Summary
Our improved understanding of how innate immune responses can be initiated and how they can shape adaptive B- and T-cell responses is having a significant impact on vaccine development by directing the development of defined adjuvants. Experience with first generation vaccines, as well as rapid advances in developing defined vaccines containing Toll-like receptor ligands (TLRLs), indicate that an expanded number of safe and effective vaccines containing such molecules will be available in the future. In this review, we outline current knowledge regarding TLRs, detailing the different cell types that express TLRs, the various signaling pathways TLRs utilize, and the currently known TLRLs. We then discuss the current status of TLRLs within vaccine development programs, including the importance of appropriate formulation, and how recent developments can be used to better define the mechanisms of action of vaccines. Finally, we introduce the possibility of using TLRLs, either in combination or with non-TLRLs, to synergistically potentiate vaccine-induced responses to provide not only prophylactic, but therapeutic protection against infectious diseases and cancer.
Keywords: Toll-like receptors/pattern recognition receptors, vaccination, dendritic cells, T cells, infectious diseases, B cells
Introduction
The development of next generation vaccines will increasingly depend on the identification and production of relevant antigen targets and, in many cases, particularly for vaccines for which T-cell responses are critical, formulating such antigens with adjuvants. The identification and characterization of adjuvant molecules and increased understanding of the importance of how such molecules are formulated have enabled the design of safe and effective immune responses, antigen sparing, dose sparing, and immune response broadening.
The mechanisms by which adjuvants achieve these effects include the generation of antigen depots, enhancement of presentation of vaccine antigens by activating antigen-presenting cells (APCs), and induction of appropriate costimulatory molecules to help direct the immune response. The immune system recognizes pathogen-associated molecular patterns (PAMPs) by means of pathogen-recognition receptors (PRRs), including the Toll-like receptors (TLRs), Nod-like receptors (NLRs), and RIG-I-like receptors (RLRs). First generation vaccines, including those consisting of inactivated or attenuated virus, bacteria, or toxin may contain inherent adjuvant activity by possessing molecules that can bind these receptors (Fig. 1 and Table 1). For example, because bacterial cell walls, bacterial DNA, and viral RNA can engage distinct TLRs and activate the TLR-expressing APCs, TLR ligands (TLRLs) have been unwittingly used in vaccines for decades.
Fig. 1. Vaccine development and implications for TLRL use.

Table 1.
Immune responses triggered by immunostimulants
| Immunostimulant | Interaction | Type of immune response |
|---|---|---|
|
|
|
|
| TLRL | ||
| Bacterial lipopeptide, lipoprotein, and lipoteichoic acid; mycobacterial lipoglycan; yeast zymosan, porin | TLR-2, 2⁄1, 2⁄6 | Th1, Ab, NK |
| Viral double stranded RNA | TLR-3 | NK |
| Lipopolysaccharide, lipid A, monophosphoryl lipid A (MPL®), AGPs, GLA | TLR-4 | Strong Th1, Ab |
| Flagellin | TLR-5 | Th1, CTL, Ab |
| Viral single stranded RNA, imidazoquinolines | TLR-7⁄8 | Strong Th1, CTL |
| Bacterial DNA, CpG DNA, hemozoin | TLR-9 | Strong Th1, CTL, and Ab; NK |
| Uropathogenic bacteria, protozoan profilin | TLR-11 | Th1 |
| Other | ||
| Saponins (Quil-A, QS-21, Tomatine, ISCOM, ISCOMATRIX) | Antigen processing | Strong Th1, CTL, and Ab; long term memory |
| Cytokines: GM-CSF, IL-2, IFN-α, Flt-3L | Cytokine receptors | Th1, Ab |
| Bacterial toxins (CT, LT) | ADP ribosylating factors | Ab |
Over the past two decades, an increased understanding of the cellular and molecular nature of innate immunity and the signals that trigger particular responses from APCs has allowed the design of new defined adjuvants. For next generation vaccines, the addition of well characterized adjuvant(s) has been and will continue to be necessary. To date, few recombinant protein-based vaccines have been licensed. The two most important of these consist of virus-like particles adsorbed to alum. These vaccines, which target hepatitis B virus and human papilloma virus (HPV), induce high antibody titers. Recently, vaccines containing a combination of alum and monophosphoryl lipid A (MPL), which is the first defined TLRL in licensed vaccines, have been approved for the above indications. The inclusion of MPL reportedly increased vaccine immunogenicity and produced a broader immune response to vaccine antigens. In this review, we outline the past and future use of TLRLs as adjuvants within vaccines.
Background
The benefits of adjuvants
The inclusion of adjuvants within vaccines can enhance vaccine-induced protection by providing strong and long-term immune responses, broadening protection, or providing cross-protection against related pathogens, improving immune responses in poorly responsive populations and potentially reducing the amount of antigen required. These properties enable an increase in the manufacturing capacity and offer a greater distribution potential for the given vaccine. This review describes the potential of targeting TLRs to promote immune responses and outline the progress being made toward the development of TLR-based adjuvants for use in clinical-grade vaccines.
PRRs
Understanding how immune responses are naturally initiated and directed by pathogens can provide valuable insight for vaccine development. The type of immune response that is naturally protective against a given pathogen can direct the choice of adjuvants that are likely to induce similar protection in response to vaccine. The quality of the immune response to a potential pathogen may be determined by engagement of specific PRRs expressed by cells at the infection site. PRRs recognize PAMPs, small molecular motifs of bacterial or viral origin that are not commonly found in eukaryotic organisms [e.g. double stranded RNA (dsRNA) or lipopolysaccharide (LPS)]. PRRs can also recognize endogenous signals released during host cell stress or death, such as fragments of extracellular matrix proteins or heat shock proteins [commonly referred to as damage-associated molecular patterns (DAMPs)]. PRRs are located extracellularly on the cell membrane or intracellularly within the endosome or the cytosol (1), and engagement typically causes cytokine and chemokine production, facilitating the recruitment and activation of APCs and the subsequent priming of adaptive B- and T-cell responses.
Discovery of TLRs
TLRs are among the best characterized PRRs. The transmembrane-located Toll receptor was first identified in the early 1980s in Drosophila, where its function is required for responses to fungal and Gram-positive bacterial infections (2). In the 1990s, TLRs were identified in mammals, as was the Toll⁄interleukin (IL)-1 receptor (TIR) domain that is a shared signaling motif of the IL-1 receptor and TLRs (3). Five TLR⁄TIR signaling adapters that are recruited to the TLR⁄TIR interface have been identified: MyD88, MyD88-adapter-like (MAL⁄TIRAP), TIR-domain-containing adapter protein inducing interferon (IFN)-β (TRIF) (which is also known as TICAM1), TRIF-related adapter molecule (TRAM) (which is also known as TICAM2), and sterile α-and armadillo-motif containing protein (SARM). The described functions of these TLR signaling adapters have been reviewed extensively (3, 4).
TLR signaling
The known TLR family has expanded greatly over the last few years, with 13 TLRs now described, 10 of which are found in humans. The signaling pathways involved in translating receptor engagement to gene expression have been thoroughly investigated. TLR2, TLR4, TLR5, TLR7, TLR8, and TLR9 signal through an IL-1R-associated kinase (IRAK) signaling cascade governed through the MyD88 signaling adapter, which in turn activates various transcription factors (reviewed in 5). TLR2 and TLR4 also require activation of the MAL signaling adapter for effective activation of the IRAK signaling cascade through MyD88. Signaling through MyD88 by TLRs can result in the induction of type I IFN (IFN-α and IFN-β), which are required for control of viral infections, and of pro-inflammatory cytokines such as tumor necrosis factor (TNF) and IL-6 (3). While signaling through TLR2, TLR5, TLR7, TLR8, and TLR9 is lost in the absence of MyD88, TLR4 can signal through an alternative TIR domain signaling adapter pathway governed by TRIF and TRAM. TLR3 is the only known TLR that signals solely through TRIF⁄TRAM (3). Thus, TLR4 is unique in its ability to signal through both the MyD88 and TRIF signaling pathways. Like MyD88, signaling through TRIF induces activation of NF-κB and expression of IFN-β. The relative roles of the MyD88⁄-MAL and TRIF signaling pathways in TLR4 signaling, and whether the MyD88 and TRIF pathways can be initiated through the same receptor simultaneously is of interest but is not fully understood. The fifth TIR associated signaling adapter, SARM, is the most recently identified and least well characterized. It is suggested that SARM blocks or negatively regulates signaling through the TRIF pathway (6).
TLR: expression and characteristics
Although signaling through TLRs alone is sufficient for the initiation of adaptive immune responses by APCs (7), the specific cell types expressing TLRs as well as cellular location of TLRs are worthy of consideration when determining which adjuvant formulations to include within particular vaccines. The best characterized response to TLRLs is the shaping of the immune response by engagement of TLRs expressed by macrophages and dendritic cells (DCs) (7), but members of the TLR family are differentially expressed by a wide variety of hematopoietic and non-hematopoietic cell types and can be located on the cell surface and within endosomal compartments (4, 7, 8) (Fig. 2). It has also been demonstrated that signaling directly through TLRs expressed on B or T cells can support antibody class-switching, activation of memory B cells, suppression of the T-regulatory cell response, and the survival of effector T cells.
Fig. 2. Examples of the differential expression of PRRs by human cells.
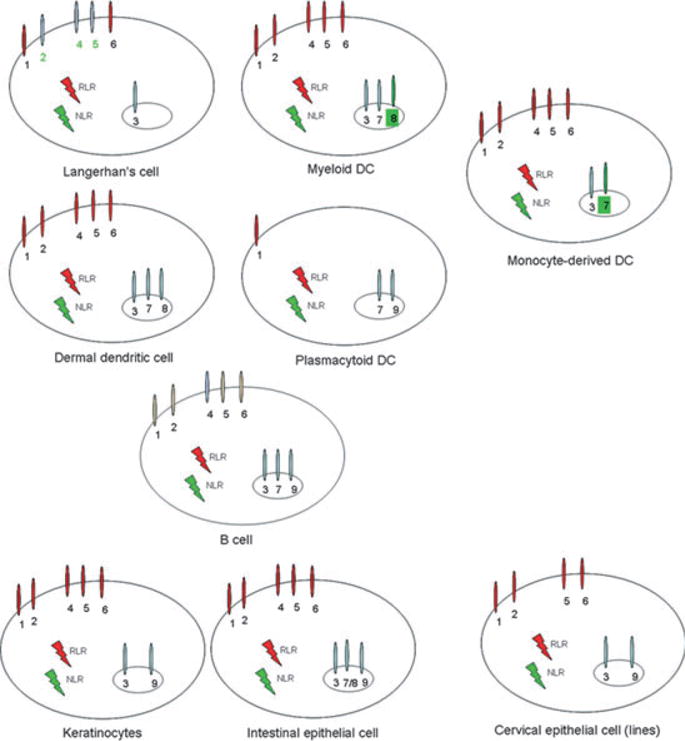
TLR2
TLR2 can be found on the surface of neutrophils, macrophages, monocytes, basophils, T cells, B cells, natural killer (NK) cells, and immature DCs (9). TLR2 specificity and activity is conferred through its ability to form heterodimers with either TLR1 or TLR6 in mice and TLR1, TLR6 or TLR10 in humans (10–12). Natural TLR2 ligands include peptidoglycan (PGN) from the cell walls of Gram-positive bacteria, bacterial glycolipids and lipopeptides, mycobacterial lipoarabinomannan, and glycophosphatidylinositol (GPI)-anchored structures from trypanosomes. Studies in mice suggest that TLR2 participates in the control of numerous bacterial infections.
