Abstract
Degenerative Cervical Myelopathy (DCM) is the most common form of spinal cord impairment in adults and results in disability and reduced quality of life. DCM can present with a wide set of clinical and imaging findings, including: 1) pain and reduced range of motion of the neck, and motor and sensory deficits on clinical exam, and 2) cord compression due to static and dynamic injury mechanisms resulting from degenerative changes of the bone, ligaments, and intervertebral discs on MRI. The incidence and prevalence of DCM has been estimated at a minimum of 4.1 and 60.5 per 100,000, respectively, but surgical trends and an aging population suggest these numbers will rise in the future. The diagnosis of DCM is based on clinical examination, with a positive Hoffmann’s sign and hand numbness typically appearing in the upper limbs, and gait abnormalities such as difficulty with tandem gait serving as sensitive diagnostic findings. Loss of bladder function may also occur in patients with severe DCM. The degree of neurological impairment can be measured using the modified Japanese Association Scale (mJOA) or Nurick grade. Non-operative management has a limited role in the treatment, while surgical management has been shown to both be safe and effective for halting disease progression and improving neurological function. Predictors of surgical outcome include age and baseline severity, indicating that early recognition of DCM is important for ensuring an optimal surgical outcome.
Keywords: Cervical Spondylotic Myelopathy, sensation, pain, compressive myelopathy
Introduction
Degenerative cervical myelopathy (DCM) is an overarching term encompassing several etiologies including cervical spondylotic myelopathy (CSM), ossification of the posterior longitudinal ligament (OPLL), ossification of the ligamentum flavum (OLF), and degenerative disc disease (DDD). Together, this diverse group of pathologies represent the most common cause of degenerative, non-traumatic spinal cord impairment in the adult population [1,2]. While each of these entities present unique pathophysiology, much of the pathogenesis is interrelated, often blending together into a complex myelopathic picture.
In this overview, we discuss the natural history of DCM and its symptomatology; specifically focusing on the alterations in sensorium, unique neuropathic manifestations, and nociceptive changes that occur as the cervical spinal cord undergoes chronic, progressive compression. Characteristic imaging features are presented in the context of patient presentation, emphasizing anatomical correlation with both subjective and objective clinical findings. Expectant and surgical treatment of DCM are reviewed, including the anticipated disease trajectory in both avenues of patient management.
Topics
Epidemiology
The incidence and prevalence of DCM in North America has been estimated at a minimum of 4.1 and 60.5 per 100,000, respectively [2], but strong epidemiological data has been difficult to obtain likely due to the complex multifactorial etiology of the disease process [3]. New et al. [4] estimated that degenerative diseases of the spine comprise 59 percent of non-traumatic spinal cord injury in Japan, 54 percent in the United States, 31 percent in Europe, 22 percent in Australia, and up to 30 percent in Africa. The authors also proposed the regional incidence to be 76, 26, and 6 per million in North America, Europe, and Australia, respectively. Although this data is not limited to cervical spinal cord injury and many patients with less severe symptoms were excluded from several studies included in the review, it is reasonable to infer that since DCM is one of the most common causes of non-traumatic cervical spinal cord injury, it represents a formidable problem in the aging North American population. More in-depth analyses of the epidemiological trends in individual etiologies contributing to DCM are available in recent reviews by Nouri et al. [2] and Davies et al. [5].
