Abstract
The study of cancer has represented a central focus in medical research for over a century. The great complexity and constant evolution of the pathology require the use of multiple research model systems and interdisciplinary approaches. This is necessary in order to achieve a comprehensive understanding into the mechanisms driving disease initiation and progression, to aid the development of appropriate therapies. In recent decades, the fruit fly Drosophila melanogaster and its associated powerful genetic tools have become a very attractive model system to study tumour-intrinsic and non-tumour-derived processes that mediate tumour development in vivo. In this review, we will summarize recent work on Drosophila as a model system to study cancer biology. We will focus on the interactions between tumours and their microenvironment, including extrinsic mechanisms affecting tumour growth and how tumours impact systemic host physiology.
1. Introduction
Despite being the most studied human disease, cancer remains a leading cause of mortality worldwide. Nearly 1 in 6 deaths in 2015 was attributable to cancer, according to the World Health Organization, with an increase of 70% of new cases projected within the next two decades [1]. The seemingly restricted success in controlling and reducing the devastating outcomes of this disease is due, to a great extent, to the high complexity and variable nature of the pathology. The current limited understanding of many aspects of cancer biology is partly imposed by limitations in conventional animal models of research.
The organismal implications and ultimate outcome of tumour burden in patients are undoubtedly determined by a combination of tumour-intrinsic mechanisms and interactions between tumours and proximal, as well as distal tissues [2–4]. While cancer research has classically focused on identifying tumour autonomous processes, there is a recent growing interest in understanding the nonautonomous mechanisms that control tumour progression [5]. Indeed, pioneering work dating back to the 19th century established the notion that distant tissues influence tumour growth and metastasis, when in 1896 Sir Beatson published a report on the treatment of inoperable cases of breast carcinomas through ovariectomy [2]. More recently, many molecular mechanisms have been identified highlighting the importance of the tumour microenvironment (TME) in cancer progression [5]. The crosstalk between tumour cells and their microenvironment often resembles normal physiological responses: for example, interactions between cancer cells and the immune system imitate various aspects of host-pathogen interaction [6]. In such a context, the body can detect cancer cells and react by mounting an immune response, to fight abnormal cell behaviours associated with the presence of a tumour. However, tumour cells appear to evolve to turn on new or divert existing physiological programs in order to evade the action of the immune system [6, 7]. The end result of such a power struggle between cancer cells and the surrounding tissues will ultimately determine the outcome of the tumour and its host. Targeting nontumoural tissues to counteract cancer growth is becoming a prime therapeutic strategy, which takes advantage of the higher genetic stability and lesser susceptibility of normal cells to escape drug treatments [8]. Hence, the discovery of novel non-tumour autonomous mechanisms to fight cancer progression is a promising area of research. However, the physiological complexity and limitations in the genetic accessibility of mammalian models systems render in vivo studies of non-tumour autonomous processes difficult to accomplish in conventional whole animal model systems.
Drosophila melanogaster remains the most powerful genetic model in research. During the last decades, the development of various tumour models, including leukaemia, neuroblastoma, glioblastoma, colorectal, and ovarian cancer, has made the fruit fly an attractive in vivo model system to decipher tumour intrinsic (i.e., tumour cell-autonomous) and extrinsic (i.e., non-tumour autonomous) molecular mechanisms mediating tumour growth and metastasis [9, 10]. Such studies have revealed astonishing conservation in the processes driving cancer development between flies and humans [10]. The ability to spatially and temporally regulate gene expression in tumour-bearing animals, as well as the low genetic redundancy, is particularly useful for the study of non-tumour autonomous mechanisms. Major advances in the understanding of these tumour-extrinsic mechanisms have been provided through the use of models based on loss of cell polarity, utilising mutants of the scribble-group of tumour suppressors genes (scribble: scrib, lethal giant larvae: lgl, and disc large: dlg), which encode key components of the basolateral polarity complex [11]. These mutations induce transformation of larval epithelial tissues, called imaginal discs, into “benign” neoplastic tumours. In this context, activation of proto-oncogenes, such as Ras or Src, drives tumour cell proliferation, spreading to distant tissues [12, 13]. During the years following the discovery of scrib-group genes as tumour suppressors in Drosophila, research has provided growing evidence that these models are directly relevant to human conditions. Indeed, scrib and dlg proteins are known targets of several oncogenic viruses, such as Human Papillomavirus, the main agent of cervical cancers. These viruses induce the degradation of the polarity complex proteins, comprising a key part of the process of malignant transformation in these conditions [11, 14, 15]. Loss of scrib has also been shown to work as a tumour suppressor in human breast, liver, skin, and lung cancers [16–19]. The loss of the human homolog of the Lgl protein has been involved in colorectal cancer [20] and hepatocarcinoma [21] and is associated with an increased risk of metastasis in endometrial cancer [22]. Moreover, similar to its Drosophila homolog, scrib also cooperates with the Ras oncogene to promote tumour cell invasion [12, 23].
Here, we discuss recent discoveries in Drosophila that have shed light into how extrinsic signals influence tumours, as well as mechanisms that mediate the systemic impact of tumours in the host. We focus on new findings highlighting the influence of immunity and metabolism in cancer progression and cancer-related disorders.
2. Cellular and Systemic Immunity Influence Tumour Growth and Cell Death
2.1. The Immune System: A Double-Edged Sword
Work in mammals has highlighted the immune system as a key component of the tumour microenvironment (TME), which plays a critical role in defining tumour outcome. While early studies on cancer patients support anticancer activity of the immune system [24], recent research has revealed that immunity can also promote tumour growth and metastasis [25]. However, deciphering the mechanisms of this dual immune function is a challenging task, mostly due to the complex cellular and molecular composition of the mammalian immune system [5]. For the past 15 years, the development of cancer models in Drosophila has allowed the discovery of molecular mechanisms mediating both pro- and antitumoural immunity. In contrast to mammals, which possess both innate and adaptive immunity, Drosophila only relies on innate immunity to fight against pathogens and tumours. Additionally, while mammals have numerous types of white blood cells, the cellular arm of Drosophila innate immunity includes only three main cells types—plasmatocytes, lamellocytes, and crystal cells—commonly called haemocytes. Only plasmatocytes have been currently reported to be associated with tumours [26]; however, a possible diversity within the haemocyte population bound to tumours cannot be excluded. Even if such macrophage-like cells were unable to infiltrate tumours as macrophages do, they could still produce a cocktail of mammalian-like cytokines leading to inflammation. While short-term inflammation can be beneficial to protect the host from challenges, such as those posed by pathogenic infection, chronic inflammation is associated with tumour initiation and metastasis in both Drosophila and mammals [27–29].
Tumour Necrosis Factor alpha (TNF-α) is a major proinflammatory cytokine produced within the TME, which was originally characterised for its ability to induce tumour death [30]. Consistently, TNF-α's discovery led to great expectations for its use as a therapeutic target for cancer. However, further experiments have revealed a dual role for TNF-α as both an anti- and protumour factor [31]. The molecular bases of TNF-α's antagonistic actions were poorly understood. However, recent research in Drosophila has highlighted some key molecular aspects underlying this dual action of the cytokine. Drosophila possesses a single TNF-α homolog called Eiger (Egr) [32, 33], whose role as an immune proinflammatory cytokine is conserved [34]. The importance of Egr in the TME has been highlighted in Drosophila tumour models through the use of mutants of the scrib-group of tumour suppressors genes. Egr expression is induced in tumours and tumour-associated immune cells [35, 36], much like mammalian TNF-α, which is detected in tumour cells as well as macrophages and T lymphocytes [31]. Given the focus of this review, we will only discuss the extrinsic role of Egr here. However, a tumour-intrinsic role of the cytokine has also been previously demonstrated [35, 37].
2.2. Cellular Arm of the Immune System and Associated Cytokines
Experimental evidence showed that immune cell-derived Egr has antitumoural activity. Patches of scrib, lgl, or dlg mutant cells generated in imaginal discs, delaminate, and are mostly removed from the epithelia through cell competition [12, 37–40]. However, in Egr mutant animals elimination of polarity deficient clones is abolished, and this effect can be recapitulated by knocking down Egr specifically within haemocytes, highlighting a conserved non-tumour autonomous anticancer function of Egr in Drosophila [35, 36] (Figure 1). Complementarily, loss of the TNF-α receptor Grindelwald (Grnd) in scrib mutant cells suppressed their removal from the epithelia [41]. In those cases, where a group of mutant cells is generated in a wild-type background, the elimination of mutant cells through cell competition relies on Egr-dependent JNK activation, which subsequently restricts cell proliferation and the survival of mutant cells [35, 38, 42]. This JNK-dependent toxic effect of TNF-α is conserved in mammals, as TNF-α induces cell death through TNFR1 and subsequent JNK signalling activation [29]. Recent discoveries of new molecules driving cell competition in Drosophila, including immune response proteins, may uncover new mechanisms involved in the elimination of cancer cells from a healthy tissue [43–45]. Egr has also been shown to exert antitumoural effects independently of cell competition. Full mutants animals for scrib-group genes, where neoplastic tumours develop from the whole imaginal disc, also show dependency from haemocyte-derived Egr to trigger JNK activation and tumour cell death [36, 46] (Figure 1). These studies highlight the importance of the TME and demonstrate a conserved antitumoural function of TNF-α-dependent inflammation in Drosophila models of cancer.