TLR3
TLR3 is located within endosomes where it recognizes dsRNA produced during viral replication within an infected cell. As already mentioned, TLR3 is unique among the known TLRs in that it signals solely through the TRIF⁄TRAM to activate NF-κB-regulated gene expression. This signaling cascade results in a T-helper 1 (Th1) polarizing immune response that will be protective against viral infection, making TLR3 agonists attractive adjuvant candidates within vaccines designed to combat intracellular pathogens (1). The dsRNA mimic poly(I:C) induces NF-κB activation and results in IFN-β induction through TLR3 ligation (13). Interestingly, TLR3 ligation may also be required for activation of RIG-I, which is a cytosolic PRR that similarly recognizes dsRNA and responds by induction of IFN-β (7). This provides complementary pathways for detecting and responding to viral products within the cell.
TLR4
TLR4 can be expressed on the plasma membrane of various cell types, including neutrophils, monocytes, eosinophils, basophils, T cells, B cells, NK-cell, immature DCs, and endothelial cells (4, 9). Importantly, however, expression of mature TLR4 in humans is limited to macrophages and dendritic cells. TLR4 recognizes a variety of PRRs (both PAMPs and DAMPs), most notably LPS complexed with the adapter proteins MD-2 and CD14 but also Streptococcus pneumoniae pneumolysin, Chlamydia pneumoniae HSP60, mouse mammary tumor virus encoded envelope proteins, plant ligands, several heat shock proteins, components of the extracellular matrix such as hyaluronan, and fragments of fibronectin (1, 3, 4, 14). TLR4 naturally participates in control of infection with Neisseria meningitidis, Escherichia coli, Haemophilus influenzae, Klebsiella pneumoniae, Brucella abortus, Streptococcus pneumoniae, and Mycobacterium tuberculosis (1).
TLR4 is unique among the known TLRs that in that it is the only one that engages both the MyD88⁄MAL and TRIF⁄TRAM signaling pathways. Although signaling through either MyD88⁄MAL or TRIF⁄TRAM is well understood, their relative roles in response to TLR4 ligation have not been well defined. It is not yet known whether a single TLR4 molecule can signal through one or both pathways at the same time, although it has been suggested that MyD88⁄MAL is required for the rapid activation of TLR4 signaling and for the production of TNF, while TRAM stimulates a more sustained induction of NF-κB by the MyD88 independent pathway (3). Recent work demonstrates that stimulation of DCs with poly(I:C), which signals only through TRIF⁄TRAM, does not induce IL-12 production, while DCs stimulated with ligands for TLR that signal through MyD88⁄MAL produce high amounts of IL-12, suggesting that MyD88⁄MAL signaling is responsible for IL-12 production and the resulting IFN-γ characterized, Th1-type immune response (15). On the other hand, IL-6 is induced in response to LPS stimulation of MyD88-deficient DCs but not TRIF-deficient DCs, suggesting that IL-6 is induced by the TRIF⁄TRAM pathway (15). Further work defining the roles of these two signaling pathways in TLR4 activation is required for a full understanding of TLR4 signaling.
TLR5
TLR5 is expressed on the plasma membrane of neutrophils, monocytes, T cells, and endothelial cells (4). Signaling through TLR5 requires MyD88⁄MAL recruitment to the TIR and induces high levels of NF-κB. TLR5 is the sole known TLR that recognizes only a protein ligand: the conserved region of bacterial flagellin (16). Ligation of TLR5 with flagellin increases expression of monocyte activation markers such as CD80 and CD25 on human monocytes, and flagellin-expressing vectors can induce a local inflammatory response in the skin, causing induction of a humoral response and the expression of IFN-γ in response to flagellin expression (17).
TLR7 and TLR8
TLR7 and TLR8 are localized on endosomes within neutrophils, monocytes, eosinophils (TLR7 only), T cells (TLR8 only), B cells (TLR7 only), plasmacytoid DCs (TLR7 only), and endothelial cells (1, 9). TLR7 and TLR8 recognize RNA segments enriched for GU or poly-U sequences and both signal through MyD88⁄MAL, activating both NF-κB and the IRF-controlled genes. Imidazoquinolinamines, a class of small molecules including imiquimod and resiquimod, can bind TRL7⁄8 and initiate immune responses (18, 19). While humans express both TLR7 and TLR8, there is debate as to whether mice express a functional TLR8, complicating the translation of findings from studies of TLR7⁄8 acting molecules in mice to human vaccines (20–23).
TLR9
TLR9 is located in the endosome of neutrophils, monocytes, eosinophils, T cells, B cells, NK cells, plasmacytoid DCs, and endothelial cells (9). TLR9 signals through the MyD88⁄MAL pathway following recognition of unmethylated CpG oligodeoxynucleotides (CpG ODN), which can be found in the viral and bacterial genome, but are absent from the mammalian genome (1). TLR9 ligation induces expression of type I IFN and is required for control of viral replication. In the absence of TLR9 expression, mice are also susceptible to parasitic infections such as with Toxoplasma gondii due to the absence of induction of IFN-γ expression (24).
TLRLs in adjuvant development
Important considerations regarding TLR expression
Distinctions in TLR expression that can complicate the interpretation of data across species need to be considered during the adjuvant development process. For example, important differences have been shown in the expression and function of TLR in rodent models compared with humans. Human B cells are poor responders to direct TLR4 ligation whereas mouse B cells respond by releasing cytokines (Fig. 5). While B cells are the principal TLR9-expressing cells in humans, but in mice, TLR9 is expressed on B cells, monocyte⁄macrophages, and plasmacytoid DCs. These differences can have importance consequences on the induced responses. Injection of normal human volunteers with CpG 7909, a B-class CpG ODN, induces systemic innate immune cell activation manifested by expression of a Th1-like pattern of cytokines and IFN-inducible chemokines (25). This pattern of chemokine and cytokine induction is markedly different from that reported following CpG ODN administration to rodents.
Fig. 5. Use of various TLRL to generate IFNγ-producing antigen-specific CD4+ T cells.
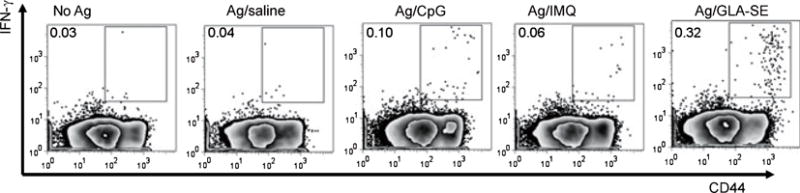
Mice were immunized by subcutaneous injection of the Mycobacterium leprae antigen ML0276 in the presence of CpG, IMQ, or GLA-SE at biweekly intervals, for a total of three immunizations. Spleens were collected 1 month after the third immunization, single cell suspensions prepared, and cells cultured with antigen and BD Golgi STOP (to prevent secretion) overnight, then fixed and stained for flow cytometry to determine the percentage CD3+CD4+CD44hi IFNγ cells. Data were originally published in (63).
Another important consideration regarding the use of TLRL in vaccines is the influence of age on TLR responsiveness. Many vaccines are currently provided to children in their first year and reports indicate that neonatal and adult APCs respond differently to TLRLs. Analysis of ex vivo stimulated blood collected regularly from children throughout the first year of life and compared with healthy adults reveals the development of TLR responses. Following stimulation of umbilical cord blood with TLRLs, production of the Th1-polarizing cytokine IL-12p70 and IFN-α was generally impaired compared with stimulation of adult whole blood. Responses to TLR3, TLR7, and TLR9 agonists rapidly increase to adult levels during the first month of life, but TLR4 responsiveness in neonates increases slowly, with low LPS-induced IL-12p70 production and high IL-10 production up to 1 month of the age (26). When stimulated with either a TLR4 agonist (LPS) or a TLR9 agonist (CpG ODN), surface expression of CD80 and HLA-DR reaches adult levels within the first 3 months of life on myeloid DC and by 6–9 months of life on monocytes and plasmacytoid DCs. Production of TNF, IP-10, and IL-12p70 also reaches adult levels between 6–9 months of life. In response to TLR9 ligation, production of the type I IFN-dependent chemokines IP-10 and CXCL9 gradually increases with age but is still limited in 1-year-old infants as compared with adult controls (27). The first year of life therefore represents a critical period during which adult-like levels of TLR responses are reached for most but not all cytokine responses, making the selection of appropriate TLRLs critical for effective vaccination of infants.
While neonates and infants mature in their responsiveness to TLRLs, responsiveness through TLR wanes with aging. Most data on TLR function in the context of human aging have focused on responses through TLR4, where conflicting observations have been made (28). Our data clearly demonstrate responsiveness to TLR4 agonists in both aged humans and mice (unpublished data). Substantial decreases in responsiveness through other TLRs with age, however, have been observed. An evaluation of TLR1⁄2 function in monocytes from older adults found that cytokine production is substantially lower than younger adults. The upregulation of costimulatory molecules on monocytes from older adults was less for all TLR ligands tested than that observed for cells from young individuals (29). Following TLR1⁄2, TLR2⁄6, TLR3, TLR5, and TLR8 engagement on distinct DC subsets, lower cytokine production was detected in older adults (>65 years) compared with younger adults (age 21–30 years) (30). The consequences of aging on human TLR function, along with other factors influencing adaptive responses, may impair activation of the immune response and contribute to poorer vaccine responses in older adults. Impaired TLR function in the aging immune response could explain, for example, defects in cytokine production that are strongly associated with poor antibody responses to influenza immunization. As the Lyme disease vaccine is dependent on intact TLR1⁄2 signaling, this may also explain the decreased efficacy of the Lyme disease vaccine in those aged over 60 (31, 32).
The unwitting use of TLRL in known vaccines
Despite our increasing understanding of adaptive immune responses and the role of the innate immune system in their modulation, the mode of action of many vaccines that have been empirically successful are only now being revealed. Several widely used vaccines may have TLR activating components. For example, it has been recognized since 1893 that a mixture of bacterial cell lysate (Coley’s toxin) exhibits immunostimulatory properties that can ameliorate the progression of some carcinomas. It was not until 1983 that bacterial DNA was identified as the underlying component within the lysate that elicited the response, and then later that the response was found to be mediated through TLR9. A recent evaluation of vaccines as a possible source of TLR ligands to induce DC maturation identified a cocktail of commonly used preventive vaccines (BCG-SSI, Influvac, and Typhim) that contains TLR ligands capable of optimally generating Th1-inducing mature DCs (33).
