Abstract
Purpose
Information on processes for trials assessing investigational therapeutics is sparse. We assessed the trial development processes within the Department of Investigational Cancer Therapeutics (ICT) at MD Anderson Cancer Center and analyzed their effects on the trial activation timeline and enrollment.
Experimental Design
Data were from a prospectively maintained registry that tracks all clinical studies at MD Anderson. From this database we identified 2,261 activated phase I-III trials; 221 were done at the ICT. ICT trials were matched to trials from other MD Anderson departments by phase, sponsorship, and submission year. Trial performance metrics were compared with paired Wilcoxon signed rank tests.
Results
We identified 3 facets of the ICT research infrastructure: parallel processing of trial approval steps; a physician-led research team; and regular weekly meetings to foster research accountability. Separate analyses were conducted stratified by sponsorship (industry [133 ICT and 133 non-ICT trials] or institutional [68 ICT and 68 non-ICT trials]). ICT trial development was faster from IRB approval to activation (median difference of 1.1 months for industry-sponsored trials vs. 2.3 months for institutional) and from activation to first enrollment (median difference of 0.3 months for industry vs. 1.2 months for institutional) (all matched P<0.05). ICT trials also accrued more patients (median difference of 8 participants for industry vs. 33.5 for institutional) quicker (median difference 4.8 participants/year for industry vs. 11.1 for institutional) (all matched P<0.05).
Conclusions
Use of a clinical research–focused infrastructure within a large academic cancer center was associated with efficient trial development and participant accrual.
Keywords: Clinical trial design, investigational therapeutics, early phase clinical trials
Introduction
Innovations in molecular and cancer biology have led to exponential growth in the development of cancer therapeutics.(1) A critical step in translating these agents from benchtop to clinic is early phase clinical trials.(2–4) However, despite the importance of such trials, little work has focused on their optimization.(5) The few studies available on trial development and performance have generally concentrated on nationally sponsored trials.(6–9) Those studies identified deficiencies such as long intervals from concept review to trial activation and significant proportions of trials failing to meet minimum accrual goals.(6, 8)
The Department of Investigational Cancer Therapeutics (ICT), created at The University of Texas MD Anderson Cancer Center in 2004, focuses on early phase clinical trials to assess investigational therapeutics.(2) One of the largest such departments, the ICT has developed a focused clinic workflow and research infrastructure that facilitates rapid development of such trials and patients accrual. A significant impetus for the formation of this department was ambiguity in which disease-specific department would enroll patients to “all comers” trials. Furthermore, industry sponsored enrollment spots are competitive and often require quick activation and accrual. It was felt by departmental and institutional leadership that a “capitalist” research model may facilitate activation and enrollment, especially given the unmet need for a platform for disease-site agnostic clinical trials. Thus the ICT department was formed.
Because of the department’s unique mission, patients with a diagnosis of any type of advanced cancer are referred to it after receiving standard-of-care options. Patients are then treated sequentially on early phase trials, enrolling in subsequent trials after completing the first ones. Here we describe the infrastructure that this department uses to facilitate efficient trial activation and patient accrual. We further compare trials development times and patient accrual in the ICT protocols with similar trials in other MD Anderson departments with the goal of demonstrating feasibility of the presented clinical research infrastructure.
Materials and Methods
The Clinical Oncology REsearch (CORe) database is a prospectively maintained institutional registry for clinical studies at MD Anderson. Since 1984, all clinical studies must be registered in CORe, where each protocol is tracked for completion of approval hurdles, participant accrual, and study closure. The current analysis was limited to activated trials, those submitted after January 1, 2004 (as 2004 was the year in which the ICT was founded), and phase I-III studies. A total of 2,261 trials met these criteria, 221 of which were done in the ICT. This study was reviewed by the Institutional Review Board (IRB) and deemed exempt.
Statistical Analyses
Trials conducted through the Department of ICT (i.e., ICT trials) were individually matched to trials conducted through other departments at MD Anderson by trial phase (I-III), study source (non-industry externally funded [e.g. CTEP, NCI, etc…], industry, institutional, or national cooperative group), and year in which the trial was submitted to initiate the institutional trail review process (2004–2014). Accrual metrics and the development timelines were compared between ICT vs. non-ICT trials by using paired Wilcoxon signed rank tests. For medians, 95% confidence intervals (CIs) were calculated by a distribution-free method. All tests were 2-sided with α <0.05 considered significant. Statistical analyses were conducted with SAS version 9.4 (SAS institute Inc., Cary, NC).
