proliferative diabetic retinopathy (PDR) or central retinal vein occlusion, or after retinal surgeries such as 360-degree laser retinopexy, scleral buckles and encircling bands, and rectinectomy with endolaser, with neurotrophic corneal ulcerations sometimes developing as a consequence [76-79]. However, some studies have suggested that reduction in corneal sensitivity or nerve density after PRP treatment may be attributable to the effect of diabetes in PDR patients [80,81].
Glaucoma Medical Therapy
Decreased corneal sensation has been reported in glaucoma patients treated with medical therapy [82], particularly with topical beta-adrenergic antagonists [83,84]. Many of these adverse effects have been linked to benzalkonium chloride (BAC), the most commonly used preservative in topical antiglaucoma preparations [85,86]. Ocular surface disease has a prevalence of 59 percent in glaucoma cases, with higher prevalence in patients using BAC-containing antiglaucoma medications [87]. Patients may experience dryness, foreign body sensation, tearing, burning, and redness. Other side effects include chronic conjunctival inflammation, tear film alterations, medically resistant herpetic keratitis, corneal erosions, and impaired wound healing [88-91]. Of note, topical medication-related ocular surface disease results in worse symptoms, poorer compliance to treatment, and decreased quality of life in glaucoma patients [92,93].
Decreased SBNP density and increased number of nerve beadings and tortuosity may be associated with alteration of corneal sensation in patients treated with topical antiglaucoma medications [94-96]. Conversely, Rossi et al. revealed the potential benefits of using preservative-free drops, reporting that naïve glaucoma patients did not show significant changes when on a preservative-free formula, whereas previously treated patients had an improvement in number of corneal nerves, decreased number of bead-like formations, and nerve tortuosity after 1 year of treatment [97].
Aging
Corneal sensation appears to decrease with age [98,99], including thermal sensitivity to a cooling stimulus, regardless of gender or diabetic status [100,101]. However, current research yields conflicting results regarding the correlation of aging with changes in SBNP density, most likely due to differences in imaging techniques and analysis. Data from tandem-scanning and slit-scanning confocal microscopy suggest that SBNP density is maintained in an age-independent manner [102,103], whereas laser-scanning confocal microscopy reveal a pronounced loss of corneal epithelial nerve terminals and SBNP density with age [104], with a corresponding decline in SBNP density of 0.25 percent to 0.9 percent per year [105]. Increased nerve tortuosity has also been observed with advancing age [103,106].
Diabetes Mellitus
The examination of corneal nerves and sensation has also been pursued in the context of early detection and assessment of systemic diseases associated with peripheral neuropathies, such as diabetes. Currently, nerve electrophysiology, sural nerve, and skin punch biopsy are the gold standards for diagnosing and quantifying diabetic peripheral neuropathy [107]. However, these tests often detect diabetic peripheral neuropathy only when the neuropathy becomes well established, and have limited sensitivity for detecting early diabetic peripheral neuropathy [108]. Nerve biopsies are also invasive and expensive, limiting repeat testing and adoption as a routine diagnostic tool [109].
Unmyelinated C-class and Aδ small nerve fibers are adversely affected in diabetic peripheral neuropathy, contributing to paresthesias and loss of pain and temperature sensation [110]. Small corneal nerve fibers also can be affected at this early stage, leading to changes that might be noninvasively and rapidly identified using IVCM [111]. Patients with type 1 or type 2 diabetes exhibit a marked reduction in corneal SBNP density, decreased nerve branching, and increased nerve tortuosity compared to healthy corneas [112,113]. While these abnormal corneal SBNP changes are associated with a reduction in corneal sensation [114], importantly, they can precede any clinical signs and symptoms of neuropathy, retinopathy, or microalbuminuria, or impairment of corneal sensitivity in patients with diabetes [115-117]. Interestingly, they are also seen in patients with impaired glucose tolerance, who do not yet meet clinical criteria for type 2 diabetes mellitus [118]. In addition to a moderate-to-high sensitivity and specificity of IVCM for diagnosis of diabetic neuropathy [119], a correlation between the loss of corneal nerve fibers and clinical and electrophysiological assessment of the severity of diabetes has been demonstrated; patients with worsened symptoms of peripheral neuropathy or diabetic retinopathy exhibit decreased SBNP density [119,120]. Finally, recovery of the corneal SBNP along with improving neuropathy has been shown in patients receiving simultaneous pancreas and kidney transplantation, suggesting the potential application of monitoring corneal nerves for therapeutic response and recovery in diabetes [121,122].