Several investigators are now examining how widely used vaccines that were developed by empiric means actually operate. With a 75 year history of use and its provision to over 500 million people, the live attenuated yellow fever vaccine 17D (YF-17D) is one of the most effective vaccines available. It is now known that YF-17D activates multiple DC subsets via TLR2, TLR7, TLR8, and TLR9 to elicit a pro-inflammatory response involving IL-12p40, IL-6, and IFN-α (34). Notably, TLR4 does not appear to be engaged by YF-17D. The resulting adaptive immune responses are characterized by a mixed Th1⁄Th2 cytokine profile and antigen-specific CD8+ T cells. The Th1⁄Th2 balance appears to be differentially controlled by distinct TLRs; thus, while vaccinated MyD88−⁄− mice demonstrate a profound impairment of the Th1 response, vaccinated TLR2−⁄− mice demonstrate an enhancement of Th1 and Tc1 responses. Adverse events following receipt of YF-17D are rare, although anaphylactic shock has been reported. This is thought to be a reaction to egg proteins because the attenuated virus is raised in eggs and not the result of a generalized toxicity phenomenon (35). The vast majority of adverse events reported following vaccination are a mild fever and headache, which occur in about 25% of those receiving the vaccine, but this subset of individuals also possess the highest titers of yellow fever-neutralizing antibodies (35). In fact, 99% of those immunized with a single dose of YF-17D have sufficient circulating antibodies for protection from yellow fever, and although the current recommendation for re-vaccination is every 10 years, circulating antibodies have been found in individuals immunized up to 35 years prior (36). The mechanism of action and safety profile of YF-17D encourages the further use of vaccination strategies to stimulate tailored immune responses through the use combinations of different TLR ligands.
Vaccinia virus (VV) has been used extensively as a vehicle in the clinical application of vaccines for infectious diseases and cancer. Studies suggested that the unique potency of VV-based vaccine lies in its effective activation of the innate immune system; however, as with YF-17D, until recently, the innate pathways evoked by VV remained largely unknown. It turns out that both TLR-dependent and -independent pathways are required for activating innate and adaptive immunity to VV (37, 38). The TLR-dependent response is mediated through TLR2 and MyD88, leading to the production of pro-inflammatory cytokines, whereas activation of a TLR-independent pathway results in IFN-β secretion (37). While it is thought that control of adaptive T-cell responses is mainly achieved by the maturation and function of APCs subsequent to the engagement of TLRs, during VV infection direct signaling in CD8+ T cells through TLR2–MyD88 also promotes their survival and differentiation into long-lived memory T cells (39).
Defined TLR-based adjuvants in practice⁄development
There has been an increased demand for the development of novel vaccine adjuvants that lead to enhanced protection against infection and the development of long-lasting immunological memory. Given our increased knowledge regarding the distinct TLRs, the cell types and cellular location in which they are expressed, and the signaling pathways they engage, several well defined and characterized TLRLs are now being selectively developed with the primary purpose to serve as adjuvant components within vaccines.
TLR2 agonists
As stand-alone TLR2-based adjuvants, triacylated lipopeptides, such as Pam3CysSerLys4 (Pam3CSK4) and their derivatives, have been used in several experimental vaccines models. Administration of TLR2⁄TLR1 agonists supports B-cell responses and T-cell stimulation. An example of this is shown by adjuvanting tetanus toxoid with of the derivatives of poly-saccharide from Group B Streptococcus, which results in strong T-cell priming and high tetanus-specific antibody titers (39). Recent publications have also demonstrated that, when used as adjuvants, derivatives of the TLR2⁄6 ligand Mycoplasma fermentans macrophage activating lipopeptide (MALP-2) also stimulate strong T-cell and antibody responses. Intranasal administration of synthetic MALP-2 with enterohemorragic E. coli proteins EspB and C-280 γ-Intimin generates high levels of both circulating serum IgG and mucosal IgA (40). In addition to the strong antibody response, antigen-specific recall of spleen cells collected from mice that received antigens and synthetic MALP-2 produced the cytokines IFN-γ, IL-2, and IL-4 (41). Immunization of mice with S-[2,3-bispalmitoyiloxy-(2R)-propyl]-R-cystenyl-amido-monomethoxyl polyethylene glycol (BPP) (a synthetic derivative of MALP-2) enhances the cytotoxic T-cell response to antigen by increasing cross presentation by DCs to CD8+ T cells (42). BPP retains activity in human lung tissue culture, inducing expression of TNF, IL-10, and MIP-1β. Thus, synthetic derivatives of MALP-2 support the generation of antibody and cytotoxic T-cell responses in experimental models and demonstrate adjuvant-like properties in the clinical setting. TLR2 ligand agonists have not been approved for administration in humans.
TLR2 agonists do, however, hold promise for the development of novel adjuvant administration strategies, as they may also be conjugated directly to antigens. A lipid core peptide that mimics Pam3CSK has been recently developed, allowing simultaneous manufacture of an adjuvant⁄antigen combination (42). The likelihood of co-administration of antigen and adjuvant to the same cell increases, providing what would be considered the optimal delivery of stimulatory and specific signals. Delivery of adjuvant with lipid core peptide increases IL-12 secretion by DCs, resulting in highly proliferating T cells in vitro (42). In general, adjuvant-antigen complexes may, however, have practical limitations due to the inability to independently vary concentrations of the respective components.
TLR4 agonists
Given the natural role of TLR4 in controlling a large number of infectious diseases and its ability to utilize both the MyD88 and TRIF signaling pathways, it is not surprising that TLR4 agonists are the most developed adjuvants. Monophosphoryl lipid A (MPL™), the TLR4 interactive portion of LPS, is derived from Salmonella minnesota LPS. The clinical-grade version of MPL was originally developed by Ribi and is currently manufactured by GlaxoSmithKline Biologicals (43). Studies examining MPL have been conducted for over 30 years, and over 100 000 doses of MPL have been administered to human subjects worldwide. The reported safety profile of MPL is similar to that of alum, the adjuvant delivery system that has been used for over 70 years and is used in over 80% of vaccines administered worldwide (reviewed in 43). With approximately 0.1% the toxicity of LPS, properly formulated MPL enhances antibody responses, facilitates T-cell expansion, and induces recall responses against antigens without causing excessive inflammatory side effects (43). Administration of MPL in combination with the aluminum salt (AS04 GSK Adjuvant Systems 04) results in induction of TNF secretion by monocytes and a Th1 response in the effector arm of the immune system, but this outcome is dependent upon both the nature of the antigen with which it is administered and the route of administration (43, 44). The MPL-containing vaccines FENDrix and Cervarix are registered for use in many countries, and in late 2009, the FDA approved the administration of Cervarix the United States (http://www.cancer.gov/cancertopics/druginfo/fda-recombinant-hpv-bivalent-vaccine). This breakthrough made MPLA the first defined adjuvant to be approved for worldwide use since alum (43).
It was reported that the low toxicity of MPL is associated with a bias toward TRIF signaling, which is likely caused by the active suppression, rather than passive loss, of pro-inflammatory activity (45). It was suggested that synthetic derivatives of MPL retain a TRIF bias as compared with synthetic diphosphoryl lipid A, indicating a change in a single phos-phoryl group is sufficient for TRIF-biased TLR4 stimulation. However, some TRIF-associated genes, such as MCP-1, are only weakly expressed in response to MPL, and some MyD88-associated inflammatory and anti-inflammatory cytokines, such as TNF and IL-10, are strongly activated by MPL stimulation (45). If there is a TRIF-signaling bias following MPL engagement, it is not complete. The low toxicity of MPL, compared with LPS, may also be explained by its ability to induce high IL-10 expression that could counter-balance the pro-inflammatory response (43, 45). This hypothesis is supported by data indicating the reduction in toxicity of the immune response against a respiratory syncytial virus (RSV) vaccine. In the absence of MPL a highly inflammatory response that causes lung pathology is induced, but when administered with MPL, RSV vaccination induced IL-10 that reduces the inflammatory reaction (43).
We have employed MPL within many of our vaccine development programs. Our results demonstrate that MPL effectively promotes a Th1 response against various co-formulated recombinant antigens, and these responses protect against subsequent challenge with the relevant pathogen (leishmanial or M. tuberculosis) (46–52). Such data as well the extensive portfolio generated by GlaxoSmithKline Biologicals have supported the advancement of MPL-based vaccines into higher species where protective immune responses are similarly induced and into clinical trials where Th1 and delayed-type hypersensitivity responses are safely promoted (48, 53–57). In addition to skewing the response to Th1 in the context of prophylactic immunization, in comparison to treatment with standard meglumine antimoniate chemotherapy, the provision of IDRI’s LEISH-F1 + MPL-SE vaccine with chemotherapy provides effective immune priming and speeds the cure of cutaneous leishmaniasis (58). Treatment of dogs exhibiting mild symptoms of visceral leishmaniasis with LEISH-F1 + MPL-SE indicates that the vaccine alone provides effective immune therapy and cure (59, 60). IDRI has recently developed a synthetic lipid A derivative that eases manufacture and provides a highly consistent end product. Like MPL, glycopyranosyl lipid adjuvant (GLA) also signals through TLR4 (61, 62). When formulated with SE, an oil-in-water emulsion, and mixed with antigen, the resulting Ag⁄GLA-SE induces potent Th1 immune response in mice (63–65) (Figs 3 and 4). Early results from trials providing GLA-incorporating vaccines to higher species indicate excellent safety profiles that are accompanied by the enhancement of antigen-specific responses (66).
Fig. 3. Differential use of TLRs by human and mouse B cells.
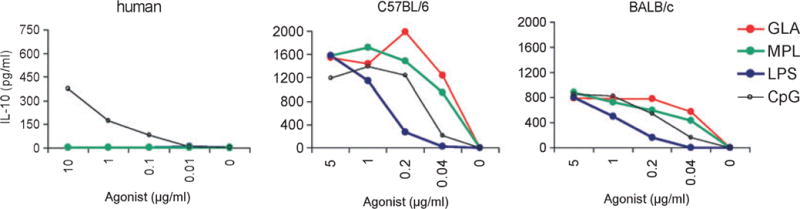
B cells from a human donor or from two mouse strains were stimulated with tittered doses of TLR4 (MPL, GLA, LPS) or TLR9 (CpG) ligands, then secretion of IL-10 measured by ELISA.
Fig. 4. The GLA-SE adjuvant promotes high quality Th1 responses characterized by the generation of antigen-specific pluripotent CD4+ T cells.
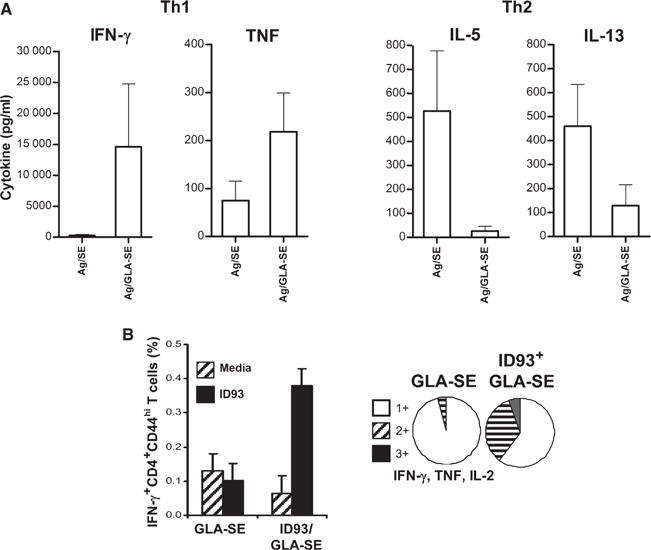
Mice were immunized by injection of the Mycobacterium tuberculosis fusion antigens in the presence of GLA-SE. Spleens were collected, single cell suspensions prepared and cells cultured with antigen. (A) Cytokines secreted into the culture supernatant were measured by ELISA. (B) Cells were cultured with antigen and BD Golgi STOP (to prevent secretion) overnight, then fixed and stained for flow cytometry. Activated CD4+ T cells were identified based on CD3 and CD4 expression, and further gated as CD44hi. Graphs depict the proportion of activated CD4+ T cells expressing one, two, or the three cytokines (IFN-γ, TNF, and IL-2). Data originally published in (65).
