Abstract
Whereas the adequate intake of potassium is relatively high in healthy adults, i.e., 4.7 g per day, a dietary potassium restriction of usually less than 3 g per day is recommended in the management of patients with reduced kidney function, especially those who tend to develop hyperkalaemia including patients who are treated with angiotensin pathway modulators. Most potassium-rich foods are considered heart-healthy nutrients with high fibre, high anti-oxidant vitamins and high alkali content such as fresh fruits and vegetables; hence, the main challenge of dietary potassium management is to maintain high fibre intake and a low net fixed-acid load, because constipation and metabolic acidosis are per se major risk factors for hyperkalaemia. To achieve a careful reduction of dietary potassium load without a decrease in alkali or fibre intake, we recommend the implementation of certain pragmatic dietary interventions as follows: Improving knowledge and education about the type of foods with excess potassium (per serving or per unit of weight); identifying foods that are needed for healthy nutrition in renal patients; classification of foods based on their potassium content normalized per unit of dietary fibre; education about the use of cooking procedures (such as boiling) in order to achieve effective potassium reduction before eating; and attention to hidden sources of potassium, in particular additives in preserved foods and low-sodium salt substitutes. The present paper aims to review dietary potassium handling and gives information about practical approaches to limit potassium load in chronic kidney disease patients at risk of hyperkalaemia.
Keywords: chronic kidney disease, dialysis, end-stage renal disease, hyperkalaemia, potassium, diet, nutrition, fibre
1. Introduction
Hyperkalaemia is a common electrolyte abnormality which occurs most frequently in patients with decreased kidney function, with the highest prevalence observed in patients with end-stage renal disease (ESRD). Severe hyperkalaemia is a medical emergency, as high serum potassium levels or its abrupt excursions may be a cause of sudden cardiac death [1,2]. Besides a decrease in potassium excretion by the kidneys (as seen in chronic kidney disease (CKD) or ESRD and often made worse by medications such as inhibitors of the renin-angiotensin-aldosterone system (RAAS)), hyperkalaemia may also be exacerbated by an abnormal redistribution between the intracellular and extracellular space and by increased dietary potassium intake [3,4].
In the early stages of CKD, even very high potassium intake is not sufficient to cause hyperkalaemia and external potassium balance is generally neutral, unless therapies reducing net intracellular shift or renal excretion capacity are administered. This is an important consideration since high potassium diets are useful in patients with CKD because they have been associated with favourable cardiovascular and renal outcomes [5,6]. However, in advanced stages of CKD and in ESRD a positive external potassium balance, namely a dietary input that surpasses output, has a crucial role in engendering hyperkalaemia [3,4], and its prevention requires (among others) a balanced management of dietary potassium load [7,8].
The present paper aims to review dietary potassium handling and gives information about practical approach to limit potassium load in CKD patients at risk of hyperkalaemia.
2. Dietary Potassium Intake
Whereas the US Food and Nutrition Board of the Institute of Medicine has set an adequate intake for potassium relatively high in healthy adults, i.e. 4.7 g (120 mmol) per day, the World Health Organization (WHO) recommends a dietary potassium intake of 3.9 g (100 mmol) per day or at least 90 mmol/day (3510 mg/day), to reduce blood pressure and the risk of cardiovascular damage, stroke and coronary heart disease [9]. In patients with non-dialysis dependent (NDD) CKD stages 1–5, the National Kidney Foundation (NKF) suggests an unrestricted potassium intake unless the serum potassium level is elevated. In hemodialysis patients, potassium intake should be up to 2.7–3.1 g/day and in peritoneal dialysis patients close to 3–4 g/day; in both cases, adjustments based on serum potassium levels are crucial [10]. A recent comprehensive review paper on nutritional management of CKD by Kalantar-Zadeh and Fouque [11] has suggested an intake of 4.7 g/day in the early stages of CKD without risk of hyperkalaemia, but a dietary potassium restriction of less than 3 g (less than 77 mmol) per day in CKD patients who tend to develop hyperkalaemia (serum potassium levels >5.3 mEq/L). A low potassium diet is defined as a dietary intake of 2–3 g/day (approximately 51–77 mmol/day) as shown in Table 1.
Table 1.
Recommended dietary potassium intake at different Stages of chronic kidney disease in adults. Adapted from Table 2 in Kalantar-Zadeh K and Fouque D [11].
| Normal kidney function (eGFR ≥ 60 *) and no proteinuria but at higher CKD risk, e.g., diabetes, hypertension, or solitary kidney | Mild to moderate CKD (eGFR 30 < 60 *) without substantial proteinuria (<0.3 g/day) | Advanced CKD (eGFR < 30 *) or any CKD with substantial proteinuria (>0.3 g/day) | Prevalent dialysis therapy, or any CKD stage with existing or imminent PEW | |
|---|---|---|---|---|
| Dietary Potassium (g/day) | Same as recommended for the general population (4.7 g/day). | Same as the general population unless frequent or severe hyperkalaemia excursions. | <3 g/day if hyperkalaemia occurs frequently while maintaining high fibre intake. | <3 g/day target high fibre intake |
* The unit for eGFR is mL/min/1.73 m2 body surface area (BSA). Abbreviations: CKD: chronic kidney disease, d: per day (such as in g/kg/day), eGFR: estimated glomerular filtration rate in mL/min/1.73 m2, PEW: protein energy wasting.
2.1. Renal Regulation of Potassium Balance
The majority of the regulation of potassium balance occurs at the renal level. Following a dietary potassium load, renal excretion increases after a few minutes reaching maximum levels after 2 h [12], thus preventing hyperkalaemia. This occurs by means of increased aldosterone production. Potassium secretion may also be facilitated by a recently postulated enteric sensor that reduces sodium reabsorption in the proximal tubule and facilitates potassium secretion by increased delivery of sodium to the distal tubule [3]. Additional renal responses to potassium loading include reduced sodium reabsorption and increased potassium-channel conductance [3,4]. In patients with advanced CKD the kidneys’ ability to adapt to increased potassium intake diminishes and in ESRD the renal mechanisms of potassium excretion often become negligible, making these patients extremely prone to hyperkalaemia.
2.2. The Role of the Gastrointestinal Tract in Regulating Potassium Balance
Dietary potassium is absorbed mostly in the duodenum and jejunum and the net intestinal potassium absorption is approximately 90%. Under physiologic circumstance faecal excretion is quite constant at about 10 mmol/day, with a maximum level of 15–20 mmol/day. The capacity of the colon/rectum to secrete potassium is inversely related to residual kidney function and becomes the main route of potassium excretion in patients with ESRD [13,14].
Potassium concentration in the faeces is very high (83–95 mmol/L), so that diarrhoea (more than 300 cc of fecal volume a day) may lead to profound hypokalemia. Hence, it is conceivable that slow faecal transit time along the intestinal tract favours potassium absorption, whereas faster intestinal transit time reduces potassium absorption [15]. This suggests that constipation, instead of potassium dietary load, is the main determinant of hyperkalaemia in CKD and ESRD patients [16].
