Abstract
Background
Persistent human papillomavirus infection in human immunodeficiency virus (HIV)-infected individuals has been strongly associated with anal squamous cell carcinoma. The incidence of anal squamous cell carcinoma continues to increase in this population despite advances in HIV therapy. There are few studies describing the prevalence of anal cancer precursors, treatment outcomes, and associated factors among HIV-infected populations in the southern United States.
Methods
A retrospective chart review was performed on 355 HIV-infected patients from a Southern HIV clinic who were 18 years or older and had received at least one anal Pap smear. Demographic and clinical variables were collected. Descriptive statistics, single variable, and multivariate logistic regression analysis were performed to evaluate for predictors of high-grade squamous intraepithelial lesion (HSIL). Odds ratios and 95% confidence intervals were constructed for independent measures.
Results
After the first anal Pap smear, 38.3% (70/183) of patients with abnormal results were lost to follow-up. Comparing patients with biopsy proven HSIL versus low-grade squamous intraepithelial lesions, patients were less likely to have HSIL if they had a higher CD4 count (odds ratio, 0.81; 95% confidence interval, 0.72–0.93; P = 0.0022). Treatment success after the first round of treatment for topical and infrared coagulation therapy was 36.7% (18/49, all therapy types), and of those who achieved biopsy proven treatment success at follow-up screening, 94.4% (17/18) required subsequent therapy.
Conclusions
Patients with a higher CD4 count were less likely to have HSIL. CD4 nadir, number of sexual partners, and race/ethnicity were not significantly associated with the presence of HSIL.
Among men who have sex with men (MSM), the incidence of anal carcinoma is approximately 35 cases per 100,000 population, and rates of anal cancer in human immunodeficiency virus (HIV)-positive MSM approach 128/100,000 population in some studies, likely representing human papillomavirus (HPV) and HIV coinfection.1–5
Based on the cervical cancer screening paradigm, anal Pap smears (APS) have been used in some settings. Essentially all of the anal Pap smear performance and outcomes research has been derived from patient populations outside the southern United States. Important cultural, behavioral, and genetic differences exist between patients served in the study clinic, which has larger minority representation than previous study populations. It is unclear how these differences contribute to the natural history of HPV-mediated anal SIL and progression to carcinoma in these populations. This study represents a first step in the process to define the natural history of HPV-mediated anal SIL in a southern clinic population.
METHODS
Clinic Organization
The study institution implemented yearly APS testing in HIV-infected MSM as part of routine clinical care. Results of APSs graded atypical cells of undetermined significance (ASCUS) or higher prompted referral to the high-resolution anoscopy (HRA) clinic. If high-grade squamous intraepithelial lesion (HSIL) was confirmed via biopsy, treatment was pursued through three possible modalities: imiquimod (5%) or 5-fluorouracil (5-FU) cream applied intra-anally for up to 16 weeks or HRA-directed infrared coagulation therapy (IRC).
Study Organization
A retrospective chart review was performed on 355 HIV-positive patients from a North Carolina-based HIV clinic who were 18 years or older and had received at least 1 APS between January 1, 2008, and August 31, 2013. Multiple demographic and disease-related data were collected. Subsequent encounters after the initial HRA were reviewed for lesion regression after treatment. CD4 and HIV viral load values were taken as the values in closest proximity to either the HRA or APS dates of interest. A suppressed viral load was defined as <200 copies/mL. The HSIL via biopsy was defined as any anal intraepithelial neoplasia (AIN2) or higher (regardless of P16 status as the Lower Anogenital Squamous Terminology Consensus had not been fully adopted in our pathology group).6 The APS cytology results of ASCUS or higher were considered abnormal. Treatment success by biopsy (after either 5-FU/Imiquimod/IRC) was defined as regression from AIN2 or higher (this includes AIN2 regardless of P16 status and AINIII/CIS, as the higher values) to AIN1 or lower. Treatment failure was defined as follow-up HRA with biopsy showing continued the presence of AIN2 or higher.
Statistical Analysis
There were only 7 women in the study, and they were excluded from the statistical analysis. Findings from the first APS were evaluated, and for those with abnormal results, rates of follow-up for HRA evaluation were analyzed using Fisher exact test.