The bacterium N. meningitidis is the causative agent of meningitis and sepsis. A generally effective vaccine against N. meningitidis serogroup B is not yet available, but outer membrane vesicle vaccines are in development. These vaccines contain N. meningitidis wildtype LPS, however, making them controversial because of the associated high toxicity. The adjuvant activity of a panel of different TLRL in combination with LPS-deficient meningococcal outer membrane complexes demonstrate that TLR3, TLR4, TLR7, and TLR9 agonists enhance immune responses against LPS-deficient outer membrane complexes. Adjuvant activity was characterized by higher levels of antigen-specific IgG, a higher IgG2a⁄IgG1 ratio and higher serum bactericidal antibody titers compared with LPS-deficient outer membrane complexes alone (67).
TLR5 agonists
The D1 portion of bacterial flagellin contains the TLR5-binding region and this can be expressed in a fusion protein with selected vaccine antigens. Such a fusion protein co-delivers antigen and TLR5 agonist to the APC, yielding immune responses superior to the simple mixing of antigen and agonist. The molar ratio of adjuvant to antigen can therefore be very low compared with many other formulations, providing the benefit of more efficient antigen use within vaccines. It has been demonstrated that TLR5 ligation enhances intranasal influenza vaccination in mice and rabbits by increasing antibody production (68, 69). Further development of flagellin as a vaccine adjuvant for widespread use may, however, be restricted by its proteinaceous nature and the possible presence of anti-flagellin immune responses in humans.
TLR7⁄8 agonists
While TLR7 or TLR8 agonists have not yet been approved for administration with vaccines, TLR7⁄8 agonists such as imiquimod and resiquimod have been approved by the FDA for use as stand-alone entities (70). Imiquimod is approved for topical administration for the treatment of basal cell carcinoma in immunocompetent adults and demonstrates efficacy for treatment of non-genital warts caused by HPV, molluscum contagiosum, squamous cell carcinoma, and lentigo maligna (68). As predicted for a TLR7⁄8 agonist, imiquimod stimulates the local induction of IFN-α, TNF, IL-6, and IL-12 at the administration site and induces a cytotoxic T-cell response (68). Although imiquimod and resiquimod are currently approved only for topical administration, studies in mice suggest that subcutaneous or intramuscular immunization with either of these TLR7⁄8 agonists enhances the immune response to co-administered antigens (69, 70). Imiquimod has also demonstrated an ability to activate macrophage killing of Leishmania species, and in an open-label, prospective study combining imiquimod with standard chemotherapy for leishmaniasis, 90% of cutaneous leishmaniasis patients who had not previously responded to drug treatment were found to be cured with this combination therapy at the 6-month follow-up period (71).
TLR9 agonists
Certain CpG motif-containing ODN that engage TLR9 enhance immune responses to co-delivered antigens in animal models. These are now being developed for clinical use as either vaccine adjuvants or immune therapeutics by Coley Pharmaceuticals (Pfizer) and Dynavax Technologies, among others. Different classes of CpG ODN that mimic the immunostimulatory activity of bacterial DNA and are recognized by TLR9 have been described (72). All classes stimulate TLR9-dependent signaling, but with strikingly different dose-response relationships. A-class ODN were found to promote the highest degree of NK cell stimulation, as well as IFN-α secretion by plasmacytoid DCs. B-class ODN stimulate strong B-cell and NK-cell activation and cytokine production. C-class ODN combine the effects of A- and B-class CpG ODN. C-class ODN strongly stimulate B-cell and NK-cell activation and IFN-α production. C-class CpG are a novel class of CpG motif-containing ISS that includes both a 5′-TCG element and a CpG-containing palindrome. C-class CpG retain activity in an in vitro primate system and induce the expression of several cytokines and IFN-α-inducible genes when administered to mice and primates, indicating that this novel class is thus an attractive candidate for therapeutic strategies (73). In vivo studies demonstrate that C-class ODN are very potent Th1 adjuvants that could represent new therapeutic drugs for broad applications in infectious disease or cancer therapy and administration of CpG ODN via the pulmonary route for treatment of allergic indications has proven to be safe in primates and humans (74).
Subcutaneous injection of normal human volunteers with a B-class CpG ODN, CpG 7909, induces systemic innate immune activation manifested by expression of a Th1-like pattern of cytokines and IFN-inducible chemokines (25). Consistent with the increased chemokine expression, subcutaneous CpG 7909 injection induces transient shifts in blood neutrophils, lymphocytes, and monocytes. A second subcutaneous CpG 7909 injection administered 2 weeks after the first elicits similar immune responses, demonstrating safety and efficacy of repeated TLR9 stimulation. In a similar trial with an alternate CpG (CPG 10101, also known as ACTILON), a maximum tolerated dose was not reached even after two 20 mg injections or when a 4 mg dose was administered twice weekly for 4 weeks (75). The limited number of adverse events associated with ACTILON was consistent with data obtained with CpG 7909, in that the events consisted mostly of dose-dependent but transient injection site reactions and flu-like symptoms, with no evidence of organ toxicity or systemic autoimmunity.
In a clinical evaluation of immunogenicity, CpG 7909 was added to a commercial hepatitis B vaccine (Engerix-B; GlaxoSmithKline) and administered by intramuscular injection to healthy volunteers at 0, 4, and 24 weeks. HBsAg-specific antibody responses appeared significantly earlier and were significantly higher in CpG 7909 recipients compared to various control subjects. Strikingly, most individuals receiving CpG 7909-adjuvanted vaccines developed protective levels of anti-HBs IgG within just 2 weeks of the priming dose and a trend towards higher rates of cytotoxic T-cell lymphocyte responses in the higher dose groups of CpG was also observed (76). Increased anti-HBsAg antibody levels were associated with higher CpG 7909 doses, such that after the first dose, a seroprotective titer (>10 mIU⁄ml) was noted in an increasing percentage of subjects with as adjuvant doses were escalated. After a second dose, most subjects given ISS plus HBsAg, with the exception of a few individuals in the group receiving the lowest dose, were seropositive for HBsAg antibodies (77, 78). Dynavax has begun a phase 3 trial of its CpG-containing HEPLISAV™ hepatitis B vaccine. Of additional note, when included as an adjuvant in a pneumococcal conjugate vaccine, CpG 7909 significantly increased the proportion of vaccine high responders among HIV-infected individuals (77). Thus, TLR9 agonists demonstrate significant promise for inclusion within vaccines intended for immune suppressed individuals such as HIV patients or the elderly, and further development along these lines appears warranted.
In animal models, administration of immunostimulatory DNA sequences preferentially elicits Th1 dominated immunity. While free CpG ODN are potent immunomodulators that can drive Th1 responses to co-administered antigens or allergens, the direct linkage of CpG ODN to a protein can enhance this response (79). Conjugation of CpG ODN to the major short ragweed allergen Amb a 1 results in enhanced immunotherapeutic potential in mice. The Amb a 1-CpG ODN conjugate induced a Th1 response (IFN-γ secretion), whereas Amb a 1 not conjugated to the adjuvant induced a Th2 response (IL-5 secretion). Immunostimulatory DNA sequences can also inhibit developing or ongoing Th2 (type 2) responses. In mice primed for a Th2 response, injection with Amb a 1-CpG ODN conjugate induced a de novo Th1 response and suppressed IgE antibody formation after challenge with Amb a 1 (79). Amb a 1-CpG ODN conjugate also induced high anti-Amb a 1 IgG antibody titers in rabbits and cynomolgus monkeys, whereas Amb a 1 alone or Amb a 1 coinjected with CpG ODN did not induce a detectable response. In a randomized, third party-blinded, placebo-controlled, proof-of-concept study conducted entirely in the winter in adults with ragweed allergy, Dynavax administered six subcutaneous injections of purified Amb a 1 linked to the 22-base-long immunostimulatory phosphorothioate oligodeoxyribonucleotide 1018 [Amb a 1-immunostimulatory DNA sequence conjugate (AIC)]. Data indicated this approach was safe, with no clinically significant systemic or local allergic reactions were associated with AIC or placebo injections. More importantly, Dynavax demonstrated that in vivo human ragweed-specific Th2 responses could be selectively redirected toward Th1 responses, with significant increases in IFN-γ expression and significant decreases in IL-5 expression after the sixth injection. Responses to the unrelated bacterial antigen streptokinase and the global capacity to mount immune responses upon polyclonal activation with PHA were not altered, suggesting the effect was Amb a 1 antigen specific (80). This approach highlights the potential for induction of potent Th1 responses and even immune redirection in human diseases.
Evaluations of the effect of the number of CpG ODN bound to antigen has on immunogenicity and allergenicity indicate that both antibody induction in vivo and antibody recognition in vitro are highly sensitive to the number of CpG ODN linked. At the highest CpG ODN to protein ratios, antigen-specific antibody induction was very low. Moderate CpG ODN to protein ratios induced high antibody responses in which IgG2a (BALB⁄c mice) generally predominated. Low ISS to protein ratios produced the highest overall antibody responses in which IgG1 predominated (81). In contrast, as demonstrated in both in vivo mouse and in vitro human PBMC studies, varied CpG ODN to protein ratios did not affect T-cell responses. All CpG ODN to protein ratios evaluated induced similar responses represented by high levels of IFN-γ and low levels of Th2 cytokines. These studies indicated that by controlling the number of CpG ODN molecules bound to a protein, antibody production can be selectively manipulated, while the potent Th1 properties of a CpG ODN-linked protein are retained.
A novel vaccine adjuvant comprising a complex containing CpG ODN and the synthetic cationic innate defense regulator peptide HH2 that has enhanced immune modulating activities has also been developed (80). The complex of HH2 and the CpG ODN 10101 is a potent inducer of cytokine⁄chemokine expression and upregulates surface marker expression on DCs. Immunization of mice with a co-formulation of the HH2-CpG complex and pertussis toxoid significantly enhanced the induction of toxoid-specific antibodies when compared with toxoid alone, inducing high titers of IgG1 and IgG2a, typical of a balanced Th1⁄Th2 response, and also led to high IgA titers (80).
Adjuvant formulations
Vaccine antigens and adjuvants can be made more effective by employing appropriate particulates, as well as delivery systems, to increase cell uptake and provide sustained release of antigen and the active pharmaceutical ingredients. Certain structural formulations such as liposomes, aluminum gels, and aqueous suspensions are more amenable to uptake by APCs. Association of recombinant antigens and adjuvants with particles provides a sustained release mechanism, allows for multimeric presentation to TLRs and APCs, and provides a delivery vehicle for otherwise insoluble components. In our laboratory, combinations of TLRLs with phospholipids in liposomal formulations or aqueous suspensions are of particular interest, because it has been demonstrated that MPL-like molecules are more effective when delivered as aggregates instead of monomers and that the structural geometry and phase of these molecules and aggregates is critical for biological activity (67, 82). Thus, combining phospholipid molecules with TLRL in liposomes or suspensions may demonstrably improve the structure and biological activity of the adjuvant. An example of this approach is an adjuvanted seasonal flu vaccine, Fluad® (Novartis), which contains MF59, an oil-in-water (o⁄w) emulsion that accelerates antigen uptake by APCs or prolongs APC survival (83, 84). A diverse range of oils may be used without dramatically altering the physicochemical properties between each formulation although squalene is the most widely used, as it allows the creation of smaller size emulsions and potentially provides greater stability than most other oils tested (85, 86). The addition of stable emulsions (SE) stimulates antibody responses but does not improve T-cell responses (87–90).
