Abstract
Background: American College of Emergency Physicians (ACEP) [1] recommends that patients presenting with acute non-traumatic headache concerning for subarachnoid hemorrhage (SAH) undergo lumbar puncture (LP) when non-contrast head computed tomography (CT) is negative. The diagnostic yield of this approach is unknown. Objective: Evaluate the diagnostic yield, lengths of stay and complication rates of LPs in patients undergoing Emergency Department (ED) evaluation for aneurysmal SAH. Methods: Multi-center, retrospective, hypothesis-blinded, explicit chart review of patients undergoing ED-based lumbar puncture between 2007 and 2012. Charts of neurologically intact patients presenting with headache that had a negative head CT and underwent LP primarily to rule out SAH were reviewed. Trained data abstractors blinded to study hypothesis used standardized data forms with predefined terms for chart abstraction. We re-abstracted and assessed inter-rater agreement for 20 percent of charts with a 100 percent inter-rater agreement. Data were descriptive, using 95 percent confidence intervals. Results: 1,282 LPs were performed, and 342 patients met inclusion criteria but only 1 percent were deemed positive for SAH in the chart. No aneurysm or vascular malformation was identified in those with positive LPs for SAH. Complications were in 4 percent and xanthochromia was found in 13 percent. Total length of stay was 7.8 hours (0.95 CI; 7.5 – 8.2). No patient discharged from the ED after a negative workup for SAH was re-admitted for SAH or underwent a neurosurgical procedure during a three-month follow-up period. Conclusions: LP in our cohort of neurologically intact CT-negative ED headache patients did not identify any cases of aneurysmal SAH but was associated with serious complications, a significant false positive rate, and extended ED length of stay.
Keywords: Subarachnoid hemorrhage, Lumbar puncture, Headache, Head CT, Emergency Department evaluation, utility and yield of lumbar puncture
Introduction
Aneurysmal SAH despite being a low incidence condition is associated with a high rate of mortality and morbidity [2]. Moreover, missed SAH is a common concern among emergency providers [3]. The current American College of Emergency Physicians guidelines recommend that “in patients presenting to the Emergency Department with sudden-onset, severe headache and a negative non-contrast head CT scan result, LP should be performed to rule out SAH” [1].
High CT sensitivity [4], increasing availability of advanced imaging with expedited angiographic testing [5], and high quality, prospective, ED-based studies of acute headache [4] have all contributed to the debate [6-20] over the added utility and diagnostic yield of LP after a negative non-contrast head CT in the specific subset of neurologically intact patients.
We reviewed the ED visits and clinical course of neurologically intact patients presenting with an acute non-traumatic headache to our two-hospital health system and undergoing LP after a negative non-contrast head CT for possible SAH between 2007 and 2012. We aimed to determine the incidence of CT-negative, LP-positive SAH in this cohort and whether this cohort received any significant neurosurgical interventions for SAH secondary to a vascular etiology such as aneurysms or arteriovenous malformations. We hypothesized that LP would be of low diagnostic yield for aneurysmal SAH.
Methods
We conducted a multi-center, hypothesis-blinded retrospective chart review of patients undergoing ED-based lumbar puncture between September 2007 and September 2012 at two academic emergency departments (Yale New Haven Hospital and Shoreline Medical Center, which is a subsidiary of the Yale New Haven Hospital) with a combined annual census of 105,000 patients. Board certified emergency physicians assisted by emergency medicine residents and emergency physician extenders (this includes physician assistants and advanced practice registered nurses) staffed both departments continuously. Emergency physicians and extenders were only those affiliated with the Yale New Haven Hospital system and its satellite facility–Shoreline Medical Center. The Institutional Review Board at Yale New Haven Hospital governs research conducted at both sites; both hospitals approved the study.