Conclusions and Outlook
Corneal nerves play a vital role in maintaining and protecting corneal integrity and sensation, and consequently, preserving ocular health and vision. Furthermore, the study of altered corneal innervation and sensation has implications beyond diseases of the eye; it can offer exciting insight into the clinical diagnosis or management of a wide variety of diseases that would otherwise require invasive testing or waiting for irreversible neuropathy to manifest. Additional investigation into the numerous factors essential for corneal nerve re-innervation and recovery of corneal sensation, beyond that in herpetic disease or ocular surgery, is needed. This, along with the continued development and validation of methods by which we analyze alterations in corneal nerve morphology and function, may someday lend significant therapeutic benefit to patients whom we may be currently limited in helping.
Glossary
- HZO
Herpes zoster ophthalmicus
- IOP
Intraocular pressure
- IVCM
in vivo confocal microscopy
- LASIK
Laser-assisted in situ keratomileusis
- PKP
penetrating keratoplasty
- PRK
photorefractive keratectomy
- PRP
panretinal photocoagulation
- SBNP
subbasal nerve plexus
References
- Bonini S, Rama P, Olzi D, et al. Neurotrophic keratitis. Eye (Lond). 2003;17:989–95. [DOI] [PubMed] [Google Scholar]
- Kitano S. An embryologic study of the human corneal nerves. Jpn J Ophthalmol. 1957;1:48–55. [Google Scholar]
- Marfurt CF, Kingsley RE, Echtenkamp SE. Sensory and sympathetic innervation of the mammalian cornea. A retrograde tracing study. Invest Ophthalmol Vis Sci. 1989;30(3):461–72. [PubMed] [Google Scholar]
- Müller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76(5):521–42. [DOI] [PubMed] [Google Scholar]
- Cruzat A, Qazi Y, Hamrah P. In vivo confocal microscopy of corneal nerves in health and disease. Ocul Surf. 2017;15(1):15–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004;78:513–25. [DOI] [PubMed] [Google Scholar]
- Millodot M. A review of research on the sensitivity of the cornea. Ophthalmic Physiol Opt. 1984;4(4):305–18. [PubMed] [Google Scholar]
- Zander E, Weddell G. Observations on the innervation of the cornea. J Anat. 1951;85:68–99. [PMC free article] [PubMed] [Google Scholar]
- Belmonte C. Eye dryness sensations after refractive surgery: impaired tear secretion or “phantom” cornea? J Refract Surg. 2007;23:598–602. [DOI] [PubMed] [Google Scholar]
- Belmonte C, Giraldez F. Responses of cat corneal sensory receptors to mechanical and thermal stimulation. J Physiol. 1981;321:355–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen KH, Reeh PW. Sustained graded pain and hyperalgesia from harmless experimental tissue acidosis in human skin. Neurosci Lett. 1993;154:113–6. [DOI] [PubMed] [Google Scholar]
- Acosta MC, Tan ME, Belmonte C, et al. Sensations evoked by selective mechanical, chemical, and thermal stimulation of the conjunctiva and cornea. Invest Ophthalmol Vis Sci. 2001;42:2063–7. [PubMed] [Google Scholar]
- Tanelian DL, Beuerman RW. Responses of rabbit corneal nociceptors to mechanical and thermal stimulation. Exp Neurol. 1984;84:165–78. [DOI] [PubMed] [Google Scholar]
- Stern ME, Gao J, Siemasko KF, et al. The role of the lacrimal functional unit in the pathophysiology of dry eye. Exp Eye Res. 2004;78(3):409–16. [DOI] [PubMed] [Google Scholar]
- Nishida T. Neurotrophic mediators and corneal wound healing. Ocul Surf. 2005;3(4):194–202. [DOI] [PubMed] [Google Scholar]