We are currently examining various formulations for efficient and effective use of TLRL-based adjuvants. As an amphiphilic molecule, the TLR4 agonist GLA self-assembles into aggregates upon exposure to aqueous medium. A high energy input by sonication or microfluidization in the presence of small amounts of surfactant enables a reduction in particle size to form a nanosuspension of GLA aggregates. These relatively simple aqueous formulations may be sufficient for extended use in some cases, as they have demonstrated equivalent immunogenic activity compared with more complex formulations.
Adsorption of TLR4 agonists onto aluminum hydroxide (alum) particles is another means for effectively delivering adjuvant with antigen. These alum particles consist of nanometer crystals that assemble into aggregates of several micrometers and provide a stable particulate formulation for the sustained release of adjuvant. Whereas alum alone induces a Th2 response, the inclusion of TLR4 agonist can alter the quality of the immune response to a balanced Th1⁄Th2 response. An MPL-alum formulation is used within GSK’s cervical cancer vaccine Cervarix®.
Adjuvant efficacy can similarly be enhanced by incorporation within an o⁄w emulsion or within bilayers. Emulsion droplets approximately 100 nm in diameter are stable for years and emulsions have been shown to effectively and safely induce immune responses to influenza antigens and facilitate dose sparing of antigen. The o⁄w formulations MF59 and AS03 are already approved for influenza vaccines in Europe (91, 92). The synergistic activity of a TLR4 agonist (MPL) upon the addition of QS21, from the saponin Quil-A, has been demonstrated with GSK’s well-known adjuvant formulations AS01 and AS02 (92). These formulations employ liposome and emulsion platforms, respectively.
Tools for investigating the TLR mechanisms of action
TLR signal adapter molecule-deficient mice
The generation of MyD88−⁄− and TRIF−⁄− mice has been a critical breakthrough in the study of TLR mechanisms and their involvement in various processes, including vaccination. As discussed above, these mouse strains are commonly used as the first line of investigation to establish a TLR-dependent mechanism that can then be further delineated using mice deficient in specific TLR.
The most scrutinized TLR pathway is arguably TLR4. LPS potentiates antigen-specific T-cell survival and Th1 differentiation by stimulating both MyD88 and TRIF signaling pathways. The contribution of these signaling molecules appears to be regulated in a temporal fashion related to TLR endocytosis (81), and each contributes in a different manner to the overall immune response. LPS induces long-term survival of superantigen (SEA)-stimulated CD4+ and CD8+ T cells in a MyD88-dependent manner (93), suggesting that MyD88-mediated responses plays a role in the T-cell longevity. However, although LPS increases the accumulation of T cells in the spleens of TRIF-deficient mice, fewer T cells are recovered from liver and lung in comparison with wildtype mice (82), suggesting that TRIF signaling may also play a partial role in T-cell survival. Most of the primed T cells in TRIF-deficient mice fail to upregulate CXCR3 and produce less IFN-γ compared with control mice, suggesting that TRIF-dependent signals participate in drawing T cells to the site of infection and shifting the immune response toward Th1. Boosting with a CD40 agonist in addition to LPS restored the effector CD8+ T-cell response in TRIF-deficient livers but only partially restored CD4 + T cells, suggesting that LPS primes CD8+ and CD4+ T-cell immunity through different mechanisms. Although some light has been shed on the roles of MyD88 and TRIF in stimulating the immune response following TLR stimulation, further work is required to determine additional roles for these signaling adapters in the immune response to vaccination and subsequent infections.
Targeted immune cell-deficient mice
APCs play pivotal roles in promoting and directing immune responses, and, as such, they are likely key targets for vaccination. Attempts to identify the definitive functions of DC or macrophages during vaccination of mice have previously been complicated by problems in specifically depleting individual populations without affecting other cell types. Transgenic systems with which to determine if particular cell types are critical for vaccination have, however, emerged in the last few years.
Mouse cells are naturally resistant to diphtheria toxin (DT), because they do not express the DT receptor (DTR). Mice can be engineered to render them DT sensitive, however, by expressing a DTR transgene under the control of the selected gene promoters. Transgenic introduction of DTR with either the CD11c, CD11b, or langerin promoters has allowed the specific depletion of DCs, macrophages, and Langerhans cells, respectively (94–97). Injection of DT into DTR transgenic mice results in the rapid ablation of targeted cells from the otherwise intact animal, allowing the assessment of function in a variety of physiologic processes, including vaccination (94). Using CD11c-DTR mice, the enhancing effects of alum on both cellular and humoral immunity were completely abolished when CD11c+ monocytes and DCs were conditionally depleted during immunization (98). Depletion of CD11chigh cells does not affect LPS-driven specific T-cell survival, but conventional DCs are paramount for effector T-cell differentiation as measured by IFN-γ potential (93). Selective depletion of CD11chigh DCs indicates that these cells are also required for activation of CD4+ T cells after oral and nasal immunization following administration of antigen with or without the potent mucosal adjuvant cholera toxin (99). Giving a very high dose of antigen to DC-ablated mice, however, does result in proliferation of antigen-specific CD4+ T cells, indicating that DC dependency may be overcome with a very high dose of antigen.
CD11c-DTR mice do not tolerate prolonged DC ablation, because repetitive DT application results in adverse side effects, diminishing their use in long term or repeated depletion studies (100). This problem can be overcome, however, by the use of mixed bone marrow (BM) chimeras generated by reconstitution of lethally irradiated wild type mice with CD11c-DTR transgenic BM (101). The resultant syngeneic (CD11c-DTR × wildtype) BM chimeras can be treated with DT for prolonged periods of time without any adverse side effects. Moreover, mixed BM chimeras with wildtype, mutant, and CD11c-DTR transgenic BM can serve as a powerful means of investigating the DC-intrinsic contributions to vaccination. In an example of such an approach, Bates and colleagues (102) used a combination of CD4+ T-cell adoptive transfers and BM chimera mice in which the presence or absence of TLR5+⁄+CD11c+ cells was controlled by DT injection to determine whether flagellin, in the context of intramuscular immunization with a flagellin-OVA fusion protein, was acting directly on DCs. The antigen-specific CD4+ T-cell response in mice with CD11c+ cells from a TLR5−⁄− background and all other cells of wildtype background was dramatically reduced in comparison to mice that had DCs from mixed TLR5−⁄− and wildtype backgrounds. Immunization of TLR5+⁄+MyD88−⁄− mice revealed that the enhanced response following immunization with flagellin-OVA is dependent on signaling via the TLR5-MyD88 pathway as well as enhanced antigen uptake and processing resulting from antigen targeting via TLR5. These data indicate that the direct stimulation of conventional DCs and signaling through TLR5 are necessary for the adjuvant activity of a flagellin fusion protein.
Future directions
Synergy of adjuvants
Single TLRLs are currently being developed and utilized for vaccination and tumor immunotherapy. Certain combinations of adjuvant molecules could have advantages over use of a single agonist, however, as there is evidence that the use of multiple TLRLs or individual TLRLs in combination with non-TLRLs may more efficiently generating or effectively directing immune responses.
TLR⁄TLR synergy
TLRs recognize specific molecular signatures of pathogens using two independent signaling pathways (MyD88 and TRIF) (Fig. 6). Several evolutionarily conserved TLRLs are expressed by pathogens, leading to the possibility that targeting of different TLRs might enhance immune responses by signaling via both adapter pathways. Ghosh and colleagues (103) compared cytokine responses of all the possible tandem combinations of known TLRLs in human PBMCs with a focus on pro-inflammatory cytokine production. TLR4 plus TLR7⁄8 combinations synergistically upregulated IFN-γ and IL-12, enhanced IFN-α production, and also moderately induced TNF, while TLR2 plus TLR7⁄8 synergistically upregulated IFN-γ but not IL-12 expression. TLR9 agonist CpG2216 produced high IFN-α but failed to up regulate IFN-γ singly or in tandem. Furthermore, TLR9-induced type-1 IFN was downregulated in combination with TLR7 or TLR8 agonists. TLR3 induced significant IFN-α⁄-β responses when used in a complex with a membrane permeability enhancer and additively enhanced response with agonists to TLR2, TLR5, TLR7⁄8, and TLR8.
Fig. 6. PRRs use different adapter molecules, allowing the use of agonist combinations to provide synergy in responses.
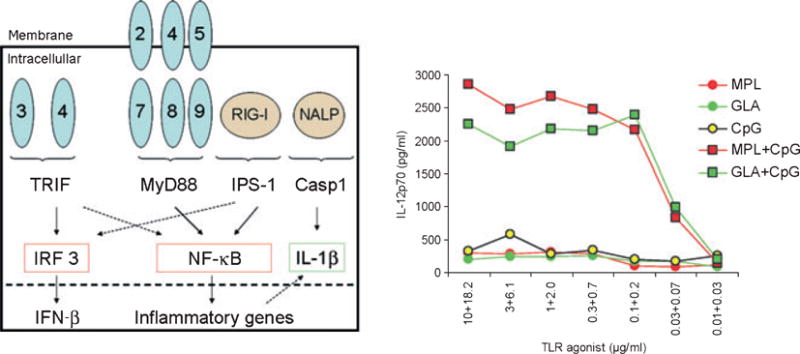
The cellular locations, adapter molecules, and final genes targeted by different PRRs, along with an example of the responses elicited when DCs are stimulated through TLR4 (GLA, MPL), TLR9 (CpG), or both TLR4 and TLR9 are depicted.
TLR4 ligation naturally induces both MyD88 and TRIF recruitment, but monocyte-derived DCs can also combine and integrate TRIF and MyD88 signals received via different TLR (for example, TRIF following of engagement of TLR3 with polyI:C and MyD88 following ligation of TLR2 with PGN, TLR2⁄TLR6 with zymosan or TLR5 with flagellin). The combination of both pathways with these generally low IL-12p70 inducers resulted in cytokine levels similar to LPS, which acts via TLR4. The combination of TLR3 (poly I:C) or TLR4 (LPS) engagement with TLR8 (R848) ligation induced synergistic effects on cytokine production, highlighted by enhanced IL-12p70 secretion. Enhanced phosphorylation in TLR3 and TLR8-activated DCs revealed the critical contribution of p38 MAPK to synergistically inducing IL-12p70 (104). Primary and recall CD8+ T-cell responses to the synergistically activated DCs were superior compared with LPS or R848 alone, demonstrating improved function. The results indicate that DC process, combine, and integrate signals delivered by pathogens to launch effective adaptive immune responses and highlight the potential of using TLRL in combination to create potent adjuvant formulations (Fig. 6).