Patient Selection and Definition of Terms
Using a single, linked, universal medical record system (LYNX Medical systems, Bellevue, WA) encompassing both hospitals we searched ICD-9 codes to identify lumbar punctures performed during the study time period. Based on a comprehensive system for billing catchment and quality oversight, lumbar puncture was a procedure typically documented and reported by ICD-9 codes in our hospital system. We did not have an administrative procedure or oversight system for capture and identification of non-billed lumbar puncture procedures; however, we were not aware of this occurring with any frequency. Additionally, there were internal departmental procedures in place within our billing office to capture missed billing opportunities.
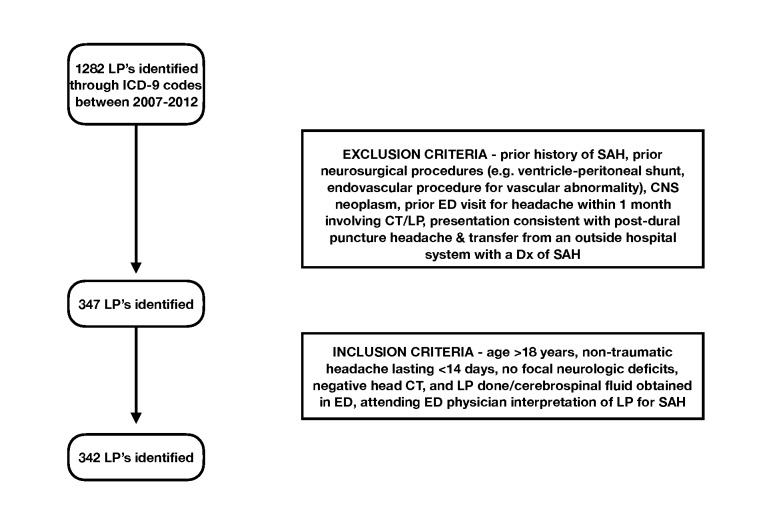
From the initial group of lumbar punctures identified through ICD-9 codes, we reviewed charts by hand for inclusion and exclusion criteria (Figure 1). Inclusion criteria were age greater than 18 years, non-traumatic headache lasting less than 14 days, no focal neurologic deficits, negative computed tomography imaging of the head, and cerebrospinal fluid successfully obtained in the ED. Exclusion criteria were prior history of SAH, prior neurosurgical procedures (e.g. ventriculo-peritoneal shunt, endovascular procedure for vascular abnormality), central nervous system neoplasm, prior ED visit for headache within last one month involving head CT/lumbar puncture, presentation consistent with post-dural puncture headache, and transfer from an outside hospital system with a known diagnosis of SAH.
Figure 1.
Inclusion and exclusion criteria for enrollment of study patients.
We defined all terms prior to database search and chart data abstraction. A non-traumatic headache was defined as neither fall nor direct trauma to the head in the previous seven days. Head CTs were deemed negative for SAH if an attending radiologist report was documented indicating no hemorrhagic findings. For a designation of a non-focal neurologic examination, we required attending physician documentation of a non-focal neurologic examination. In order to avoid any personal judgment of data abstractors, all LPs were deemed positive, negative, or traumatic for SAH based on the final interpretation of the ED attending of record as articulated in the chart. This interpretation was typically narrative but occasionally documented via an available checkbox option on the LP procedure note in our charting system.
Follow-up was conducted through chart review only. Charts of patients admitted were examined for neurosurgical interventions of any kind and for final diagnoses. Charts of patients discharged were followed forward for a period of three months using the health system’s database, specifically seeking documentation of repeat evaluation of headache, or any morbidity/mortality potentially related to missed SAH. Our hospital system is the only tertiary care referral center for neurosurgical diseases in a large geographical catchment area and manages all patients requiring endovascular (diagnostic and interventional), and/or operative management pertaining to cerebral aneurysms, vascular malformations, or intracranial hemorrhages. Return visit diagnoses over a three-month period were reviewed; however specific data points from these patient encounters subsequent to initial evaluation in our two EDs were not abstracted into our database unless missed SAH appeared to be a possible explanation.