2.3. Sources, Composition of Diet and Hyperkalaemia
Potassium is present in a large variety of foods, both from animal and plant sources. Potassium is found mainly intracellularly in animals, where it has a crucial role in determining the electric potential of cell membranes and then the excitability of nervous system and muscle cells. Hence it is not surprising that food rich in cells, such as meat or fish, are relevant sources of potassium. Indeed a recent study showed that high protein intake in maintenance dialysis patients has direct correlation with hyperkalaemia [17]. In this study higher dietary potassium intake was associated with increased death risk in long-term haemodialysis patients, even after adjustments for serum potassium level; dietary protein; energy and phosphorus intake; and nutritional and inflammatory marker levels. The potential role of dietary potassium in the high mortality rate of dialysis patients warrants clinical trials.
Notwithstanding the importance of protein intake, fruits and vegetables are supplying the majority of dietary potassium in most diets [18]. Potassium is crucial for many processes in a plant’s life cycle, with its importance considered second only to nitrogen for plant growth and composition (1 to 3% by weight) [19]. Plants require potassium ions for protein synthesis, enzyme activation and maintenance of cation/anion balance in the cytosol. Potassium is also involved in the opening and closing of stomata regulating proton pumps; it plays an important role in photosynthesis and in photo-protection and it takes part in protein synthesis and in downward solute transport from the leaves [19,20]. Potassium is important for crop yield as well as for the quality of edible parts of crops; its deficiency has a strong impact on plant metabolism. Plant responses to low potassium involve changes in the concentrations of many metabolites as well as alteration in the transcriptional levels of many genes and in the activity of many enzymes [19]. Potassium levels in plants are also associated with disease resistance through its effects on decreased cell permeability and decreased susceptibility to tissue penetration. When adequate levels of potassium are present a greater amount of silica is incorporated into the cell walls, strengthening the epidermal layer which represent a physical barrier to pathogens. Moreover, potassium seems to directly contribute to an adequate thickening of cell walls [21]. Purely plant-based LPD (Low Protein Diet) may or may not lead to a more consistent potassium load than animal-based LPD.
Therefore, in the case of a need for a protein restricted diet in advanced CKD, animal-based LPD could be favoured over vegetarian LPD [22,23], combined with educational strategies to reduce the effective potassium load and close monitoring of serum potassium level. However, such a strategy entails a therapeutic compromise, as it would abandon the additional cardiovascular benefits of a plant-based diet. Plant-based foods have favourable effects on systemic hypertension, on glomerular hemodynamics and perm-selectivity, leading to reduction of proteinuria. They supplylessbio-available phosphorus (in the form of phytate) with favourable effect on the CKD mineral and bone disease (MBD) and they are associated with effective renal protection probably by means of the reduced acid load [24].
However, plant-based foods also supply a high content of fibres and alkali as well as anti-oxidant vitamins and trace elements. Of consequence, a lower net acid load and favourable effects on intestinal motility and microbiota are expected. Prevention or correction of metabolic acidosis and of constipation represent mechanisms that counteract the hyperkalaemia-inducing effects of high potassium intake, and may explain why vegetarian diets, more or less associated with a reduction in protein intake did not induce increase of serum potassium or overt hyperkalaemia in CKD patients [25,26,27,28]. Similarly, during high-fruit intake, no changes in serum potassium levels were reported [23,24,25,27].
Fibre intake has a major role in the modulation of intestinal microbiota, with high-fibre diets promoting the growth of bacteria with saccharolytic metabolism and lowering proteolytic-derived uremic toxins [29,30,31,32] and also leading to faster bowel transit time. Conversely, reduced bowel motility and constipation can induce dysbiosis of the intestinal microbiota, contributing to uremic intoxication and increase net absorption of potassium, leading to hyperkalaemia [16]. Therefore, a high fibre content in the diet should be preserved even when the potassium intake is to be lowered.
3. Dietary Intervention to Limit Potassium Intake
In clinical practice, a common dilemma in the management of advanced CKD patients with chronic hyperkalaemia is the patients’ deprivation of the beneficial effects of RAAS inhibitors or of the favourable effects of vegetarian diets [33] in order to control hyperkalaemia. A solution may be the use of intestinal potassium binders [34,35]. However, the capacity of intestinal potassium -binders to remove potassium is limited and they could be expensive in the long run. Therefore, a careful control of the dietary potassium load is in an important aspect of the management of CKD and heart failure patients with, or at risk of hyperkalaemia.
In patients with stage 4–5 NDD CKD and ESRD, dietary potassium management also has to be synchronized with additional nutritional goals, namely the amount of protein intake (restricted in NDD-CKD or increased in ESRD), high fibre intake, reduced net fixed acid production and cardiovascular effects and the favouring of a heart-healthy diet (typically consisting of fruits and vegetables) [36].
All of these goals are of clinical relevance while also useful to counteract the risk of hyperkalaemia. To obtain a reduction of potassium load without inducing a decrease in alkali or fibre intake, we recommend the implementation of certain pragmatic interventions:
-
(a)
Knowledge and education about the type of foods which contain excess potassium (per serving or per unit of weight), about the foods needed for proper nutrition in CKD and ESRD, and that supply a low potassium load.
-
(b)
Classification of foods based on their potassium content normalized per unit of fibre.
-
(c)
Education about the use of cooking procedures (especially boiling) in order to achieve demineralization and in particular for removing potassium before eating.
-
(d)
Attention to hidden sources of potassium (e.g., food additives and low-sodium salt substitutes).
The first step is the provision of information about the type of foods which contain excess potassium, which should be avoided. However, potassium is almost ubiquitous, which makes it very difficult for patients to make dietary choices based on general guidance alone. Skilled dietitians are required to properly select foods (Table 2 and Table 3) to individualize dietary programs with low potassium content and to realize intensive educational programs and regular counselling. This activity is time and money consuming and it is difficult to realize in the common clinical practice without the support of dedicated and qualified health professionals.
Table 2.