Continuous variables were described using means and standard deviations, whereas categorical variables were described using counts and proportions. For each first HRA evaluation and biopsy, patients with HSIL and low-grade squamous intraepithelial lesion (LSIL) were compared to ascertain if various factors (CD4 count, CD4 nadir, and so on) were associated with HSIL or LSIL. Differences in continuous variables were compared using Kruskal-Wallis tests and differences in categorical variables were compared using Fisher exact tests.
In patients, who completed an HRA, were referred to treatment, completed treatment, and had a follow-up HRA, we compared various dichotomous HRA outcomes from the patient’s first diagnostic HRA to the patient’s post-therapeutic follow-up HRA using Fisher exact tests. Outcomes included treatment failure versus treatment success, as defined above, stratified by treatment modality.
Logistic regression analysis was performed on those who were found to have HSIL on their first HRA to predict treatment failures. Models were adjusted for race, current smoking, whether patients were actively on antiretroviral therapy (ART), CD4 value, number of years since HIV diagnosis, and suppressed viral load. Odds ratios (OR) and 95% confidence intervals (CIs) were constructed for single and multivariable models.
All analyses were performed in SAS 9.4 (Cary, NC), and all hypothesis testing was two sided at the 0.05 significance level.
RESULTS
Study Participants
Of the 355 patient charts reviewed, 348 (98.0%) were men, and of this group, 318 (91.4%) were MSM (Table 1). The median age was 42 (18–75), the majority of patients were black (60%) or white (33.3%), and almost half (45.9%) reported a smoking history before their first APS.
TABLE 1.
Study Population Demographics
| Males | Females | |
|---|---|---|
| Total | 348 | 8 |
| Age: median (range), y | 42(18–75) | 42.5 |
| Age: mean (SD) | 40.5 (11.7) | 41.8 |
| Race | ||
| African American (%) | 209 (60.1) | 7 (87.5) |
| White (not Hispanic) (%) | 116 (33.3) | 1 (12.5) |
| White (Hispanic) (%) | 17 (4.9) | 0 (0) |
| Asian (%) | 3 (0.9) | 0 (0) |
| American Indian/Alaska Native (%) | 2 (0.6) | 0 (0) |
| Multiracial (%) | 1 (0.29) | 0 (0) |
| Sexual behavior | ||
| Men who have sex with men (%) | 318 (91.4) | N/A |
| Men who have sex with men and women (%) | 15 (4.3) | N/A |
| Men who have sex with women (%) | 15 (4.3) | N/A |
| Women who have sex with men (%) | N/A | 6 (75) |
| Women who have sex with women (%) | N/A | 1 (12.5) |
| Women who have sex with men and women (%) | N/A | 1 (12.5) |
| Other | ||
| Smokers before first anal pap smear (%) | 157 (45.9) | 3 (37.5) |
Anal Pap Smear Follow-Up Outcomes
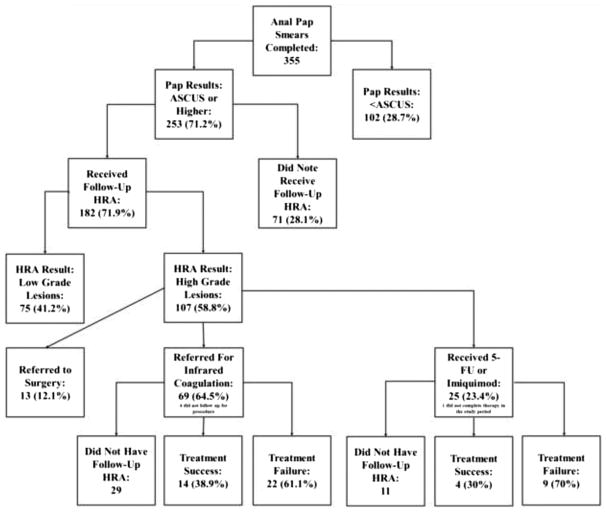
Over two thirds of the study population (N = 253 [71.2%]) had an abnormal APS. Twenty-eight percent (71/253) of all patients who were found to have ASCUS or higher on the initial APS failed to follow-up for HRA despite attempts at scheduling these visits (Table 2). When this cohort is further subdivided by ethnic groups (Table 2), African American males were less likely than white males to follow-up for an HRA procedure (65.4% vs 83.3%, respectively, P = 0.002). Of the 182 individuals who had a follow-up HRA, 75 (41.2%) were found to have only LSIL, and 107 (58.8%) were found to have HSIL.