- Beuerman RW, Schimmelpfennig B. Sensory denervation of the rabbit cornea affects epithelial properties. Exp Neurol. 1980;69(1):196–201. [DOI] [PubMed] [Google Scholar]
- Nishida T, Chikama T, Sawa M, et al. Differential contributions of impaired corneal sensitivity and reduced tear secretion to corneal epithelial disorders. Jpn J Ophthalmol. 2012;56(1):20–5. [DOI] [PubMed] [Google Scholar]
- Nakumura M, Nishida T, Ofuji C, et al. Synergistic effect of substance P with epidermal growth factor on epithelial migration in rabbit cornea. Exp Eye Res. 1997;65:321–9. [DOI] [PubMed] [Google Scholar]
- Yamada N, Matsuda R, Morishige N, et al. Open clinical study of eye-drops containing tetrapeptides derived from substance P and insulin-like growth factor-1 for treatment of persistent corneal epithelial defects associated with neurotrophic keratopathy. Br J Ophthalmol. 2008;92:896–900. [DOI] [PubMed] [Google Scholar]
- Mikulec AA, Tanelian DL. CGRP increases the rate of corneal re-epithelialization in an in vitro whole mount preparation. J Ocul Pharmacol Ther. 1996;12:417–23. [DOI] [PubMed] [Google Scholar]
- Chaudhary S, Namavari A, Yco L, et al. Neurotrophins and nerve regeneration-associated genes are expressed in the cornea after lamellar flap surgery. Cornea. 2012;31(12):1460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard M, Hovakimyan M, Guthoff RF, et al. In vivo visualisation of murine corneal nerve fibre regeneration in response to ciliary neurotrophic factor. Exp Eye Res. 2014;120:20–7. [DOI] [PubMed] [Google Scholar]
- You L, Kruse FE, Volcker HE. Neurotrophic factors in the human cornea. Invest Ophthalmol Vis Sci. 2000;41:692–702. [PubMed] [Google Scholar]
- Cellini M, Bendo E, Bravetti GO, et al. The use of nerve growth factor in surgical wound healing of the cornea. Ophthalmic Res. 2006;38:177–81. [DOI] [PubMed] [Google Scholar]
- Tan MH, Bryars J, Moore J. Use of nerve growth factor to treat congenital neurotrophic corneal ulceration. Cornea. 2006;25:352–5. [DOI] [PubMed] [Google Scholar]
- Cohen A, Bray GM, Aguayo AJ. Neurotrophin-4/5 (NT-4/5) increases adult rat retinal ganglion cell survival and neurite outgrowth in vitro. J Neurobiol. 1994;25(8):953–9. [DOI] [PubMed] [Google Scholar]
- Kobayashi NR, Fan DP, Giehl KM, et al. BDNF and NT-4/5 prevent atrophy of rat rubrospinal neurons after cervical axotomy, stimulate GAP-43 and Tα1-tubulin mRNA expression, and promote axonal regeneration. J Neurosci. 1997;17(24):9583–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Castro F, Silos-Santiago I, De Armentia MI, et al. Corneal innervation and sensitivity to noxious stimuli in trkA knockout mice. Eur J Neurosci. 1998;10(1):146–52. [DOI] [PubMed] [Google Scholar]
- Marfurt CF, Cox J, Deek S, et al. Anatomy of the human corneal innervation. Exp Eye Res. 2010;90:478–92. [DOI] [PubMed] [Google Scholar]
- Al-Aqaba MA, Alomar T, Miri A, et al. Ex vivo confocal microscopy of human corneal nerves. Br J Ophthalmol. 2010;94:1251–7. [DOI] [PubMed] [Google Scholar]
- Müller LJ, Vrensen GF, Pels L, et al. Architecture of human corneal nerves. Invest Ophthalmol Vis Sci. 1997;38:985–94. [PubMed] [Google Scholar]
- Webb RH, Hughes GW, Delori FC. Confocal scanning laser ophthalmoscope. Appl Opt. 1987;26:1492–9. [DOI] [PubMed] [Google Scholar]
- Minsky M. Memoir on inventing the confocal microscope. Scanning. 1988;10:128–38. [Google Scholar]