Results
ICT Trial Demographics
Of the 2,261 phase I-III activated trials submitted after January 1, 2004, 221 were conducted by ICT. The number of trials submitted from ICT increased gradually from 2004 to 2014, with a median of 20 submitted trials per year (range 8 [in 2004] to 29 [in 2013]) (Suppl. Fig. S1). Over this period, 13 ICT investigators opened a median 16 trials per investigator (range 6–45). Characteristics and trial development timelines for ICT trials are presented in Table 1. Most ICT trials were phase I (78%) and sponsored by industry (60%). The median time from trial submission to activation was 4.3 months (interquartile range [IQR] 3.0–6.0).
Table 1.
Characteristics of Trials Conducted in the Department of Investigative Cancer Therapeutics at MD Anderson Cancer Center
| Study Characteristics | ICT Trials (n=221) |
|---|---|
| Trial phase designation | |
| Phase I | 173 (78%) |
| Phase I–II | 23 (10%) |
| Phase II | 21 (10%) |
| Phase III | 4 (2%) |
| Trial source | |
| Non-Industry externally funded* | 8 (4%) |
| Industry | 133 (60%) |
| Institution | 77 (35%) |
| National cooperative group | 3 (1%) |
| Trial timeframes: median (IQR), months | |
| Submission to activation | 4.3 (3.0–6.0) |
| Submission to IRB approval | 1.2 (1.0–1.8) |
| IRB approval to activation | 2.6 (1.6–4.4) |
| Activation to first patient enrolled** | 0.4 (0.1–1.1) |
| Trial accrual: median (IQR) | |
| Accrual rate: participants/year | 15.3 (9.6–26.1) |
| Total accrual: participants | 30 (12–57) |
| Trials activated per investigator: median (range) | 16 (6–45) |
Abbreviations: ICT, investigational cancer therapeutics; IRB, Institutional Review Board; IQR, interquartile range
Funding sources include: National Institutes of Health [NIH], Department of Defense [DoD], National Cancer Institute [NCI], and Cancer Therapy Evaluation Program [CTEP].
Includes only trials that accrued at least one participant.
Parallel Processing of Steps in the Trial Approval Process
The number of major trial approval steps required by MD Anderson was identified as being 16 for institutional-sponsored trials and 13 for industry-sponsored trials (Fig. 1). Although institutional policy mandates that specific approval steps occur in series, to the greatest extent possible, the ICT emphasizes parallel approval (Fig. 1). Upon submission of the protocol to initiate institutional review, investigators within the ICT simultaneously assign trial staff, submit the protocol for approval by the Clinical and Translational Research Center, and initiate contract and budgeting activities (Fig. 1).
Fig. 1.
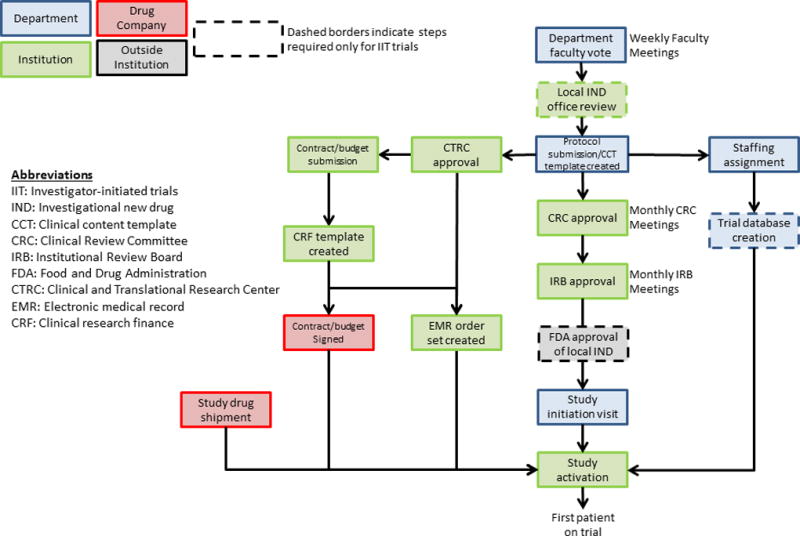
Schematic of core processes necessary for trial activation at MD Anderson Cancer Center and the general flow within the Department of Investigational Cancer Therapeutics to achieve each step.