Potassium content of animal-origin food, beverages, sugar and sweets and fats.
| Potassium (mg) | Potassium (mg) | ||||||
|---|---|---|---|---|---|---|---|
| 100 g | Serving | 100 kcal | 100 g | Serving | 100 kcal | ||
| Meat | Milk and dairy | ||||||
| Chicken breast | 370 | 370 | 370 | Milk | 150 | 188 | 234 |
| Chicken thigh | 355 | 355 | 332 | Yogurt | 150 | 188 | 170 |
| Duck | 290 | 290 | 182 | Brie | 100 | 50 | 31 |
| Lamb | 350 | 350 | 220 | Cheddar | 120 | 60 | 31 |
| Liver | 320 | 320 | 225 | Cottage cheese | 89 | 89 | 77 |
| Pork | 290 | 290 | 185 | Cream cheese | 150 | 150 | 84 |
| Rabbit | 360 | 360 | 261 | Emmenthal cheese | 107 | 54 | 27 |
| Turkey breast | 320 | 320 | 221 | Gouda cheese | 89 | 45 | 26 |
| Turkey thigh | 310 | 310 | 167 | Parmesan cheese | 120 | 60 | 30 |
| Beef | 330 | 330 | 206 | Pecorino cheese | 94 | 47 | 24 |
| Veal | 360 | 360 | 391 | Ricotta cheese | 119 | 119 | 82 |
| Preserved meat | Spreadable cheese | 108 | 54 | 35 | |||
| Bresaola | 505 | 253 | 334 | Stracchino cheese | 62 | 62 | 21 |
| Canned meat | 140 | 140 | 226 | Fats | |||
| Cooked ham | 227 | 114 | 106 | Butter | 15 | 2 | 2 |
| Ham | 454 | 227 | 203 | Cream | 91 | 9 | 44 |
| Mortadella | 130 | 65 | 41 | Margarine | 5 | 1 | 1 |
| Salami | 473 | 237 | 123 | Olive oil | 0 | 0 | 0 |
| Sausage | 130 | 65 | 33 | Sugar and Sweets sand | |||
| Wurstel | 140 | 70 | 52 | Dark chocolate | 300 | 30 | 55 |
| Fish | Fruit ice cream | 180 | 72 | 101 | |||
| Anchovies | 278 | 417 | 290 | Honey | 51 | 3 | 17 |
| Carpa | 286 | 429 | 204 | Marmalade | 100 | 5 | 45 |
| Hake | 320 | 480 | 451 | Milk chocolate | 420 | 42 | 74 |
| Herring | 320 | 480 | 148 | Milk ice cream | 110 | 44 | 46 |
| Mussel | 320 | 480 | 381 | Sugar | 2 | 0 | 1 |
| Salmon | 310 | 465 | 168 | Beverages | |||
| Shrimp | 266 | 399 | 375 | Beer | 35 | 116 | 78 |
| Sole | 280 | 420 | 326 | Cola | 1 | 3 | 3 |
| Trout | 429 | 644 | 364 | Orange juice | 150 | 300 | 417 |
| Egg | Red wine | 110 | 138 | 145 | |||
| Egg white | 135 | 95 | 314 | Tea | 0 | 0 | 0 |
| Whole egg | 133 | 67 | 104 | Wine | 61 | 76 | 86 |
| Yolk | 90 | 31 | 28 | ||||
Table 3.
Potassium content of plant-based foods.
| Potassium (mg) | Potassium (mg) | |||||||
|---|---|---|---|---|---|---|---|---|
| 100 g | Serving | 100 kcal | 100 g | Serving | 100 kcal | |||
| Cereals and tubers | Pulses | |||||||
| Barley | 120 | 60 | 38 | Beans | 650 | 650 | 625 | |
| Buckwheat | 220 | 176 | 60 | Dry beans | 1445 | 723 | 465 | |
| Corn flakes | 99 | 45 | 27 | Dry chickpeas | 800 | 400 | 239 | |
| Pasta | 160 | 128 | 45 | Dry lentils | 980 | 490 | 302 | |
| Rice | 110 | 88 | 30 | Dry soy beans | 1740 | 870 | 437 | |
| Rye bread | 190 | 95 | 86 | Lupine | 351 | 351 | 308 | |