Chart Review
Medical record numbers obtained via ICD-9 codes from our billing database search were abstracted into a separate database developed and managed by our research group. We categorized data points as falling into three main categories: visit analysis, LP analysis, and patient characteristics. Medical record numbers were subsequently converted into unique study IDs prior to data abstraction and statistical analysis.
Data was abstracted by one researcher and a research assistant with concomitant faculty oversight. We designed a two-week training process for our research assistant, including methods of data extraction using a pre-made data form and a glossary of all terms. The research assistant was kept blinded to the hypothesis of the study and was unaware of any specific data point as a primary outcome variable. Data from the most recent year in our database (2011-2012) was abstracted during the two-week training period. This was done twice, sequentially, once by the researcher and once by the research assistant for the purposes of inter-rater reliability examination and remediation as needed. Inter-rater reliability in this phase of the research was 100 percent. Data for subsequent years was extracted primarily by the research assistant with 20 percent random re-abstraction of charts by the researcher for ongoing assessments of accuracy. There was 100 percent inter-rater reliability in this process as well.
Statistical Analysis
Data were primarily descriptive and 95 percent confidence intervals were used.
Results
Of 1,282 LPs performed in the five-year time period, 935 were performed on patients that met exclusion criteria and thus not considered (see Figure 1). During data abstraction five charts were additionally excluded based on missing data: one because a neurologic examination was not documented, one because attending interpretation of the LP was not documented, one because of missing laboratory data from cerebrospinal fluid analysis, one because no attending radiologist interpretation was documented, and one because the indication for LP was not SAH. Among the remaining 342 charts, seven charts had isolated data fields missing that did not impact final analysis or inclusion (four charts did not document the presence or absence of syncope and three did not document neck pain).
Of the final study cohort, 293 were evaluated at the main campus (Yale New Haven Hospital), 44 at the satellite ED (Shoreline Medical Center), and five initially at the satellite campus and later transferred to the main campus. Table 1 lists characteristics of patients in the cohort. Sixty-nine percent classified their headache as the worst of life and roughly half (48 percent) reported maximal intensity within the first hour.
Table 1. Breakdown of key demographic and clinical presentation characteristics per year of presentation.
| Year | # of male | # of female | Mean age | Mean SBP | Headache as worst ever as % | % with max intensity within one hour | Mean time between onset and presentation (hrs) | Pos fam HX% | % of smokers | % with HTN | % with syncope | % with neck pain |
| 2007-2008 | 25 | 46 | 40.2 | 138 | 68 | 32 | 98 | 4 | 13 | 20.0 | 9.30 | 20.0 |
| 2008-2009 | 25 | 31 | 43.4 | 139 | 59 | 43 | 72.2 | 14 | 18 | 20.0 | 4.0 | 14.3 |
| 2009-2010 | 33 | 19 | 40 | 138 | 73 | 60 | 60.6 | 4 | 13.40 | 23.0 | 2.0 | 19.0 |
| 2010-2011 | 53 | 16 | 40.3 | 165 | 75 | 64 | 30.4 | 13 | 23 | 33.0 | 4.3 | 24.6 |
| 2011-2012 | 34 | 60 | 42.5 | 135 | 71 | 41 | 37 | 6 | 14.50 | 21.0 | 3.0 | 23.5 |
| Total | 170 | 172 | 41.28 | 143 | 69 | 48 | 59.64 | 8 | 16 | 23.0 | 4.5 | 20.0 |
| 95% CI | 39.34 to 43.21 | 127.62 to 158.38 | 61.43 to 76.97 | 31.23 to 64.77 | 25.64 to 93.63 | 2.09 to 14.31 | 11.17 to 21.58 | 16.56 to 30.23 | 1.01 to 8.02 | 15.21 to 25.34 |
Of the 342 patients, final diagnoses (admitting diagnosis specified from ED) were SAH (n=5), headache not otherwise specified (n=224), migraine headache (n=25), tension headache (n=9), meningitis including both viral and bacterial (n=41), and “other” (n=38), a category including sinusitis, atypical migraine, pseudotumor cerebri, cavernous sinus thrombosis, optic neuritis, carbon monoxide poisoning, hypertensive urgency, viral illness, transient ischemic attack, vertigo, syncope, and cervicalgia.