Pathogenesis: Disc Degeneration and Vertebral Restructuring
In general, degeneration of the cervical spine occurs over time as a result of structural load, repetitive microtrauma, and age-related changes to bone, muscle, and intervertebral disc physiology. The degenerative process typically begins with wear of the disc, which normally acts to distribute pressure forces evenly on vertebral endplates and facet joints. Through the loss of proteoglycans and water, discs lose their elastic and supportive nature and begin to levy uneven pressure forces on adjacent vertebrae, which subsequently results in osteophyte development. Vertebrae also progressively lose their height and progressively widen. The culmination of these processes converge in the form of spinal canal stenosis leading to chronic compression of the spinal cord and eventual development of myelopathy [2]. For this reason, patients with either a narrow spinal canal or large spinal cord (cord-canal mismatch) are at increased risk for developing DCM over their lifetime [6,7]. In addition to static injury, these gross anatomical changes may also result in increased mobility or spondylolisthesis which may be stable or unstable. When unstable, increased range of motion can result in dynamic injury and repetitive minor trauma [8-11]. From a pathophysiological perspective, the injury to the spinal cord eventually disrupts the blood-spinal cord barrier resulting in neuroinflammation, ischemia, and apoptosis, which collectively contribute to demyelination, astrogliosis, and axonal degeneration [12-15]. This eventually culminates in the manifestation of symptomatic myelopathy with characteristic clinical findings (Table 1).
Table 1. Typical clinical signs and symptoms seen in patients with DCM.
| Symptoms | Signs |
| Motor deficits | Inverted brachioradialis reflex |
| Numbness of hands | Hoffmann’s sign |
| Thenar atrophy | Ankle clonus |
| Hyperreflexia | Babinski Sign |
| Spasticity | Romberg Sign |
| Impairment of gait | Lhermitte’s phenomenon |
| Incontinence | |
| Clumsy hands | |
| Weakness | |
| Paresthesias |
Pathogenesis: Hypertrophy and Ossification of the Spinal Canal Ligaments
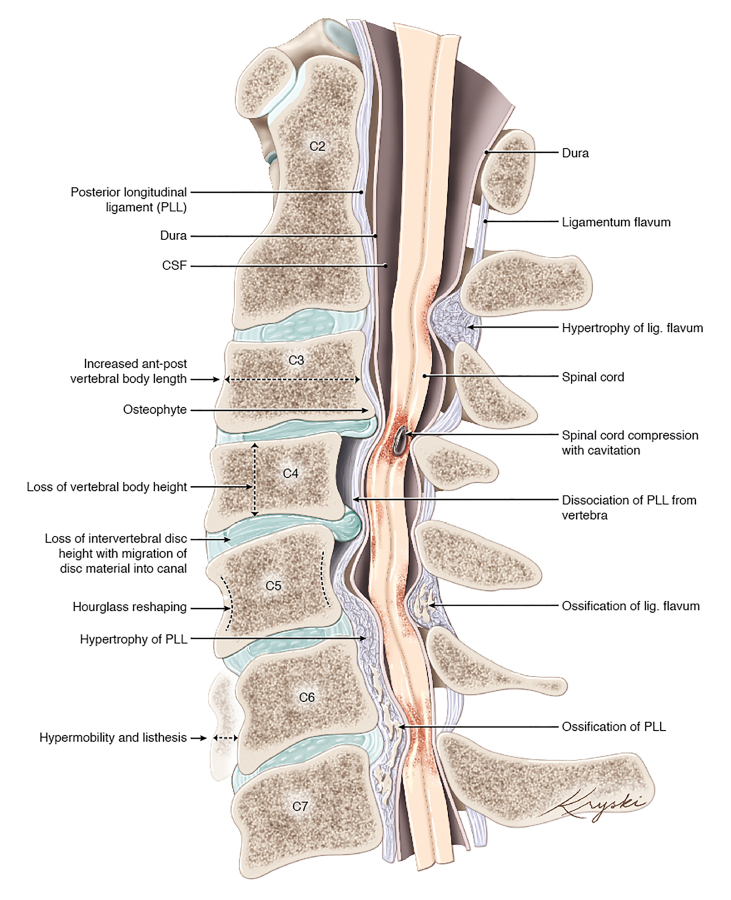
Spinal cord compression from degenerative changes can also occur due to the enlargement and ossification of spinal canal ligaments, specifically the posterior longitudinal ligament (PLL) and the ligamentum flavum (LF) (Figure 1). The enlargement of the ligaments may occur as a result of (1) disc bulging into the canal causing enlargement of the PLL, sometimes progressing to reactive ossification of the PLL, and (2) loss in disc height, frequently resulting in inbuckling and rarely ossification of the LF and compression of the spinal cord from the posterior [16]. Genetic factors have also been implicated in the development of ossification of these spinal ligaments, and these patients may not exhibit clear degenerative findings. Patients from East Asia have been shown to be particularly afflicted by OPLL [2].