| Toasted bread | 140 | 35 | 34 | Peas | 202 | 202 | 266 | |
| White Bread | 176 | 88 | 64 | Fruits | ||||
| Whole bread | 210 | 105 | 86 | Apple | 120 | 180 | 267 | |
| Whole rice | 250 | 200 | 70 | Apricot | 320 | 480 | 1143 | |
| Potatoes | 570 | 1140 | 671 | Banana | 350 | 525 | 530 | |
| Sweet potatoes | 370 | 740 | 425 | Blackberry | 260 | 390 | 722 | |
| Vegetables | Blueberry | 160 | 240 | 640 | ||||
| Asparagus | 240 | 480 | 828 | Cherry | 229 | 344 | 603 | |
| Basil | 300 | 600 | 769 | Fig | 270 | 405 | 574 | |
| Beetroot | 300 | 600 | 1579 | Grape | 192 | 288 | 315 | |
| Broccoli | 340 | 680 | 1259 | Grapefruit | 230 | 345 | 885 | |
| Carrot | 220 | 440 | 667 | Kiwi | 400 | 600 | 909 | |
| Cauliflower | 350 | 700 | 1400 | Lemon | 140 | 210 | 298 | |
| Celery | 280 | 560 | 1400 | Mango | 250 | 375 | 472 | |
| Chard | 286 | 572 | 1682 | Melon | 333 | 500 | 1009 | |
| Cucumber | 140 | 280 | 1000 | Orange | 200 | 300 | 588 | |
| Eggplant | 184 | 368 | 1227 | Peach | 260 | 390 | 963 | |
| Fennel | 276 | 552 | 3067 | Pear | 130 | 195 | 325 | |
| Green beans | 280 | 560 | 1556 | Pineapple | 250 | 375 | 625 | |
| Leeks | 310 | 620 | 1069 | Pomegranate | 290 | 435 | 460 | |
| Lettuce | 240 | 192 | 1263 | Raspberry | 220 | 330 | 647 | |
| Mushrooms | 235 | 470 | 870 | Strawberry | 160 | 240 | 593 | |
| Olives | 432 | 130 | 304 | Tangerine | 210 | 315 | 292 | |
| Onions | 140 | 280 | 538 | Watermelon | 280 | 420 | 1867 | |
| Peeled tomatoes | 230 | 460 | 1095 | Dried fruits and nuts | ||||
| Pepperoni | 210 | 420 | 955 | Dried figs | 1010 | 303 | 395 | |
| Pumpkin | 202 | 404 | 1122 | Dried plum | 824 | 247 | 375 | |
| Red radish | 180 | 360 | 1385 | Almond | 860 | 258 | 159 | |
| Rocket salad | 369 | 295 | 1476 | Cashew nuts | 565 | 170 | 104 | |
| Spinach | 530 | 1060 | 1710 | Nuts | 368 | 110 | 56 | |
| Zucchini | 210 | 420 | 1909 | Peanuts | 680 | 204 | 114 | |
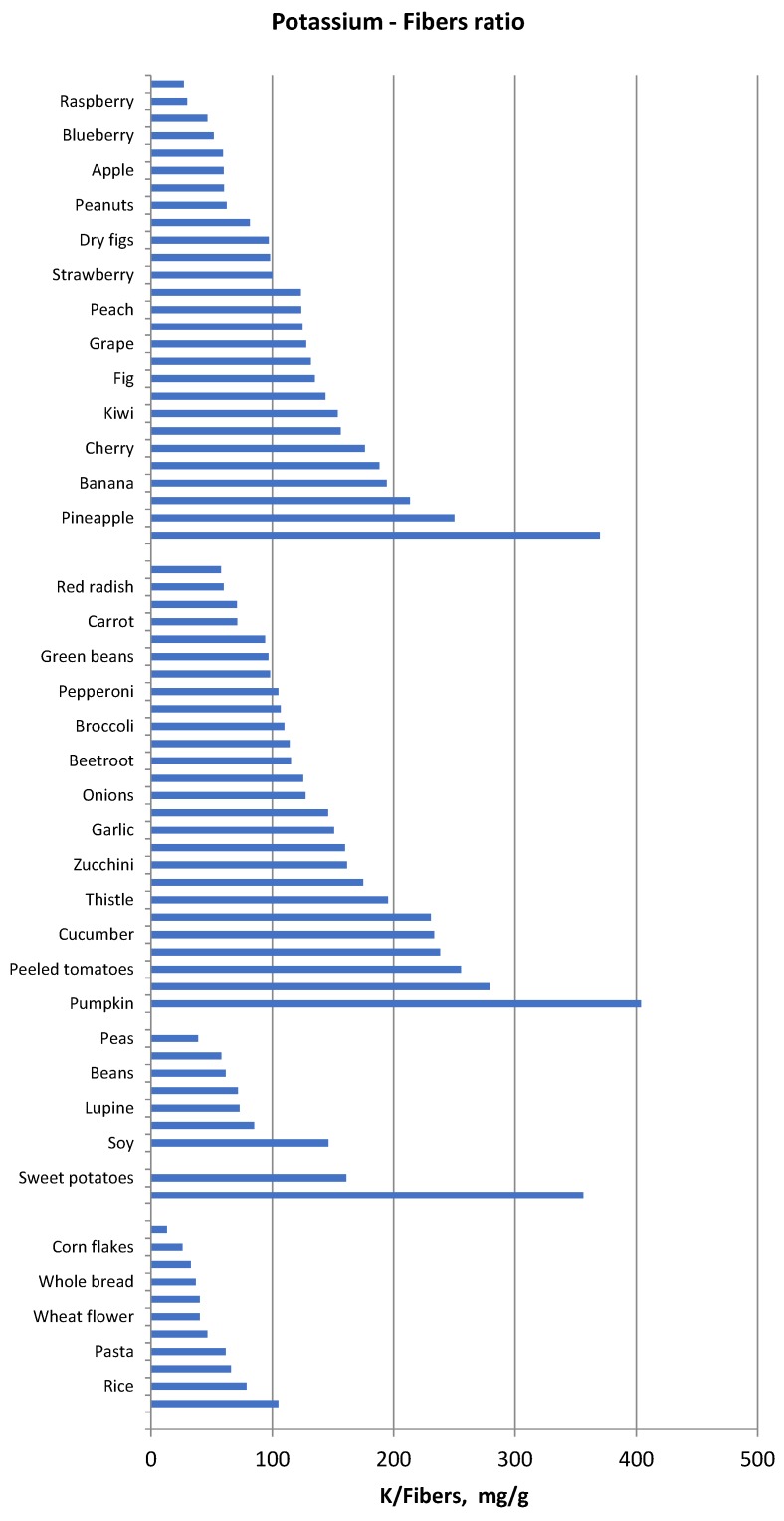
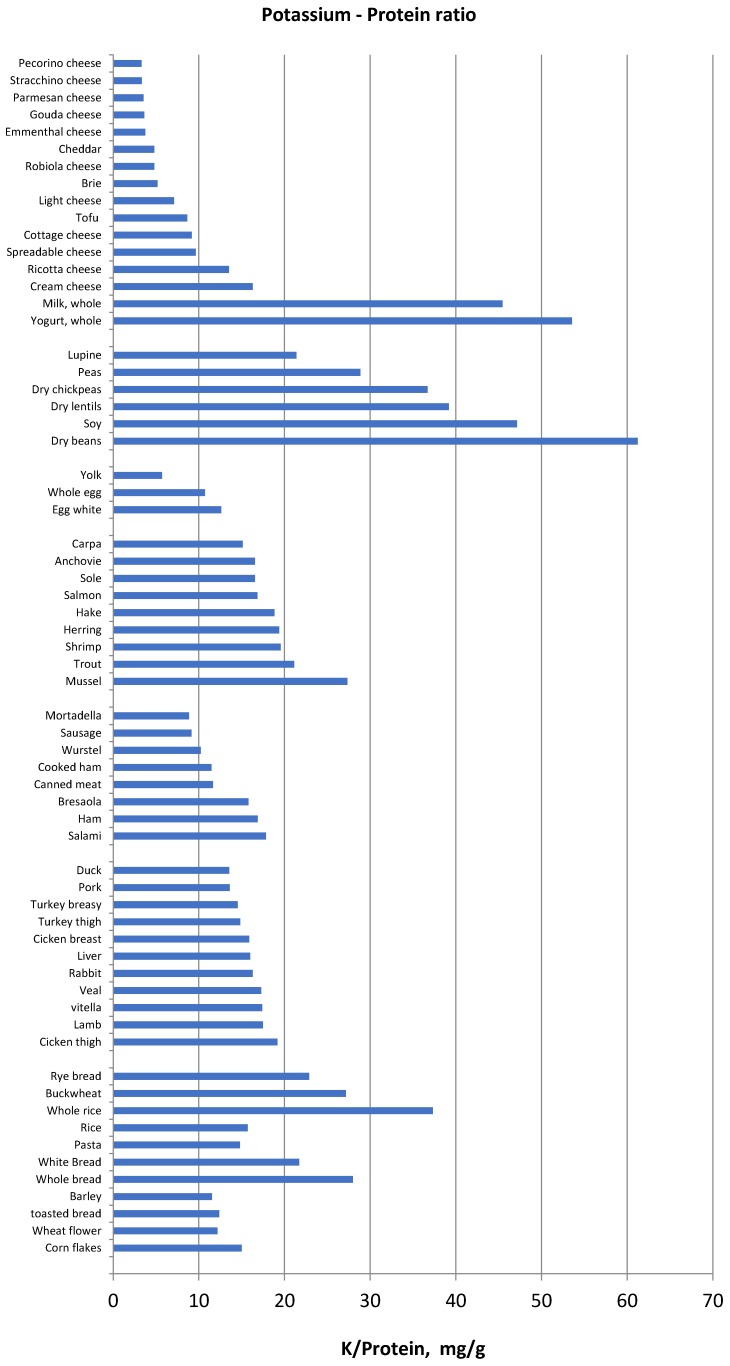
Another method of food selection may be based on the potassium content normalized for unit of fibre, namely the reporting of the potassium content of vegetables and fruits also as “mg per 1 g fibre”. Foods with low potassium to fibre ratio may be allowed whereas foods with very high potassium to fibre ratio should be avoided (Figure 1). Similarly, since protein intake must be increased in haemodialysis and peritoneal dialysis patients, food selection should be addressed to reduce potassium intake without reducing dietary protein intake. Hence, reporting potassium intake per unit (g) of protein may be another method that can make it easier to limit the intake of foods that supply more potassium for the same protein intake (Figure 2) in ESRD patients.