- Patel DV, Ku JY, Johnson R, et al. Laser scanning in vivo confocal microscopy and quantitative aesthesiometry reveal decreased corneal innervation and sensation in keratoconus. Eye (Lond). 2009;23:586–92. [DOI] [PubMed] [Google Scholar]
- Alhatem A, Cavalcanti B, Hamrah P. In vivo confocal microscopy in dry eye disease and related conditions. Semin Ophthalmol. 2012;27(5–6):138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrah P, Cruzat A, Dastjerdi MH, et al. Unilateral herpes zoster ophthalmicus results in bilateral corneal nerve alteration: an in vivo confocal microscopy study. Ophthalmology. 2013;120(1):40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum E, Golebiowski B, Swarbrick HA. Mapping the corneal sub-basal nerve plexus in orthokeratology lens wear using in vivo laser scanning confocal microscopy. Invest Ophthalmol Vis Sci. 2012;53:1803–9. [DOI] [PubMed] [Google Scholar]
- Mantopoulos D, Cruzat A, Hamrah P. In vivo imaging of corneal inflammation: new tools for clinical practice and research. Semin Ophthalmol. 2010;25(5–6):178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervo TM, Moilanen JA, Rosenberg ME., et al. In vivo confocal microscopy for studying corneal diseases and conditions associated with corneal nerve damage. Adv Exp Med Biol. 2002;506(Pt A):657–665. [DOI] [PubMed] [Google Scholar]
- Labbé A, Alalwani H, Van Went C, et al. The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Invest Ophthalmol Vis Sci. 2012;53(8):4926–31. [DOI] [PubMed] [Google Scholar]
- Winter K, Scheibe P, Kohler B, et al. Local variability of parameters for characterization of the corneal subbasal nerve plexus. Curr Eye Res. 2016;41:186–98. [DOI] [PubMed] [Google Scholar]
- Patel DV, McGhee CN. Quantitative analysis of in vivo confocal microscopy images: a review. Surv Ophthalmol. 2013;58:466–75. [DOI] [PubMed] [Google Scholar]
- Meijering E, Jacob M, Sarria JC, et al. Design and validation of a tool for neurite tracing and analysis in fluorescence microscopy images. Cytometry A. 2004;58:167–76. [DOI] [PubMed] [Google Scholar]
- Sindt CW, Lay B, Bouchard H, et al. Rapid image evaluation system for corneal in vivo confocal microscopy. Cornea. 2013;32:460–5. [DOI] [PubMed] [Google Scholar]
- Cochet P, Bonnet R. L’esthesie corneenne. Clin Ophthalmol. 1960;4:3–27. [Google Scholar]
- Belmonte C, Acosta MC, Schmelz M, et al. Measurement of corneal sensitivity to mechanical and chemical stimulation with a CO2 esthesiometer. Invest Ophthalmol Vis Sci. 1999;40:513–9. [PubMed] [Google Scholar]
- Shaikh S, Ta CN. Evaluation and management of herpes zoster ophthalmicus. Am Fam Physician. 2002;66:1723–30. [PubMed] [Google Scholar]
- Liesegang TJ. Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology. 2008;115:S3–12. [DOI] [PubMed] [Google Scholar]
- Ragozzino MW, Melton LJ, Kurland LT, et al. Population-based study of herpes zoster and its sequelae. Medicine. 1982;61:310–6. [DOI] [PubMed] [Google Scholar]
- Patel DV, McGhee CN. Laser scanning in vivo confocal microscopy demonstrating significant alteration of human corneal nerves following herpes zoster ophthalmicus. Arch Neurol. 2010;67:640–1. [DOI] [PubMed] [Google Scholar]
- Cavalcanti BM, Cruzat A, Sahin A., et al. In vivo confocal microscopy detects bilateral changes of corneal immune cells and nerves in unilateral herpes zoster ophthalmicus. Ocul Surf. 2017;S1542-0124(17)30130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesegang TJ. Corneal complications from herpes zoster ophthalmicus. Ophthalmology. 1985;92:316–24. [DOI] [PubMed] [Google Scholar]