Physician-Research Team Structure
In contrast to the centralized staffing model used in other departments at MD Anderson, the ICT uses an investigator-centered staffing model in which each investigator manages a separate research team (Fig. 2). These teams include data coordinators, research coordinators, and research nurses; a specific protocol administrator and financial analyst are also assigned to each team (Fig. 2a). Although no uniform workload metrics exist within the institution, a senior research coordinator is expected to follow approximately 10–20 active patients and enroll 3–4 patients per month. In this staffing model, each investigator supports the salaries of all team members through departmental funds and grants/contracts and therefore makes hiring decisions. Critically, each investigator decides whether sufficient staffing is available for new clinical trials based on their assessment of the capacity of their research team and the current project load. One potential benefit of such a model is greater ability to foster team camaraderie and moral thus potentially improving performance; however, this particular aspect is heavily dependent on the managerial style of the overseeing investigator.
Fig. 2.
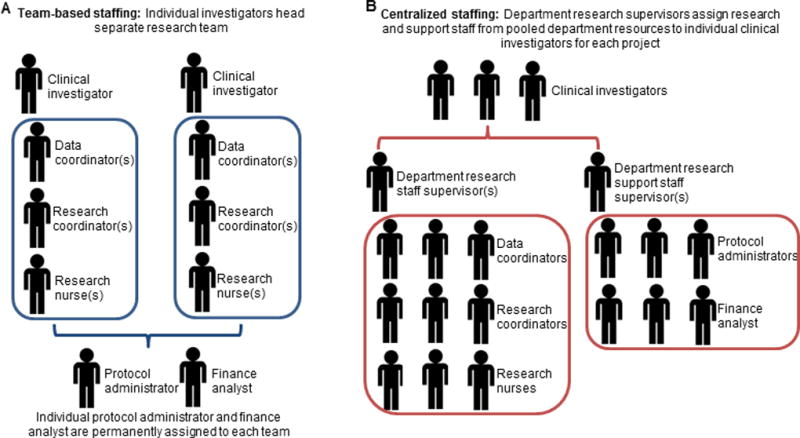
Schematic of team-based staffing models used at (A) the Department of Investigational Therapeutics and (B) a more traditional centralized staffing model.
Given the intensive research requirement of the ICT department, most investigators start with a time commitment approximately 60% clinical and 40% research, such that 2 full days of clinic are generally scheduled. Although investigators are expected to “break even” and cover their research effort and salaries of research staff with grants and contracts, it is not uncommon to receive support from the institution or department. With regards to individual trials, the primary financial factors considered are budget per patient, allotted accrual, and anticipated effort of the research staff. However, it should be emphasized that scientific merit is the primary driver of protocol consideration within the ICT department while financial factors represent the last consideration.
In contrast, in the centralized staffing model used by most other MD Anderson departments, a department pool of research staff is overseen by supervisors who assign members of that staff to individual investigators on a project-by-project basis (Fig. 2b). Under this model, departmental research is maintained by central oversight that determines hiring decisions and is supported by pooled grant and department funds. Department leadership and supervisors thus determine whether adequate staffing is available to initiate a new clinical trial.
Fostering an Atmosphere of Research Accountability
Because the staffing model used by the ICT is decentralized, mechanisms constructed to ensure research accountability include 4 weekly department meetings: faculty meetings, timeline meetings, regulatory manager meetings, and grand rounds. During faculty meetings, new study concepts are presented by the investigator and then vetted by members of the department. New trial concepts must be deemed of scientific merit and to not substantially compete with an existing trial at these faculty meetings before the trial can be submitted to the CORe database. During the department timeline meetings, research staff, investigators, and both department and institutional regulatory personnel review all protocols that are currently undergoing approval, and the time to complete each regulatory step is tracked. If a trial is held up at any step, the meeting participants provide advice on how to circumvent that roadblock. A weekly meeting is held solely by department regulatory managers to review each protocol. Finally, each newly activating trial is presented in departmental grand rounds to ensure that all faculty and advanced practice providers are trained, and that the entire department is familiar with the Department’s clinical trial portfolio.