Figure 1.
Potassium to fibre ratio (mg/g) in several food categories.
Figure 2.
Potassium to protein ratio (mg/g) in several food categories.
Additional aspects useful to limit effective potassium intake is education about the use of cooking procedures (as soaking or boiling) in order to obtain food demineralization [37,38,39,40,41]: boiling is able to remove up to 60–80% of the potassium content of several raw foods (Table 4)
Table 4.
Potassium removal by home-based cooking methods.
| Food Group/Item | Type of Treatment or Food Processing | % Potassium Content Reduction |
|---|---|---|
| Vegetables (15 different varieties) |
Each food was placed in 2 liters of hot tap water (100–110 °F), stirred vigorously for 15–20 s and allowed to stand for a predetermined time period. Ham and hot dogs (meat group) were placed in boiling water bath, stirred and allowed to boil for 3 min. Avocado and banana from the fruit group were placed in cold tap water, stirred gently and allowed to stand for the predetermined time period [37]. | 59 ± 40 |
| Fruits (8 different varieties) |
43 ± 16 | |
| Legumes (5 different varieties) |
78.5 ± 20.5 | |
| Meats (7 different varieties) |
57 ± 41 | |
| Tuberous root vegetables * | Soaking [38] | 8% |
| Tuberous root vegetables * | Double cooking (boil, rinse and boil again) [38] | 46% |
| White Potato (Solanum tuberosum) |
Leaching overnight after cubing [41] | 0–4% |
| White Potato (Solanum tuberosum) |
Leaching overnight after shredding [41] | 2–17% |
| White Potato (Solanum tuberosum) |
Boiling after cubing [41] | 50% |
| White Potato (Solanum tuberosum) |
Boiling after shredding [41] | 69–75% |
| Banana (Matooke) | Soaking | No significant reduction |
| Banana (Matooke) | Boiling 60 min at 200 °C [39] | 37% |
| Chocolate | Soaking [40] | 16% |
| Potato | 16% | |
| Apple | 26% | |
| Tomato | 37% | |
| Banana | Soaking [40] | 41% |
* Fresh and sweet batata, cocomalanga, dasheen, eddo, black yam, white yam, yellow yam, yampi, malanga, red yautia, white yautia and yucca.
Jones analyzed the mineral content of a wide variety of foods after different processing procedures. Food samples were subjected to aqueous mineral extraction after a pre-treatment which was different depending on the food group (i.e., cleaning, peeling, cutting into slices or strips, shredding etc.). They were then exposed to different water temperatures and time depending on cell type and the initial state (raw, dried etc.): for example, vegetables were placed in 2 liters of hot tap water (100–110 °F), stirred vigorously for 15–20 s and allowed to stand for a predetermined time period. Ham and hot dogs (meat group) were placed in boiling water bath, stirred and allowed to boil for 3 min. Avocado and banana from the fruit group were placed in cold tap water, stirred gently and allowed to stand for the predetermined time period. The reduction range for potassium was 59% ± 40% for vegetables, 78.5% ± 20.5% for legumes, 57% ± 41% for meats, 94% ± 3% for flours, 99% for cheddar cheese and 43% ± 16% for fruits.
Burrows and Ramer confirmed these results, finding that soaking was not effective in the leaching of significant amounts of potassium from tuberous root vegetables while the double cooking method (boil, rinse, boil again) leached more potassium than did the normal cooking method (boiling) [38]. Similar results were found also by Aiimwe et al. who showed that soaking did not change potassium content in a particular type of banana while boiling at 200 °C reduced potassium concentration from 1.4 ppm to 1 ppm after 60 min [37]. Poor results with soaking were found also by Picq et al. with various types of foods (Table 4) [40]. Preparation of food seems to be important: boiling potatoes after cubing or shredding results in a much greater loss of potassium [41].
These procedures are generally considered “negatives” as they can affect food nutritional properties, taste and appearance but giving patients appropriate instructions on how to process food after boiling (e.g., adding flavouring herbs) this obstacle can be overcome with the advantage that many restricted foods become permissible.
Finally, attention should be paid to hidden sources of potassium, such as salt substitutes and certain food additives. The former contain potassium instead of sodium and are usually recommended in hypertensive patients to reduce sodium intake and to increase potassium intake. However, in the case of treatment with RAASi and/or in patients with reduced renal function the risk of hyperkalaemia from these agents may be considerable [42]. Two categories of salt substitutes are available on the market: low-sodium salts and sodium-free salts. In the low-sodium salt the sodium chloride content must not exceed 35% (corresponding to a sodium content not exceeding 13.6 g%) and cannot be less than 20% (corresponding to a sodium content not less than 7.8%) with a potassium: sodium ratio of at least 1.5:1. In the sodium-free salts potassium content ranges between 20% and 30% and sodium content is fixed at a maximum value of 0.12%. Hence, for instance, 5–6 g of a current salt substitute can supply from 1000–1200 mg to 1500–1800 mg of extra-potassium. This represents a significant potassium load on top of the potassium derived from food intake, which is of concern in patients with reduced capacity of potassium excretion. Special attention should be paid to the use of these salt substitutes because people believe that they are safer than regular salt and they tend to use greater amounts of them. The effect of potassium-based additives on potassium burden is not widely recognized, with limited literature. Sherman and Mehta [43] found that potassium content in foods with additives varied widely and that uncooked, enhanced meat and poultry products had potassium levels up to threefold greater than similar unenhanced food products. The use of additives in packaged poultry, fish or meat foods can increase the effective dietary potassium load and of special concerns in patients with CKD, are sodium-reduced products [44,45].
For instance, additive-free products had an average potassium content <387 mg/100 g, whereas five of the 25 products with additives analyzed in that investigation contained at least 692 mg/100 g with a maximum of 930 mg/100 g [43]. Table 5 reports the most frequently used potassium-based additives and their acceptable daily intake (ADI) [43]. A temporary ADI of 3 mg/kg is currently established, while an ADI of 25 mg/kg for potassium sorbate is under evaluation [46]. Paying attention to the food labels may be useful although the quantity of potassium added as an additive is not generally available.
Table 5.