Figure 1.
Illustration of the various gross anatomic pathophysiologic changes that contribute to degenerative cervical myelopathy. PLL, posterior longitudinal ligament; CSF, cerebrospinal fluid. Originally published by Nouri et al. (2015) [2], medical illustration by Diana Kryski (Kryski Biomedia).
Sensory Changes and Clinical Findings in DCM
Although the natural history of DCM varies greatly, the classic subjective findings present while eliciting the history of afflicted patients are neck pain, diminished hand dexterity, balance difficulties, paresthesias, weakness, and in severe cases, bowel/bladder dysfunction. There are several classification systems based on these physical findings including Nurick grade [17], Japanese Orthopedia Association scale (JOA) [18], and modified Japanese Orthopedic Association scale (mJOA) [19]. The Nurick grade focuses on the degree of mobility, and ability to carry out daily functions and employment, while the JOA and mJOA incorporates assessment of upper motor, lower motor, sensory and bladder function. Of note, radiculopathic symptoms can be present as a confounding factor in DCM. A recent retrospective study by Choi et al. [20] of 127 patients found that 66 (51.9 percent) had combined cervical radiculopathy and myelopathy; therefore, it is important to evaluate for both upon investigation of a patient’s presentation. The finding of concomitant radiculopathy can also complicate the clinical evaluation as radiculopathy typically presents with hypo-reflexia, while myelopathy presents with hyper-reflexia.
Myelopathic signs on physical examination have varying sensitivity and specificity: hyperreflexia (72 percent and 43 percent) with biceps representing the most sensitive at 62 percent and brachioradialis representing the most specific at 89 percent, Hoffmann sign (59 percent and 84 percent), an inverted brachioradialis reflex (51 percent and 81 percent), clonus (13 percent and 100 percent), and Babinski (13 percent and 100 percent) [21]. Despite the sub-optimal diagnostic value of many of these provocative signs, DCM patients demonstrate at least 1 myelopathic sign 79 percent of the time vs. 57 percent in controls (comprised of patients with neck pain or radicular symptoms, but not myelopathy), and if cord signal changes or myelomalacia are present these numbers jump to 95 percent vs. 63 percent. In addition to the aforementioned signs, sensory testing – sensation to pain, light touch, deep pressure, two-point discrimination, vibration, and proprioception – should be performed in both the upper and lower extremities. Tests of hand dexterity, such as the “15 second grip-and-release” exam, may also be useful (normal patients can grip/release 25 to 30 times in 15 seconds, which may be reduced in DCM) [22,23]. Nerve conduction studies including somatosensory evoked potentials, motor evoked potentials, and electromyography can be useful in differentiating between peripheral and central pathology in addition to potentially allowing differentiation between dorsal/ventral cord compression signs [24].