Because TLR3 signals solely through the TRIF signaling pathway, combining a TLR3 ligand adjuvant with an adjuvant that targets a MyD88-signaling TLR may enhance adjuvant activity, yielding a synergistic effect that could increase generation of protective memory responses. In fact, stimulation of TLR3 by dsRNA and TLR2 by zymosan demonstrates a synergistic effect of the combined agonists on production of serum IgG and mucosal IgA in response to intranasal vaccination with influenza vaccine A⁄PR8 HA (105). Production of pro-inflammatory cytokines TNF, IL-6, and IFN-β were also synergistically increased by co-administration of the TLR3 and TLR2 ligands. Co-administration of a TLR3 agonist with the TLR2 agonist MALP-2 similarly resulted in a synergistic effect, resulting in induction of CD8+ T cells that produced high levels of IFN-γ upon restimulation ex vivo in a model of HIV vaccination (106). Administration of TLR2 and TLR3 with a TLR9 ligand further enhanced the synergistic effect, resulting in highly functional cytotoxic CD8+ T cells in mice that received ligands to all three TLR simultaneously (106). While TLR3 binds the synthetic mimetic of dsRNA poly(I:C), it has also been demonstrated that another type of dsRNA, polyadenylic–polyuridylic acids [poly(A:U)], can also act as an adjuvant. Poly(A:U) functions mainly through TLR3 and TLR7, inducing both IFN-α and IL-12p40 from mouse BM-derived DC. It was discovered that poly(A:U)-induced IFN-α production depended on plasmacytoid DCs and required TLR7, while IL-12p40 induction resulted from two cDC subsets, CD24high DCs and CD11bhigh DCs in a TLR3-and TLR7-dependent manner, respectively (107). In vivo injection of poly(A:U) with antigen led to clonal expansion of and IFN-γ production from antigen-specific CD8+ T cells, and both TLR3 and TLR7 expression with required for the clonal T-cell expansion (107).
Our recent work further indicates the importance of using TLRLs in a synergistic fashion to promote strong adaptive immune responses. While significant levels of IFN-γ CD4 T cells were raised following immunization of uninfected mice with a leishmanial vaccine candidate antigen L110 in conjunction with a variety of TLRLs, we found that combinations of TLR4 and TLR9 agonists were required to elicit protective responses in Leishmania major-infected mice (64) (Fig. 7). These results indicate that underlying infections may complicate the selection of vaccine adjuvants and demonstrate the importance of considering the population that will ultimately be administered vaccination throughout the development process.
Fig. 7. Impact of infection on vaccines engaging different TLRs.
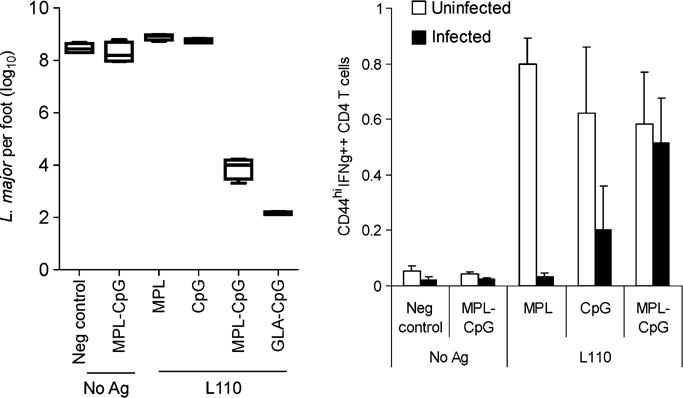
Parasite burden was reduced in the Leishmania major-infected footpads of mice treated with a combination of TLR4 and TLR9 agonists and this corresponded with the ability to prime antigen-experienced CD4+ T cells capable of producing IFN-γ. While single TLRLs could raise these responses in the spleens of uninfected mice, the combination of TLR4 and TLR9 agonists was required to do so in L. major-infected mice. Data originally published in (64).
TLR⁄non-TLR synergy
Recent evidence suggests that some adjuvants activate the innate immune system in a TLR-independent manner. Additional PRRs, such as the nucleotide-binding domain (NOD), leucine rich repeat containing family [Nod-like receptors (NLRs)] and the retinoic-acid inducible protein 1 [RIG-I-like receptors (RLRs)], are now being identified (108, 109). Activation of these additional PRRs by their cognate ligands leads to production of inflammatory cytokines, upregulation of MHC molecules and costimulatory signals on APCs, activation of NK cells, and priming and amplification of antigen-specific T- and B-cell responses. Thus, like TLRs, NLRs and RLRs also link innate and adaptive immunity and their ligands could potentially be used as adjuvants. Although some specific NLR-activating ligands have already been described, most NLR do not have known or characterized ligands.
Muramylpeptides released from bacterial PGN into the intracytoplasmic compartment are among the few known NLR ligands (110, 111). Activation of NOD1 alone appears to permit the priming of predominantly antigen-specific Th2 responses and provides caspase-1 activation to permit the generation of functional IL-1β and IL-18 (112). Recognition of PGN by Nod1 synergizes with TLR2 signaling and results in the secretion of IL-12 and IL-23 that promote Th1 and Th17 responses (113).
Expression of either RIG-I and melanoma-differentiation associated gene-5 is triggered by dsRNA or by synthetic RNA analogs. Natural RIG-I agonists are single-stranded RNA viral genomes bearing 50-triphosphates that trigger cell-intrinsic innate immune responses during infection (114). As such, viral vectors used for delivery of vaccine could induce both TLRs and RLR pathways. Ligation of RIG-I leads to signals that induce type I IFN-mediated immunity that could also play a role in cross-presentation of antigens by MHC class I molecules. Interplay between RIG-I and TLRs has recently been suggested, with stimulation through TLR4-inducing RIG-I expression in macrophages. Depletion or ablation of RIG-I inhibited the cellular processes involved in phagocytosis and prevented TLR4-induced phagocytosis of bacteria (115). As NLR and RLR ligands become known and the interplay between these and TLRLs can be examined, it is likely that novel adjuvant formulations can be created to capitalize on synergistic activities between these molecules.
Conclusions
It is possible to shape desired immune responses by formulating TLRLs, individually, in combination, or potentially with non-TLRLs, for use in the development of next generation vaccines. We have shown that antigens of either Leishmania or M. tuberculosis, formulated in o⁄w emulsions, stimulate primarily Th2 responses and confer no protection against infection whereas the same antigens, formulated in the same emulsions but with the addition of MPL or GLA, lead to dominant Th1 responses that do provide protection. In addition to skewing the response to Th1 in the context of prophylactic immunization, the TLR4 agonist MPL can alter the course of an active immune response. Thus, MPL has been used in allergy desensitization (Allergy Therapeutics), and we have begun trials using MPL-adjuvanted antigens, in combination with chemotherapy, to treat human leishmaniasis (58). The future will see expanded use of formulated TLRLs as important components of prophylactic and therapeutic vaccines for infectious diseases and cancer. Advances in formulation development and improvements in the molecular composition of TLRLs are leading to the development of adjuvants capable of inducing directed CD4+ or CD8+ T-cell responses, as well as affecting the quality and quantity of specific antibodies. From previous experience with first generation vaccines as well as rapid advances in developing defined vaccines containing TLRLs, it is apparent that an expanded number of safe and effective vaccines containing such molecules will be available in the future.
Acknowledgments
Infectious Disease Research Institute has a mission of developing products for the prevention, detection and treatment of infectious diseases of poverty. IDRI’s vaccine programs are funded by, among others, American Leprosy Missions, Bill and Melinda Gates Foundation and National Institute for Health. Immune Design Corporation is a privately held company with a goal of developing therapeutic vaccines for the treatment of infectious and malignant disease.
References
- 1.O’Neill LA, Bryant CE, Doyle SL. Therapeutic targeting of Toll-like receptors for infectious and inflammatory diseases and cancer. Pharmacol Rev. 2009;61:177–197. doi: 10.1124/pr.109.001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imler JL, Zheng L. Biology of Toll receptors: lessons from insects and mammals. J Leukoc Biol. 2004;75:18–26. doi: 10.1189/jlb.0403160. [DOI] [PubMed] [Google Scholar]
- 3.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 4.Gribar SC, Richardson WM, Sodhi CP, Hackam DJ. No longer an innocent bystander: epithelial toll-like receptor signaling in the development of mucosal inflammation. Mol Med. 2008;14:645–659. doi: 10.2119/2008-00035.Gribar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostuni R, Zanoni I, Granucci F. Deciphering the complexity of Toll-like receptor signaling. Cell Mol Life Sci. 2010;67:4109–4134. doi: 10.1007/s00018-010-0464-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carty M, Goodbody R, Schroder M, Stack J, Moynagh PN, Bowie AG. The human adaptor SARM negatively regulates adaptor protein TRIF-dependent Toll-like receptor signaling. Nat Immunol. 2006;7:1074–1081. doi: 10.1038/ni1382. [DOI] [PubMed] [Google Scholar]
- 7.Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunol Rev. 2009;227:221–233. doi: 10.1111/j.1600-065X.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- 8.Chang ZL. Important aspects of Toll-like receptors, ligands and their signaling pathways. Inflamm Res. 2010;59:791–808. doi: 10.1007/s00011-010-0208-2. [DOI] [PubMed] [Google Scholar]
- 9.Dasari P, Nicholson IC, Hodge G, Dandie GW, Zola H. Expression of toll-like receptors on B lymphocytes. Cell Immunol. 2005;236:140–145. doi: 10.1016/j.cellimm.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 10.Ozinsky A, et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci USA. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeuchi O, et al. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol. 2001;13:933–940. doi: 10.1093/intimm/13.7.933. [DOI] [PubMed] [Google Scholar]
- 12.Hasan U, et al. Human TLR10 is a functional receptor, expressed by B cells and plasmacytoid dendritic cells, which activates gene transcription through MyD88. J Immunol. 2005;174:2942–2950. doi: 10.4049/jimmunol.174.5.2942. [DOI] [PubMed] [Google Scholar]
- 13.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 14.Bulut Y, et al. Mycobacterium tuberculosis heat shock proteins use diverse Toll-like receptor pathways to activate pro-inflammatory signals. J Biol Chem. 2005;280:20961–20967. doi: 10.1074/jbc.M411379200. [DOI] [PubMed] [Google Scholar]
- 15.Krummen M, et al. Release of IL-12 by dendritic cells activated by TLR ligation is dependent on MyD88 signaling, whereas TRIF signaling is indispensable for TLR synergy. J Leukoc Biol. 2010;88:189–199. doi: 10.1189/jlb.0408228. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi F, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 17.Applequist SE, et al. Activation of innate immunity, inflammation, and potentiation of DNA vaccination through mammalian expression of the TLR5 agonist flagellin. J Immunol. 2005;175:3882–3891. doi: 10.4049/jimmunol.175.6.3882. [DOI] [PubMed] [Google Scholar]
- 18.Smith KJ, Hamza S, Skelton H. The imidazoquinolines and their place in the therapy of cutaneous disease. Expert Opin Pharmacother. 2003;4:1105–1119. doi: 10.1517/14656566.4.7.1105. [DOI] [PubMed] [Google Scholar]