Most frequently used potassium-based food additives and the corresponding acceptable daily intake (ADI).
| Categories | Chemical Name | E Number (Europe) | ADI | Where to Find Them |
|---|---|---|---|---|
| Preservatives | Potassium sorbate | E202 | 3 mg/kg | Pre-cooked or long-lasting foods, powder dressings, nuts, sauces, preserved meats, stuffed pasta (tortellini, ravioli), jellies, concentrated fruit juices, processed cheeses, wine, margarine |
| Potassium metabisulphite | E224 | 0.35 mg/kg | ||
| Potassium nitrate | E252 | 5 mg/kg | ||
| Antioxidants and acidity regulators | Potassium citrate | E332 | No limit | |
| Potassium tartrate | E336 | 30 mg/kg | ||
| Stabilizers, emulsifier, thickeners | Potassium alginate | E402 | 50 mg/kg | |
| Potassium diphosphate | E450 | 70 mg/kg | ||
| Potassium triphosphate | E451 | 70 mg/kg | ||
| Flavour enhancer | Potassium glutamate | E622 | Not defined | |
| Potassium guanylate | E628 | |||
| Potassium inosinate | E632 |
4. Low Potassium Regimens for Pragmatic Management of CKD Patients with Hyperkalaemia
Nutritional therapy in CKD is very complex, as it has to consider concomitantly the intake of protein, energy, sodium, phosphorus and potassium. Individualized nutritional education programs and regular counselling are all important aspects of clinical management, which also look to improve patients’ lifestyle. Dietary interventions require the active participation of patients and their relatives and caregivers. Recommendations or prescriptions must be simple, understandable and easy to implement in daily life by most patients.
In the Appendix, we provide a leaflet as a guide for education and counselling in the field of hyperkalaemia for patients and professionals. In our experience the use of educational tools such as brochures with food images and inserts with “traffic light” colors can be useful during counselling to summarize some essential aspects of nutritional therapy and to help patients remember instructions. Several foods within the various food groups have been color-coded. The color green identifies foods that can be safely consumed even in hyperkalaemia, orange means foods that can be consumed with caution in hyperkalaemia, while in red are foods that should be avoided in hyperkalaemic patients if possible. This results in 3 columns, which allow users to build 3 kinds of diets characterized by mild, moderate or severe potassium restriction (up to 2 g per day), recommended in the case of mild, moderate or severe chronic hyperkalaemia, respectively (Figure S1). In addition, we also provide several pragmatic suggestions meant to facilitate the application of potassium restricted diets in daily life (Figure S2). Obviously this tool, such as any other visual and practical tool, can only help in achieving excellent results if it is used within nutritional education programs appropriately applied by skilled and well trained health professionals who adapt the nutritional intervention to the individual patient. As we described above, the leaflets report a selection of foods from various food groups. It is possible that some foods are missing but boxes can be filled with other foods as needed, to adapt the tool to patients’ habits, culture, traditions and needs.
5. Conclusions
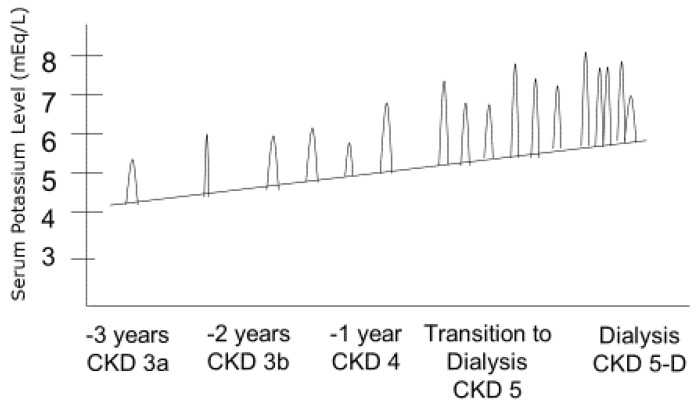
Whereas the adequate intake of potassium is relatively high in healthy adults, i.e., 4.7 g per day, dietary potassium restriction of usually less than 3 g per day is recommended in the management of patients with CKD, especially those who tend to develop hyperkalaemia. Proper dietary counseling is imperative in CKD and ESRD patients with chronic or recurrent hyperkalaemia or those at risk of hyperkalaemia with sporadic hyperkalaemic surges as renal failure progresses (Figure 3). A more balanced and realistic approach to such overzealous restrictions would mean choosing the most beneficial sources of potassium which is only possible through a concerted education effort [46].
Figure 3.
Acute on chronic and recurrent hyperkalaemia episodes by stages of chronic kidney disease (CKD).
Special attention must be paid to avoid excess potassium intake, but together with maintenance of a high fibre intake and a low net fixed-acid load. This is an important point since constipation and metabolic acidosis are major risk factors for chronic hyperkalaemia.
To achieve a reduction of potassium load without causing a decrease in alkali or fibre intake, we recommend avoiding foods which contain excessive amount of potassium, favouring foods with low potassium content relative to fibre and protein content, providing education about the use of cooking procedures (especially boiling and soaking) in order to achieve demineralization and increasing attention to hidden sources of potassium (e.g., food additives and low-sodium salt substitutes). Using these principles, a pragmatic educational tool can be prepared to make the implementation of diets with limited potassium content and more patient-friendly in the management for CKD and ESRD patients with chronic or recurrent hyperkalaemia.
Abbreviations
| CKD | Chronic Kidney Disease |
| RAAS | Renin-Angiotensin-Aldosterone System |
| ESRD | End Stage Renal Disease |
| RDA | Recommended Daily Allowance |
| NDD | Non-Dialysis Dependent |
| NKF | National Kidney Foundation |
| MBD | Mineral Bone Disease |
| HF | Heart Failure |
| LPD | Low Protein Diet |
| ADI | Acceptable Daily Intake |
| BSA | Body Surface Area |
| DPI | Dietary Protein Intake |
| EAA | Essential Amino-Acids |
| eGFR | estimated Glomerular Filtration Rate |
| HBV | High Biologic Value (referred to protein) |
| HTN | Hypertension |
| IBW | Ideal Body Weight |
| KA | Ketoacids (keto-analogues of amino-acids) |
| P | Phosphorus |
| PD | Peritoneal Dialysis |
| RKF | Residual Kidney Function |
| sHPT | secondary Hyperparathyroidism |
Supplementary Materials
The following are available online at http://www.mdpi.com/2072-6643/10/3/261/s1. Figure S1: Pragmatic dietary approaches to mild, moderate and severe chronic hyperkalaemia; Figure S2: General advices for dietary management of hyperkalaemia.
Author Contributions
A.C. designed project conception, wrote the paper and had primary responsibility for final content, wrote and edited the paper; C.P.K. made substantial contributions to conception and design and he revised the manuscript critically for important intellectual content; C.D’A. drafted the manuscript and supplemental Figures, contributed to project conception; K.K.-Z made substantial contributions to conception and design and he edited the manuscript critically for important intellectual content.