Diagnostic Imaging of the Cervical Spine in DCM
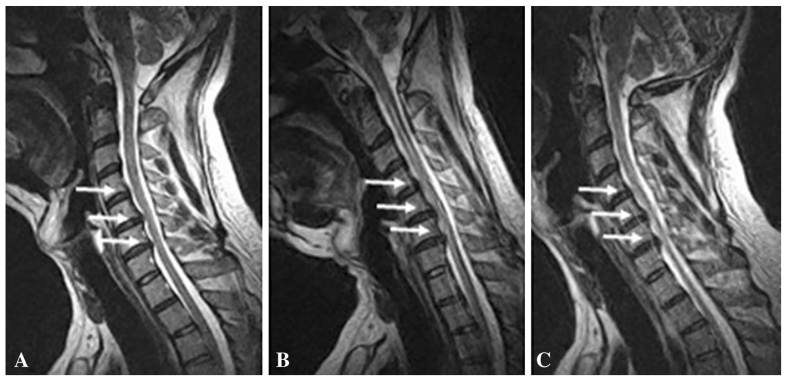
Although signs of a reduced spinal canal diameter and calcified changes can be observed on computed tomography (CT) of the cervical spine, magnetic resonance imaging (MRI) remains the imaging modality of choice for evaluation of DCM. CT myelogram offers an alternative method of assessing cervical cord compression in patients who are unable to undergo MRI [25]. MRI allows for assessment of vertebral bone changes, intervertebral disc degeneration, PLL/LF changes, structural deformities, characterization of surrounding soft-tissue structures, and visualization of spinal cord morphology and injury [16]. In patients with spondylolisthesis, suspected instability, or movement-dependent cord compression, the use of flexion and extension radiographs or MRI are also useful (Figure 2).
Figure 2.
Cervical disc bulge in a 40-year-old man. In the neutral position (A), T2-weighted sagittal MR image shows C4/5, C5/6, and C6/7 levels disc bulge. In flexion (B) and extension (C), the disc bulge is increased, especially in the C6/7 level. Adapted with permission, Springer Nature. European Spine Journal. Missed cervical disc bulges diagnosed with kinematic magnetic resonance imaging. Lao L, Daubs MD, Scott TP, Phan KH, Wang JC. Copyright 2014.
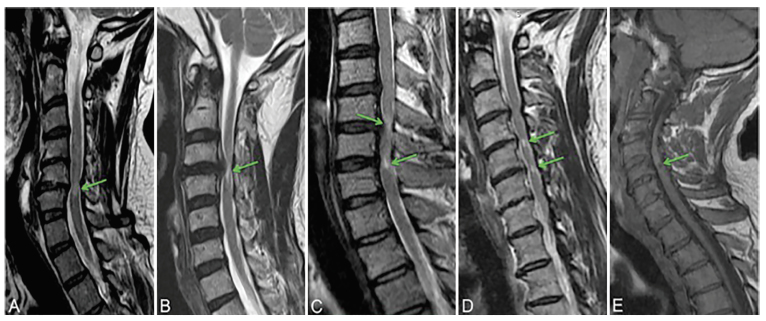
Signal changes seen on T1- and T2-weighted MRI imaging of the spinal cord have also shown a moderate ability to predict outcomes after surgical intervention [26-29]. In general, T2 weak signal hyperintensity (more intense than normal spinal cord but less intense than CSF) that appears diffuse without clear bordering has been associated with potentially reversible changes such as edema, Wallerian degeneration, demyelination, and ischemia. T2 imaging showing substantial hyperintensity with sharp bordering and T1 hypointensity represent changes considered to be irreversible such as cavitation, neural tissue loss, myelomalacia, necrosis, and spongiform changes in gray matter (Figure 3) [30,31]. In addition, myelopathic signs as discussed previously have been shown to be significantly more common in patients with cord signal changes suggestive of myelomalacia [21].
Figure 3.
Types of signal changes that can appear in patients with DCM. A-D: Sagittal T2WI. A: Type I, diffuse and faint hyperintensity. B: Type II, focal and sharp hyperintensity. C: Type III, both Type I (higher arrow) and Type II (lower arrow) hyperintensity characteristics are present. D: Two discontinuous focal hyperintensities are present. E: Sagittal MRI with T1WI showing a focal hypointensity. Originally published in Nouri et al. (2016) [16]. Reprinted with permission from Neurosurgical Focus.