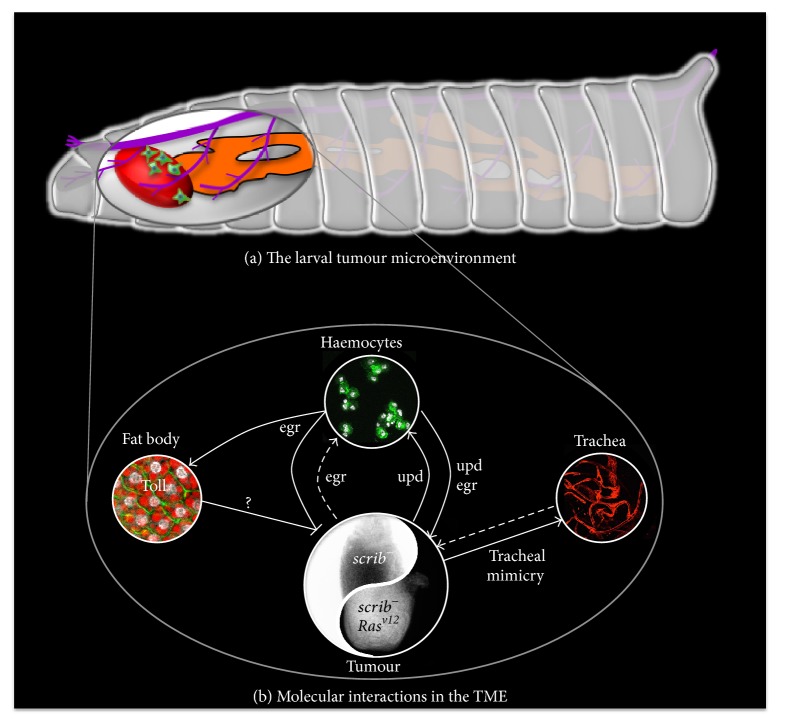
Figure 1.
Immune interactions between larval tumours and their microenvironment (TME). (a) In Drosophila larvae, where tumours are generated in imaginal discs (tumour in red), the TME consists mostly of immune cells (in green), the fat body (in orange), and the trachea (in purple). (b) The molecular interactions within the TME are represented in this figure. Positive effects on growth and/or proliferation are highlighted by lines ending in arrowheads, while lines ending in bars show negative effects, mostly represented by increased cell death. Solid lines indicate demonstrated interactions and dashed lines potential ones. Both the immune cells and the tumour produce the fly TNF homolog Egr. It acts as a double-edge sword depending on the context of the tumour, represented as the Ying-Yang paradigm. Egr is antitumour in scrib-group mutant contexts, while being protumour and prometastatic when Rasv12 is present in the scrib-group mutant genetic background. The effect of tumour-derived Egr on immune cells is still an open question. Egr is required to activate the Toll pathway in the fat body, which subsequently promotes tumour cell death in combination with Egr itself, through an unknown signal (question mark). The interleukin homolog Upd3 produced by the tumour induces immune cells proliferation, while immune cell-derived Upd3 promotes tumour proliferation and invasion. While tumour can promote tracheogenesis through incorporation of tumour cells into the tracheal wall (tracheal mimicry), the effects of trachea on tumour growth and metastasis remain elusive.
In contrast to the described antitumoural functions, Drosophila TNF-α can also exert protumoural effects. Evidence for such a role is provided by studies on tumours where scrib-complex mutations are associated with a constitutively active form of Ras (Rasv12). Ras is a conserved proto-oncogene mutated in many cancer types, with a 16% overall incidence rate in all analysed human tumours [47]. In Drosophila, clones of cells mutated for scrib-complex proteins and overexpressing Rasv12 fail to be eliminated by surrounding epithelial cells. Instead, they form neoplastic tumours that can invade distant tissues [12, 48]. While JNK is required for cell death in scrib mutant clones, cooperation with Rasv12 in these clones diverts the function of JNK pathway activation toward tumour cell proliferation and invasion [39, 49]. In this context, haemocyte-derived Egr has also been shown to promote JNK activation, as knockdown of Egr specifically in immune cells abolished JNK activation and restricted the ability of scrib, Rasv12 mutant cells to grow and invade. Strikingly, transplantation of Egr-wild-type immune cells could rescue the progression of scrib, Rasv12 tumours, as well as JNK activation, providing the final demonstration that Drosophila TNF shares protumour effects with its mammalian counterpart [36] (Figure 1). This is further supported by observations of high expression levels of Grnd in scrib, Rasv12 tumours and by data showing that Grnd knockdown in those tumours also disrupts their growth and invasive properties [41]. Interestingly, tumours display increased levels of ROS, which have been reported to promote haemocyte-dependent Egr secretion and subsequent JNK-induced proliferation in response to apoptosis, suggesting a protumoural feedback loop mechanism [50]. Further insights into the mechanisms mediating this protumorigenic role of Egr come from a recent demonstration that caspase-dependent ROS production in cancer cells is required for the recruitment of macrophages into scrib, Rasv12 tumours [51]. This work demonstrates that Rasv12-driven tumour progression requires the activation of Caspases, which function as tumour promoters. This mechanism is suggested to be one of the key mediators of the switch of Egr from an antitumour to a protumour cytokine by Ras. The protumoural function of TNF-α produced by immune cells is highly reminiscent to the one described in mammalian systems. In a mouse model of skin carcinogenesis where loss of TNF-α suppresses tumour formation [52], transplantation of B-cells from TNF-α competent mice is sufficient to restore tumour formation. However, this effect appears to be indirectly mediated through TNF-α-dependent regulation of T-cell number [53]. A more direct parallel between TNF-α-dependent antitumoural responses in flies and humans comes from work on Kras-dependent intrahepatic cholangiocarcinoma. In this context, TNF-α produced by Kuppfer cells (liver-specific myeloid cells) drives preneoplastic lesions through JNK signalling pathway activation [54].
The demonstration of antagonistic actions of TNF-α in Drosophila and mammalian tumours suggests that the successful use of antitumoural immunity as a cancer therapy may strongly depend on, and must take into consideration, the genetic composition of the tumour. This is further supported by data showing that not all neoplastic tumours are sensitive to Egr. The neoplastic growth induced upon knockdown of avalanche (avl), a Syntaxin involved in the fusion of endocytic vesicles to the early endosome, is dependent on Grnd but escapes the need for Egr [41]. Interestingly, avl tumours produced high levels of Wingless (Wg) protein, which is a known target of JNK pathway activation and a key driver of compensatory proliferation, which is linked to cancer progression [55, 56]. It is therefore conceivable that the genetic properties and/or tissue location of a tumour dictate its sensitivity to different signalling pathways. A recent study in Drosophila showed that Wg dependent tumours proliferate independently of the TME and TNF-α/Grnd [57]. Similarly, tumours bearing combined loss of Rasv12 and hyperactivation of the nonreceptor tyrosine kinase Src, which also feature Wg overexpression [58], are largely insensitive to Egr loss (J.B.C. personal communication). High Wg activity could therefore be one of the factors rendering tumours insensitive to TNF-α. The expression of growth factors and activation of downstream signalling pathways in epithelial tissues in general and in Drosophila imaginal discs in particular are usually restricted to certain tissue locations [59, 60]. Recent work in Drosophila identified the presence of “tumour hot-spots.” Tumour hot-spots are defined as locations within tissues where neoplastic mutations are more likely to result in successful tumoural growths capable of invading normal tissues and it is a process involving differential activation of JAK/STAT signalling [61]. It is likely that additional spatially restricted factors, including graded morphogens, such as Wg, Decapentaplegic (Dpp), or Hedgehog (Hh), may influence “tumour hot-spots” and, therefore, the potential impact of TNF-α in this context.
A key phenotypic feature of scrib-group mutants is the loss of epithelial cell polarity. In tumours lacking lgl, knockdown of the JNK pathway rescues loss of cell polarity [62]. Loss of cell polarity is required for epithelial-mesenchymal transition (EMT), which drives tumour progression, including invasion [63, 64]. Given that Egr is a major driver of JNK pathway activation, the fly TNF-α may be a determinant in the loss of cell polarity in tissues carrying these neoplastic transformations. Indeed, Egr regulates asymmetric localisation of determinants of asymmetric division, Miranda and Prospero, in neuroblasts, supporting a role for Egr in cell polarity determination [65]. Interestingly, TNFα-dependent loss of cell polarity has been reported upon induction of chronic inflammation in the mouse intestine [66]. Likewise, a recent report shows that TNFα-dependent EMT increases lung cancer metastasis [67]. This possible relationship between TNF-α and cell polarity could also be the driving force for TNF-α's protumour effect on Rasv12 expressing cells, as Ras hyperactivation facilitates the prosurvival function of JNK signalling.