- Cruzat A, Hamrah P, Cavalcanti BM, et al. Corneal reinnervation and sensation recovery in patients with herpes zoster ophthalmicus: an in vivo and ex vivo study of corneal nerves. Cornea. 2016;35:619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao K, Leveque C, Pflugfelder SC. Corneal nerve regeneration in neurotrophic keratopathy following autologous plasma therapy. Br J Ophthalmol. 2010;94:584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini S, Lambiase A, Rama P., et al. Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology. 2000;107:1347–1351. [DOI] [PubMed] [Google Scholar]
- Waldock A, Cook SD. Corneal transplantation: how successful are we? Br J Ophthalmol. 2000;84:813–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhaas M. Corneal sensation after cataract and refractive surgery. J Cataract Refract Surg. 1998;24:1399–409. [DOI] [PubMed] [Google Scholar]
- Niederer RL, Perumal D, Sherwin T, et al. Corneal innervation and cellular changes after corneal transplantation: an in vivo confocal microscopy study. Invest Ophthalmol Vis Sci. 2007;48:621–6. [DOI] [PubMed] [Google Scholar]
- Rao GN, John T, Ishida N, et al. Recovery of corneal sensitivity in grafts following penetrating keratoplasty. Ophthalmology. 1985;92:1408–11. [DOI] [PubMed] [Google Scholar]
- Richter A, Slowik C, Somodi S, et al. Corneal reinnervation following penetrating keratoplasty correlation of esthesiometry and confocal microscopy. Ger J Ophthalmol. 1996;5:513–7. [PubMed] [Google Scholar]
- Mathers WD, Jester JV, Lemp MA. Return of human corneal sensitivity after penetrating keratoplasty. Arch Ophthalmol. 1988;106:210–1. [DOI] [PubMed] [Google Scholar]
- Feiz V, Mannis MJ, Kandavel G, et al. Surface keratopathy after penetrating keratoplasty. Trans Am Ophthalmol Soc. 2001;99:159–68. [PMC free article] [PubMed] [Google Scholar]
- Kumar RL, Koenig SB, Covert DJ. Corneal sensation after descemet stripping and automated endothelial keratoplasty. Cornea. 2010;29:13–8. [DOI] [PubMed] [Google Scholar]
- Ceccuzzi R, Zanardi A, Fiorentino A, et al. Corneal sensitivity in keratoconus after penetrating and deep anterior lamellar keratoplasty. Ophthalmologica. 2010;224:247–50. [DOI] [PubMed] [Google Scholar]
- Campos M, Hertzog L, Garbus JJ, et al. Corneal sensitivity after photorefractive keratectomy. Am J Ophthalmol. 1992;114:51–4. [DOI] [PubMed] [Google Scholar]
- Erie JC, McLaren JW, Hodge DO, Bourne WM. Recovery of corneal subbasal nerve density after PRK and LASIK. Am J Ophthalmol. 2005;140:1059–64. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Jin KK, Kyung YS, et al. Comparison of corneal nerve regeneration and sensitivity between LASIK and laser epithelial keratomileusis (LASEK). Am J Ophthalmol. 2006;141:1009–15. [DOI] [PubMed] [Google Scholar]
- Lee BH, McLaren JW, Erie JC, et al. Reinnervation in the cornea after LASIK. Invest Ophthalmol Vis Sci. 2002;43:3660–4. [PubMed] [Google Scholar]
- Benitez-del-Castillo JM, del Rio T, Iradier T, et al. Decrease in tear secretion and corneal sensitivity after laser in situ keratomileusis. Cornea. 2001;20:30–2. [DOI] [PubMed] [Google Scholar]
- Gallar J, Acosta MC, Moilanen JA, et al. Recovery of corneal sensitivity to mechanical and chemical stimulation after laser in situ keratomileusis. J Refract Surg. 2004;20:229–35. [DOI] [PubMed] [Google Scholar]
- Chao C, Golebiowski B, Stapleton F. The role of corneal innervation in LASIK-induced neuropathic dry eye. Ocul Surf. 2014;12:32–45. [DOI] [PubMed] [Google Scholar]
