Abstract
Background & Aims
Sleep disturbances are common in patients with cirrhosis, but their determinants and effects on health-related quality of life are not well understood. We investigated the prevalence of disturbed sleep in these patients, factors associated with sleep disruption, and effects on quality of life.
Methods
We performed a prospective, cross-sectional study of 193 stable ambulatory patients with cirrhosis (154 with decompensated cirrhosis). Participants completed the Pittsburgh sleep questionnaire index (to assess sleep quality), the chronic liver disease questionnaire (CLDQ), muscle cramp questionnaires, and underwent neurocognitive testing. Actigraphy was performed in a subset of patients with normal and disturbed sleep. We collected serum samples from subjects with normal and disturbed sleep and performed non-targeted metabolomic analyses.
Results
Of the study subjects, 157 (81%) had disturbed sleep, with Pittsburgh sleep questionnaire index scores >5. Disturbed sleep was associated with muscle cramps, daytime somnolence, and decreased quality of life, based on CLDQ scores. Factors independently associated with disturbed sleep in logistic regression analysis included hypoalbuminemia, opiate therapy, and muscle cramps. Disturbed sleep was independently associated with CLDQ score (correlation parameter, −36.6; 95% CI, −24 to −49; P<.001) on linear regression. Disturbed sleep was associated with neurocognitive impairment and with significantly delayed bedtime and decreased total sleep time, measured by actigraphy. Disturbed sleep was associated with metabolome signatures of alterations to the intestinal microbiome and lipid, arginine, and urea cycle metabolism.
Conclusion
Most patients (81%) with advanced cirrhosis have disturbed sleep. This has negative effects on quality of life and is associated with disruptions of several metabolic pathways, including metabolism by the intestinal microbiota.
Keywords: PSQI, CLDQ, metabolome, actigraphy
Introduction
Patients with cirrhosis experience a decrease in health-related quality of life, associated with ascites, hepatic encephalopathy (HE), debilitation, fatigue, muscle cramps and other complications of liver disease. 1 Sleep disturbances have been reported to impact the quality of life in patients with cirrhosis.2 Sleep disturbances in patients with cirrhosis include difficulty with falling asleep, fragmented night-time sleep and increased daytime somnolence.3
These symptoms are commonly attributed to the presence of HE. Reversal of the sleep-wake cycle is considered an early sign of HE, and is associated with impaired daytime functioning. 3 In addition, HE has been associated with disordered circadian cycle, melatonin profile and sleep quality in animal models and human studies.4–8 Sleep is known to transiently worsen after transjugular intrahepatic shunting and induced hyperammonemia9,10 In addition self-reported sleep is described to improve after lactulose initiation for minimal HE.11 On the other hand, the prevalence of insomnia is not related to psychometric performance in cirrhosis,12 and the severity of HE was not associated with insomnia.2 Sleep quality may be impaired for other reasons in cirrhosis, and observational studies on sleep quality indicate an increase in the prevalence of sleep disturbances in patients with cirrhosis, even in the in the absence of minimal or overt HE. 2,12 The association between HE and disturbed sleep remains unclear.
A number of tools have been developed and validated to assess subjective sleep quality, including the Pittsburgh Sleep Quality Index (PSQI) for night time sleep and the Epworth Sleepiness Scale (ESS) for daytime somnolence.13,14 Sleep quality has also been objectively assessed in liver and other disease states using actigraphy. This utilizes an accelerometer equipped, wrist worn device that sensitively tracks periods of immobility reflecting duration and quality of sleep (supplemental figure 3). This allows assessment of sleep in the home environment and daily routine. Actigraphy measured sleep and self-reported sleep varied by health and sleep characteristics in young adults.15 In other studies on non-clinical samples of young and older adults, there was no correlation of actigraphy measurements with PSQI measures.16 Sleep studies in patients with cirrhosis using actigraphy have demonstrated delayed time to bed, increased night-time activity and fragmented sleep.6 It is not clear how well actigraphy derived sleep parameters would correlate with standardized sleep questionnaires in patients with cirrhosis.
The prevalence of sleep disturbances in selected patients with cirrhosis are estimated at 48% to 55%. 3,12 Anecdotally, the majority of patients with cirrhosis report some degree of difficulty with sleep, but the prevalence of sleep disorders in large academic hepatology practices is undefined. Additionally, studies on the correlates of disturbed sleep in cirrhosis have rarely described the impact on quality of life. 3,6,9,12,17 The aims of this study were to describe the prevalence of disturbed sleep in patients with cirrhosis in a large hepatology practice, and assess its impact on health-related quality of life. Additional aims of the study were to; i) describe the differences in neurocognitive function associated with disturbed sleep, ii) compare actigraphy results in subjectively normal and disturbed sleep, and iii) conduct an exploratory metabolomic analyses in a subset of patients with and without disturbed sleep.
Materials and Methods
Subjects
Adults with cirrhosis seen in the hepatology outpatient clinics at Indiana University between December 2012 and January 2014 were eligible for this prospective study, with a target enrollment of 200 subjects. The study was approved by the Indiana University Institutional Review Board. Cirrhosis was defined based on clinical and imaging (and with histological features where available). A priori exclusion criterion was overt HE (West Haven score ≥ 2) as judged clinically by investigators at the time of enrollment and study questionnaires. Patients attending both general hepatology and transplant hepatology clinics were invited by study personnel to participate in this study. Consenting subjects completed questionnaires to assess their sleep quality, daytime somnolence and quality of life. Data collected included demographics, etiology, severity and complications of liver disease, comorbidities, known sleep disorders and medication use. Patients with known sleep disorders such as obstructive sleep apnea (OSA) or restless leg syndrome (RLS) were not excluded, but were accounted for in descriptive and subgroup analysis. Similarly, patients reporting active alcohol or psychoactive/sedating drug use were not excluded since they represent a significant subset of patients with end stage liver disease. Aliquots of serum were frozen at −80°C.