TABLE 2.
Anal Pap Smear Follow-Up Stratified by Pap Smear Result
| Results of APS | Number (%) Who Did Not Have Subsequent HRA | Number (%) Who Had Subsequent HRA | ||||
|---|---|---|---|---|---|---|
| ASCUS (N = 126) | 44 (34.9%) | 82 (65.1%) | ||||
| LSIL (N = 110) | 23 (20.9%) | 87 (79.1%) | ||||
| ASCUS-H (N = 12) | 3 (25.0%) | 9 (75.0%) | ||||
| HSIL/Carcinoma in situ (N = 5) | 1 (20.0%) | 4 (80.0%) | ||||
| Carcinoma (N = 0) | 0 | 0 | ||||
| Total Number (N = 253) | 71 (28.1%) | 182 (71.9%) | ||||
|
| ||||||
| Anal Pap smear results stratified by age | ||||||
|
| ||||||
| Age, y | ||||||
| 18–25 | 26–35 | 36–45 | 45–55 | 56–65 | 65+ | |
| ASCUS (N = 126) | 11 | 38 | 26 | 38 | 11 | 2 |
| LSIL (N = 110) | 14 | 42 | 25 | 22 | 7 | 0 |
| ASCUS-H (N = 12) | 3 | 3 | 0 | 5 | 1 | 0 |
| HSIL/Carcinoma in situ (N = 5) | 1 | 1 | 1 | 0 | 2 | 0 |
| Carcinoma (N = 0) | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||
| Follow-Up from First Anal Pap Smear (With ASCUS or Higher) Stratified by Race | ||||||
|
| ||||||
| Race | Number (%) Who Did Not Have Subsequent HRA | Number (%) Who Had Subsequent HRA | ||||
| African American | 53 (34.6%) | 100 (65.4%) | ||||
| White | 16 (16.7%) | 80 (83.3%) | ||||
Factors Predicting Presence of HSIL
When comparing all patients who were diagnosed with HSIL and LSIL by HRA-guided biopsy (Table 3), we found that individuals with higher CD4 counts were less likely to have HSIL (OR, 0.81; 95% CI, 0.72–0.93; P = 0.0022). It should be noted that the mean time between CD4 and viral load testing and HRA was 2.3 months. Although not significant, having a suppressed viral load was found to approach statistical significance as a factor that was protective against HSIL (OR, 0.54; 95% CI, 0.26–1.11; P = 0.094), and being a cigarette smoker was found to approach statistical significance as well (OR, 1.59; 95% CI, 0.89–2.94; P = 0.13) as a factor that increased the odds of having HSIL. CD4 nadir, number of lifetime sexual partners, African American race, white race, and number of years since diagnosis with HIV were not found to significantly impact the odds of developing HSIL.
TABLE 3.
Risk Factors for HSIL at First HRA Single-Variable Analysis
| Category | OR | 95% CI | P |
|---|---|---|---|
| Race (AA to white, not Hispanic) | 1.06 | (0.58–1.95) | 0.85 |
| No. years since HIV diagnosis | 0.97* | (0.78–1.19) | 0.75 |
| Age, y | 0.90* | (0.79–1.03) | 0.12 |
| Mean CD4 value | 0.81† | (0.72–0.93) | 0.0022 |
| Mean CD4 nadir | 0.90† | (0.79–1.03) | 0.13 |
| On ART | 1.51 | (0.61–3.85) | 0.37 |
| Suppressed viral load | 0.54 | (0.26–1.11) | 0.094 |
| Cigarette smokers | 1.59 | (0.89–2.94) | 0.13 |
| Lifetime partners >11 | 0.68 | (0.29–1.60) | 0.38 |
Odds ratio per 5 year increase.
Odds ratio per 100 unit increase.
In the multivariate logistic regression analysis of individuals predicting HSIL on the first HRA (Table 4), increased CD4 values were associated with decreased odds of HSIL (OR, 0.82; 95% CI, 0.68–0.97; P = 0.022). Cigarette smoking approached statistical significance with increased odds of developing HSIL (OR, 1.72, 95% CI, 0.89–3.32; P = 0.11).
TABLE 4.