Conflicts of Interest
A.C. received consulting fees from Vifor Fresenius, Shire, Abbott. C.P.K. received consulting fees from Abbott, Abbvie, Amgen, Bayer, Keryx and Sanofi-aventis and research support from Shire. K.K.-Z. received commercial honoraria and/or support from Abbott, Abbvie, Alexion, Amgen, Astra-Zeneca, Aveo, Chugai, DaVita, Fresenius, Genentech, Haymarket Media, Hospira, Kabi, Keryx, Novartis, Pfizer, Relypsa, Resverlogix, Sandoz, Sanofi, Shire, Vifor, UpToDate and ZS-Pharma. (US government agencies (such as NIH) and non-for profit foundations or societies (such as NKF) are not listed, while K.K.-Z. have also received additional honoraria from such entities).
References
- 1.Kovesdy C.P., Regidor D.L., Mehrotra R., Jing J., McAllister C.J., Greenland S., Kopple J.D., Kalantar-Zadeh K. Serum and dialysate potassium concentrations and survival in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2007;2:999–1007. doi: 10.2215/CJN.04451206. [DOI] [PubMed] [Google Scholar]
- 2.Pun P.H., Lehrich R.W., Honeycutt E.F., Herzog C.A., Middleton J.P. Modifiable risk factors associated with sudden cardiac arrest within hemodialysis clinics. Kidney Int. 2011;79:218–227. doi: 10.1038/ki.2010.315. [DOI] [PubMed] [Google Scholar]
- 3.Gumz M.L., Rabinowitz L., Wingo C.S. An Integrated View of Potassium Homeostasis. N. Engl. J. Med. 2015;373:60–72. doi: 10.1056/NEJMra1313341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmer B.F., Clegg D.J. Physiology and pathophysiology of potassium homeostasis. Adv. Physiol. Educ. 2016;40:480–490. doi: 10.1152/advan.00121.2016. [DOI] [PubMed] [Google Scholar]
- 5.Palmer B.F., Clegg D.J. Achieving the Benefits of a High-Potassium, Paleolithic Diet, without the Toxicity. Mayo Clin. Proc. 2016;91:496–508. doi: 10.1016/j.mayocp.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Burnier M. Should we eat more potassium to better control blood pressure in hypertension? Nephrol. Dial. Transplant. 2018 doi: 10.1093/ndt/gfx340. [DOI] [PubMed] [Google Scholar]
- 7.Kovesdy C.P. Management of hyperkalaemia in chronic kidney disease. Nat. Rev. Nephrol. 2014;10:653–662. doi: 10.1038/nrneph.2014.168. [DOI] [PubMed] [Google Scholar]
- 8.Kovesdy C.P., Appel L.J., Grams M.E., Gutekunst L., McCullough P.A., Palmer B.F., Pitt B., Sica D.A., Townsend R.R. Potassium homeostasis in health and disease: A scientific workshop cosponsored by the National Kidney Foundation and the American Society of Hypertension. J. Am. Soc. Hypertens. 2017;11:783–800. doi: 10.1016/j.jash.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization (WHO) Guideline: Potassium Intake for Adults and Children. WHO; Geneva, Switzerland: 2012. [PubMed] [Google Scholar]
- 10.K/DOQI, National Kidney Foundation Clinical Practice guidelines for nutrition in chronic renal failure. Am. J. Kidney Dis. 2000;35:S1–S140. doi: 10.1053/ajkd.2000.v35.aajkd03517. [DOI] [PubMed] [Google Scholar]
- 11.Kalantar-Zadeh K., Fouque D. Nutritional Management of Chronic Kidney Disease. N. Engl. J. Med. 2017;377:1765–1776. doi: 10.1056/NEJMra1700312. [DOI] [PubMed] [Google Scholar]
- 12.Rabelink T.J., Koomans H.A., Hené R.J., Dorhout Mees E.J. Early and late adjustment to potassium loading in humans. Kidney Int. 1990;38:942–947. doi: 10.1038/ki.1990.295. [DOI] [PubMed] [Google Scholar]
- 13.Hayes C.P., Jr., Robinson R.R. Fecal potassium excretion in patients on chronic intermittent hemodialysis. Trans. Am. Soc. Artif. Intern. Organs. 1965;11:242–246. doi: 10.1097/00002480-196504000-00046. [DOI] [PubMed] [Google Scholar]
- 14.Hayes C.P., Jr., McLeod M.E., Robinson R.R. An extrarenal mechanism for the maintenance of potassium balance in severe chronic renal failure. Trans. Assoc. Am. Physicians. 1967;80:207–216. [PubMed] [Google Scholar]
- 15.Agarwal R., Afzalpurkar R., Fordtran J.S. Pathophysiology of potassium absorption and secretion by the human intestine. Gastroenterology. 1994;107:548–571. doi: 10.1016/0016-5085(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 16.St-Jules D.E., Goldfarb D.S., Sevick M.A. Nutrient Non-equivalence: Does Restricting High-Potassium Plant Foods Help to Prevent Hyperkalemia in Hemodialysis Patients? J. Ren. Nutr. 2016;26:282–287. doi: 10.1053/j.jrn.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noori N., Kalantar-Zadeh K., Kovesdy C.P., Murali S.B., Bross R., Nissenson A.R., Kopple J.D. Dietary potassium intake and mortality in long-term hemodialysis patients. Am. J. Kidney Dis. 2010;56:338–347. doi: 10.1053/j.ajkd.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.USDA National Nutrient Database for Standard Reference, Release 28. [(accessed on 15 December 2016)]; Available online: https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-human-nutrition-research-center/nutrient-data-laboratory/docs/usda-national-nutrient-database-for-standard-reference/
- 19.Prajapati K., Modi H.A. The importance of potassium in plant growth—A review. Indian J. Plant Sci. 2012;1:177–186. [Google Scholar]
- 20.Gupta S., Yadav B.S., Raj U., Freilich S., Varadwaj P.K. Transcriptomic analysis of soil grown T. aestivum cv. root to reveal the changes in expression of genes in response to multiple nutrients deficiency. Front. Plant Sci. 2017;8:1025. doi: 10.3389/fpls.2017.01025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y., Wu W.H. Regulation of potassium transport and signaling in plants. Curr. Opin. Plant Biol. 2017;12:123–128. doi: 10.1016/j.pbi.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Aparicio M., Bellizzi V., Chauveau P., Cupisti A., Ecder T., Fouque D., Garneata L., Lin S., Mitch W.E., Teplan V., et al. Keto acid therapy in predialysis chronic kidney disease patients: Final consensus. J. Ren. Nutr. 2012;22:S22–S24. doi: 10.1053/j.jrn.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Cupisti A., D’Alessandro C., Gesualdo L., Cosola C., Gallieni M., Egidi M.F., Fusaro M. Non-Traditional Aspects of Renal Diets: Focus on Fiber, Alkali and Vitamin K1 Intake. Nutrients. 2017;9:444. doi: 10.3390/nu9050444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovesdy C.P. Metabolic acidosis and kidney disease: Does bicarbonate therapy slow the progression of CKD? Nephrol. Dial. Transplant. 2012;27:3056–3062. doi: 10.1093/ndt/gfs291. [DOI] [PubMed] [Google Scholar]