The discovery of other immune-derived cytokines may have implications on their role in cancer progression through the TME. Haemocyte-derived Dpp, the fly homolog of Bone Morphogenetic Protein 2/4 (BMP2/4), a member of the Transforming Growth Factor beta (TGF-β) signalling family, can promote intestinal stem cell (ISC) proliferation in response to infection [68]. A similar effect has been reported in response to both septic and aseptic injuries for hemocyte-derived unpaired 2 and 3 (Upd 2/3), the Drosophila interleukin homologs that function as ligands of the JAK/STAT pathway [69]. Consistently, in scrib mutant larvae Upd 3 produced by the tumour induces JAK/STAT activation in the immune tissues (fat body and haemocytes), leading to a positive feedback loop that increases Upd 3 levels in haemocytes, which is required for JAK/STAT-induced proliferation of haemocyte and subsequent tumour suppression [26] (Figure 1). On the other hand, Upd3 can also impact JAK/STAT activation within scrib/Rasv12 tumour, where it cooperates with JNK to promote growth and metastasis [48] (Figure 1). A protumour effect of JAK/STAT signalling is also reported in fly leukaemia model, as its activation is sufficient to drive Drosophila blood cell neoplasia [70].
2.3. The Humoral Immune Response to Tumours
While the local immune response to tumours is receiving great interest for the design of new immunotherapies, the role of systemic immunity in mammals remains elusive. However, recent advances are highlighting the importance of systemic immunity to drive successful immunotherapy [71]. Pioneering work done in Drosophila has demonstrated a role of systemic or humoral innate immunity in the impairment of tumourigenesis. The main organ involved in humoral immunity in Drosophila is the fat body, which processes analogous functions to the mammalian liver and adipose tissues. Several conserved immune signalling pathways are activated in the fat body upon infection, including Toll, immune deficiency (Imd), and JAK/STAT signalling [72]. Activation of those pathways leads to the expression of downstream effectors (antimicrobial peptides, turandots, clotting factors, serine proteases, TEPs, serpins, and cytokines), which act by clearing the underlying infection and promoting recovery of infected tissues [73]. Interestingly, tumour-bearing animals show activation of the humoral immune response [46]. Unexpectedly, activation of the Toll signalling pathway in the fat body of tumour-bearing animals could be prevented by knocking-down the Toll ligand Spaetzle (spz) in haemocytes or by removing Egr from tumours, suggesting that Egr produced by the tumour promotes Spz production by haemocytes, which in turn activates the Toll pathway in the fat body [46]. Toll knockdown in the fat body leads to increased tumour size and decreased tumour cell death. Conversely, Toll overexpression is sufficient to induce tumour cell death and decrease tumour size, a process that requires haemocyte-derived Egr [46]. All together, evidence shows that TNF-α-dependent activation of systemic Toll signalling is an important component of a nonautonomous tumour suppressor program (Figure 1). The exact mechanisms of Toll activation, as well as the downstream effector(s) of the Toll pathway in tumour-bearing animals, remain elusive. Interestingly, downstream Toll targets expressed following infection include antimicrobial peptides (AMPs), which have been reported to exert antitumoural activity in vitro [74].
It is worth mentioning recent technical advances in flies that have provided new means to study the interactions between the tumour and the TME or more distant tissues. Tumour allografts have been a powerful technique to assess some physiological aspects of tumour growth and metastasis [57, 75–77], permitting independent genetic manipulation of tumours and non-tumour host tissues. Furthermore, it is likely that the use of new genetic tools that allow manipulation of gene expression independently from the widely used Gal4 system, such as the LexA/LexAop and QF/QS/QUAS systems [78, 79], will be extremely useful to study the influence of distant tissues on tumours. However, to this end the development of new fly lines is required, in order to establish these alternative gene-driving systems for use in large/unbiased screening of processes involved in tumourigenesis in Drosophila.
2.4. The Tracheal System and Its Role in Tumourigenesis
The vascular system of vertebrates is known to play a critical role in the tumour microenvironment, through interaction with the tumour and the immune system. Indeed, blood vessels deliver oxygen and nutrients, as well as immune cells, to all tissues. The fast-growing properties of cancers lead to the development of some hypoxic areas that are not vascularised. As a result, angiogenesis is required, in order to sustain the high demand for oxygen and nutrients necessary to ensure tumour growth. This therefore constitutes an attractive target for interfering with tumour development [80]. In Drosophila, oxygen is provided by the tracheal system that spreads throughout the animal, thus providing an analogous system to the vertebrate vasculature. Moreover, the Drosophila tracheal epithelium is also important in immunity, as it constitutes a physical barrier to the external milieu and is able to produce defence proteins [73]. Interestingly, a recent study showed that tracheogenesis occurs in the TME of hypoxic tumours in Drosophila. Strikingly, tumour cells undertake a trachea-specific developmental program and become incorporated into existing tracheal walls [81] (Figure 1). This data is reminiscent of the vascular mimicry process described in several mammalian cancer types, where tumour cells form functional blood vessel-like structures that can provide oxygen and nutrients to the tumour [82]. However, while tracheal derived Dpp is shown to influence ISC proliferation in the fly adult gut [83], the contribution of tracheogenesis to larval tumour growth and cell death and its possible contribution to antitumoural immunity remains an open question.
The studies described above highlight the importance of cellular and systemic immunity in shaping the tumour outcome. Critically, they reveal the existence of anti- and protumour mechanisms mediated by the immune system that are conserved between flies and humans and also uncover novel interactions between tumours and the immune system (Figure 1). However, even in a “simple” model system, interactions between tumour and immune cells are extremely complex. Future work in Drosophila will help to better understand how the global immune response shapes the TME, and how tumours are able to influence the antitumoural immune response via interactions with their microenvironment.
3. Interactions between Host Metabolism and Tumours
3.1. Tumours Impact Systemic Metabolism
One of the striking effects of tumour burden is the alteration in host metabolism that occurs as a direct consequence of tumour development. The origins of the understanding that metabolism is altered in cancer patients can be traced back to the identification of glucose intolerance as the first systemic metabolic abnormality linked to the presence of a tumour [84]. This was followed by Warburg's discovery of the abnormal metabolism of glucose into lactate in tumours, which occurred even in the presence of oxygen [85]. Later discoveries have revealed a large panel of metabolic dysfunctions within tumours, which sustain further growth and proliferation of tumour cells. The high nutritional demand of tumours can influence nutrient availability in the TME, as demonstrated by recent work in mouse models showing that glucose restriction within the TME inhibits antitumour T-cell function [86, 87]. Furthermore, the high levels of hormone, peptides, and cytokine secretion observed during early tumour formation also affect metabolic pathways in distant tissues, leading to the hypothesis that tumours behave as “metabolic dictators” [88]. The biological complexity and limited genetic tools available in mammalian models, as well as the lack of physiological relevance of cell culture models to questions of interorgan communication, have largely hindered the investigation of altered host and tumour metabolism. As a model system, Drosophila has proven very relevant to the investigation of the links between tumour burden and altered systemic metabolism and the effects that this can have on both the tumour and host [89] (Figure 2).
Figure 2.
Metabolic interactions between tumours and their microenvironment (TME). Interactions between the tumour, the TME, and other environmental factors are represented in this figure. Solid arrows indicate demonstrated interactions, while dashed lines with question marks designate putative ones. Nonautonomous metabolic changes in the TME can affect both the TME and the tumour and are generated through various means. High levels of dietary sugar promote tumour growth and induce systemic insulin resistance in the TME. Tumours can also perturb TME insulin signalling by the secretion of an insulin-signalling antagonist, ImpL2. Autophagy in the TME promotes tumour growth through the recycling of amino acids from the TME into the tumour. Expression of the amino acid transporter slif in the tumour is necessary for this protumour effect. TME autophagy can be triggered by tumour-derived ROS and may also be driven by cytokine signalling or direct competition with the tumour for nutrients. Both, autophagy and impaired insulin signalling can contribute to tissue wasting and cancer cachexia. The causes of wasting in the TME and the effects of wasting in these tissues are an increasing research focus. However, the effects of TME wasting on the tumour remain an open question.