Of the five patients diagnosed with SAH, none received any neurosurgical interventions (Figure 2). One was initially diagnosed as SAH with grossly bloody cerebrospinal fluid in the setting of active anti-coagulation for valvular disease with an INR of 3.0. This subject was later determined to have no aneurysm or vascular malformations by conventional angiography. This patient’s clinical course was also complicated by an epidural hematoma at the spinal level of the LP. The remaining four patients also underwent angiographic imaging showing no aneurysmal source of bleeding. Each was discharged within 48 hours of admission after admission and observation in the neurological intensive care unit.
Figure 2.
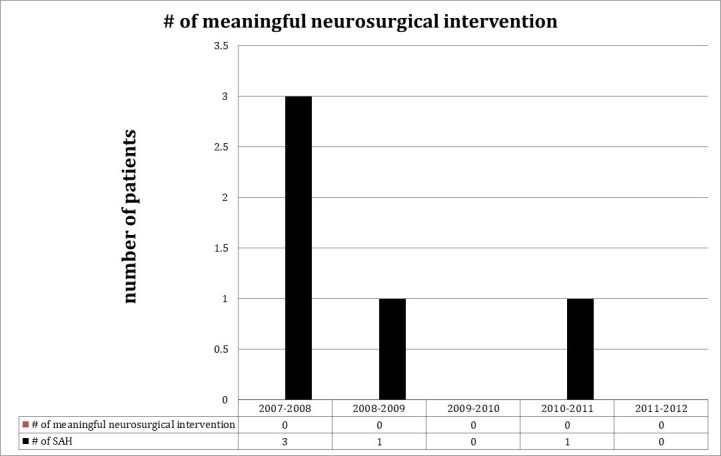
Yearly breakdown of the number of neurosurgical interventions undertaken amongst those patients who had a negative head CT but a LP considered by the emergency medicine provider to be positive for SAH.
Of the 342 patients, 81 (24 percent) were admitted and 261 (76 percent) were discharged. None of the 261 discharged patients was noted to have a return visit (in the entire hospital system) in the next three months that included a diagnosis of SAH or cerebral aneurysm. Of the 81 patients admitted to the hospital, none underwent any neurosurgical intervention for cerebral aneurysm or SAH.
As per visit analyses (Figure 3), the mean length of stay for these patients in the ED was 7.8 hours (95 percent CI of 7.5 to 8.2 hours). This is the time interval between the first set of triage vitals being documented in the chart to documentation in the medical record of an admission or discharge order. The mean time between patient arrival and head CT was 2.6 hours (95 percent CI of 2.2 to 2.9 hrs). This is the time interval between the first set of triage vitals being documented in the chart to documentation per the radiologist of the final head CT read. The mean time interval between arrival and cerebrospinal fluid (CSF) collection was 5.3 hours (95 percent CI of 5.0 to 5.6 hrs). This is the time interval between the first set of triage vitals being documented in the chart to documentation of the final CSF results. The mean time interval between CSF collection and disposition was 3.6 hours (95 percent CI of 3.1 to 4.2 hrs). This is the time interval between the documentation of the final CSF results in the electronic medical record to documentation of an admission or discharge order.
Figure 3.
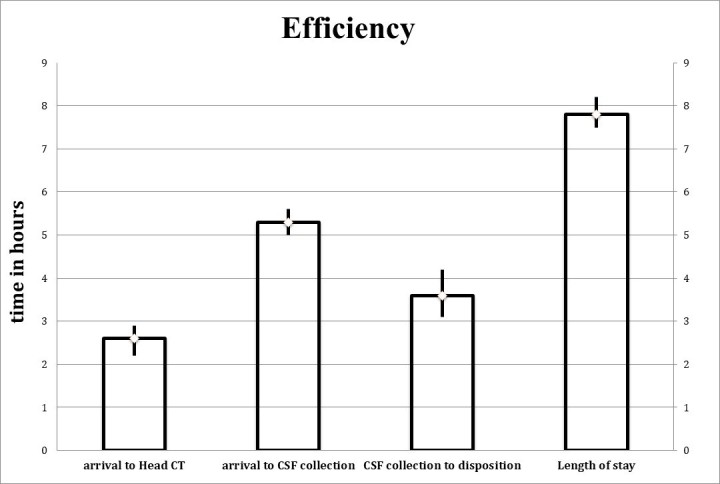
Average times for head CT, CSF collection, CSF collection to disposition and overall length of stay to describe overall efficiency associated with the CT-LP workflow.