- 25.Barsotti G., Morelli E., Cupisti A., Meola M., Dani L., Giovannetti S. A low-nitrogen low-phosphorus vegan diet for patients with chronic renal failure. Nephron. 1996;74:390–394. doi: 10.1159/000189341. [DOI] [PubMed] [Google Scholar]
- 26.Cupisti A., Morelli E., Meola M., Barsotti M., Barsotti G. Vegetarian diet alternated with conventional low-protein diet for patients with chronic renal failure. J. Ren. Nutr. 2002;12:32–37. doi: 10.1053/jren.2002.29595. [DOI] [PubMed] [Google Scholar]
- 27.Di Iorio B.R., Di Micco L., Marzocco S., De Simone E., De Blasio A., Sirico M.L., Nardone L., On Behalf Of Ubi Study Group Very Low- protein Diet (VLPD) Reduces Metabolic Acidosis in subjects with Chronic Kidney Disease: The “Nutritional Light Signal” of the RenalAcid Load. Nutrients. 2017;17:69. doi: 10.3390/nu9010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moorthi R.N., Armstrong C.L., Janda K., Ponsler-Sipes K., Asplin J.R., Moe S.M. The effect of a diet containing 70% protein from plants on mineral metabolism and musculoskeletal health in chronic kidney disease. Am. J. Nephrol. 2014;40:582–591. doi: 10.1159/000371498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meijers B.K., De Pretern V., Verbeke K., Vanrenterghem Y., Evenepoel P. p-Cresyl sulfate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol. Dial. Transplant. 2010;25:219–224. doi: 10.1093/ndt/gfp414. [DOI] [PubMed] [Google Scholar]
- 30.Salmean Y.A., Segal M.S., Langkamp-Henken B., Canales M.T., Zello G.A., Dahl W.J. Foods with added fiber lower serum creatinine levels in patients with chronic kidney disease. J. Ren. Nutr. 2013;23:e29–e32. doi: 10.1053/j.jrn.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 31.De Angelis M., Montemurno E., Vannini L., Cosola C., Cavallo N., Gozzi G., Maranzano V., Di Cagno R., Gobbetti M., Gesualdo L. Effect of whole-grain barley on the human fecal microbiota and metabolome. Appl. Environ. Microbiol. 2015;81:7945–7956. doi: 10.1128/AEM.02507-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cosola C., De M., Rocchetti M.T., Montemurno E., Maranzano V., Dalfino G., Manno C., Zito A., Gesualdo M., Ciccone M.M., et al. Beta-glucans supplementation associates with reduction in p-cresyl sulfate levels and improved endothelial vascular reactivity in healthy individuals. PLoS ONE. 2017;12:e0169635. doi: 10.1371/journal.pone.0169635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goraya N., Simoni J., Jo C.H., Wesson D.E. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin. J. Am. Soc. Nephrol. 2013;8:371–381. doi: 10.2215/CJN.02430312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weir M.R., Bakris G.L., Bushinsky D.A., Mayo M.R., Garza D., Stasiv Y., Wittes J., Christ-Schmidt H., Berman L., Pitt B. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N. Engl. J. Med. 2015;372:211–221. doi: 10.1056/NEJMoa1410853. [DOI] [PubMed] [Google Scholar]
- 35.Packham D.K., Rasmussen H.S., Lavin P.T., El-Shahawy M.A., Roger S.D., Block G., Qunibi W., Pergola P., Singh B. Sodium zirconium cyclosilicate in hyperkalemia. N. Engl. J. Med. 2015;372:222–231. doi: 10.1056/NEJMoa1411487. [DOI] [PubMed] [Google Scholar]
- 36.Bellizzi V., Cupisti A., Locatelli F., Bolasco P., Brunori G., Cancarini G., Caria S., De Nicola L., Di Iorio B.R., Di Micco L., et al. “Conservative Treatment of CKD” study group of the Italian Society of Nephrology. Low-protein diets for chronic kidney disease patients: The Italian experience. BMC Nephrol. 2016;17:77. doi: 10.1186/s12882-016-0280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones W.L. Demineralization of a wide variety of foods for the renal patient. J. Renal Nutr. 2001;11:90–96. doi: 10.1016/S1051-2276(01)38751-4. [DOI] [PubMed] [Google Scholar]
- 38.Burrowes J.D., Ramer N.J. Removal of potassium from tuberous root vegetables by leaching. J. Ren. Nutr. 2006;16:304–311. doi: 10.1053/j.jrn.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 39.Asiimwe J., Sembajwe L.F., Senoga A., Bakiika E., Muwonge H., Kalyesubula R. Overnight soaking or boiling of “Matooke” to reduce potassium content for patients with chronic kidney disease: Does it really work? Afr. Health Sci. 2013;13:546–550. doi: 10.4314/ahs.v13i3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Picq C., Asplanato M., Bernillon N., Fabre C., Roubeix M., Ricort J.M. Effects of water soaking and/or sodium polystyrene sulfonate addition on potassium content of foods. Int. J. Food Sci. Nutr. 2014;65:673–677. doi: 10.3109/09637486.2014.908172. [DOI] [PubMed] [Google Scholar]
- 41.Bethke P.C., Jansky S.H. The effects of boiling and leaching on the content of potassium and other minerals in potatoes. J. Food Sci. 2008;73:H80–H85. doi: 10.1111/j.1750-3841.2008.00782.x. [DOI] [PubMed] [Google Scholar]
- 42.Kovesdy C.P. Updates in hyperkalemia: Outcomes and therapeutic strategies. Rev. Endocr. Metab. Disord. 2017;18:41–47. doi: 10.1007/s11154-016-9384-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sherman R.A., Mehta O. Phosphorus and potassium content of enhanced meat and poultry products: Implications for patients who receive dialysis. Clin. J. Am. Soc. Nephrol. 2009;4:1370–1373. doi: 10.2215/CJN.02830409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parpia A.S., L’Abbé M., Goldstein M., Arcand J., Magnuson B., Darling P. The Impact of Additives on the Phosphorus, Potassium, and Sodium Content of Commonly Consumed Meat, Poultry, and Fish Products Among Patients With Chronic Kidney Disease. J. Ren. Nutr. 2017;28:83–90. doi: 10.1053/j.jrn.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 45.Parpia A.S., Goldstein M.B., Arcand J., Cho F., L’Abbé M.R., Darling P.B. Sodium-reduced Meat and Poultry Products Contain a Significant Amount of Potassium from Food Additives. J. Acad. Nutr. Diet. 2018 doi: 10.1016/j.jand.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 46.Dietary Guidelines Advisory Committee . The Report of the Dietary Guidelines Advisory Committee on Dietary Guidelines for Americans. Department of Health and Human Services and Department of Agriculture; Washington, DC, USA: 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.