- 19.Shukla NM, Malladi SS, Mutz CA, Balakrishna R, David SA. Structure-activity relationships in human toll-like receptor 7-active imidazoquinoline analogues. J Med Chem. 2010;53:4450–4465. doi: 10.1021/jm100358c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemmi H, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 21.Jurk M, et al. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat Immunol. 2002;3:499. doi: 10.1038/ni0602-499. [DOI] [PubMed] [Google Scholar]
- 22.Gorden KK, Qiu XX, Binsfeld CC, Vasilakos JP, Alkan SS. Cutting edge: activation of murine TLR8 by a combination of imidazoquinoline immune response modifiers and polyT oligodeoxynucleotides. J Immunol. 2006;177:6584–6587. doi: 10.4049/jimmunol.177.10.6584. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Xu C, Hsu LC, Luo Y, Xiang R, Chuang TH. A five-amino-acid motif in the undefined region of the TLR8 ectodomain is required for species-specific ligand recognition. Mol Immunol. 2010;47:1083–1090. doi: 10.1016/j.molimm.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foureau DM, et al. TLR9-dependent induction of intestinal alpha-defensins by Toxoplasma gondii. J Immunol. 2010;184:7022–7029. doi: 10.4049/jimmunol.0901642. [DOI] [PubMed] [Google Scholar]
- 25.Krieg AM, Efler SM, Wittpoth M, Al Adhami MJ, Davis HL. Induction of systemic TH1-like innate immunity in normal volunteers following subcutaneous but not intravenous administration of CPG 7909, a synthetic B-class CpG oligodeoxynucleotide TLR9 agonist. J Immunother. 2004;27:460–471. doi: 10.1097/00002371-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Belderbos ME, et al. Skewed pattern of Toll-like receptor 4-mediated cytokine production in human neonatal blood: low LPS-induced IL-12p70 and high IL-10 persist throughout the first month of life. Clin Immunol. 2009;133:228–237. doi: 10.1016/j.clim.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen M, et al. Acquisition of adult-like TLR4 and TLR9 responses during the first year of life. PLoS ONE. 2010;5:e10407. doi: 10.1371/journal.pone.0010407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Duin D, Shaw AC. Toll-like receptors in older adults. J Am Geriatr Soc. 2007;55:1438–1444. doi: 10.1111/j.1532-5415.2007.01300.x. [DOI] [PubMed] [Google Scholar]
- 29.van Duin D, et al. Age-associated defect in human TLR-1⁄2 function. J Immunol. 2007;178:970–975. doi: 10.4049/jimmunol.178.2.970. [DOI] [PubMed] [Google Scholar]
- 30.Panda A, et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol. 2010;184:2518–2527. doi: 10.4049/jimmunol.0901022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexopoulou L, et al. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat Med. 2002;8:878–884. doi: 10.1038/nm732. [DOI] [PubMed] [Google Scholar]
- 32.Sigal LH, et al. A vaccine consisting of recombinant Borrelia burgdorferi outer-surface protein A to prevent Lyme disease. Recombinant Outer-Surface Protein a Lyme Disease Vaccine Study Consortium. N Engl J Med. 1998;339:216–222. doi: 10.1056/NEJM199807233390402. [DOI] [PubMed] [Google Scholar]
- 33.Schreibelt G, et al. Commonly used prophylactic vaccines as an alternative for synthetically produced TLR ligands to mature monocyte-derived dendritic cells. Blood. 2010;116:564–574. doi: 10.1182/blood-2009-11-251884. [DOI] [PubMed] [Google Scholar]
- 34.Querec T, et al. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med. 2006;203:413–424. doi: 10.1084/jem.20051720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Domingo C, Niedrig M. Safety of 17D derived yellow fever vaccines. Expert Opin Drug Saf. 2009;8:211–221. doi: 10.1517/14740330902808086. [DOI] [PubMed] [Google Scholar]
- 36.Roukens AH, Visser LG. Yellow fever vaccine: past, present and future. Expert Opin Biol Ther. 2008;8:1787–1795. doi: 10.1517/14712598.8.11.1787. [DOI] [PubMed] [Google Scholar]
- 37.Zhu J, Martinez J, Huang X, Yang Y. Innate immunity against vaccinia virus is mediated by TLR2 and requires TLR-independent production of IFN-beta. Blood. 2007;109:619–625. doi: 10.1182/blood-2006-06-027136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delaloye J, et al. Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS Pathog. 2009;5:e1000480. doi: 10.1371/journal.ppat.1000480. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Gallorini S, et al. Toll-like receptor 2 dependent immunogenicity of glycoconjugate vaccines containing chemically derived zwitterionic polysaccharides. Proc Natl Acad Sci USA. 2009;106:17481–17486. doi: 10.1073/pnas.0903313106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cataldi A, et al. Efficient immune responses against Intimin and EspB of enterohaemorragic Escherichia coli after intranasal vaccination using the TLR2⁄6 agonist MALP-2 as adjuvant. Vaccine. 2008;26:5662–5667. doi: 10.1016/j.vaccine.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 41.Prajeeth CK, et al. The synthetic TLR2 agonist BPPcysMPEG leads to efficient cross-priming against co-administered and linked antigens. Eur J Immunol. 2010;40:1272–1283. doi: 10.1002/eji.200939790. [DOI] [PubMed] [Google Scholar]
- 42.Phillipps KS, Wykes MN, Liu XQ, Brown M, Blanchfield J, Toth I. A novel synthetic adjuvant enhances dendritic cell function. Immunology. 2009;128:e582–e588. doi: 10.1111/j.1365-2567.2008.03038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casella CR, Mitchell TC. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell Mol Life Sci. 2008;65:3231–3240. doi: 10.1007/s00018-008-8228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Didierlaurent AM, et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol. 2009;183:6186–6197. doi: 10.4049/jimmunol.0901474. [DOI] [PubMed] [Google Scholar]
- 45.Cekic C, Casella CR, Eaves CA, Matsuzawa A, Ichijo H, Mitchell TC. Selective activation of the p38 MAPK pathway by synthetic monophosphoryl lipid A. J Biol Chem. 2009;284:31982–31991. doi: 10.1074/jbc.M109.046383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coler RN, et al. Immunization with a poly-protein vaccine consisting of the T-Cell antigens thiol-specific antioxidant, Leishmania major stress-inducible protein 1, and Leishmania elongation initiation factor protects against leishmaniasis. Infect Immun. 2002;70:4215–4225. doi: 10.1128/IAI.70.8.4215-4225.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skeiky YA, et al. Protective efficacy of a tandemly linked, multi-subunit recombinant leishmanial vaccine (Leish-111f) formulated in MPL adjuvant. Vaccine. 2002;20:3292–3303. doi: 10.1016/s0264-410x(02)00302-x. [DOI] [PubMed] [Google Scholar]
- 48.Skeiky YA, et al. Differential immune responses and protective efficacy induced by components of a tuberculosis polyprotein vaccine, Mtb72F, delivered as naked DNA or recombinant protein. J Immunol. 2004;172:7618–7628. doi: 10.4049/jimmunol.172.12.7618. [DOI] [PubMed] [Google Scholar]
- 49.Reed SG, Coler RN, Campos-Neto A. Development of a leishmaniasis vaccine: the importance of MPL. Expert Rev Vaccines. 2003;2:239–252. doi: 10.1586/14760584.2.2.239. [DOI] [PubMed] [Google Scholar]
- 50.Coler RN, Goto Y, Bogatzki L, Raman V, Reed SG. Leish-111f, a recombinant polyprotein vaccine that protects against visceral Leishmaniasis by elicitation of CD4+ T cells. Infect Immun. 2007;75:4648–4654. doi: 10.1128/IAI.00394-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goto Y, Bogatzki LY, Bertholet S, Coler RN, Reed SG. Protective immunization against visceral leishmaniasis using Leishmania sterol 24-c-methyltransferase formulated in adjuvant. Vaccine. 2007;25:7450–7458. doi: 10.1016/j.vaccine.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar R, Goto Y, Gidwani K, Cowgill KD, Sundar S, Reed SG. Evaluation of ex vivo human immune response against candidate antigens for a visceral leishmaniasis vaccine. Am J Trop Med Hyg. 2010;82:808–813. doi: 10.4269/ajtmh.2010.09-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brandt L, et al. The protective effect of the Mycobacterium bovis BCG vaccine is increased by coadministration with the Mycobacterium tuberculosis 72-kilodalton fusion polyprotein Mtb72F in M. tuberculosis-infected guinea pigs. Infect Immun. 2004;72:6622–6632. doi: 10.1128/IAI.72.11.6622-6632.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reed S, Lobet Y. Tuberculosis vaccine development; from mouse to man. Microbes Infect. 2005;7:922–931. doi: 10.1016/j.micinf.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 55.Tsenova L, et al. Evaluation of the Mtb72F polyprotein vaccine in a rabbit model of tuberculous meningitis. Infect Immun. 2006;74:2392–2401. doi: 10.1128/IAI.74.4.2392-2401.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reed SG, et al. Defined tuberculosis vaccine, Mtb72F⁄AS02A, evidence of protection in cynomolgus monkeys. Proc Natl Acad Sci USA. 2009;106:2301–2306. doi: 10.1073/pnas.0712077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Velez ID, et al. Safety and immunogenicity of a defined vaccine for the prevention of cutaneous leishmaniasis. Vaccine. 2009;28:329–337. doi: 10.1016/j.vaccine.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 58.Nascimento E, et al. A clinical trial to evaluate the safety and immunogenicity of the LEISH-F1 + MPL-SE vaccine when used in combination with meglumine antimoniate for the treatment of cutaneous leishmaniasis. Vaccine. 2010;28:6581–6587. doi: 10.1016/j.vaccine.2010.07.063. [DOI] [PubMed] [Google Scholar]
- 59.Miret J, et al. Evaluation of an immunochemotherapeutic protocol constituted of N-methyl meglumine antimoniate (Glucantime) and the recombinant Leish-110f + MPL-SE vaccine to treat canine visceral leishmaniasis. Vaccine. 2008;26:1585–1594. doi: 10.1016/j.vaccine.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trigo J, et al. Treatment of canine visceral leishmaniasis by the vaccine Leish-111f + MPL-SE. Vaccine. 2010;28:3333–3340. doi: 10.1016/j.vaccine.2010.02.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anderson RC, et al. Physicochemical characterization and biological activity of synthetic TLR4 agonist formulations. Colloids Surf B Biointerfaces. 2010;75:123–132. doi: 10.1016/j.colsurfb.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 62.Baldwin SL, et al. Enhanced humoral and Type 1 cellular immune responses with Fluzone adjuvanted with a synthetic TLR4 agonist formulated in an emulsion. Vaccine. 2009;27:5956–5963. doi: 10.1016/j.vaccine.2009.07.081. [DOI] [PubMed] [Google Scholar]