Predicting High-Grade Dysplasia Using Multivariate Logistic Regression Analysis on HRA 1
| Category | OR | 95% CI | P |
|---|---|---|---|
| Race (AA to white, not Hispanic) | 1.29 | (0.66–2.52) | 0.45 |
| No. years since HIV diagnosis | 1.16* | (0.88–1.53) | 0.29 |
| Age, y | 0.88* | (0.74–1.05) | 0.17 |
| Mean CD4 value | 0.82† | (0.68–0.97) | 0.022 |
| Mean CD4 nadir | 0.99† | (0.82–1.21) | 0.97 |
| On ART | 1.11 | (0.31–4.05) | 0.87 |
| Suppressed viral load | 0.87 | (0.34–2.22) | 0.78 |
| Cigarette smokers | 1.72 | (0.89–3.32) | 0.11 |
OR per 5 year increase.
OR per 100 unit increase.
Treatment Outcomes
Topical therapy (5-FU or imiquimod (5%) creams) or IRC was offered to patients based on the location and extent of HSIL found on HRA biopsies. Among those who underwent a follow-up HRA posttreatment (N = 49/94), treatment success for all therapies combined was 36.7% (18/49). With regard to IRC, 61.1% (22/36) of patients treated required repeat therapy compared to 69.2% (9/13) of those treated with 5-FU or imiquimod.
DISCUSSION
Patients with a higher CD4 count before HRA-guided biopsy were found to be less likely to have HSIL, suggesting a protective effect. In contrast to past studies, no statistically significant effect was found from CD4 nadir, number of sexual partners, length of time infected with HIV, or race/ethnicity on rates of biopsy-proven HSIL.7–11 A nonstatistically significant trend was found for individuals with suppressed viral load on the single variable analysis (Table 3) to have a reduced odds of having HSIL (OR, 0.54; 95% CI, 0.26–1.11; P = 0.094), which would have been consistent with prior data.12 Additionally, cigarette smoking was found to have a nonstatistically significant trend toward increased odds of developing HSIL in both the single variable and multivariable logistic regression analysis of the data (Tables 3 and 4), and this was consistent with prior literature.7
The study data echoes prior studies showing that treatment modalities were often suboptimal in the short term.13 In this population, IRC was found to have a treatment failure rate of 61.1% (22/34) after 1 treatment, and this was consistent with previous results.14 The topical treatments had the lowest rate of treatment success after the first round of therapy at only 30.8% (4/13) which was consistent with prior data.15 Both the imiquimod and 5-fluorouracil therapies require multiple weeks of digital intra-anal application of the creams, and there is no way to control for administration. Also, these medications were used for diffuse high-grade AIN, whereas IRC was used for more limited disease. With regard to the IRC therapies, regions which were biopsied previously and found to have HSIL were treated, and on the follow-up HRA, these areas were preferentially biopsied again to assure pathological disease regression. Overall, the relatively high rate of recurrence of HSIL may be partially mediated by the presence of metachronous lesions.16–18
Limitations of this study include the retrospective nature of the study and the high rates of loss to follow-up (28%), which was most marked among African American participants (35%). Unfortunately, the sexual partner data was not collected routinely until after September 28, 2012. Although this study’s sample size is comparable to or greater than past studies, it remains a relatively small group of patients. Many questions remain regarding the natural history of HPV-induced anal SIL, the effectiveness of the APS screening process, and the long-term efficacy of present treatment modalities.
Figure.
Breakdown of results.
Acknowledgments
The authors wish to acknowledge the support of the Biostatistics and Bioinformatics Shared Resource, Comprehensive Cancer Center of Wake Forest Baptist Medical Center and NCI Cancer Center Support Grant P30 CA012197.
Footnotes
Conflict of Interest: None declared.