Comparison of Clinical Trial Development Timelines
Among all trials within the ICT, the median time from protocol submission to IRB approval was 1.2 months (IQR 1.0–1.8); from IRB approval to activation, 2.6 months (IQR 1.6–4.4); and from activation to enrollment of the first patient, 0.4 months (IQR 0.1–1.1) (Table 1). Given the differences in logistics and the protocol approval process according to sponsorship (Fig. 1), we next analyzed institution- and industry-sponsored trials separately.
Among institution-sponsored trials, the matched analysis compared 68 ICT trials that were individually matched to non-ICT trials. No significant differences were found for time from study submission to IRB approval (median 1.3 mo for ICT trials vs. 1.2 mo for non-ICT trials, matched P=0.10). Time from IRB approval to activation was shorter for ICT trials (median 2.9 mo for ICT vs. 5.1 mo for non-ICT, matched P=0.001), as was time from study activation to first patient enrolled (median 0.5 mo for ICT vs. 1.2 mo for non-ICT, matched P<0.001) (Fig. 3a).
Fig. 3.
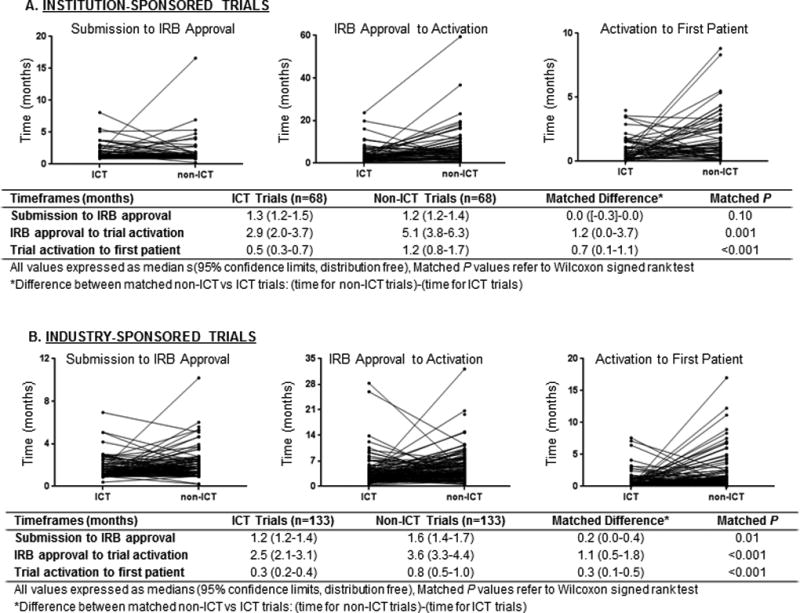
Matched pair analysis comparing trials done within the Department of Investigational Cancer Therapeutics (ICT) and outside that department (non-ICT), analyzing times to complete trial approval hurdles stratified by institutional (A) and industry (B) sponsorship.
Similar patterns were observed with the 133 matched industry-sponsored trials. The time from submission to IRB approval was shorter for the ICT trials (median 1.2 mo for ICT trials vs. 1.6 for non-ICT trials, matched P=0.01), as was time from IRB approval to trial activation (median 2.5 mo ICT vs. 3.6 mo non-ICT, matched P<0.001), and time from trial activation to first patient enrolled (median 0.3 mo ICT vs. 0.8 mo non-ICT, matched P<0.001) (Fig. 3b).
Comparison of Patient Accrual
Among all ICT trials, the median number of participants enrolled in a trial was 30 (IQR 12–57), with an accrual rate of 15.3 per year (IQR 9.6–26.1). As was done for the trial development timelines, separate matched analyses were done for institution- and industry-sponsored trials.