3.2. The Effects of Diet on Tumour Burden
Obesity and type 2 diabetes are common comorbidities in modern society and are characterised by systemic insulin resistance and hyperglycaemia. These conditions are associated with an increased risk of developing cancer and are a risk factor for cancer mortality [90–94]. Insulin resistance can be modelled in Drosophila through the use of a high sugar diet, generating phenotypes that recapitulate the human condition [95]. In this context, small clones of noninvasive tumours cells transform into highly proliferative, metastatic tumours, due to the ability of these tumours to evade diet-induced systemic insulin resistance [58]. Tumours retain sensitivity to insulin signalling due to the overexpression of insulin receptor, as a result of elevated expression of Wg. This allows them to exploit the elevated levels of circulating glucose present in the context of the high sugar diet and peripheral tissue insulin resistance (Figure 2). It was later demonstrated [81] that activation of salt-inducible kinase in tumours from animals fed a high sugar diet functions to inhibit Hippo signalling, which facilitates the increase in Wg signalling that mediates the evasion of insulin resistance by these tumours. However, it is unclear whether nutrient availability has a universal impact on tumour growth, or whether any such dependency also relies on the genetic makeup of the tumour. An additional example of nutrient dependency can be identified in cells bearing a loss of function mutation in the tumour suppressor gene PTEN, which is commonly mutated across a broad range of cancers [96]. Under normal conditions, PTEN mutant clones in epithelial wing disc tissue show increased cell size but do not overgrow or disrupt tissue architecture. However, upon systemic nutrient restriction PTEN mutant cells display a proliferative advantage over wild-type cells, which is dependent on the function of the amino acid transporter slimfast (slif) [97]. Interestingly, overgrowth of PTEN mutant cells in the context of nutrient restriction was sufficient to induce systemic nonautonomous effects, decreasing the size of other tissues in the organism. PTEN mutant cells are suggested to outcompete distant wild-type cells for access to nutrients, as genetically driving growth in PTEN-competent peripheral tissues reduced the overgrowth observed in PTEN mutant cells [97]. Interestingly, the TOR pathway, a nutrient-dependent regulator of tissue growth, promotes the activity of Yki in wing discs [98], which is a known promoter of tumour growth [99–101]. This may therefore represent a possible mechanism by which increased nutrient availability promotes tumour growth in these Drosophila models. These findings demonstrate the drastic effect that the perturbation of host metabolism by extrinsic factors can have on tumour growth, how tumours exert systemic effects on distant tissues, and how the genetic properties of the tumour itself are critical in mediating this crosstalk.
Parallels can be drawn between the results observed in these Drosophila models and those found in vertebrates. Preexisting obesity and diabetes promoted tumour growth in a rat cancer model [94], while a study of over one million patients over 26 years identified diabetes as a predictor of both cancer development and cancer death [102]. Drosophila cancer models involving diet and obesity are therefore particularly relevant to the human condition, as the protumour effects demonstrated in the contexts of these studies appear to be conserved in higher organisms, and the mediating factors are environmental influences that are very common in developed societies. The studies discussed here highlight new aspects of tumour physiology, suggesting that tumours are direct competitors to host tissues for nutrients and are frequently able to outcompete them for access to metabolic resources through various means (Figure 2). This induces nonautonomous metabolic effects in host tissues, which are likely to be beneficial to the tumour.
3.3. Non-Tumour Autonomous Autophagy and Tumour Growth
Macroautophagy is the process of bulk degradation of cytoplasmic components, facilitating the removal of defective organelles and the recycling and remobilising of cellular resources in times of stress [103]. While intratumour autophagy has been shown to act as a tumour suppressor, Rasv12 tumour cells in larval wing discs activate autophagy nonautonomously in the wild-type cells of the disc, demonstrating the ability of tumours to affect the TME in this manner [104]. This was further confirmed by another study reporting systemic non-cell-autonomous autophagy in animals bearing invasive neoplastic scrib/Rasv12 tumours [77]. Moreover, this study demonstrated that autophagic activity in tissues both local and distal to the tumour promoted tumour growth. Inhibition of autophagy in the local TME is sufficient to significantly inhibit tumour growth and invasion, an effect that is further enhanced when autophagy is also blocked in all peripheral tissues. These results directly demonstrate that non-cell-autonomous autophagy in local and distant nontumour tissues contributes to tumour growth and invasion [77]. These data are relevant to vertebrate models, as autophagy in pancreatic stellate cells has been demonstrated to promote tumour growth in a pancreatic cancer cell line implanted into mice [105]. Drosophila studies have also suggested that microenvironmental autophagy fuels tumour growth through the mobilisation of nutrients from these local and peripheral nontumour tissues (Figure 2). It has been proposed that, in starvation conditions, autophagy induced by Desat1-dependent Myc activity may act in a non-cell-autonomous manner to promote tumour growth [106], while decreased amino acid transport, by the targeted knockdown of slif in the tumour, results in a dramatic loss of tumour growth [77]. In human cell culture models, microenvironmental autophagy has also been shown to metabolically support human pancreatic ductal adenocarcinoma in a non-cell-autonomous manner, through the provision of Alanine as a carbon source [107]. This shows that the data presented in these Drosophila studies is highly relevant to the vertebrate condition.
The tumour-derived factor(s) that drive the onset of microenvironmental autophagy are not yet fully defined; however, ROS signalling is an excellent candidate for further investigation (see Filomeni et al. [108] for a comprehensive review of ROS and autophagy). Starvation-induced autophagy is mediated by mitochondrially generated ROS, via the activation of the TOR pathway [109], while ROS are elevated in scrib/Rasv12 tumours, and the generation of mitochondrial ROS is sufficient to induce local autophagy in wing discs [77]. Manent et al. [104] provide evidence that ROS derived from tumour cells is sufficient to induce autophagy nonautonomously in the local microenvironment and that this also activates protumour JNK signalling in these cells. Altogether, these studies suggest that tumour-derived ROS might act as a convergent signal that triggers non-cell-autonomous microenvironmental autophagy and JNK signalling in the TME, both of which are protumour events (Figure 2). There is also some evidence in mouse models to support the idea that ROS may play an important role in TME autophagy. Fibroblasts that suffer oxidative stress induced by ROS and hypoxia in the TME undergo autophagy, which acts to degrade mitochondria. This alters the metabolism of these cells towards aerobic glycolysis, which, combined with autophagic degradation, is suggested to provide recycled nutrients from the TME to the tumour to fuel growth [110]. The transfer of energy between tumour and the TME in the form of metabolites is suggested to maintain the TME in a protumour setting [88]. Another recent work performed in cell culture and mouse models suggests that tumour-derived IL-6 may be a candidate for inducing autophagy in more tissues distal tissues from the tumour [111]. This work may represent an interesting novel target for the focus of research on the effects of peripheral tissue autophagy in Drosophila cancer models, as the expressions of IL-6-like Upd ligands are elevated in Drosophila neoplastic tumours [112]. There is little work exploring the potential interactions between ROS, autophagy, and Il-6 signalling in the context of the TME, and given the studies discussed here, Drosophila may represent a suitable model for further work into the interactions between these factors and their combined impact on the tumour and the TME. The importance of Drosophila studies on microenvironmental autophagy is reinforced by the apparent conservation of mechanisms in human patients and other vertebrate models systems. Further work in Drosophila is likely to be invaluable in improving our understanding of how metabolic changes in TME may affect tumours and shape tumour-host interactions.
3.4. Cancer-Associated Cachexia
One of the best-recognised outcomes of altered host metabolism in the context of tumour burden is the condition of cancer cachexia, a paraneoplastic syndrome that results in the dramatic loss of muscle and adipose tissue [113]. Cachexia is a highly multifactorial condition with numerous metabolic aberrations implicated in the onset of the condition, including perturbed insulin signalling, systemic hypercatabolism, inflammatory and immune responses, and deregulation of muscle homeostasis [114–117]. Cachexia is a highly deleterious condition, as it decreases patient tolerance to cancer therapies, negatively affects quality of life, and increases the risk of mortality, with up to 30% of cancer patient deaths occurring as a direct result of cachexia [118, 119]. Importantly, there is no clear therapeutic gold standard for the treatment of cachectic patients, in part due to the poorly understood aetiology of the condition. Cancer cachexia represents an extreme example of the effect a tumour can have on the host, as the presence of the tumour generates such a strong alteration of the host's metabolic state that it leads to the development of a novel pathology. There are unanswered questions about the systemic effects of cachexia beyond the direct effects of the wasting itself, including whether cachexia has a functional role that affects the tumour or other tissues.
Two independent models of cancer cachexia have shown the utility of Drosophila in this field of research [76, 120]. Both reports demonstrated that tumours secrete high levels of imaginal morphogenesis protein-Late 2 (ImpL2), a secreted insulin-signalling antagonist that functions by direct binding to Dilp2 [121]. These studies also showed that tumour-bearing flies developed systemic insulin-resistance phenotypes in tissues distal from the tumour. This insulin resistance promoted tissue wasting, a process that is also likely to occur in human patients and other animal models [122–126] (Figure 2). RNAi knockdown of ImpL2 in the tumour was sufficient to reduce the systemic insulin resistant phenotype and thus partially rescue the wasting phenotypes observed in peripheral tissues, without impacting the growth of the tumour [76, 120]. This work provides an excellent example of the use of Drosophila cancer models in the field of tumour-microenvironment interactions. Research into cachexia is an emergent field, and the identification of a tumour-derived factor that mediates a systemic effect on host tissue metabolism is an important example of the ability of Drosophila models to recapitulate and dissect complex phenotypes. Interestingly, autophagy is one of the main mechanisms of tissue degradation during cancer cachexia [111, 127, 128]. There are direct associations between whether tumours are cachectogenic and their ability to induce autophagy [111]. Together, these studies raise an interesting open question as to the functional nature of cachexia, namely, whether the process is not just deleterious to the host, but whether it is also beneficial to the tumour, due to the mobilisation of metabolites from muscle and adipose tissues. There are also questions as to whether tumour-inherent properties drive cachexia, and thus whether genetic factors can be established that mediate cachexia. Data from human patients suggest this may be the case, as pancreatic and gastric cancers have a much higher incidence rate of cachexia when compared to other tumour types [129, 130]. The Drosophila models discussed here represent a good opportunity to answer some of these important open questions.