Treating emergency providers interpreted 301 LPs to be negative, and 36 to be traumatic, while reporting 14 complications (13 post-dural headaches, one epidural hematoma, Figure 4). These complications were identified by either a listing of complications in the procedure note or during review of ED visits subsequent to the initial date of LP by the research team. As seen in Figure 5, a total of 44 LPs were noted to be positive for xanthochromia (all assessed per visual inspection by lab tech), inclusive of the five patients diagnosed with SAH with the remaining 39 deemed positive for xanthochromia but traumatic/negative for SAH. To clarify, the presence/absence of xanthochromia was by visual inspection by the lab tech and the positive/negative/traumatic designation of the LP for SAH was that of the ED attending on record as listed in the chart.
Figure 4.
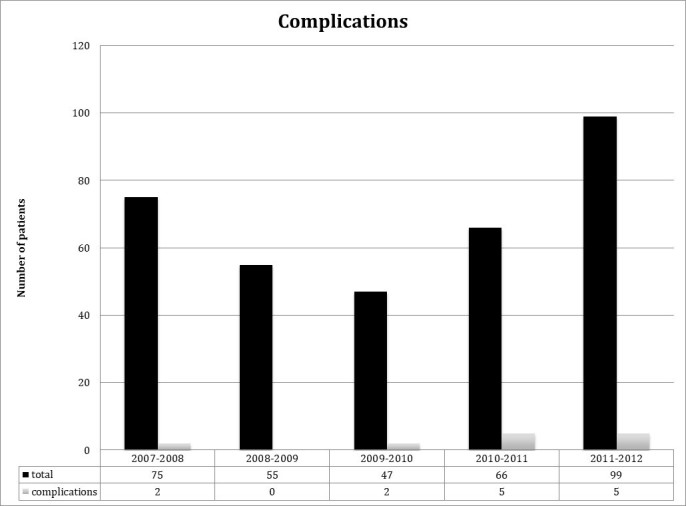
Yearly complication rate for all LPs performed.
Figure 5.
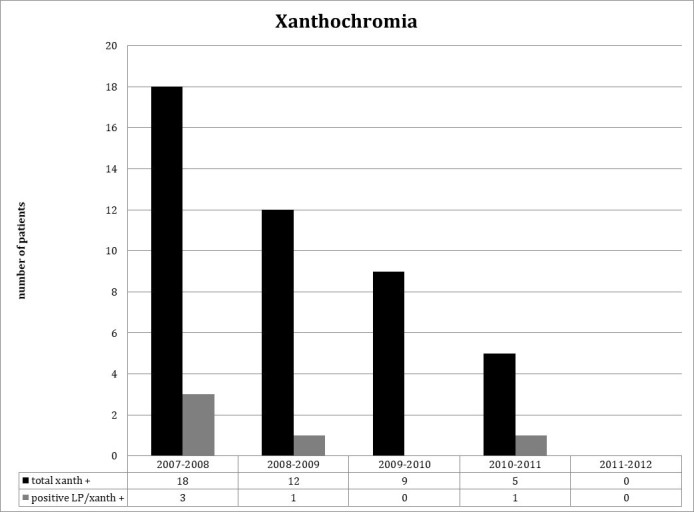
The yearly occurrence of all LPs that reported xanthochromia compared with those LPs that were considered positive for SAH and had xanthochromia.