Among the institution-sponsored ICT trials, greater numbers of patients were accrued to ICT trials (median 52 participants for ICT trials vs. 15.5 for non-ICT trials) at faster accrual rates (median 17.6 participants/year for ICT vs. 6.4 for non-ICT) (both matched P<0.001) (Fig. 4a). Similar patterns were observed for industry-sponsored trials, for which both total accrual and accrual rate were higher for the ICT trials (total accrual: median 18 participants for ICT trials vs. 7 for non-ICT trials; accrual rate: median 14/year for ICT vs. 7.5 for non-ICT) (both matched P<0.001) (Fig. 4b).
Fig. 4.
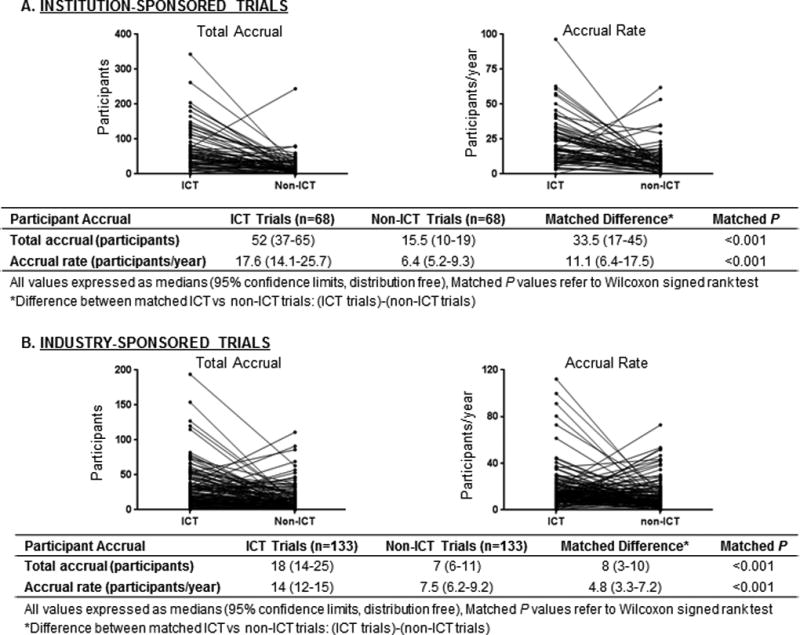
Matched pair analysis comparing trials done within the Department of Investigational Cancer Therapeutics and outside that department (non-ICT), analyzing participant accrual stratified by institutional (A) and industry (B) sponsorship.
Discussion
We present an outline of the research infrastructure at a dedicated department of investigational therapeutics and assess the development and performance of trials under this infrastructure. Notably, the goals of the ICT are fundamentally different from those of traditional departments: whereas a traditional department treats most patients with standard-of-care and not on clinical trials, an ICT goal is to treat all patients on-protocol with investigational agents. Strict comparisons between trials from ICT with those from other departments are fraught with bias. As such, the comparisons presented here are not meant to demonstrate superiority but rather to demonstrate that such a non-traditional department infrastructure can perform at least as well as more traditional departments with regard to clinical trial metrics. It should also be noted that although early phase trials represent the majority of trials within the ICT department, the focus of the department is on developing investigational therapeutics. Increasingly later stage trials have been activated. One example is basket trials targeting specific mutations across cancer sites. The activation of such later phase trials is more recent and thus not reflected in the current analysis which is limited to trials submitted up to 2014.
Two studies have investigated similar trial parameters from cancer centers within the United States. Wang-Gillam et al. analyzed 83 thoracic oncology trials from Washington University in St. Louis (WUSM), and Dilts et al. analyzed 218 oncology trials from Vanderbilt-Ingram Cancer Center and its affiliated network sites (VICC/VICCAN).(7, 10) The median total accrual rates were reported to be 8.7 patients/year for VICC/VICCAN and 7.4 patients/year for WUSM, and the total times from trial submission to activation were 5.7 months for VICC/VICCAN and 5.4 months for WUSM.(7, 10) Although a direct comparison between a cancer center and a single department should not be made, these data help to provide context to the reported total accrual per trial (30 patients) and time from submission to activation (4.3 months) observed for ICT.