References
- 1.Kreuter A, Potthoff A, Brockmeyer NH, et al. Anal carcinoma in human immunodeficiency virus-positive men: results of a prospective study from Germany. Br J Dermatol. 2010;162:1269–1277. doi: 10.1111/j.1365-2133.2010.09712.x. [DOI] [PubMed] [Google Scholar]
- 2.Amirian ES, Fickey PA, Jr, Scheurer ME, et al. Anal cancer incidence and survival: comparing the greater San-Francisco Bay area to other SEER cancer registries. PLoS One. 2013;8:e58919. doi: 10.1371/journal.pone.0058919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silverberg MJ, Lau B, Justice AC, et al. Risk of anal cancer in HIV-infected and HIV-uninfected individuals in North America. Clin Infect Dis. 2012;54:1026–1034. doi: 10.1093/cid/cir1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grulich AE, van Leeuwen MT, Falster MO, et al. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 5.Bedimo RJ, McGinnis KA, Dunlap M, et al. Incidence of non-AIDS-defining malignancies in HIV-infected versus noninfected patients in the HAART Era: impact of immunosuppression. JAIDS J Acquir Immune Defic Syndr. 2009;52:203–208. doi: 10.1097/QAI.0b013e3181b033ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darragh TM, Colgan TJ, Thomas Cox J, et al. The lower anogenital squamous terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Int J Gynecol Pathol. 2013;32:76–115. doi: 10.1097/PGP.0b013e31826916c7. [DOI] [PubMed] [Google Scholar]
- 7.Fagan SP, Bellows CF, 3rd, Albo D, et al. Length of human immunodeficiency virus disease and not immune status is a risk factor for development of anal carcinoma. Am J Surg. 2005;190:732–735. doi: 10.1016/j.amjsurg.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Daling JR, Madeleine MM, Johnson LG, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004;101:270–280. doi: 10.1002/cncr.20365. [DOI] [PubMed] [Google Scholar]
- 9.Conley L, Bush T, Darragh TM, et al. Factors associated with prevalent abnormal anal cytology in a large cohort of HIV-infected adults in the United States. J Infect Dis. 2010;202:1567–1576. doi: 10.1086/656775. [DOI] [PubMed] [Google Scholar]
- 10.D’Souza G, Wiley DJ, Li X, et al. Incidence and epidemiology of anal cancer in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2008;48:491–499. doi: 10.1097/QAI.0b013e31817aebfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crum-Cianflone NF, Hullsiek KH, Marconi VC, et al. Anal cancers among HIV-infected persons: HAART is not slowing rising incidence. AIDS. 2010;24:535–543. doi: 10.1097/QAD.0b013e328331f6e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathews WC, Agmas W, Cachay ER, et al. Natural history of anal dysplasia in an HIV-infected clinical care cohort: estimates using multi-state Markov modeling. PLoS One. 2014;9:e104116. doi: 10.1371/journal.pone.0104116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nathan M, Hickey N, Mayuranathan L, et al. Treatment of anal human papillomavirus-associated disease: a long term outcome study. Int J STD AIDS. 2008;19:445–449. doi: 10.1258/ijsa.2007.007290. [DOI] [PubMed] [Google Scholar]
- 14.Richel O, de Vries HJC, van Noesel CJM, et al. Comparison of imiquimod, topical fluorouracil, and electrocautery for the treatment of anal intraepithelial neoplasia in HIV-positive men who have sex with men: an open-label, randomised controlled trial. Lancet Oncol. 2013;14:346–353. doi: 10.1016/S1470-2045(13)70067-6. [DOI] [PubMed] [Google Scholar]
- 15.Snyder SM, Siekas L, Aboulafia DM. Initial experience with topical fluorouracil for treatment of HIV-associated anal intraepithelial neoplasia. J Int Assoc Physicians AIDS Care (Chic) 2011;10:83–88. doi: 10.1177/1545109710382578. [DOI] [PubMed] [Google Scholar]
- 16.Goldstone SE, Kawalek AZ, Huyett JW. Infrared coagulator: a useful tool for treating anal squamous intraepithelial lesions. Dis Colon Rectum. 2005;48:1042–1054. doi: 10.1007/s10350-004-0889-0. [DOI] [PubMed] [Google Scholar]
- 17.Marks DK, Goldstone SE. Electrocautery ablation of high-grade anal squamous intraepithelial lesions in HIV-negative and HIV-positive men who have sex with men. JAcquir Immune Defic Syndr. 2012;59:259–265. doi: 10.1097/QAI.0b013e3182437469. [DOI] [PubMed] [Google Scholar]
- 18.Goldstone RN, Goldstone AB, Russ J, et al. Long-term follow-up of infrared coagulator ablation of anal high-grade dysplasia in men who have sex with men. Dis Colon Rectum. 2011;54:1284–1292. doi: 10.1097/DCR.0b013e318227833e. [DOI] [PubMed] [Google Scholar]