Discussion
Our review of 342 neurologically intact patients presenting to the ED with an acute non-traumatic headache revealed only five patients with a negative head CT and an LP consistent with SAH. None were found to have a vascular etiology (aneurysm or AVM) to this SAH or experienced SAH-related mortality. The use of lumbar puncture in this cohort, however, was associated with both morbidity and substantially prolonged ED length of stay. None of those patients admitted for a false positive or equivocal LP or for observation were determined to have aneurysmal SAH. Within 90 days no discharged patients were re-admitted with a diagnosis of SAH, and none were treated at our regional neurosurgical center for SAH.
Acute care medical providers are often challenged when considering the diagnosis of SAH. Classical teaching, as suggested by the ACEP clinical policy [1], has traditionally been that in cases where the diagnosis is seriously considered, LP should be performed. The yield of this approach, however, is unknown. Recent prospective, high-quality studies of acute headache patients have found the yield of LP to be extremely low [4,11], but have not focused on, or specifically reported, the detection of aneurysmal rupture as an underlying etiology among the few SAH patients identified.
More specifically, we found that none of our subjects with a positive LP after negative head CT had significant neurosurgical interventions and all remained neurologically intact. In our estimation this finding could have three explanations. SAH events in our cohort may have been non-aneurysmal, or may have been small and self-limited, or positive LP findings in our cohort were falsely positive. Regardless of explanation all of these subjects seem to have had a benign hospital course with good neurologic outcomes. Importantly, these findings also led to invasive and aggressive testing and prolonged ICU stays with no health benefit. In effect, the LP was of negligible diagnostic yield and no therapeutic yield. These findings raise the question of whether routine LP is, overall, more beneficial or more harmful in such patients.
While aneurysmal SAH did not occur in our study cohort our data also raises questions about the overall utility of LP. Multiple alternate diagnoses were made in our cohort including a > 10 percent rate of meningitis, a condition requiring LP for identification. However, on further unstructured, non-blinded review of these charts, a significant number of subjects (75/342; 22 percent) were febrile or exhibited findings on physical examination, such as papilledema. This suggests that while LP was performed and SAH was a diagnostic consideration, meningitis and other diagnoses may have been additional, or even primary, conditions of diagnostic pursuit during ED evaluation.
In our study a significant number of LPs exhibiting xanthochromia were ultimately determined not to be associated with a diagnosis of SAH. While all LPs considered positive for SAH exhibited xanthochromia, it is intriguing that 39 other subjects with xanthochromia were ultimately deemed to have either incidental traumatic blood or cerebrospinal fluid analysis not consistent with xanthochromia due to SAH. Possible explanations include a remote non-aneurysmal SAH event ultimately identified through fluid analysis, the presence of contaminant red blood cells, and laboratory error. Clinical outcomes in this group were excellent, a finding that may raise questions about the legitimacy of xanthochromia as a proper criterion standard for diagnosis of SAH in a neurologically intact, CT-negative cohort.
It is also notable that LP testing in our data was associated with significantly increased ED length of stay. In specific, the mean time to a completed and interpreted head CT was 2.6 hours. The mean time to perform and result an LP was 5.3 hours. Both these aforementioned times are with respect to the same starting time, i.e, when the first triage vitals were documented in the medical record. This sums to an additive clinical time of 2.7 hours. In the setting of zero yield, as well as multiple studies finding increased mortality rates associated with ED crowding, the roughly 2.7 aggregate additional hours of bed occupancy associated with LP raises a major question about efficiency as well.