- Bitirgen G, Belviranli S, Malik RA, et al. Assessment of Corneal Sensation, Innervation and Retinal Nerve Fiber Layer in Patients Treated with Multiple Intravitreal Ranibizumab Injections. PLoS One. 2017;12(1):e0170271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cillà S, Fogagnolo P, Sacchi M, et al. Corneal involvement in uneventful cataract surgery: an in vivo confocal microscopy study. Ophthalmologica. 2014;231:103–10. [DOI] [PubMed] [Google Scholar]
- Babu K, Narasimha MK, Ramachandra MK. Wavelike epitheliopathy after phacoemulsification: role of in vivo confocal microscopy. Cornea. 2007;26:747–8. [DOI] [PubMed] [Google Scholar]
- Misra SL, Goh YW, Patel DV, et al. Corneal microstructural changes in nerve fiber, endothelial and epithelial density after cataract surgery in patients with diabetes mellitus. Cornea. 2015;34:177–81. [DOI] [PubMed] [Google Scholar]
- Banerjee PJ, Chandra A, Sullivan PM, et al. Neurotrophic corneal ulceration after retinal detachment surgery with retinectomy and endolaser: a case series. JAMA Ophthalmol. 2014;132:750–2. [DOI] [PubMed] [Google Scholar]
- Gibson RA. Reduction of corneal sensitivity after retinal detachment surgery. Br J Ophthalmol. 1981;65(9):614–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menchini U, Scialdone A, Pietroni C, et al. Argon versus krypton panretinal photocoagulation side effects on the anterior segment. Ophthalmologica. 1990;201(2):66–70. [DOI] [PubMed] [Google Scholar]
- Bouheraoua N, Hrarat L, Parsa CF, et al. Decreased corneal sensation and subbasal nerve density, and thinned corneal epithelium as a result of 360-degree laser retinopexy. Ophthalmology. 2015;122:2095–102. [DOI] [PubMed] [Google Scholar]
- Neira-Zalentein W, Holopainen JM, Tervo TM. Corneal sensitivity in diabetic patients subjected to retinal laser photocoagulation. Invest Ophthalmol Vis Sci. 2011;52:6043–9. [DOI] [PubMed] [Google Scholar]
- Misra S, Ahn HN, Craig JP, et al. Effect of panretinal photocoagulation on corneal sensation and the corneal subbasal nerve plexus in diabetes mellitus. Invest Ophthalmol Vis Sci. 2013;54:4485–90. [DOI] [PubMed] [Google Scholar]
- Van Went C, Alalwani H, Brasnu E, et al. Corneal sensitivity in patients treated for glaucoma or ocular hypertension. J Fr Ophtalmol. 2011;34:684–90. [DOI] [PubMed] [Google Scholar]
- Kitazawa Y, Tsuchisaka H. Effects of timolol on corneal sensitivity and tear production. Int Ophthalmol. 1980;3:25–9. [DOI] [PubMed] [Google Scholar]
- Van Buskirk EM. Corneal anesthesia after timolol maleate therapy. Am J Ophthalmol. 1979;88:739–43. [DOI] [PubMed] [Google Scholar]
- Noecker RJ, Herrygers LA, Anwaruddin R. Corneal and conjunctival changes caused by commonly used glaucoma medications. Cornea. 2004;23:490–6. [DOI] [PubMed] [Google Scholar]
- Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative-free glaucoma medication. Br J Ophthalmol. 2002;86:418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung EA, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17(5):350–5. [DOI] [PubMed] [Google Scholar]
- Baudouin C, Pisella PJ, Fillacier K, et al. Ocular surface inflammatory changes induced by topical antiglaucoma drugs: human and animal studies. Ophthalmology. 1999;106:556–63. [DOI] [PubMed] [Google Scholar]