Questionnaires and Neurocognitive testing
Quality of sleep was assessed by the Pittsburg Sleep Quality Index (PSQI) was used to assess the quality of sleep. 13 The Epworth Sleepiness Scale was used to assess daytime somnolence or likelihood of falling asleep in different situations of daily living.14 The Chronic Liver Disease Questionnaire (CLDQ) was be used to assess the health related quality of life in subjects.18,19 Muscle cramps were recently shown to be independently associated with reduced quality of life in cirrhosis. 20 Therefore, supplemental questions were included to assess for muscle cramps in the last 24 hours, and to describe the time of day they occurred. The Number Connection Test (NCT) A and B and the Inhibitory Control Test (ICT) were applied to all subjects at the time of completion of study questionnaires have been used to detect covert HE.21,22 (Details of questionnaire definitions and neurocognitive testing are described in the supplemental data)
Actigraphy
A subset of enrolled subjects, with good sleep and poor sleep, completed actigraphy. Subjects were instructed to wear the actigraphy wrist device (Actiwatch 2®, Philips Respironics, Andover, MA) for 2 weeks. Data were analyzed using the automated settings (Actiware®, Philips Respironics), with standardized reporting of time to sleep, time of waking, total time in bed and sleep time, sleep latency, wake time after sleep onset and number of awakenings per night.
Metabolomics
The mechanism by which sleep is disturbed in cirrhosis is not well understood. However, given the potential association with HE, we borrowed from recent insights into the pathogenesis of HE relating to alterations in the intestinal metabiome and gut-brain axis.23,24 We postulated that disturbed sleep in patients with cirrhosis may be associated with alterations in metabolic pathways including gut metabiome. Non-targeted metabolic profiling was conducted on stored serum obtained from cirrhotic patients reporting good sleep (PSQI 0–5) (n=26), and poor sleep (PSQI>5) (n=54). Out of 54 patients with poor sleep, 20 had mildly disturbed sleep (PSQI 6–8) whereas 34 had severely disturbed sleep (PSQI >11). One-way ANOVA was used to compare groups and p <0.05 was considered significant. Random Forest analysis was used to identify top ranking metabolites of importance to the classification scheme.
Statistical analysis
Descriptive analysis was performed to determine prevalence of poor sleep in the study cohort, and comparison of demographic and clinical variables in good and poor sleepers. In addition simple and multiple logistic regression for predictors of poor sleep, and linear regression for predictors of quality of life by CLDQ were performed. Factors associated with P < .1 were included in the multiple regression analysis. Analysis was performed with SPSS 21 (International Business Machines, New York). All tests were 2 tailed with a threshold of significance at P <.05.
Results
A total of 200 subjects were enrolled during the study period. Seven subjects were excluded from the analysis due to non-completion of the PSQI instrument (3), withdrawal from the study (1), unconfirmed diagnosis of cirrhosis (1), alternating third work shift schedule (1) and interferon based therapy (1). Thirty-six (18.6%) of the 193 studied subjects had PSQI ≤5 (mean 3±1) i.e. subjectively good sleep, and 157 (80.9%) had PSQI>5 (mean 12±4) i.e. poor sleep. Eight patients did not complete all parts of the PSQI, but had a score>5 based on available data and were categorized as having poor sleep.
Demographic and clinical variables were compared in subjects reporting good vs. poor sleep (Table 1). The prevalence of poor sleep in subjects with Childs class A, B and C disease was 71%, 85% and 93% respectively (p = 0.02), and in patients with compensated and decompensated cirrhosis was 70% and 84% respectively (P = 0.04). Muscle cramps were reported to occur in the nighttime in 38%, daytime in 2%, and both day and nighttime in 56% of subjects (4% did not report the time of cramps). Eighteen subjects reported OSA (3 also reported RLS), and OSA was more frequent in patients with poor sleep (table 1). A small number (7) of subjects reported a history of RLS (6 with poor sleep and 1 with good sleep), which limited meaningful analysis of RLS as a covariate. Patients with poor sleep had increased daytime somnolence as rated by the ESS, and worse quality of life as indicated by a lower mean CLDQ score (table 1).
Table 1.
Demographic and clinical variables in the study cohort (N=193), comparing subjects with good and poor sleep. Data are reported as mean ± standard deviation or percentages.