Overall, our study is yet another external validation of the low utility of LP in the evaluation of a neurologically intact patient with a headache (low risk) and concern for aneurysmal SAH. In particular, this study looks at the specific characteristics of the LP itself and not assume the proxy outcome of the LP being of less value with improved modern day CT technology [4]. Our study reports LP specific outcomes on 100 percent of patients studied. We report zero yield in this low risk group, i.e. the number of neurosurgical interventions in the subset that were LP positive for SAH but CT negative and neurologically intact. We also report the inefficiency and specifically the 2.7 extra hours for each patient evaluated which is important in light of the aforementioned poor yield. We also report the complication rate and the relative inaccuracy of xanthochromia as a clinical indicator of the presence of SAH in these low risk subsets. These specific LP characteristics are important to appreciate as completing a double-blinded, randomized, placebo-controlled trial where an LP is not offered is not the standard of care and is likely a study that may never be conducted. Our study also had higher resolution CT scanners (64-slice) that are more reflective of modern day practice and theoretically more robust of a sensitivity analysis of both the CT and by extension the LP. Our enrollment was up to 14 days making this data more generalizable to a greater ED population.
Recent studies [4,10] provide a pooled analysis of CT sensitivity within 6 hours or the 6 hour miss rate of modern day CT scanners. Our study adds to that data by giving 100 percent of the LP characteristics and the clinical implications of yield, efficiency, and complication rate. This LP data allows concrete data points for an emergency medicine practitioner for informed consent and shared decision making discussions with patients.
The design of the study followed the patients after discharge and even when they were admitted and assessed for clinical and patient specific/relevant outcomes. As no harm occurred to those patients that were discharged or those that were just admitted for observation of their headache this data suggests that perhaps an approach of just observation and serial neurological exams may be just as beneficial as doing the LP upfront in this low risk patient population. It is, of course, hard to identify true causality due to the retrospective nature of this study but the study is the first to suggest this alternative management approach as being safe.
Limitations
Our study was a chart review and is therefore challenged by the methodology, confounding, and potential biases inherent to chart review reports. We attempted to address these challenges through explicit methodology and minimization of potential bias through rigorous data collection methodology. In addition, our follow-up mechanism did not allow for identification of neurosurgical or SAH cases that may have occurred outside of the health care system catchment area. We also set strict criteria for inclusion, and excluded charts with “missing data,” as well as relying on billing codes corresponding to the performance of an LP. This approach may have limited power and may also have excluded cases of aneurysmal SAH falling under irregular final diagnosis codes or chart designations, or cases for which the procedure was not documented. Our evaluation of xanthochromia was via visual inspection alone and not spectrophotometry. This was a matter of institutional choice and not author preference. However, visual inspection for xanthochromia is a common practice in many institutions and may have more of a real-world application.
Conclusions
Our review of 342 ED-based LPs performed over a five-year period to identify potential SAH in neurologically intact patients with acute headaches after a negative head CT identified zero cases of aneurysmal SAH. In our setting, LP following negative CT imaging of the brain was therefore a test of extremely low yield, utility, and also associated with both increased morbidity and ED length of stay. Xanthochromia was diagnostically unhelpful in identifying SAH.
Acknowledgments
This group is thankful to the Connecticut College of Emergency Physicians for their research grant (award number R11679) and the funding resulting from the same that was instrumental to this project.