4. Concluding Remarks
The studies discussed here demonstrate that Drosophila is a relevant model for studying cancer and its interactions with the TME, with many parallels to orthologous vertebrate conditions. Research utilising Drosophila as a model system has shown that immune and metabolic processes induced in a nonautonomous manner by the presence of the tumour are sufficient to feed back to the tumour and alter its characteristics. This can be shown well in the studies of microenvironmental autophagy, which is induced in the TME by the tumour, and serves to support tumour growth and metastasis [77, 104], and in the dual role of haemocyte-derived Egr, which can promote or suppress tumour growth depending on the tumour context [36, 46] (Figures 1 and 2).
Given the effects observed in response to the tumour there are likely to be interactions between the immune system and metabolism in this context. Indeed, nutrient restriction in larvae inhibits TOR signalling in the fat body, leading to increased levels of circulating Egr. Egr binds to insulin-producing cells in the brain and suppresses the production and secretion of progrowth Dilp2 and Dilp5 [131]. As previously discussed, Egr is also a mediator of tumour-induced immunity with context-dependent pro- or antitumour function [36, 41, 46]. There is therefore the potential for crosstalk between host tissues with tumour-derived metabolic derangement and immune pathways in Drosophila. Work in human cell culture and mouse models has demonstrated that tumours can alter host immunity via directly influencing immune cell metabolism. Lactic acid secreted by the tumour into the TME changes macrophages metabolism, polarising them towards a tumour-promoting state [132, 133]. These macrophages produce ARG1, a metabolic enzyme that generates polyamines (metabolites essential for cell division), which promote tumour growth in this context [134]. Another example is given by the direct competition for glucose between tumours and TME T cells, which is also sufficient to alter T-cell metabolism, suppressing antitumour responses and highlighting how the Warburg effect is used to escape the immune system [86, 87].
However, there is a lack of comprehensive understanding as to how different factors such as diet and metabolism, immune responses, and tumours interact and cooperate or synergise when presented together. This is often the case in the human condition, for example, in the case of a cancer patient with diabetes. Drosophila cancer models represent an excellent basis for the study of the roles these factors may play, both individually and combined together, and how nonautonomous signalling inputs might influence both tumour and host tissue responses. There are likely to be inevitable questions about the ability of simple Drosophila tumour models with one or two genetic drivers to fully recapitulate the complexity of tumour burden in higher animals, including human patients. However, the simplicity of these models is likely to prove advantageous when attempting to dissect the contributing roles of the multiple interacting factors that comprise tumour-TME interactions. There is also interesting work on the generation of Drosophila “avatars,” fly lines that can generate close homologs of tumours from specific patients, including the numerous genetic aberrations that drive a particular type of tumour in humans [135]. Such avatars may represent an excellent opportunity to test principles uncovered in more simple Drosophila cancer models, in order to investigate whether these discoveries still hold in a more complex tumour setting, including tumour-TME responses.
The mechanisms mediating the crosstalk between tumours and local and distal tissues are still being uncovered. Improving the understanding of the signalling pathways that may link together the complex interactions between host metabolism, immunity, and tumour growth is an essential aspect towards the unravelling of such crosstalk. Drosophila models represent an excellent platform for the continued investigation of these complex interactions, thanks to the multiple advantages of the model system. Low genetic redundancy, powerful genetic tools, and the possibility of tightly controlling not only the genetics of the tumour but also various aspects of the tumour micro- and macroenvironment render Drosophila a strong paradigm for further work into these complex interactions that impact human health and disease.
Acknowledgments
The authors thank all their colleagues, whose work has contributed to this review. Jean-Philippe Parvy and Joseph A. Hodgson are funded by institutional CRUK core funding. Julia B. Cordero is a Sir Henry Dale Fellow jointly funded by the Wellcome Trust and the Royal Society (Grant no. 104103/Z/14/Z).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Cancer. Cancer. World Health Organization, World Health Organization. 2017, http://www.who.int/mediacentre/factsheets/fs297/en/
- 2.Beatson G. On The Treatment of Inoperable Cases of Carcinoma of The Mamma: Suggestions for A New Method of Treatment, with Illustrative Cases. The Lancet. 1896;148(3803):162–165. doi: 10.1016/S0140-6736(01)72384-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norum J. H., Andersen K., Sørlie T. Lessons learned from the intrinsic subtypes of breast cancer in the quest for precision therapy. British Journal of Surgery. 2014;101(8):925–938. doi: 10.1002/bjs.9562. [DOI] [PubMed] [Google Scholar]
- 4.Choi J. The role of tumor-associated macrophage in breast cancer biology. Histology and Histopathology. 2017:p. 11916. doi: 10.14670/HH-11-916. [DOI] [PubMed] [Google Scholar]
- 5.Balkwill F. R., Capasso M., Hagemann T. The tumor microenvironment at a glance. Journal of Cell Science. 2012;125(23):5591–5596. doi: 10.1242/jcs.116392. [DOI] [PubMed] [Google Scholar]
- 6.Hotchkiss R. S., Moldawer L. L. Parallels between cancer and infectious disease. The New England Journal of Medicine. 2014;371(4):380–383. doi: 10.1056/NEJMcibr1404664. [DOI] [PubMed] [Google Scholar]
- 7.Marcus A., Gowen B. G., Thompson T. W., et al. Recognition of tumors by the innate immune system and natural killer cells. Advances in Immunology. 2014;122:91–128. doi: 10.1016/B978-0-12-800267-4.00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palumbo A., de Oliveira Meireles Da Costa N., Bonamino M. H., Ribeiro Pinto L. F., Nasciutti L. E. Genetic instability in the tumor microenvironment: A new look at an old neighbor. Molecular Cancer. 2015;14(1, article no. 145) doi: 10.1186/s12943-015-0409-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tipping M., Perrimon N. Drosophila as a Model for Context-Dependent Tumorigenesis. Journal of Cellular Physiology. 2014;229(1):27–33. doi: 10.1002/jcp.24427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miles W. O., Dyson N. J., Walker J. A. Modeling tumor invasion and metastasis in Drosophila. DISEASE MODELS & MECHANISMS. 2011;4(6):753–761. doi: 10.1242/dmm.006908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Humbert P., Russell S., Richardson H. Dlg, scribble and Lgl in cell polarity, cell proliferation and cancer. BioEssays. 2003;25(6):542–553. doi: 10.1002/bies.10286. [DOI] [PubMed] [Google Scholar]
- 12.Pagliarini R. A., Xu T. A Genetic Screen in Drosophila for Metastatic Behavior. Science. 2003;302(5648):1227–1231. doi: 10.1126/science.1088474. [DOI] [PubMed] [Google Scholar]
- 13.Vidal M., Larson D. E., Cagan R. L. Csk-deficient boundary cells are eliminated from normal drosophila epithelia by exclusion, migration, and apoptosis. Developmental Cell. 2006;10(1):33–44. doi: 10.1016/j.devcel.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Kiyono T., Hiraiwa A., Fujita M., Hayashi Y., Akiyama T., Ishibashi M. Binding of high-risk human papillomavirus E6 oncoproteins to the human homologue of the Drosophila discs large tumor suppressor protein. Proceedings of the National Acadamy of Sciences of the United States of America. 1997;94(21):11612–11616. doi: 10.1073/pnas.94.21.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakagawa S., Huibregtse J. M. Human scribble (Vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin-protein ligase. Molecular and Cellular Biology. 2000;20(21):8244–8253. doi: 10.1128/mcb.20.21.8244-8253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhan L., Rosenberg A., Bergami K. C., et al. Deregulation of Scribble Promotes Mammary Tumorigenesis and Reveals a Role for Cell Polarity in Carcinoma. Cell. 2008;135(5):865–878. doi: 10.1016/j.cell.2008.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapil S., Sharma B. K., Patil M., et al. The cell polarity protein Scrib functions as a tumor suppressor in liver cancer. Oncotarget . 2017;8(16):26515–26531. doi: 10.18632/oncotarget.15713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearson H. B., McGlinn E., Phesse T. J., et al. The polarity protein Scrib mediates epidermal development and exerts a tumor suppressive function during skin carcinogenesis. Molecular Cancer. 2015;14(1, article no. 169) doi: 10.1186/s12943-015-0440-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elsum I. A., Yates L. L., Pearson H. B., et al. Scrib heterozygosity predisposes to lung cancer and cooperates with KRas hyperactivation to accelerate lung cancer progression in vivo. Oncogene. 2014;33(48):5523–5533. doi: 10.1038/onc.2013.498. [DOI] [PubMed] [Google Scholar]