In addition to the limited reports focusing on trial performance at single cancer centers, recent analyses focusing on national organizations identified high rates of poor trial accrual (18% to 71%)(8, 9, 11–13) and slow concept development times.(11) An alarming statistic reported by Stensland et al. was the enrollment of 48,000 patients on failed NCI cooperative group studies over a 7-year period.(14) Partially at fault are the overly complex trial development processes, with reports of up to 110 steps to open a trial.(7, 10) Not only do long development times directly result in longer time for trial completion, but they also are associated with failure to achieve accrual goals due to fatigue among the research team or the possibility of the original research question becoming irrelevant.(8, 9) To address this, our group previously reported on a limited strategic alliance with a drug company to expedite trial approval, known as project “Zero Delay.”(2) Zero Delay resulted in an activation time of only 46 days for one such study, the processes from which serve as the basis for much of the clinical trials infrastructure presented here.(2)
Not all aspects of the research infrastructure within ICT are applicable to many academic departments, and not all institutions are able to support a department focused on investigational therapeutics. MD Anderson is a high-volume center that attracts a significant proportion of patients seeking investigational therapeutics. As noted earlier, a goal of the ICT is to treat all patients on trials investigating an experimental therapeutic, whereas more traditional departments treat most patients off-protocol with standard-of-care therapies. As such, the ICT has developed a specialized research infrastructure to streamline trial approval and patient accrual. The structure of physician-led and -funded research teams requires the full dedication of investigators. Significant commitments by department faculty are also required to attend the weekly meetings necessary to foster an environment of accountability. Despite these limitations, dedicated departments focused on investigational therapeutics may be viable in large institutions. Furthermore, aspects of the presented infrastructure may be applied to smaller units or even individual investigators within a cancer center especially as the need to recruit from multiple disease-sites is likely to increase.
Weaknesses of the current analysis deserve mention. First, this was a retrospective review from a single institution, with the associated inherent biases. Second, only those trials that were eventually activated were analyzed, and thus we ignored trials that were submitted and never activated. Finally, to allow exact matching, we used only three known variables. On the other hand, strengths of this study included its unique scope, which focuses on the infrastructure of a department dedicated to investigational therapeutics. The matched analysis between departments from a single institution reduce confounding, especially given that all trials must complete the same institutional approval steps and enroll participants from the same general population. Further context is provided by the fact that phase I trial designs are generally more complicated than phase II and III trials, often requiring complex patient selection, frequent accrual holds to monitor toxicity, close IND oversight, and numerous blood draws for pharmacokinetic and pharmacodynamic analyses.
In conclusion, we have presented key elements of a clinical research infrastructure for a dedicated investigational therapeutics department that focuses on treating patients with advanced disease. ICT seeks to provide quality care for such patients by giving access to an array of investigational therapies that might otherwise be unavailable. In addition to caring for patients, a primary go of ICT is to contribute to the scientific literature and facilitate benchtop-to-clinic translation. As noted previously, the aim of this analysis was not to directly compare clinical trial metrics between departments but rather to show the viability of a dedicated investigational therapeutic department with respect to trial activation and participant accrual. We believe that the need to develop investigational cancer therapeutics will only increase in the future, and thus the creation of focused departments or multidepartment units tasked to develop these trials is a viable approach to efficiently meet this demand.
Supplementary Material
Statement of Translational Relevance.
There is an increasing need for the efficient development and implementation of early phase clinical trials especially given the recent increase in cancer drug discovery. We report on the key elements of the clinical research infrastructure in the department of Investigational Cancer Therapeutics (ICT) at MD Anderson Cancer Center. To assess the performance of trials under this infrastructure we present a matched analysis comparing trials within ICT versus those from other departments at MD Anderson. Given the unique goals of this department, the purpose of this analysis was not to demonstrate superiority of a research infrastructure over another but to assess the feasibility of such a clinical research model.
Acknowledgments
This work was funded in part with NCI Core Grant CA016672, NIH clinical and translational science award UL1 RR024148, and by the NIH Cancer Center Support Grant (CCSG) award CA016672. The authors would like to acknowledge Christine Wogan for her assistance in preparing the manuscript.