Treatment
DCM is almost always considered a surgical problem, with between 20 to 62 percent of patients deteriorating at 3 to 6 years of follow-up when managed expectantly [32]. Furthermore, in patients presenting asymptomatically with cord compression, the incidence of development of symptomatic myelopathy is approximately 8 percent at 1 year and nearly 23 percent at 4 years of follow-up [33]. A recent study by Zhang et al. [34] showed improvement in all age groups managed surgically, with significant recovery within 1 week and at 6 months following surgery, and no difference between ages in postoperative complications. Based on these findings, and those of several other studies including one by Fehlings et al. [35,36] which investigate outcomes in surgical decompression, it is nearly universally recommended that DCM be intervened on surgically at the earliest possible opportunity to prevent progression and allow maximum potential for recovery. Surgical approaches to decompress the spinal cord can be undertaken by removing the offending compressive pathology, expanding the spinal canal through removal or manipulation of the poster lamina of the vertebrae. Anterior approaches are typically favored in patients with cervical kyphosis and in those with large anterior pathology, while posterior approaches are favored with multilevel cervical compression or OPLL [37].
Ongoing clinical trials suggest that there may be a role for adjunct pharmacologic treatment, such as addressing glutamate-induced excitotoxicity with riluzole, in conjunction with surgical decompression [32]. Further investigation is needed in this area, but future therapeutic regimens will likely involve a multi-faceted approach in addition to traditional surgical intervention.
Conclusions
While DCM can substantially impact neurological function and result in disability, early recognition and treatment can prevent patients from further deterioration and can help most patients recover some neurological function. Clinical diagnosis relies heavily on characteristic symptoms and signs elicited during history and physical exam which prompt further investigation with cervical spine imaging. It is therefore imperative that clinicians remain vigilant in considering DCM as a differential diagnosis when symptoms appear, particularly in the elderly population who often attribute their function decline to advancing age.
Glossary
- DCM
Degenerative Cervical Myelopathy
- mJOA
modified Japanese Association Scale
- CSM
cervical spondylotic myelopathy
- OPLL
ossification of the posterior longitudinal ligament
- OLF
ossification of the ligamentum flavum
- DDD
degenerative disc disease
- PLL
posterior longitudinal ligament
- LF
ligamentum flavum
- JOA
Japanese Orthopedia Association
- CT
computed tomography
- MRI
magnetic resonance imaging
References
- Kalsi-Ryan S, Karadimas SK, Fehlings MG. Cervical spondylotic myelopathy: the clinical phenomenon and the current pathobiology of an increasingly prevalent and devastating disorder. Neuroscientist. 2013;19(4):409–21. [DOI] [PubMed] [Google Scholar]
- Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG. Degenerative Cervical Myelopathy: Epidemiology, Genetics, and Pathogenesis. Spine (Phila Pa 1976). 2015;40(12):E675–93. [DOI] [PubMed] [Google Scholar]
- Wu JC, Ko CC, Yen YS, Huang WC, Chen YC, Liu L, et al. Epidemiology of cervical spondylotic myelopathy and its risk of causing spinal cord injury: a national cohort study. Neurosurg Focus. 2013;35(1):E10. [DOI] [PubMed] [Google Scholar]
- New PW, Cripps RA, Bonne Lee B. Global maps of non-traumatic spinal cord injury epidemiology: towards a living data repository. Spinal Cord. 2014;52(2):97–109. [DOI] [PubMed] [Google Scholar]