- Wand M, Gilbert CM, Liesegang TJ. Latanoprost and herpes simplex keratitis. Am J Ophthalmol. 1999;127:602–4. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Yokoi N, Kinoshita S. Comparison of the effects of topical levobunolol and timolol solution on the human ocular surface. Cornea. 2003;22:709–15. [DOI] [PubMed] [Google Scholar]
- Pisella PJ, Debbasch C, Hamard P, et al. Conjunctival proinflammatory and proapoptotic effects of latanoprost and preserved and unpreserved timolol: an ex vivo and in vitro study. Invest Ophthalmol Vis Sci. 2004;45:1360–8. [DOI] [PubMed] [Google Scholar]
- Skalicky SE, Goldberg I, McCluskey P. Ocular surface disease and quality of life in patients with glaucoma. Am J Ophthalmol. 2012;153:1–9. [DOI] [PubMed] [Google Scholar]
- Fechtner RD, Godfrey DG, Budenz D, et al. Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure lowering medications. Cornea. 2010;29:618–21. [DOI] [PubMed] [Google Scholar]
- Labbe A, Alalwani H, Van Went C, et al. The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Invest Ophthalmol Vis Sci. 2012;53:4926–31. [DOI] [PubMed] [Google Scholar]
- Baratz KH, Nau CB, Winter EJ. Effects of glaucoma medications on corneal endothelium, keratocytes, and subbasal nerves among participants in the ocular hypertension treatment study. Cornea. 2006;25:1046–52. [DOI] [PubMed] [Google Scholar]
- Martone G, Frezzotti P, Tosi GM. An in vivo confocal microscopy analysis of effects of topical antiglaucoma therapy with preservative on corneal innervation and morphology. Am J Ophthalmol. 2009;147:725–35. [DOI] [PubMed] [Google Scholar]
- Rossi GC, Blini M, Scudeller L, et al. Effect of preservative-free tafluprost on keratocytes, sub-basal nerves, and endothelium: a single-blind one-year confocal study on naive or treated glaucoma and hypertensive patients versus a control group. J Ocul Pharmacol Ther. 2013;29:821–5. [DOI] [PubMed] [Google Scholar]
- Millodot M. The influence of age on the sensitivity of the cornea. Invest Ophthalmol Vis Sci. 1977;16:240–2. [PubMed] [Google Scholar]
- Roszkowska AM, Colosi P, Ferreri FM, et al. Age-related modifications of corneal sensitivity. Ophthalmologica. 2004;218:350–5. [DOI] [PubMed] [Google Scholar]
- Murphy PJ, Patel S, Kong N, et al. Noninvasive assessment of corneal sensitivity in young and elderly diabetic and nondiabetic subjects. Invest Ophthalmol Vis Sci. 2004;45(6):1737–42. [DOI] [PubMed] [Google Scholar]
- Roszkowska AM, Colosi P, Ferreri FM, et al. Age-related modifications of corneal sensitivity. Ophthalmologica. 2004;218(5):350–5. [DOI] [PubMed] [Google Scholar]
- Erie JC, McLaren JW, Hodge DO, et al. The effect of age on the corneal subbasal nerve plexus. Cornea. 2005;24(6):705–9. [DOI] [PubMed] [Google Scholar]
- Patel DV, Tavakoli M, Craig JP, et al. Corneal sensitivity and slit scanning in vivo confocal microscopy of the subbasal nerve plexus of the normal central and peripheral human cornea. Cornea. 2009;28:735–40. [DOI] [PubMed] [Google Scholar]
- Niederer RL, Perumal D, Sherwin T, et al. Age-related differences in the normal human cornea: a laser scanning in vivo confocal microscopy study. Br J Ophthalmol. 2007;91(9):1165–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parissi M, Karanis G, Randjelovic S, et al. Standardized baseline human corneal subbasal nerve density for clinical investigations with laser-scanning in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 2013;54:7091–102. [DOI] [PubMed] [Google Scholar]
- Lagali N, Poletti E, Patel DV, et al. Focused tortuosity definitions based on expert clinical assessment of corneal subbasal nerves. Invest Ophthalmol Vis Sci. 2015;56:5102–9. [DOI] [PubMed] [Google Scholar]