| Good sleepers (n=36) | Poor sleeper (n=157) | P | |
|---|---|---|---|
|
| |||
| Age (years) | 59±8 | 57±10 | .4 |
|
| |||
| Gender (male) | 67% | 56% | .2 |
|
| |||
| Race | .16 | ||
| White | 83% | 92% | |
| Black | 11% | 5% | |
| Hispanic | None | 2% | |
| Other | 6% | 1% | |
|
| |||
| Etiology of liver disease | |||
| Viral hepatitis | 31% | 41% | .3 |
| Alcohol | 14% | 24% | .2 |
| Non-alcoholic fatty liver disease | 33% | 29% | .6 |
|
| |||
| BMI(kg/m2) | 30.6±7.7 | 31±6.6 | .4 |
|
| |||
| Serum albumin (g/dL) | 3.8±0.4 | 3.5±0.6 | .002 |
|
| |||
| MELD | 11.7±4.7 | 12.7±5.3 | .3 |
|
| |||
| History of overt hepatic encephalopathy | 34% | 55% | .03 |
|
| |||
| History of ascites | 40% | 48% | .8 |
|
| |||
| Childs Pugh score | 6.4 ± 1.7 | 7.7 ± 2.1 | .001 |
|
| |||
| Childs class | .02 | ||
| A | 58% | 34% | |
| B | 36% | 49% | |
| C | 6% | 17% | |
|
| |||
| Hepatocellular carcinoma | 11% | 10% | .9 |
|
| |||
| In evaluation or listed for liver transplantation | 44% | 56% | .3 |
|
| |||
| Transjugular intrahepatic portosystemic shunt | None | 4% | .2 |
|
| |||
| Reported muscle cramps in last 24 hours | 26% | 52% | .005 |
|
| |||
| Obstructive sleep apnea | 11% | 9% | .7 |
|
| |||
| Prescription opiate therapy | 11% | 36% | .003 |
|
| |||
| Benzodiazepine therapy | None | 17% | .009 |
|
| |||
| Epworth Sleepiness scale | .004 | ||
| Normal (0–9) | 78% | 47% | |
| Borderline daytime sleepiness (10–15) | 11% | 22% | |
| Abnormal daytime sleepiness (16–24) | 11% | 31% | |
|
| |||
| Chronic Liver Disease Quality of life (CLDQ) | 169±27 | 118±35 | <.001 |
Determinants of poor sleep and quality of life
Simple and multiple logistic regression analysis for the determinants of poor sleep are described in table 2. Muscle cramps, hypoalbuminemia and opiate therapy were independently associated with poor sleep. Poor sleep was associated with lower CLDQ on multiple linear regression (correlation parameter −36.6; 95% CI; −24 to −49; P <.001), as were muscle cramps and HE (table 3). Poor sleep remained an independent predictor of lower CLDQ when analyzing the 154 patients with decompensated cirrhosis (correlation parameter −38.3, 95%CI from −23 to −53.7), and the 72 patients not reporting OSA, RLS, cramps or opiate use (correlation parameter −35.4; 95% CI, −21.2 to −49.6; P <.001).
Table 2.
A summary of simple and multiple logistic regression analysis for the determinants of poor sleep as defined by a Pittsburgh Sleep Quality Index (PSQI) score >5.
| * Logistic regression analysis for predictors of poor sleep (PSQI >5) | ||||||
|---|---|---|---|---|---|---|
| Simple | Multiple | |||||
| Variable | Odds ratio | 95% CI | P | Odds ratio | 95% CI | P |
| Serum albumin (g/dL) | 0.37 | 0.2– 0.7 | <.001 | 0.4 | 0.2 – 0.8 | .01 |
| Prescription opiate therapy | 4.6 | 1.5 – 13.5 | .006 | 5 | 1.4 – 17.7 | .01 |
| Muscle cramps | 6.2 | 2.3 – 16.7 | <.001 | 5.2 | 1.8 – 14.6 | .002 |
| Hepatic encephalopathy | 2.3 | 1.1 – 5 | .03 | |||
Factors not predictive of poor sleep on simple logistic regression were; Age, gender, race, body mass index, bilirubin (mg/dL), creatinine (mg/dL), INR, ammonia (mg/dL), obstructive sleep apnea, etiology of liver disease category (viral hepatitis, alcoholic, fatty liver, cryptogenic, cholestatic or autoimmune hepatitis).
Table 3.
A summary of the simple and multiple linear regression analysis for the predictors of quality life as determined by Chronic Liver Disease Quality of life questionnaire (CLDQ). Results describe the correlation parameter (B), its 95% confidence interval, and the associated p-value.
| * Linear regression analysis for predictors of quality of life by CLDQ | ||||||
|---|---|---|---|---|---|---|
| Simple regression | Multiple regression | |||||
| Variable | B (correlation parameter) | 95% CI | P | B (correlation parameter) | 95% CI | P |
| Poor sleep (PSQI>5) | −50.6 | −38.5 _−62.7 | <.001 | −36.6 | −24 _ −49 | <.001 |
| Prescription opiate therapy | −22.8 | −11.2 _−34.2 | <.001 | −11.2 | −1.4 _ −21.1 | .03 |
| Muscle cramps | −31.6 | −21.4 _ −42 | <.001 | −18.8 | −9.5 _ −28.1 | <.001 |
| Hepatic encephalopathy | −25.2 | −14.8_−35.7 | <.001 | −16.7 | −7.7 _ −25.7 | <.001 |
| Ascites | −13.6 | −2.7_−24.5 | .01 | |||
| Age | 0.56 | −0.03_1.15 | .06 | |||
| Gender (male) | 11.1 | −0.02 _ 22.2 | .05 | |||
| Creatinine (mg/dL) | 2.7 | −0.2 _ 5.6 | .07 | |||
| Albumin (g/dL) | 17.4 | 8.7 _ 26.1 | <.001 | |||
| Obstructive sleep apnea | −17 | −1.8 _ 35.9 | .08 | −17.7 | −1.9 _ 33.5 | .03 |
Factors not predictive of CLDQ on simple logistic regression were; race (Caucasian, black or Hispanic), body mass index, bilirubin, INR, albumin, ammonia, etiology of liver disease category (viral hepatitis, alcoholic, fatty liver, cryptogenic, cholestatic or autoimmune hepatitis).