- 20.Schimanski C. C., Schmitz G., Kashyap A., et al. Reduced expression of Hugl-1, the human homologue of Drosophila tumour suppressor gene lgl, contributes to progression of colorectal cancer. Oncogene. 2005;24(19):3100–3109. doi: 10.1038/sj.onc.1208520. [DOI] [PubMed] [Google Scholar]
- 21.Lu X., Feng X., Man X., et al. Aberrant Splicing of Hugl-1 Is Associated with Hepatocellular Carcinoma Progression. Clinical Cancer Research. 2009;15(10):3287–3296. doi: 10.1158/1078-0432.CCR-08-2078. [DOI] [PubMed] [Google Scholar]
- 22.Tsuruga T., et al. Loss of Hugl-1 expression associates with lymph node metastasis in endometrial cancer. Oncology Research. 2007;16(9):431–435. doi: 10.3727/000000007783980855. [DOI] [PubMed] [Google Scholar]
- 23.Dow L. E., Elsum I. A., King C. L., Kinross K. M., Richardson H. E., Humbert P. O. Loss of human Scribble cooperates with H-Ras to promote cell invasion through deregulation of MAPK signalling. Oncogene. 2008;27(46):5988–6001. doi: 10.1038/onc.2008.219. [DOI] [PubMed] [Google Scholar]
- 24.Coley Nauts H., Swift W. E., Coley B. L. The Treatment of Malignant Tumors by Bacterial Toxins as Developed by the Late William B. Coley, M.D., Reviewed in the Light of Modern Research. Cancer Research. 1946;6(4):205–216. [PubMed] [Google Scholar]
- 25.Noy R., Pollard J. W. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pastor-Pareja J. C., Wu M., Xu T. An innate immune response of blood cells to tumors and tissue damage in Drosophila. Disease Models & Mechanisms. 2008;1(2-3):144–154. doi: 10.1242/dmm.000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanha-aho L.-M., Valanne S., Rämet M. Cytokines in Drosophila immunity. Immunology Letters. 2016;170:42–51. doi: 10.1016/j.imlet.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Shaukat Z., Liu D., Gregory S. Sterile inflammation in drosophila. Mediators of Inflammation. 2015;2015 doi: 10.1155/2015/369286.369286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradley J. R. TNF-mediated inflammatory disease. The Journal of Pathology. 2008;214(2):149–160. doi: 10.1002/path.2287. [DOI] [PubMed] [Google Scholar]
- 30.Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin induced serum factor that cuases necrosis of tumors. Proceedings of the National Acadamy of Sciences of the United States of America. 1975;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balkwill F. Tumour necrosis factor and cancer. Nature Reviews Cancer. 2009;9(5):361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 32.Igaki T., Kanda H., Yamamoto-Goto Y., et al. Eiger, a TNF superfamily ligand that triggers the Drosophila JNK pathway. EMBO Journal. 2002;21(12):3009–3018. doi: 10.1093/emboj/cdf306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreno E., Yan M., Basler K. Evolution of TNF signaling mechanisms: JNK-dependent apoptosis triggered by Eiger, the Drosophila homolog of the TNF superfamily. Current Biology. 2002;12(14):1263–1268. doi: 10.1016/s0960-9822(02)00954-5. [DOI] [PubMed] [Google Scholar]
- 34.Brandt S. M. Secreted Bacterial Effectors and Host-Produced Eiger/TNF Drive Death in aSalmonella-Infected Fruit Fly. PLoS Biol. Vol. 2. 2(12: p. e418; 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Igaki T., Pastor-Pareja J. C., Aonuma H., Miura M., Xu T. Intrinsic Tumor Suppression and Epithelial Maintenance by Endocytic Activation of Eiger/TNF Signaling in Drosophila. Developmental Cell. 2009;16(3):458–465. doi: 10.1016/j.devcel.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cordero J. B., Macagno J. P., Stefanatos R. K., Strathdee K. E., Cagan R. L., Vidal M. Oncogenic ras diverts a host TNF tumor suppressor activity into tumor promoter. Developmental Cell. 2010;18(6):999–1011. doi: 10.1016/j.devcel.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Froldi F., Ziosi M., Garoia F., et al. The lethal giant larvae tumour suppressor mutation requires dMyc oncoprotein to promote clonal malignancy. BMC Biology. 2010;8, article no. 33 doi: 10.1186/1741-7007-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brumby A. M., Richardson H. E. scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO Journal. 2003;22(21):5769–5779. doi: 10.1093/emboj/cdg548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Igaki T., Pagliarini R. A., Xu T. Loss of Cell Polarity Drives Tumor Growth and Invasion through JNK Activation in Drosophila. Current Biology. 2006;16(11):1139–1146. doi: 10.1016/j.cub.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 40.Amoyel M., Bach E. A. Cell competition: How to eliminate your neighbours. Development. 2014;141(5):988–1000. doi: 10.1242/dev.079129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andersen D. S., Colombani J., Palmerini V., et al. The Drosophila TNF receptor Grindelwald couples loss of cell polarity and neoplastic growth. Nature. 2015;522(7557):482–486. doi: 10.1038/nature14298. [DOI] [PubMed] [Google Scholar]
- 42.Chen C.-L., Schroeder M. C., Kango-Singh M., Tao C., Halder G. Tumor suppression by cell competition through regulation of the Hippo pathway. Proceedings of the National Acadamy of Sciences of the United States of America. 2012;109(2):484–489. doi: 10.1073/pnas.1113882109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rhiner C., López-Gay J. M., Soldini D., et al. Flower forms an extracellular code that reveals the fitness of a cell to its neighbors in Drosophila. Developmental Cell. 2010;18(6):985–998. doi: 10.1016/j.devcel.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 44.Meyer S. N., Amoyel M., Bergantinos C., et al. An ancient defense system eliminates unfit cells from developing tissues during cell competition. Science. 2014;346(6214) doi: 10.1126/science.1258236.1258236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamamoto M., Ohsawa S., Kunimasa K., Igaki T. The ligand Sas and its receptor PTP10D drive tumour-suppressive cell competition. Nature. 2017;542(7640):246–250. doi: 10.1038/nature21033. [DOI] [PubMed] [Google Scholar]
- 46.Parisi F., Stefanatos R. K., Strathdee K., Yu Y., Vidal M. Transformed epithelia trigger non-tissue-autonomous tumor suppressor response by adipocytes via activation of toll and eiger/TNF signaling. Cell Reports. 2014;6(5):855–867. doi: 10.1016/j.celrep.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 47.Prior I. A., Lewis P. D., Mattos C. A comprehensive survey of ras mutations in cancer. Cancer Research. 2012;72(10):2457–2467. doi: 10.1158/0008-5472.CAN-11-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu M., Pastor-Pareja J. C., Xu T. Interaction between RasV12 and scribbled clones induces tumour growth and invasion. Nature. 2010;463(7280):545–548. doi: 10.1038/nature08702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uhlirova M., Bohmann D. JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO Journal. 2006;25(22):5294–5304. doi: 10.1038/sj.emboj.7601401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fogarty C. E., Diwanji N., Lindblad J. L., et al. Extracellular Reactive Oxygen Species Drive Apoptosis-Induced Proliferation via Drosophila Macrophages. Current Biology. 2016;26(5):575–584. doi: 10.1016/j.cub.2015.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pérez E., Lindblad J. L., Bergmann A. Tumor-promoting function of apoptotic caspases by an amplification loop involving ROS, macrophages and JNK in Drosophila. eLife. 2017;6 doi: 10.7554/eLife.26747.e26747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moore R. J., Owens D. M., Stamp G., et al. ice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nature Medicine. 1999;5(7):828–831. doi: 10.1038/10552. [DOI] [PubMed] [Google Scholar]
- 53.Schioppa T., Moore R., Thompson R. G., et al. B regulatory cells and the tumor-promoting actions of TNF-α during squamous carcinogenesis. Proceedings of the National Acadamy of Sciences of the United States of America. 2011;108(26):10662–10667. doi: 10.1073/pnas.1100994108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yuan D., Huang S., Berger E., et al. Kupffer Cell-Derived Tnf Triggers Cholangiocellular Tumorigenesis through JNK due to Chronic Mitochondrial Dysfunction and ROS. Cancer Cell. 2017;31(6):771–789.e6. doi: 10.1016/j.ccell.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ryoo H. D., Gorenc T., Steller H. Apoptotic cells can induce compensatory cell proliferation through the JNK and the wingless signaling pathways. Developmental Cell. 2004;7(4):491–501. doi: 10.1016/j.devcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 56.Waghmare I., Roebke A., Minata M., Kango-Singh M., Nakano I. Intercellular cooperation and competition in brain cancers: Lessons from Drosophila and human studies. Stem Cells Translational Medicine. 2014;3(11):1262–1269. doi: 10.5966/sctm.2014-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muzzopappa M., Murcia L., Milán M. Feedback amplification loop drives malignant growth in epithelial tissues. Proceedings of the National Acadamy of Sciences of the United States of America. 2017;114(35):E7291–E7300. doi: 10.1073/pnas.1701791114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hirabayashi S., Baranski T. J., Cagan R. L. Transformed drosophila cells evade diet-mediated insulin resistance through wingless signaling. Cell. 2013;154(3):664–675. doi: 10.1016/j.cell.2013.06.030Article. doi: 10.1016/j.cell.2013.06.030Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clarke A. R. Wnt signalling in the mouse intestine. Oncogene. 2006;25(57):7512–7521. doi: 10.1038/sj.onc.1210065. [DOI] [PubMed] [Google Scholar]