Glossary
- CT
computed tomography
- LP
lumbar puncture
- ED
emergency department
- ACEP
American college of Emergency Physicians
- SAH
subarachnoid hemorrhage
- CSF
cerebrospinal fluid
References
- American College of Emergency Physicians Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute headache. Ann Emerg Med. 2008. October;52(4):407–36. [DOI] [PubMed] [Google Scholar]
- Edlow JA, Malek AM, Ogilvy CS. Aneurysmal subarachnoid hemorrhage: update for emergency physicians. J Emerg Med. 2008. April;34(3):237–51. Epub 2007 Dec 26. [DOI] [PubMed] [Google Scholar]
- Edlow JA, Caplan LR. Avoiding pitfalls in the diagnosis of subarachnoid hemorrhage. N Engl J Med. 2000;342:29–36. [DOI] [PubMed] [Google Scholar]
- Perry JJ, Stiell IG, Sivilotti ML, Bullard MJ, Emond M, Symington C, et al. Sensitivity of computed tomography performed within six hours of onset of headache for diagnosis of subarachnoid haemorrhage: prospective cohort study. BMJ. 2011. July;343:d4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack RF, Hutson A. Can computed tomography angiography of the brain replace lumbar puncture in the evaluation of acute-onset headache after a negative noncontrast cranial computed tomography scan? Acad Emerg Med. 2010. April;17(4):444–51. [DOI] [PubMed] [Google Scholar]
- Mark DG, Pines JM. The detection of nontraumatic subarachnoid hemorrhage: still a diagnostic challenge. Am J Emerg Med. 2006. November;24(7):859–63. [DOI] [PubMed] [Google Scholar]
- van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369:306–18. [DOI] [PubMed] [Google Scholar]
- Edlow JA. Diagnosis of subarachnoid hemorrhage in the emergency department. Emerg Med Clin North Am. 2003;21:73–87. [DOI] [PubMed] [Google Scholar]
- Boesiger BM, Shiber JR. Subarachnoid hemorrhage diagnosis by computed tomography and lumbar puncture: are fifth generation CT scanners better at identifying subarachnoid hemorrhage? J Emerg Med. 2005;29:23–7. [DOI] [PubMed] [Google Scholar]
- Savitz SI, Edlow J. Thunderclap headache with normal CT and lumbar puncture: further investigations are unnecessary: for. Stroke. 2008;39:1392–3. [DOI] [PubMed] [Google Scholar]
- Perry JJ, Spacek A, Forbes M, et al. Is the combination of negative computed tomography result and negative lumbar puncture result sufficient to rule out subarachnoid hemorrhage? Ann Emerg Med. 2008;51:707–13. [DOI] [PubMed] [Google Scholar]
- Wijdicks EF, Kerkhoff H, van Gijn J. Long-term follow-up of 71 patients with thunderclap headache mimicking subarachnoid haemorrhage. Lancet. 1988;2:68–70. [DOI] [PubMed] [Google Scholar]
- Landtblom AM, Fridriksson S, Boivie J, et al. Sudden onset headache: a prospective study of features, incidence and causes. Cephalalgia. 2002;22:354–60. [DOI] [PubMed] [Google Scholar]
- Harling DW, Peatfield RC, Van Hille PT, et al. Thunderclap headache: is it migraine? Cephalalgia. 1989;9:87–90. [DOI] [PubMed] [Google Scholar]
- Linn FH, Wijdicks EF, van der Graaf Y, et al. Prospective study of sentinel headache in aneurysmal subarachnoid haemorrhage. Lancet. 1994;344:590–3. [DOI] [PubMed] [Google Scholar]
- Gee C, Dawson M, Bledsoe J, Ledyard H, Phanthavady T, Youngquist S, et al. Sensitivity of newer-generation computed tomography scanners for subarachnoid hemorrhage: a Bayesian analysis. J Emerg Med. 2012. July;43(1):13–8. [DOI] [PubMed] [Google Scholar]
- Sidman R, Connolly E, Lemke T. Subarachnoid hemorrhage diagnosis: lumbar puncture is still needed when the computed tomography scan is normal. Acad Emerg Med. 1996;3:827–31. [DOI] [PubMed] [Google Scholar]
- Mehrotra P, Sookhoo S, Kolla S, Halbert H, Lavell K, England S. Investigation of subarachnoid haemorrhage: does the buck stop with CT? J Med Life. 2010. Jul-Sep;3(3):338–42. [PMC free article] [PubMed] [Google Scholar]
- Dupont SA, Wijdicks EF, Manno EM, Rabinstein AA. Thunderclap headache and normal computed tomographic results: value of cerebrospinal fluid analysis. Mayo Clin Proc. 2008. December;83(12):1326–31. [DOI] [PubMed] [Google Scholar]
- Mona Mohamed D. Cressler Heasely, Banu Yagmurlu, and David M. Yousem. Fluid-Attenuated Inversion Recovery MR Imaging and Subarachnoid Hemorrhage: not a Panacea. AJNR Am J Neuroradiol. 2004. April;25:545–50. [PMC free article] [PubMed] [Google Scholar]