Comparisons of neurocognitive tests in patients with good and poor sleep are described in supplemental table 5. Actigraphy was completed in 11 subjects with good sleep and 10 subjects with disturbed sleep over a median interval of 14 days (interquartile range 12 to 14 days) (table 4). Disturbed sleep was associated with a delayed bed time, and shorter total sleep time, but no differences in sleep latency, sleep efficiency or number of awakenings. There was no correlation between sleep efficiency or latency by actigraphy and respective parameters by PSQI.
Table 4.
Actigraphy results in subjects with good and poor sleep. Values are described as mean values with standard deviation
| Good sleep (n=11) | Poor sleep (n=10) | P | |
|---|---|---|---|
| Bed time (hour:minutes PM) | 9:10± 2:50 | 10:17±3:35 | .02 |
| Get up time (hour:minutes AM) | 7:41±1:45 | 7:42±1:09 | .5 |
| Time in bed (minutes) | 558 ± 117 | 499 ± 122 | .04 |
| Total sleep time (min) | 465 ± 77 | 423 ± 129 | .04 |
| Sleep onset latency (min) | 19±13 | 17±11 | .7 |
| Sleep efficiency (%) | 84 ± 5 | 84 ± 7 | .8 |
| Waking after sleep onset (min) | 62±33 | 48±22 | .16 |
| Number of awakenings | 25±8 | 25±14 | .9 |
Categorization of poor sleep according to PSQI
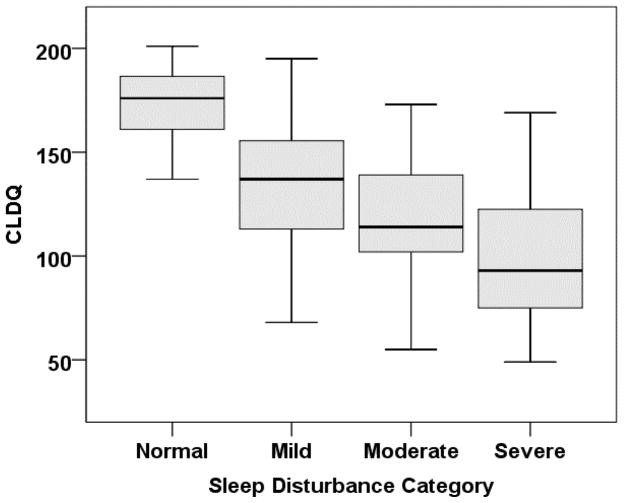
A wide distribution of PSQI and CLDQ scores in this large cohort presented an opportunity to analyze categories of sleep disturbance by PSQI. The 8 subjects who had not completed all sub-components of the PSQI were excluded in this post-hoc analysis. Sleep in the remaining 185 subjects was categorized as normal (PSQI 0–5) in 36 (20%), and the degree of sleep disturbance as mild (PSQI 6–8) in 42 (23%), moderate (PSQI 9–11) in 35 (19%) and severe (PSQI>11) in 72 (36%). Daytime somnolence was increased (ESS >9) in 22%, 40%, 51% and 61% of subjects with normal, mild, moderate and severe sleep disturbance, respectively (P =0.002). Worsening sleep category was significantly associated with progressive decrease in CLDQ (figure 1). This association held after excluding subjects with OSA, RLS, muscle cramps or opiate use (supplemental figure 4).
Figure 1.
A box plot describing Chronic Liver Disease Quality of life questionnaire (CLDQ) scores (median (bold horizontal line), interquartile range (box), and range (whiskers)) in subjects grouped according to category of sleep disturbance, including; normal, mild, moderate and severe. Comparisons of CLDQ between all categories were statistically significant, with the exception of mild versus moderate sleep disturbance.
Differences in neurocognitive testing between mild and severe sleep disturbance became more pronounced when comparing subjects without OSA, RLS, muscle cramps or opiate therapy (supplemental table 6). Notably a high proportion of subjects, even those with good sleep had >5 incorrect lure responses on the ICT, suggesting that there was likely a high prevalence of covert HE in the study group.