References
- 1.Doroshow JH. Timely completion of scientifically rigorous cancer clinical trials: an unfulfilled priority. J Clin Oncol. 2013;31:3312–4. doi: 10.1200/JCO.2013.51.3192. [DOI] [PubMed] [Google Scholar]
- 2.Kurzrock R, Pilat S, Bartolazzi M, Sanders D, Van Wart Hood J, Tucker SD, et al. Project Zero Delay: a process for accelerating the activation of cancer clinical trials. J Clin Oncol. 2009;27:4433–40. doi: 10.1200/JCO.2008.21.6093. [DOI] [PubMed] [Google Scholar]
- 3.Sung NS, Crowley WF, Jr, Genel M, Salber P, Sandy L, Sherwood LM, et al. Central challenges facing the national clinical research enterprise. JAMA. 2003;289:1278–87. doi: 10.1001/jama.289.10.1278. [DOI] [PubMed] [Google Scholar]
- 4.Bach PB. Limits on Medicare's ability to control rising spending on cancer drugs. The New England journal of medicine. 2009;360:626–33. doi: 10.1056/NEJMhpr0807774. [DOI] [PubMed] [Google Scholar]
- 5.Denicoff AM, McCaskill-Stevens W, Grubbs SS, Bruinooge SS, Comis RL, Devine P, et al. The National Cancer Institute-American Society of Clinical Oncology Cancer Trial Accrual Symposium: summary and recommendations. J Oncol Pract. 2013;9:267–76. doi: 10.1200/JOP.2013.001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dilts DM, Cheng SK, Crites JS, Sandler AB, Doroshow JH. Phase III clinical trial development: a process of chutes and ladders. Clin Cancer Res. 2010;16:5381–9. doi: 10.1158/1078-0432.CCR-10-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dilts DM, Sandler AB. Invisible barriers to clinical trials: the impact of structural, infrastructural, and procedural barriers to opening oncology clinical trials. J Clin Oncol. 2006;24:4545–52. doi: 10.1200/JCO.2005.05.0104. [DOI] [PubMed] [Google Scholar]
- 8.Cheng SK, Dietrich MS, Dilts DM. A sense of urgency: Evaluating the link between clinical trial development time and the accrual performance of cancer therapy evaluation program (NCI-CTEP) sponsored studies. Clin Cancer Res. 2010;16:5557–63. doi: 10.1158/1078-0432.CCR-10-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng SK, Dietrich MS, Dilts DM. Predicting accrual achievement: monitoring accrual milestones of NCI-CTEP-sponsored clinical trials. Clin Cancer Res. 2011;17:1947–55. doi: 10.1158/1078-0432.CCR-10-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang-Gillam A, Williams K, Novello S, Gao F, Scagliotti GV, Govindan R. Time to activate lung cancer clinical trials and patient enrollment: a representative comparison study between two academic centers across the atlantic. J Clin Oncol. 2010;28:3803–7. doi: 10.1200/JCO.2010.28.1824. [DOI] [PubMed] [Google Scholar]
- 11.Abrams JS, Mooney MM, Zwiebel JA, Korn EL, Friedman SH, Finnigan SR, et al. Implementation of timeline reforms speeds initiation of National Cancer Institute-sponsored trials. Journal of the National Cancer Institute. 2013;105:954–9. doi: 10.1093/jnci/djt137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schroen AT, Petroni GR, Wang H, Thielen MJ, Gray R, Benedetti J, et al. Achieving sufficient accrual to address the primary endpoint in phase III clinical trials from U.S. Cooperative Oncology Groups. Clin Cancer Res. 2012;18:256–62. doi: 10.1158/1078-0432.CCR-11-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennette CS, Ramsey SD, McDermott CL, Carlson JJ, Basu A, Veenstra DL. Predicting Low Accrual in the National Cancer Institute's Cooperative Group Clinical Trials. Journal of the National Cancer Institute. 2016;108 doi: 10.1093/jnci/djv324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stensland KD, McBride RB, Latif A, Wisnivesky J, Hendricks R, Roper N, et al. Adult cancer clinical trials that fail to complete: an epidemic? Journal of the National Cancer Institute. 2014;106 doi: 10.1093/jnci/dju229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