- Davies BM, McHugh M, Elgheriani A, Kolias AG, Tetreault L, Hutchinson PJ, et al. The reporting of study and population characteristics in degenerative cervical myelopathy: A systematic review. PLoS One. 2017;12(3):e0172564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita Y, Naito M, Hymanson H, Miyazaki M, Wu G, Wang JC. The relationship between the cervical spinal canal diameter and the pathological changes in the cervical spine. Eur Spine J. 2009;18(6):877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouri A, Montejo J, Sun X, Virojanapa J, Kolb LE, Abbed KM, et al. Cervical Cord-Canal Mismatch: A New Method for Identifying Predisposition to Spinal Cord Injury. World Neurosurg. 2017. December;108:112–7. [DOI] [PubMed] [Google Scholar]
- Matsunaga S, Kukita M, Hayashi K, Shinkura R, Koriyama C, Sakou T, et al. Pathogenesis of myelopathy in patients with ossification of the posterior longitudinal ligament. J Neurosurg. 2002;96(2 Suppl):168–72. [DOI] [PubMed] [Google Scholar]
- Fengbin Y, Deyu C, Xinwei W, Yu C, Jinhao M, Xinyuan L, et al. Trauma-induced spinal cord injury in cervical spondylotic myelopathy with or without lower cervical instability. J Clin Neurosci. 2013;20(3):419–22. [DOI] [PubMed] [Google Scholar]
- Fujiyoshi T, Yamazaki M, Okawa A, Kawabe J, Hayashi K, Endo T, et al. Static versus dynamic factors for the development of myelopathy in patients with cervical ossification of the posterior longitudinal ligament. J Clin Neurosci. 2010;17(3):320–4. [DOI] [PubMed] [Google Scholar]
- Jiang SD, Jiang LS, Dai LY. Degenerative cervical spondylolisthesis: a systematic review. Int Orthop. 2011;35(6):869–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadimas SK, Gatzounis G, Fehlings MG. Pathobiology of cervical spondylotic myelopathy. Eur Spine J. 2015;24 Suppl 2:132–8. [DOI] [PubMed] [Google Scholar]
- Beattie MS, Manley GT. Tight squeeze, slow burn: inflammation and the aetiology of cervical myelopathy. Brain. 2011;134(Pt 5):1259–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadimas SK, Moon ES, Yu WR, Satkunendrarajah K, Kallitsis JK, Gatzounis G, et al. A novel experimental model of cervical spondylotic myelopathy (CSM) to facilitate translational research. Neurobiol Dis. 2013;54:43–58. [DOI] [PubMed] [Google Scholar]
- Karadimas SK, Yu WR, Fehlings MG. Comrpomise of spinal cord microvasculature in cervical spondylotic myelopathy. Soc Neurosci. 2012 [Google Scholar]
- Nouri A, Martin AR, Mikulis D, Fehlings MG. Magnetic resonance imaging assessment of degenerative cervical myelopathy: a review of structural changes and measurement techniques. Neurosurg Focus. 2016;40(6):E5. [DOI] [PubMed] [Google Scholar]
- Nurick S. The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95(1):87–100. [DOI] [PubMed] [Google Scholar]
- Hukuda S, Mochizuki T, Ogata M, Shichikawa K, Shimomura Y. Operations for cervical spondylotic myelopathy. A comparison of the results of anterior and posterior procedures. J Bone Joint Surg Br. 1985;67(4):609–15. [DOI] [PubMed] [Google Scholar]
- Chiles BW, 3rd, Leonard MA, Choudhri HF, Cooper PR. Cervical spondylotic myelopathy: patterns of neurological deficit and recovery after anterior cervical decompression. Neurosurgery. 1999;44(4):762–9. [DOI] [PubMed] [Google Scholar]
- Choi BW, Kim SS, Lee DH, Kim JW. Cervical radiculopathy combined with cervical myelopathy: prevalence and characteristics. Eur J Orthop Surg Traumatol. 2017 [DOI] [PubMed] [Google Scholar]
- Rhee JM, Heflin JA, Hamasaki T, Freedman B. Prevalence of physical signs in cervical myelopathy: a prospective, controlled study. Spine (Phila Pa 1976). 2009;34(9):890–5. [DOI] [PubMed] [Google Scholar]
- Hosono N, Sakaura H, Mukai Y, Kaito T, Makino T, Yoshikawa H. A simple performance test for quantifying the severity of cervical myelopathy. J Bone Joint Surg Br. 2008;90(9):1210–3. [DOI] [PubMed] [Google Scholar]