Metabolomics
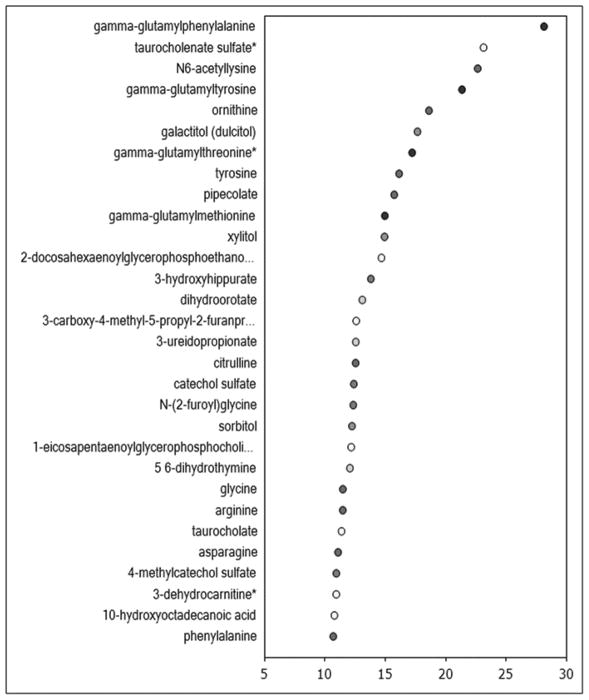
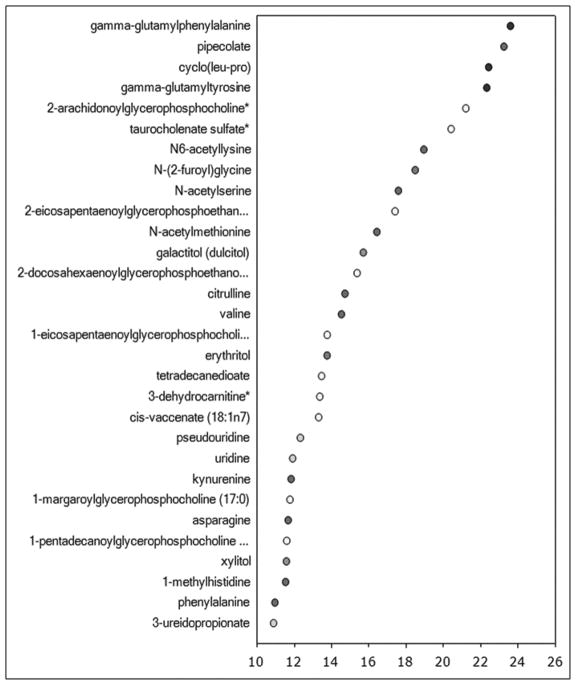
Metabolomic analysis was performed in 80 subjects, excluding OSA, RLS and opiate therapy (supplemental table 7). The biochemical profiling identified 508 serum metabolites, and of these, 45 metabolites differed significantly between patients with no and mild sleep disturbance, 54 metabolites differed between patients with no and severe sleep disturbance, and 45 metabolites differed between those with mild and severe sleep disturbance. Random Forest classification identified metabolites important to the classification scheme that spanned several different metabolic pathways (top biochemicals are shown in Figure 2). Analysis of the top biochemicals that were altered to the greatest degree (p<0.009) among the 3 groups identified several metabolites included within the Random Forest importance plot such as gamma-glutamyl amino acids, taurocholate, tyrosine, 10-hydroxyoctadecanoic acid, select lysolipids, and citrulline. Changes in biochemicals reflective of altered gut microbial metabolism, lipid metabolism, and arginine metabolism and the urea cycle were particularly pronounced. Further examination of the altered gut microbial metabolites revealed changes in several amino acid-derived (pipecolate, 3-(4-hydroxyphenyl) lactate, 3-(4-hydroxyphenyl) propionate, indoleacetate and indoleacetylglutamine), and lipid-derived (3-dehydrocarnitine and 10-hydroxyoctadecanoic acid) metabolites in individuals with mild and severe sleep disturbance. Despite elevations in the lipolysis marker glycerol in both the poor sleep and very poor sleep groups, circulating levels of free fatty acids were only substantially altered in the poor sleep group. Consistent reductions in choline-containing lysolipids were only observed in the very poor sleep group. These findings highlight a role for changes in lipid handling and phospholipid metabolism in altered sleep in patients with cirrhosis. Consistent elevations in several gamma-glutamyl amino acids, suggestive of greater activity of the gamma-glutamyltranspeptidase (GGT) enzyme associated with the gamma-glutamyl cycle, were observed in both groups of patients with poor sleep. This suggests that changes in activity of the gamma-glutamyl cycle, possibly through alterations in glutathione metabolism and oxidative stress, may play a role in underlying pathologies related to perturbations in sleep patterns in patients with cirrhosis. Interestingly, several biochemicals produced when gut flora metabolize the food additive sodium benzoate (3-hydroxyhippurate, catechol sulfate and 4-methylcatechol sulfate) were significantly lower in individuals with mild and severe sleep disturbance.
Figure 2.
The Random Forest classification and biochemical importance plot listing the metabolites in order of degree of alteration between patients with no and any sleep disturbance (2A), and between patients with no, mild and severe sleep disturbance (2B).
Only 2 metabolites were significantly associated with both muscle cramps and poor sleep (supplemental table 8). These were xylulose which is associated with pentose metabolism and oxidized cys-glycine which is associated with glutathione metabolism. While altered liver function is expected in all patients included in this study regardless of sleep status, additional changes in biochemical markers of hepatic function were observed when comparing the poor sleep and/or very poor sleep groups to the good sleep group. Accumulation of select taurine-conjugated bile acids and was noted in both groups of patients with poor sleep, suggestive of decreased hepatic function in cirrhotics with perturbed sleep patterns Notably, poor and very poor sleep were associated with significantly higher Childs Pugh score, though not MELD, (supplemental table 7), raising concern for potential confounding of the metabolomic study results by the severity of liver disease. We performed additional analysis comparing the metabolites associated with poor and very poor sleep in subgroups stratified by Childs Pugh Class (supplemental results). The majority of metabolites remained significantly different in subjects with poor and very poor sleep compared with subjects with good sleep, remained significantly different when compared in subjects within at least one Childs Pugh Class.