- 60.Kornberg T. B., Guha A. Understanding morphogen gradients: a problem of dispersion and containment. Current Opinion in Genetics & Development. 2007;17(4):264–271. doi: 10.1016/j.gde.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tamori Y., Suzuki E., Deng W.-M. Epithelial Tumors Originate in Tumor Hotspots, a Tissue-Intrinsic Microenvironment. PLoS Biology. 2016;14(9) doi: 10.1371/journal.pbio.1002537.e1002537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu M., Xin T., Weng S., et al. Activation of JNK signaling links lgl mutations to disruption of the cell polarity and epithelial organization in Drosophila imaginal discs. Cell Research. 2010;20(2):242–245. doi: 10.1038/cr.2010.2. [DOI] [PubMed] [Google Scholar]
- 63.Moreno-Bueno G., Portillo F., Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27(55):6958–6969. doi: 10.1038/onc.2008.346. [DOI] [PubMed] [Google Scholar]
- 64.Karlsson M. C., Gonzalez S. F., Welin J., Fuxe J. Epithelial-mesenchymal transition in cancer metastasis through the lymphatic system. Molecular Oncology. 2017;11(7):781–791. doi: 10.1002/1878-0261.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang H., Cai Y., Chia W., Yang X. Drosophila homologs of mammalian TNF/TNFR-related molecules regulate segregation of Miranda/Prospero in neuroblasts. EMBO Journal. 2006;25(24):5783–5793. doi: 10.1038/sj.emboj.7601461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mashukova A., Wald F. A., Salas P. J. Tumor necrosis factor alpha and inflammation disrupt the polarity complex in intestinal epithelial cells by a posttranslational mechanism. Molecular and Cellular Biology. 2011;31(4):756–765. doi: 10.1128/MCB.00811-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shang G.-S., Liu L., Qin Y.-W. IL-6 and TNF-α promote metastasis of lung cancer by inducing epithelial-mesenchymal transition. Oncology Letters. 2017;13(6):4657–4660. doi: 10.3892/ol.2017.6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ayyaz A., Li H., Jasper H. Haemocytes control stem cell activity in the Drosophila intestine. Nature Cell Biology. 2015;17(6):736–748. doi: 10.1038/ncb3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chakrabarti S., Dudzic J. P., Li X., Collas E. J., Boquete J.-P., Lemaitre B. Remote Control of Intestinal Stem Cell Activity by Haemocytes in Drosophila. PLoS Genetics. 2016;12(5) doi: 10.1371/journal.pgen.1006089.e1006089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harrison D. A., Binari R., Nahreini T. S., Gilman M., Perrimon N. Activation of a Drosophila Janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. EMBO Journal. 1995;14(12):2857–2865. doi: 10.1002/j.1460-2075.1995.tb07285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spitzer M. H., Carmi Y., Reticker-Flynn N. E., et al. Systemic Immunity Is Required for Effective Cancer Immunotherapy. Cell. 2017;168(3):487–502.e15. doi: 10.1016/j.cell.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Valanne S. Functional genomic analysis of the Drosophila immune response. Developmental & Comparative Immunology. 2014;42(1):93–101. doi: 10.1016/j.dci.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 73.Lemaitre B., Hoffmann J. The host defense of Drosophila melanogaster. Annual Review of Immunology. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 74.Deslouches B., Peter Di Y. Antimicrobial peptides with selective antitumor mechanisms: Prospect for anticancer applications. Oncotarget . 2017;8(28):46635–46651. doi: 10.18632/oncotarget.16743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rossi F., Gonzalez C. Studying tumor growth in Drosophila using the tissue allograft method. Nature Protocols. 2015;10(10):1525–1534. doi: 10.1038/nprot.2015.096. [DOI] [PubMed] [Google Scholar]
- 76.Figueroa-Clarevega A., Bilder D. Malignant drosophila tumors interrupt insulin signaling to induce cachexia-like wasting. Developmental Cell. 2015;33(1):47–56. doi: 10.1016/j.devcel.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Katheder N. S., Khezri R., O'Farrell F., et al. Microenvironmental autophagy promotes tumour growth. Nature. 2017;541(7637):417–420. doi: 10.1038/nature20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lai S.-L., Lee T. Genetic mosaic with dual binary transcriptional systems in Drosophila. Nature Neuroscience. 2006;9(5):703–709. doi: 10.1038/nn1681. [DOI] [PubMed] [Google Scholar]
- 79.Potter C. J., Tasic B., Russler E. V., Liang L., Luo L. The Q system: A repressible binary system for transgene expression, lineage tracing, and mosaic analysis. Cell. 2010;141(3):536–548. doi: 10.1016/j.cell.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Palma M., Biziato D., Petrova T. V. Microenvironmental regulation of tumour angiogenesis. Nature Reviews Cancer. 2017;17(8):457–474. doi: 10.1038/nrc.2017.51. [DOI] [PubMed] [Google Scholar]
- 81.Grifoni D., Sollazzo M., Fontana E., Froldi F., Pession A. Multiple strategies of oxygen supply in Drosophila malignancies identify tracheogenesis as a novel cancer hallmark. Scientific Reports. 2015;5, article no. 9061 doi: 10.1038/srep09061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qiao L., Liang N., Zhang J., et al. Advanced research on vasculogenic mimicry in cancer. Journal of Cellular and Molecular Medicine. 2015;19(2):315–326. doi: 10.1111/jcmm.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li Z., Zhang Y., Han L., Shi L., Lin X. Trachea-Derived Dpp Controls Adult Midgut Homeostasis in Drosophila. Developmental Cell. 2013;24(2):133–143. doi: 10.1016/j.devcel.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 84.Rohdenburg G. L., Bernhard A., Krehbiel O. Sugar tolerance in cancer. Journal of the American Medical Association. 1919;72(21):1528–1530. doi: 10.1001/jama.1919.02610210024007. [DOI] [Google Scholar]
- 85.Warburg O. The metabolism of carcinoma cells. Cancer Research. 1925;9(1):148–163. doi: 10.1158/jcr.1925.148. [DOI] [Google Scholar]
- 86.Chang C. H., Qiu J., O'Sullivan D. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162(6):1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ho P.-C., Bihuniak J. D., MacIntyre A. N., et al. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell. 2015;162(6):1217–1228. doi: 10.1016/j.cell.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee Y.-M., Chang W.-C., Ma W.-L. Hypothesis: Solid tumours behave as systemic metabolic dictators. Journal of Cellular and Molecular Medicine. 2016;20(6):1076–1085. doi: 10.1111/jcmm.12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hirabayashi S. The interplay between obesity and cancer: A fly view. Disease Models & Mechanisms. 2016;9(9):917–926. doi: 10.1242/dmm.025320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bhaskaran K., Douglas I., Forbes H., dos-Santos-Silva I., Leon D. A., Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. The Lancet. 2014;384(9945):755–765. doi: 10.1016/s0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Parkin D. M., Boyd L., Walker L. C. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. British Journal of Cancer. 2011;105:S77–S81. doi: 10.1038/bjc.2011.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.CRUK. Tipping the Scales: Why preventing obesity makes economic sense. 2016. [Google Scholar]
- 93.Orgel E., Mittelman S. D. The links between insulin resistance, diabetes, and cancer. Current Diabetes Reports. 2013;13(2):213–222. doi: 10.1007/s11892-012-0356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Honors M. A., Kinzig K. P. Diet-induced obesity and insulin resistance spur tumor growth and cancer cachexia in rats bearing the yoshida sarcoma. Nutrition and Cancer. 2014;66(5):872–878. doi: 10.1080/01635581.2014.916325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Musselman L. P. A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Disease Models Mechanisms. 2011;4(6):842–849. doi: 10.1242/dmm.007948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Milella M., Falcone I., Conciatori F., et al. PTEN: Multiple functions in human malignant tumors. Frontiers in Oncology. 2015;5, article no. 024 doi: 10.3389/fonc.2015.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nowak K., Seisenbacher G., Hafen E., Stocker H. Nutrient restriction enhances the proliferative potential of cells lacking the tumor suppressor PTEN in mitotic tissues. eLife. 2013;2013(2) doi: 10.7554/eLife.00380.e00380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hoffman N. J., et al. Gobal Phosphoproteomic Analysis of Human Skeletal Muscle Reveals a Network of Exercise-Regulated Kinases and AMPK Substrate. Cell Metab. 2015;22(5):922–935. doi: 10.1016/j.cmet.