- England JD, Gronseth GS, Franklin G, et al. Practice Parameter: evaluation of distal symmetric polyneuropathy: role of autonomic testing, nerve biopsy, and skin biopsy (an evidence-based review). Report of the American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, and American Academy of Physical Medicine and Rehabilitation. Neurology. 2009;72:177–84. [DOI] [PubMed] [Google Scholar]
- Quattrini C, Tavakoli M, Jeziorska M, et al. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes. 2007;56(8):2148–54. [DOI] [PubMed] [Google Scholar]
- Tavakoli M, Petropoulos IN, Malik RA. Corneal confocal microscopy to assess diabetic neuropathy: an eye on the foot. J Diabetes Sci Technol. 2013;7(5):1179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain P, Sachdev A, Malik RA. Early detection of diabetic peripheral neuropathy with corneal confocal microscopy. Lancet. 2005;366:1340–3. [DOI] [PubMed] [Google Scholar]
- Smith AG, Russell J, Feldman EL, et al. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care. 2006;29:1294–9. [DOI] [PubMed] [Google Scholar]
- Kallinikos P, Berhanu M, O’Donnell C, et al. Corneal nerve tortuosity in diabetic patients with neuropathy. Invest Ophthalmol Vis Sci. 2004;45:418–22. [DOI] [PubMed] [Google Scholar]
- Chang PY, Carrel H, Huang JS, et al. Decreased density of corneal basal epithelium and subbasal corneal nerve bundle changes in patients with diabetic retinopathy. Am J Ophthalmol. 2006;142:488–90. [DOI] [PubMed] [Google Scholar]
- Rosenberg ME, Tervo TM, Immonen IJ, et al. Corneal structure and sensitivity in type 1 diabetes mellitus. Invest Ophthalmol Vis Sci. 2000;41:2915–21. [PubMed] [Google Scholar]
- Mehra S, Tavakoli M, Kallinikos PA, et al. Corneal confocal microscopy detects early nerve regeneration after pancreas transplantation in patients with type 1 diabetes. Diabetes Care. 2007;30:2608–12. [DOI] [PubMed] [Google Scholar]
- Petropoulos IN, Green P, Chan AW, et al. Corneal confocal microscopy detects neuropathy in patients with type 1 diabetes without retinopathy or microalbuminuria. PLoS One. 2015;10(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitirgen G, Ozkagnici A, Malik RA, et al. Corneal nerve fibre damage precedes diabetic retinopathy in patients with Type 2 diabetes mellitus. Diabet Med. 2014;31(4):431–8. [DOI] [PubMed] [Google Scholar]
- Asghar O, Petropoulos IN, Alam U, et al. Corneal confocal microscopy detects neuropathy in subjects with impaired glucose tolerance. Diabetes Care. 2014;37(9):2643–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavakoli M, Quattrini C, Abbott C, et al. Corneal confocal microscopy: a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care. 2010;33(8):1792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messmer EM, Schmid-Tannwald C, Zapp D, et al. In vivo confocal microscopy of corneal small fiber damage in diabetes mellitus. Graefes Arch Clin Exp Ophthalmol. 2010;248:1307–12. [DOI] [PubMed] [Google Scholar]
- Tavakoli M, Mitu-Pretorian M, Petropoulos IN. Corneal confocal microscopy detects early nerve regeneration in diabetic neuropathy after simultaneous pancreas and kidney transplantation. Diabetes. 2013;62:254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro X, Sutherland DE, Kennedy WR. Long-term effects of pancreatic transplantation on diabetic neuropathy. Ann Neurol. 1997;42(5):727–36. [DOI] [PubMed] [Google Scholar]