Discussion
This study highlights the high prevalence of disturbed sleep in patients with cirrhosis (81%), relative to previous reports citing rates of 48 to 55%.3,12 This may be explained by the less stringent exclusion criteria used for enrollment (allowing patients with sleep disorders and patients using alcohol, sedatives and opiates), and the more advanced liver disease since the study was performed at a high volume liver transplant center. The size of the cohort allowed for a robust analysis of the determinants of disturbed sleep in cirrhosis. Disturbed sleep was independently associated with muscle cramps, a common problem in patients with cirrhosis that has been recently shown to independently and negatively impact quality of life.20 There is little data at present describing the predictors of poor sleep in cirrhosis. In this cohort hypoalbuminemia and opiate therapy were independent determinants of poor sleep. While opiate use may be a surrogate marker of chronic pain, which may be the underlying factor impairing sleep quality, this data only highlights the association with disturbed sleep, but not the reason for it. Notably patients with known sleep disorders such as OSA and RLS, or those using opiates, who would have been excluded in prior studies, were included here, lending broader applicability of the descriptive findings. The associations of poor sleep with quality of life persisted after sensitivity analysis excluding patients with these factors. These data suggest that disturbed sleep in cirrhosis may be multi-factorial in etiology, and that studies on sleep in cirrhosis require accurate phenotyping of not only other sleep disturbances, but also of muscle cramps, chronic pain and opiate therapy.
An important finding of this study, was that disturbed sleep was a powerful and independent predictor of decreased quality of life, even after excluding potential confounders, with a narrow range of the correlation parameter (−35.4 to −38.8) in multiple models. Therefore disturbed sleep represents an important unmet need in patients with cirrhosis.
At present the relationship of disturbed sleep and HE is unclear.2,12,25 Measures of neurocognitive function in subjects with poor sleep were notably worse than those with good sleep, particularly after excluding subjects with muscle cramps, opiate therapy, OSA or RLS. This suggests that there is an association of disturbed sleep with neurocognitive dysfunction, which may only be apparent after excluding subjects with poor sleep related to other sleep disorders or chronic pain/opiate therapy. Additional studies on sleep disturbances in patients with better characterization of covert HE are warranted to better understand that relationship.
The subjective assessments of sleep quality were compared in a subset of patients with objective measures of sleep using actigraphy. The finding of delayed sleep onset corroborates previous findings,2,6,12 which interestingly presents a pattern of disordered sleep in cirrhosis akin to delayed phase sleep disturbance.26 There were no differences in sleep efficiency, latency or number of awakenings, which have already been shown to be impaired in patients with cirrhosis compared to healthy controls.6,12,27 However, this study was intended to assess correlation of objective sleep parameters in disturbed sleep in patients with cirrhosis, and lacked a comparison group of healthy individuals without cirrhosis.
The finding of meaningful differences between the categories of disturbed sleep in relation to quality of life, daytime sleepiness and neurocognitive function, particularly for mild (PSQI=6–8) vs. severe (PSQ>11) disturbed sleep, was important. Clinical correlates have been described for similar categories of PSQI and disturbed sleep in blindness.28 Additionally a decrease in overall PSQI, rather than attaining a score ≤5, has been used and shown to correlate with therapeutic responses for disturbed sleep with a variety of associated conditions and interventions. 29–31 The present data suggests that categorizing the severity of sleep disturbance using PSQI has relevant clinical correlates in patients with cirrhosis. If confirmed, this could inform the design of studies on the pathogenesis and therapeutic endpoints of disturbed sleep in cirrhosis.
Finally, the novel metabolomic analysis suggests that cirrhotic patients with disturbed sleep (excluding those with OSA, RLS and opiate therapy) have a distinct serum metabolomic signature reflecting derangement of several metabolic pathways including gut microbial metabolism. It is not possible to establish a cause and effect association based on this data. However, these potential associations build on the growing evidence linking altered gut microbiota with disease phenotype and complications of cirrhosis, including HE.23,24 The reported impact of circadian dysregulation on altered gut microbiome and increased intestinal permeability lends further credence to the postulated association.32 The limited overlap of the significantly associated metabolites in poor sleep and muscle cramps suggests that the results of the metabolomics analysis were not significantly affected by inclusion of subjects with muscle cramps. While limited by small sample size, the metabolomics re-analysis stratified by Childs Pugh Class suggests that the observed differences in metabolites associated with disturbed sleep were not attributed to the severity of liver disease. Additional study is needed to validate the metabolomics findings in a separate cohort with minimal potential confounding from severity of liver disease and other sleep disorders. Nevertheless, this data highlights the need to describe the microbiome characteristics and gut permeability associated with disturbed sleep, and this may represent a novel therapeutic approach in selected patients.
The study has a number of limitations. It was performed at a large transplant center, consequently the subjects may represent a select group of cirrhotics with very advanced liver disease, hence the results may not reflect findings in patients with compensated cirrhosis. Few subjects reported normal sleep and the study lacked a normal control group, though the aim was not to compare cirrhotics to healthy individuals. The study did not track the number of patients refusing to participate or reasons for refusal. It did not screen for undiagnosed obstructive sleep apnea. The study also lacks data on circadian rhythm, including the diurnal preferences of subjects, and information of alterations in melatonin metabolism, although studies on association of endogenous melatonin metabolism and disturbed sleep in cirrhosis have been inconclusive and do not fully explain disturbed sleep in this population.25,26,33 The study was based on a single assessment and did not define the acuity or chronicity of disturbed sleep. Finally, the study design does not rigorously define the presence of covert hepatic encephalopathy, but supports an association with disturbed sleep which warrants further study.