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hirabayashi S., Cagan R. L. Salt-inducible kinases mediate nutrient- sensing to link dietary sugar and tumorigenesis in Drosophila. eLife. 2015;4(2015) doi: 10.7554/eLife.08501.e08501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Enomoto M., Kizawa D., Ohsawa S., Igaki T. JNK signaling is converted from anti- to pro-tumor pathway by Ras-mediated switch of Warts activity. Developmental Biology. 2015;403(2):162–171. doi: 10.1016/j.ydbio.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 101.Doggett K., Grusche F. A., Richardson H. E., Brumby A. M. Loss of the Drosophila cell polarity regulator Scribbled promotes epithelial tissue overgrowth and cooperation with oncogenic Ras-Raf through impaired Hippo pathway signaling. BMC Developmental Biology. 2011;11, article no. 57 doi: 10.1186/1471-213X-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Campbell P. T., Jacobs E. J., Newton C. C., Gapstur S. M., Patel A. V. Diabetes and cause-specific mortality in a prospective cohort of one million U.S. adults. Diabetes Care. 2012;35(9):1835–1844. doi: 10.2337/dc12-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ngabire D., Kim G. Autophagy and Inflammatory Response in the Tumor Microenvironment. International Journal of Molecular Sciences. 2017;18(9):p. 2016. doi: 10.3390/ijms18092016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Manent J., Banerjee S., de Matos Simoes R., et al. Autophagy suppresses Ras-driven epithelial tumourigenesis by limiting the accumulation of reactive oxygen species. Oncogene. 2017;36(40):5576–5592. doi: 10.1038/onc.2017.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Endo S., Nakata K., Ohuchida K., et al. Autophagy Is Required for Activation of Pancreatic Stellate Cells, Associated With Pancreatic Cancer Progression and Promotes Growth of Pancreatic Tumors in Mice. Gastroenterology. 2017;152(6):1492–1506.e24. doi: 10.1053/j.gastro.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 106.Paiardi C., Mirzoyan Z., Zola S., et al. The stearoyl-CoA desaturase-1 (Desat1) in Drosophila cooperated with Myc to induce autophagy and growth, a potential new link to tumor survival. Gene. 2017;8(5, article no. 131) doi: 10.3390/genes8050131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sousa C. M., et al. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature. 2016;536:479–483. doi: 10.1038/nature19084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Filomeni G., de Zio D., Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death & Differentiation. 2015;22(3):377–388. doi: 10.1038/cdd.2014.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li L., Chen Y., Gibson S. B. Starvation-induced autophagy is regulated by mitochondrial reactive oxygen species leading to AMPK activation. Cellular Signalling. 2013;25(1):50–65. doi: 10.1016/j.cellsig.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 110.Martinez-Outschoorn U. E., Pavlides S., Howell A., et al. Stromal-epithelial metabolic coupling in cancer: integrating autophagy and metabolism in the tumor microenvironment. The International Journal of Biochemistry & Cell Biology. 2011;43(7):1045–1051. doi: 10.1016/j.biocel.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pettersen K., Andersen S., Degen S., et al. Cancer cachexia associates with a systemic autophagy-inducing activity mimicked by cancer cell-derived IL-6 trans-signaling. Scientific Reports. 2017;7(1, article no. 2046) doi: 10.1038/s41598-017-02088-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bunker B. D., Nellimoottil T. T., Boileau R. M., Classen A. K., Bilder D. The transcriptional response to tumorigenic polarity loss in Drosophila. eLife. 2015;2015(4) doi: 10.7554/eLife.03189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fearon K., Strasser F., Anker S. D., et al. Definition and classification of cancer cachexia: an international consensus. The Lancet Oncology. 2011;12(5):489–495. doi: 10.1016/s1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 114.Porporato P. E. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis. 2016;5(2, article no. e200) doi: 10.1038/oncsis.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fearon K. C. H., Glass D. J., Guttridge D. C. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metabolism. 2012;16(2):153–166. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 116.Argilés J. M., Busquets S., Stemmler B., López-Soriano F. J. Cancer cachexia: understanding the molecular basis. Nature Reviews Cancer. 2014;14(11):754–762. doi: 10.1038/nrc3829. [DOI] [PubMed] [Google Scholar]
- 117.Aoyagi T., et al. Cancer cachexia, mechanism and treatment. World Journal of Gastrointestinal Oncology. 2015;7(4):17–29. doi: 10.4251/wjgo.v7.i4.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Couch M., Lai V., Cannon T., et al. Cancer cachexia syndrome in head and neck cancer patients: Part I. Diagnosis, impact on quality of life and survival, and treatment. Head & Neck. 2007;29(4):401–411. doi: 10.1002/hed.20447. [DOI] [PubMed] [Google Scholar]
- 119.Von Haehling S., Anker S. D. Cachexia as major underestimated unmet medical need: Facts and numbers. International Journal of Cardiology. 2012;161(3):121–123. doi: 10.1016/j.ijcard.2012.09.213. [DOI] [PubMed] [Google Scholar]
- 120.Kwon Y., Song W., Droujinine I. A., Hu Y., Asara J. M., Perrimon N. Systemic organ wasting induced by localized expression of the secreted Insulin/IGF antagonist ImpL2. Developmental Cell. 2015;33(1):36–47. doi: 10.1016/j.devcel.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Honegger B., Galic M., Köhler K., et al. Imp-L2, a putative homolog of vertebrate IGF-binding protein 7, counteracts insulin signaling in Drosophila and is essential for starvation resistance. Journal of Biology. 2008;7(3, article no. 10) doi: 10.1186/jbiol72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jasani B., Donaldson L. J., Ratcliffe J. G., Sokhi G. S. Mechanism of impaired glucose tolerance in patients with neoplasia. British Journal of Cancer. 1978;38(2):287–292. doi: 10.1038/bjc.1978.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Agustsson T., D'souza M. A., Nowak G., Isaksson B. Mechanisms for skeletal muscle insulin resistance in patients with pancreatic ductal adenocarcinoma. Nutrition Journal . 2011;27(7-8):796–801. doi: 10.1016/j.nut.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 124.Fernandes L. C., Machado U. F., Nogueira C. R., Carpinelli A. R., Curi R. Insulin secretion in Walker 256 tumor cachexia. American Journal of Physiology-Endocrinology and Metabolism. 1990;258(6):E1033–E1036. doi: 10.1152/ajpendo.1990.258.6.E1033. [DOI] [PubMed] [Google Scholar]
- 125.Noguchi Y., Yoshikawa T., Marat D., et al. Insulin resistance in cancer patients is associated with enhanced tumor necrosis factor-α expression in skeletal muscle. Biochemical and Biophysical Research Communications. 1998;253(3):887–892. doi: 10.1006/bbrc.1998.9794. [DOI] [PubMed] [Google Scholar]
- 126.Asp M. L., Tian M., Wendel A. A., Belury M. A. Evidence for the contribution of insulin resistance to the development of cachexia in tumor-bearing mice. International Journal of Cancer. 2010;126(3):756–763. doi: 10.1002/ijc.24784. [DOI] [PubMed] [Google Scholar]
- 127.Penna F., Costamagna D., Pin F., et al. Autophagic degradation contributes to muscle wasting in cancer cachexia. The American Journal of Pathology. 2013;182(4):1367–1378. doi: 10.1016/j.ajpath.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 128.Aversa Z., Pin F., Lucia S., et al. Autophagy is induced in the skeletal muscle of cachectic cancer patients. Scientific Reports. 2016;6 doi: 10.1038/srep30340.30340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dewys W. D., et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients, Eastern Cooperative Oncology Group. The American Journal of Medicine. 1980;69(4):491–497. doi: 10.1016/s0149-2918(05)80001-3. [DOI] [PubMed] [Google Scholar]
- 130.Blum D., Omlin A., Baracos V. E., et al. Cancer cachexia: a systematic literature review of items and domains associated with involuntary weight loss in cancer. Critical Review in Oncology/Hematology. 2011;80(1):114–144. doi: 10.1016/j.critrevonc.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 131.Agrawal N., Delanoue R., Mauri A., et al. The Drosophila TNF Eiger Is an Adipokine that Acts on Insulin-Producing Cells to Mediate Nutrient Response. Cell Metabolism. 2016;23(4):675–684. doi: 10.1016/j.cmet.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 132.Quatromoni J. G., Eruslanov E. Tumor-associated macrophages: function, phenotype, and link to prognosis in human lung cancer. American Journal of Translational Research. 2012;4(4):376–389. [PMC free article] [PubMed] [Google Scholar]
- 133.Fischer K., Hoffmann P., Voelkl S., et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109(9):3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 134.Colegio O. R., Chu N.-Q., Szabo A. L., et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513(7519):559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sonoshita M., Cagan R. L. Modeling human cancers in Drosophila. Current Topics in Developmental Biology. 2017;121:287–309. doi: 10.1016/bs.ctdb.2016.07.008. [DOI] [PubMed] [Google Scholar]