- Lebl DR, Hughes A, Cammisa FP, Jr, O’Leary PF. Cervical spondylotic myelopathy: pathophysiology, clinical presentation, and treatment. HSS J. 2011;7(2):170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone R, Holler Y, Brigo F, Frey VN, Lochner P, Leis S, et al. The contribution of neurophysiology in the diagnosis and management of cervical spondylotic myelopathy: a review. Spinal Cord. 2016;54(10):756–66. [DOI] [PubMed] [Google Scholar]
- Cowley P. Neuroimaging of Spinal Canal Stenosis. Magn Reson Imaging Clin N Am. 2016;24(3):523–39. [DOI] [PubMed] [Google Scholar]
- Arvin B, Kalsi-Ryan S, Mercier D, Furlan JC, Massicotte EM, Fehlings MG. Preoperative magnetic resonance imaging is associated with baseline neurological status and can predict postoperative recovery in patients with cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2013;38(14):1170–6. [DOI] [PubMed] [Google Scholar]
- Harrop JS, Naroji S, Maltenfort M, Anderson DG, Albert T, Ratliff JK, et al. Cervical myelopathy: a clinical and radiographic evaluation and correlation to cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2010;35(6):620–4. [DOI] [PubMed] [Google Scholar]
- Karpova A, Arun R, Kalsi-Ryan S, Massicotte EM, Kopjar B, Fehlings MG. Do quantitative magnetic resonance imaging parameters correlate with the clinical presentation and functional outcomes after surgery in cervical spondylotic myelopathy? A prospective multicenter study. Spine (Phila Pa 1976). 2014;39(18):1488–97. [DOI] [PubMed] [Google Scholar]
- Li F, Chen Z, Zhang F, Shen H, Hou T. A meta-analysis showing that high signal intensity on T2-weighted MRI is associated with poor prognosis for patients with cervical spondylotic myelopathy. J Clin Neurosci. 2011;18(12):1592–5. [DOI] [PubMed] [Google Scholar]
- Vedantam A, Rajshekhar V. Does the type of T2-weighted hyperintensity influence surgical outcome in patients with cervical spondylotic myelopathy? A review. Eur Spine J. 2013;22(1):96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetreault LA, Dettori JR, Wilson JR, Singh A, Nouri A, Fehlings MG, et al. Systematic review of magnetic resonance imaging characteristics that affect treatment decision making and predict clinical outcome in patients with cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2013;38(22 Suppl 1):S89–110. [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Tetreault LA, Wilson JR, Skelly AC. Cervical spondylotic myelopathy: current state of the art and future directions. Spine (Phila Pa 1976). 2013;38(22 Suppl 1):S1–8. [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Wilson JR, Yoon ST, Rhee JM, Shamji MF, Lawrence BD. Symptomatic progression of cervical myelopathy and the role of nonsurgical management: a consensus statement. Spine (Phila Pa 1976). 2013;38(22 Suppl 1):S19–20. [DOI] [PubMed] [Google Scholar]
- Zhang RJ, Shen CL, Zhang JX, Zhang XJ, Dong FL, Tao H, et al. Clinical features and surgical outcomes of cervical spondylotic myelopathy in patients of different ages: a retrospective study. Spinal Cord. 2017 [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Ibrahim A, Tetreault L, Albanese V, Alvarado M, Arnold P, et al. A global perspective on the outcomes of surgical decompression in patients with cervical spondylotic myelopathy: results from the prospective multicenter AOSpine international study on 479 patients. Spine (Phila Pa 1976). 2015;40(17):1322–8. [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Wilson JR, Kopjar B, Yoon ST, Arnold PM, Massicotte EM, et al. Efficacy and safety of surgical decompression in patients with cervical spondylotic myelopathy: results of the AOSpine North America prospective multi-center study. J Bone Joint Surg Am. 2013;95(18):1651–8. [DOI] [PubMed] [Google Scholar]
- Nouri A, Martin AR, Nater A, Witiw CD, Kato S, Tetreault L, et al. The Influence of MRI Features on Surgical-Decision Making in Degenerative Cervical Myelopathy: Results From a Global Survey of AOSpine International Members. World Neurosurg. 2017;105:864–74. [DOI] [PubMed] [Google Scholar]