In summary, we describe a high prevalence of disturbed sleep in patients with cirrhosis at a large transplant center. Disturbed sleep was predicted by muscle cramps, itself an important though poorly understood complication of end-stage liver disease. Disturbed sleep in this population appears to be multi-factorial in etiology, and may be associated with neurocognitive dysfunction. Disturbed sleep is strongly associated with decreased quality of life, and its’ severity may be meaningfully categorized based on PSQI. Further studies to elucidate the pathogenesis and therapies for disturbed sleep in patients with cirrhosis are needed in the face of this significant and unmet need.
Supplementary Material
Study highlights.
Current knowledge
The predictors and prevalence of disturbed sleep in cirrhosis are not well known
There is conflicting data on the association of disturbed sleep with hepatic encephalopathy
What is new here
Disturbed is very prevalent in cirrhosis, and is associated with hypolabuminemia, muscle cramps and opiate therapy
Disturbed sleep is significantly associated with impaired quality of life in cirrhosis
Disturbed sleep is associated with impaired cognitive function in cirrhosis
Disturbed sleep is associated with derangement of several metabolic pathways including gut microbial metabolism
Acknowledgments
This work was supported in part by K24 DK069290 to NC.
The authors thank Julie Otte RN, PhD and Edra Nordstrom for their assistance with study desgin and data collection.
Abbreviations
- CLDQ
Chronic Liver Disease Questionnaire
- ESS
Epworth Sleepiness Scale
- HE
Hepatic encephalopathy
- ICT
Inhibitory Control test
- NCT
Number Connection Test
- OSA
Obstructive sleep apnea
- PSQI
Pittsburgh Sleep Questionnaire Index
- RLS
restless leg syndrome
Footnotes
Author contributions: Marwan Ghabril; Acquisition of data, analysis and interpretation of data, drafting of manuscript, critical revision
Mollie Jackson: Acquisition of data, analysis and interpretation of data, drafting of manuscript, critical revision
Regina Weber: Acquisition of data, critical revision
Raghav Gotur: Acquisition of data, drafting of manuscript, critical revision
Eric Orman; Analysis and interpretation of data, drafting of manuscript, critical revision
Raj Vuppalanchi; Analysis and interpretation of data, drafting of manuscript, critical revision
Naga Chalasani; Analysis and interpretation of data, drafting of manuscript, critical revision
Author disclosures:
The following authors have no conflicts of interest related to this study; Marwan Ghabril, Mollie Jackson, Raghav Gotur, Edra Nordstrom, Regina Weber, Eric Orman, Raj Vuppalanchi, Naga Chalasani.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marchesini G, Bianchi G, Amodio P, et al. Factors associated with poor health-related quality of life of patients with cirrhosis. Gastroenterology. 2001;120(1):170–178. doi: 10.1053/gast.2001.21193. [DOI] [PubMed] [Google Scholar]
- 2.Montagnese S, Middleton B, Skene DJ, Morgan MY. Night-time sleep disturbance does not correlate with neuropsychiatric impairment in patients with cirrhosis. Liver Int. 2009;29(9):1372–1382. doi: 10.1111/j.1478-3231.2009.02089.x. [DOI] [PubMed] [Google Scholar]
- 3.Mostacci B, Ferlisi M, Baldi Antognini A, et al. Sleep disturbance and daytime sleepiness in patients with cirrhosis: a case control study. Neurol Sci. 2008;29(4):237–240. doi: 10.1007/s10072-008-0973-7. [DOI] [PubMed] [Google Scholar]
- 4.Zee PC, Mehta R, Turek FW, Blei AT. Portacaval anastomosis disrupts circadian locomotor activity and pineal melatonin rhythms in rats. Brain Res. 1991;560(1–2):17–22. doi: 10.1016/0006-8993(91)91209-j. [DOI] [PubMed] [Google Scholar]
- 5.Llansola M, Cantero JL, Hita-Yanez E, et al. Progressive reduction of sleep time and quality in rats with hepatic encephalopathy caused by portacaval shunts. Neuroscience. 2012;201:199–208. doi: 10.1016/j.neuroscience.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Montagnese S, Middleton B, Mani AR, Skene DJ, Morgan MY. Sleep and circadian abnormalities in patients with cirrhosis: features of delayed sleep phase syndrome? Metab Brain Dis. 2009;24(3):427–439. doi: 10.1007/s11011-009-9146-5. [DOI] [PubMed] [Google Scholar]
- 7.Montagnese S, Middleton B, Mani AR, Skene DJ, Morgan MY. Melatonin rhythms in patients with cirrhosis. Am J Gastroenterol. 2010;105(1):220–222. doi: 10.1038/ajg.2009.549. author reply 222. [DOI] [PubMed] [Google Scholar]
- 8.Sherlock S, Summerskill WH, White LP, Phear EA. Portal-systemic encephalopathy; neurological complications of liver disease. Lancet. 1954;267(6836):454–457. [PubMed] [Google Scholar]
- 9.Wiltfang J, Nolte W, von Heppe J, et al. Sleep disorders and portal-systemic encephalopathy following transjugular intrahepatic portosystemic stent shunt in patients with liver cirrhosis. Relation to plasma tryptophan. Adv Exp Med Biol. 1999;467:169–176. doi: 10.1007/978-1-4615-4709-9_22. [DOI] [PubMed] [Google Scholar]
- 10.Bersagliere A, Raduazzo ID, Nardi M, et al. Induced hyperammonemia may compromise the ability to generate restful sleep in patients with cirrhosis. Hepatology. 2012;55(3):869–878. doi: 10.1002/hep.24741. [DOI] [PubMed] [Google Scholar]