- 63.Raman VS, et al. Vaccination with the ML0276 antigen reduces local inflammation but not bacterial burden during experimental Mycobacterium leprae infection. Infect Immun. 2009;77:5623–5630. doi: 10.1128/IAI.00508-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raman VS, et al. Applying TLR synergy in immunotherapy: implications in cutaneous leishmaniasis. J Immunol. 2010;185:1701–1710. doi: 10.4049/jimmunol.1000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bertholet S, et al. A defined tuberculosis vaccine candidate boosts BCG and protects against multidrug resistant Mycobacterium tuberculosis. Sci Transl Med. 2010;2:53ra74. doi: 10.1126/scitranslmed.3001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coler RN, et al. A synthetic adjuvant to enhance and expand immune responses to influenza vaccines. PLoS ONE. 2010;5:e13677. doi: 10.1371/journal.pone.0013677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fransen F, Boog CJ, van Putten JP, van der Ley P. Agonists of Toll-like receptors 3, 4, 7, and 9 are candidates for use as adjuvants in an outer membrane vaccine against Neisseria meningitidis serogroup B. Infect Immun. 2007;75:5939–5946. doi: 10.1128/IAI.00846-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gupta AK, Cherman AM, Tyring SK. Viral and nonviral uses of imiquimod: a review. J Cutan Med Surg. 2004;8:338–352. doi: 10.1007/s10227-005-0023-5. [DOI] [PubMed] [Google Scholar]
- 69.Otero M, et al. Resiquimod is a modest adjuvant for HIV-1 gag-based genetic immunization in a mouse model. Vaccine. 2004;22:1782–1790. doi: 10.1016/j.vaccine.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 70.Zhang X, Mosser DM. Macrophage activation by endogenous danger signals. J Pathol. 2008;214:161–178. doi: 10.1002/path.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arevalo I, et al. Successful treatment of drug-resistant cutaneous leishmaniasis in humans by use of imiquimod, an immunomodulator. Clin Infect Dis. 2001;33:1847–1851. doi: 10.1086/324161. [DOI] [PubMed] [Google Scholar]
- 72.Vollmer J, et al. CpG oligodeoxynucleotides stimulate IFN-gamma-inducible protein-10 production in human B cells. J Endotoxin Res. 2004;10:431–438. doi: 10.1179/096805104225006534. [DOI] [PubMed] [Google Scholar]
- 73.Marshall JD, et al. Superior activity of the type C class of ISS in vitro and in vivo across multiple species. DNA Cell Biol. 2004;24:63–72. doi: 10.1089/dna.2005.24.63. [DOI] [PubMed] [Google Scholar]
- 74.Campbell JD, et al. CpG-containing immunostimulatory DNA sequences elicit TNF-alpha-dependent toxicity in rodents but not in humans. J Clin Invest. 2009;119:2564–2576. doi: 10.1172/JCI38294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Halperin SA, Van Nest G, Smith B, Abtahi S, Whiley H, Eiden JJ. A phase I study of the safety and immunogenicity of recombinant hepatitis B surface antigen co-administered with an immunostimulatory phosphorothioate oligonucleotide adjuvant. Vaccine. 2003;21:2461–2467. doi: 10.1016/s0264-410x(03)00045-8. [DOI] [PubMed] [Google Scholar]
- 76.Halperin SA, et al. Comparison of the safety and immunogenicity of hepatitis B virus surface antigen co-administered with an immunostimulatory phosphorothioate oligonucleotide and a licensed hepatitis B vaccine in healthy young adults. Vaccine. 2006;24:20–26. doi: 10.1016/j.vaccine.2005.08.095. [DOI] [PubMed] [Google Scholar]
- 77.Sogaard OS, et al. Improving the immunogenicity of pneumococcal conjugate vaccine in HIV-infected adults with a toll-like receptor 9 agonist adjuvant: a randomized, controlled trial. Clin Infect Dis. 2010;51:42–50. doi: 10.1086/653112. [DOI] [PubMed] [Google Scholar]
- 78.Tighe H, et al. Conjugation of protein to immunostimulatory DNA results in a rapid, long-lasting and potent induction of cell-mediated and humoral immunity. Eur J Immunol. 2000;30:1939–1947. doi: 10.1002/1521-4141(200007)30:7<1939::AID-IMMU1939>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 79.Higgins D, et al. Modulation of immunogenicity and allergenicity by controlling the number of immunostimulatory oligonucleotides linked to Amb a 1. J Allergy Clin Immunol. 2006;118:504–510. doi: 10.1016/j.jaci.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 80.Kindrachuk J, et al. A novel vaccine adjuvant comprised of a synthetic innate defence regulator peptide and CpG oligonucleotide links innate and adaptive immunity. Vaccine. 2009;27:4662–4671. doi: 10.1016/j.vaccine.2009.05.094. [DOI] [PubMed] [Google Scholar]
- 81.Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McAleer JP, Rossi RJ, Vella AT. Lipopolysaccharide potentiates effector T cell accumulation into nonlymphoid tissues through TRIF. J Immunol. 2009;182:5322–5330. doi: 10.4049/jimmunol.0803616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seubert A, Monaci E, Pizza M, O’Hagan DT, Wack A. The adjuvants aluminum hydroxide and MF59 induce monocyte and granulocyte chemoattractants and enhance monocyte differentiation toward dendritic cells. J Immunol. 2008;180:5402–5412. doi: 10.4049/jimmunol.180.8.5402. [DOI] [PubMed] [Google Scholar]
- 84.Hamilton JA, Byrne R, Whitty G. Particulate adjuvants can induce macrophage survival, DNA synthesis, and a synergistic proliferative response to GM-CSF and CSF-1. J Leukoc Biol. 2000;67:226–232. [PubMed] [Google Scholar]
- 85.Fox CB. Squalene emulsions for parenteral vaccine and drug delivery. Molecules. 2009;14:3286–3312. doi: 10.3390/molecules14093286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fox CB, et al. Effects of emulsifier concentration, composition, and order of addition in squalene-phosphatidylcholine oil-in-water emulsions. Pharm Dev Technol. 2010 doi: 10.3109/10837450.2010.495397. [DOI] [PubMed] [Google Scholar]
- 87.Guy B. The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol. 2007;5:505–517. doi: 10.1038/nrmicro1681. [DOI] [PubMed] [Google Scholar]
- 88.Kwissa M, Kasturi SP, Pulendran B. The science of adjuvants. Expert Rev Vaccines. 2007;6:673–684. doi: 10.1586/14760584.6.5.673. [DOI] [PubMed] [Google Scholar]
- 89.Mosca F, et al. Molecular and cellular signatures of human vaccine adjuvants. Proc Natl Acad Sci USA. 2008;105:10501–10506. doi: 10.1073/pnas.0804699105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fox CB, Anderson RC, Dutill TS, Goto Y, Reed SG, Vedvick TS. Monitoring the effects of component structure and source on formulation stability and adjuvant activity of oil-in-water emulsions. Colloids Surf B Biointerfaces. 2008;65:98–105. doi: 10.1016/j.colsurfb.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 91.Schultze V, D’Agosto V, Wack A, Novicki D, Zorn J, Hennig R. Safety of MF59 adjuvant. Vaccine. 2008;26:3209–3222. doi: 10.1016/j.vaccine.2008.03.093. [DOI] [PubMed] [Google Scholar]
- 92.Reed SG, Bertholet S, Coler RN, Friede M. New horizons in adjuvants for vaccine development. Trends Immunol. 2009;30:23–32. doi: 10.1016/j.it.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 93.McAleer JP, Vella AT. Understanding how lipopolysaccharide impacts CD4 T-cell immunity. Crit Rev Immunol. 2008;28:281–299. doi: 10.1615/critrevimmunol.v28.i4.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jung S, et al. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cailhier JF, et al. Conditional macrophage ablation demonstrates that resident macrophages initiate acute peritoneal inflammation. J Immunol. 2005;174:2336–2342. doi: 10.4049/jimmunol.174.4.2336. [DOI] [PubMed] [Google Scholar]
- 96.Duffield JS, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bennett CL, et al. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J Cell Biol. 2005;169:569–576. doi: 10.1083/jcb.200501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kool M, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205:869–882. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fahlen-Yrlid L, et al. CD11c(high)dendritic cells are essential for activation of CD4+ T cells and generation of specific antibodies following mucosal immunization. J Immunol. 2009;183:5032–5041. doi: 10.4049/jimmunol.0803992. [DOI] [PubMed] [Google Scholar]
- 100.Bar-On L, Jung S. Defining in vivo dendritic cell functions using CD11c-DTR transgenic mice. Methods Mol Biol. 2010;595:429–442. doi: 10.1007/978-1-60761-421-0_28. [DOI] [PubMed] [Google Scholar]
- 101.Sapoznikov A, Pewzner-Jung Y, Kalchenko V, Krauthgamer R, Shachar I, Jung S. Perivascular clusters of dendritic cells provide critical survival signals to B cells in bone marrow niches. Nat Immunol. 2008;9:388–395. doi: 10.1038/ni1571. [DOI] [PubMed] [Google Scholar]
- 102.Bates JT, Uematsu S, Akira S, Mizel SB. Direct stimulation of tlr5+⁄+ CD11c+ cells is necessary for the adjuvant activity of flagellin. J Immunol. 2009;182:7539–7547. doi: 10.4049/jimmunol.0804225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ghosh TK, et al. Toll-like receptor (TLR) 2-9 agonists-induced cytokines and chemokines: I. Comparison with T cell receptor-induced responses. Cell Immunol. 2006;243:48–57. doi: 10.1016/j.cellimm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 104.Bohnenkamp HR, Papazisis KT, Burchell JM, Taylor-Papadimitriou J. Synergism of Toll-like receptor-induced interleukin-12p70 secretion by monocyte-derived dendritic cells is mediated through p38 MAPK and lowers the threshold of T-helper cell type 1 responses. Cell Immunol. 2007;247:72–84. doi: 10.1016/j.cellimm.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 105.Ainai A, et al. Zymosan enhances the mucosal adjuvant activity of poly(I:C) in a nasal influenza vaccine. J Med Virol. 2010;82:476–484. doi: 10.1002/jmv.21694. [DOI] [PubMed] [Google Scholar]
- 106.Zhu Q, et al. Using 3 TLR ligands as a combination adjuvant induces qualitative changes in T cell responses needed for antiviral protection in mice. J Clin Invest. 2010;120:607–616. doi: 10.1172/JCI39293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sugiyama T, et al. Immunoadjuvant effects of polyadenylic:polyuridylic acids through TLR3 and TLR7. Int Immunol. 2008;20:1–9. doi: 10.1093/intimm/dxm112. [DOI] [PubMed] [Google Scholar]
- 108.Martinon F, Tschopp J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 2005;26:447–454. doi: 10.1016/j.it.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 109.Kang DC, Gopalkrishnan RV, Wu Q, Jankowsky E, Pyle AM, Fisher PB. mda-5: An interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc Natl Acad Sci USA. 2002;99:637–642. doi: 10.1073/pnas.022637199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Girardin SE, et al. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science. 2003;300:1584–1587. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- 111.Girardin SE, et al. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J Biol Chem. 2003;278:41702–41708. doi: 10.1074/jbc.M307198200. [DOI] [PubMed] [Google Scholar]
- 112.Yoo NJ, et al. Nod1, a CARD protein, enhances pro-interleukin-1beta processing through the interaction with pro-caspase-1. Biochem Biophys Res Commun. 2002;299:652–658. doi: 10.1016/s0006-291x(02)02714-6. [DOI] [PubMed] [Google Scholar]
- 113.Fritz JH, et al. Nod1-mediated innate immune recognition of peptidoglycan contributes to the onset of adaptive immunity. Immunity. 2007;26:445–459. doi: 10.1016/j.immuni.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 114.Rehwinkel J, et al. RIG-I detects viral genomic RNA during negative-strand RNA virus infection. Cell. 2010;140:397–408. doi: 10.1016/j.cell.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 115.Kong L, et al. An essential role for RIG-I in toll-like receptor-stimulated phagocytosis. Cell Host Microbe. 2009;6:150–161. doi: 10.1016/j.chom.2009.06.008. [DOI] [PubMed] [Google Scholar]