- 11.Prasad S, Dhiman RK, Duseja A, Chawla YK, Sharma A, Agarwal R. Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology. 2007;45(3):549–559. doi: 10.1002/hep.21533. [DOI] [PubMed] [Google Scholar]
- 12.Cordoba J, Cabrera J, Lataif L, Penev P, Zee P, Blei AT. High prevalence of sleep disturbance in cirrhosis. Hepatology. 1998;27(2):339–345. doi: 10.1002/hep.510270204. [DOI] [PubMed] [Google Scholar]
- 13.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 14.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15(4):376–381. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 15.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19(6):838–845. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grandner MA, Kripke DF, Yoon IY, Youngstedt SD. Criterion validity of the Pittsburgh Sleep Quality Index: Investigation in a non-clinical sample. Sleep Biol Rhythms. 2006;4(2):129–139. doi: 10.1111/j.1479-8425.2006.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hourmand-Ollivier I, Piquet MA, Toudic JP, Denise P, Dao T. Actigraphy: A new diagnostic tool for hepatic encephalopathy. World J Gastroenterol. 2006;12(14):2243–2244. doi: 10.3748/wjg.v12.i14.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Younossi ZM, Guyatt G, Kiwi M, Boparai N, King D. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut. 1999;45(2):295–300. doi: 10.1136/gut.45.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Younossi ZM, Boparai N, Price LL, Kiwi ML, McCormick M, Guyatt G. Health-related quality of life in chronic liver disease: the impact of type and severity of disease. Am J Gastroenterol. 2001;96(7):2199–2205. doi: 10.1111/j.1572-0241.2001.03956.x. [DOI] [PubMed] [Google Scholar]
- 20.Chatrath H, Liangpunsakul S, Ghabril M, Otte J, Chalasani N, Vuppalanchi R. Prevalence and morbidity associated with muscle cramps in patients with cirrhosis. Am J Med. 2012;125(10):1019–1025. doi: 10.1016/j.amjmed.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weissenborn K, Ruckert N, Hecker H, Manns MP. The number connection tests A and B: interindividual variability and use for the assessment of early hepatic encephalopathy. J Hepatol. 1998;28(4):646–653. doi: 10.1016/s0168-8278(98)80289-4. [DOI] [PubMed] [Google Scholar]
- 22.Bajaj JS, Saeian K, Verber MD, et al. Inhibitory control test is a simple method to diagnose minimal hepatic encephalopathy and predict development of overt hepatic encephalopathy. Am J Gastroenterol. 2007;102(4):754–760. doi: 10.1111/j.1572-0241.2007.01048.x. [DOI] [PubMed] [Google Scholar]
- 23.Bajaj JS. The role of microbiota in hepatic encephalopathy. Gut Microbes. 2014;5(3):397–403. doi: 10.4161/gmic.28684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bajaj JS, Heuman DM, Sanyal AJ, et al. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS One. 2013;8(4):e60042. doi: 10.1371/journal.pone.0060042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montagnese S, De Pitta C, De Rui M, et al. Sleep-wake abnormalities in patients with cirrhosis. Hepatology. 2014;59(2):705–712. doi: 10.1002/hep.26555. [DOI] [PubMed] [Google Scholar]
- 26.Montagnese S, Middleton B, Mani AR, Skene DJ, Morgan MY. On the origin and the consequences of circadian abnormalities in patients with cirrhosis. Am J Gastroenterol. 2010;105(8):1773–1781. doi: 10.1038/ajg.2010.86. [DOI] [PubMed] [Google Scholar]
- 27.Teodoro VV, Bragagnolo MA, Jr, Lucchesi LM, et al. Polysomnographic sleep aspects in liver cirrhosis: a case control study. World J Gastroenterol. 2013;19(22):3433–3438. doi: 10.3748/wjg.v19.i22.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tabandeh H, Lockley SW, Buttery R, et al. Disturbance of sleep in blindness. Am J Ophthalmol. 1998;126(5):707–712. doi: 10.1016/s0002-9394(98)00133-0. [DOI] [PubMed] [Google Scholar]
- 29.Bush AL, Armento ME, Weiss BJ, et al. The Pittsburgh Sleep Quality Index in older primary care patients with generalized anxiety disorder: psychometrics and outcomes following cognitive behavioral therapy. Psychiatry Res. 2012;199(1):24–30. doi: 10.1016/j.psychres.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campos FL, da Silva FP, Junior, de Bruin VM, de Bruin PF. Melatonin improves sleep in asthma: a randomized, double-blind, placebo-controlled study. Am J Respir Crit Care Med. 2004;170(9):947–951. doi: 10.1164/rccm.200404-488OC. [DOI] [PubMed] [Google Scholar]
- 31.Deschenes CL, McCurry SM. Current treatments for sleep disturbances in individuals with dementia. Curr Psychiatry Rep. 2009;11(1):20–26. doi: 10.1007/s11920-009-0004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voigt RM, Forsyth CB, Green SJ, et al. Circadian disorganization alters intestinal microbiota. PLoS One. 2014;9(5):e97500. doi: 10.1371/journal.pone.0097500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steindl PE, Finn B, Bendok B, Rothke S, Zee PC, Blei AT. Disruption of the diurnal rhythm of plasma melatonin in cirrhosis. Ann Intern Med. 1995;123(4):274–277. doi: 10.7326/0003-4819-123-4-199508150-00005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.