Abstract
The development of immune checkpoint inhibitors represents a major breakthrough in the field of cancer therapeutics. Pursuant to their success in melanoma and numerous solid tumor malignancies, these agents are being investigated in hematological malignancies including acute myelogenous leukemia (AML) and myelodysplastic syndromes (MDS). Although AML/MDS have traditionally been considered to be less immunogenic than solid tumor malignancies, recent pre-clinical models suggest a therapeutic role for immune checkpoint inhibition in these diseases. CTLA-4 inhibition may be especially effective in treating late post-allogeneic stem cell transplant relapse of AML in patients with limited or no graft versus host disease. Immune checkpoint inhibition, specifically PD-1 inhibition, demonstrated limited single agent efficacy in patients with relapsed AML and with MDS post-hypomethylating therapy. Rationally designed combinations of PD-1 inhibitors with standard anti-leukemic therapy are needed. Hypomethylating agents such as azacitidine, up-regulate PD-1, PD-L1, and PD-L2 in patients with AML/MDS and up-regulation of these genes was associated with the emergence of resistance. The combination of azacitidine and PD-1/PD-L1 inhibition may be a potential mechanism to prevent or overcome resistance to 5-azacitidine. A number of such combinations are being evaluated in clinical trials with early encouraging results. Immune checkpoint inhibition is also an attractive option to improve relapse-free survival or eliminate minimal residual disease post induction and consolidation by enhancing T-cell surveillance in patients with high-risk AML. The ongoing clinical trials with checkpoint inhibitors in AML/MDS will improve our understanding of the immunobiology of these diseases and guide us to the most appropriate application of these agents in the therapy of AML/MDS.
Keywords: checkpoint inhibitors, immunotherapy, acute myeloid leukemia, myelodysplastic syndrome
INTRODUCTION
T-cell mediated immunity involves a series of steps beginning with antigen peptide presentation on the major histocompatibility complex (MHC) of antigen presenting cells (APCs) to the T-cell receptor (TCR), with subsequent T-cell activation and effector response. The steps in this pathway are regulated by careful counterbalancing of the co-stimulatory and co-inhibitory signals, resulting in appropriate T-cell effector function [1]. Immune checkpoints play an important role in regulation of immune homeostasis by optimally balancing the stimulatory and inhibitory signals that mediate the T-cell immune response [2]. Under normal physiological conditions, immune checkpoints regulate self-tolerance and protect tissues from damage by restraining the immune systems response to pathogenic infection. The major receptors regulating T-cell activation, include co-stimulatory receptors such as CD28, 4-1BB, CD27, ICOS (expressed on T-cells) or CD80 and CD86 (expressed on APCs), and co-inhibitory receptors, most relevant being cytotoxic T- lymphocyte-associated-protein 4 (CTLA4) and programmed cell-death protein (PD1)(both expressed predominantly, but not exclusively on T-cells) [1, 3].
The concept of targeting the immune system, and not the tumor itself was a long conceived idea that truly came to the fore after Dr James Allison’s breakthrough discovery of CTLA-4, a receptor on the surface of T cells that blocks the immune response by inhibiting T cell activation and the subsequent development of an anti-CTLA-4 antibody, ipilimumab that blocks this “immune checkpoint” protein, thereby freeing the immune system to attack tumors[4]. Breakthrough clinical results came a decade later, when dramatic and durable responses were noted in a proportion of advanced/metastatic melanoma patients using CTLA-4 inhibitors [5, 6].
The second major approach to immune checkpoint blockade that has been clinically investigated in a large number of cancer patients involves targeting a co-inhibitory receptor-ligand system expressed on activated T cells by blocking either the co-inhibitory receptor PD-1 or its ligand PD-L1, [7]. These two major co-inhibitory checkpoint pathways (CTLA-4 and PD-1/PD-L1) operate at different stages of T cell activation and inhibit antitumor immune responses by different mechanisms of action. CTLA-4 plays a major role in regulating T cell activation during initial stages of the immune response and is expressed predominantly on the T-cells in lymph nodes, whereas PD-1/PDL-1 controls T cell function during the later phases of immune response after T cells exit the circulation and home into tumor tissues, thus playing an important role in peripheral tolerance [8–11].
Following the successes with immune checkpoint inhibitors in solid tumors, these therapies are being evaluated in hematologic malignancies, including acute myelogenous leukemia (AML) and myelodysplastic syndromes (MDS) [12]. In this context, leukemia may be viewed as a prototype for immune responsive tumors. Leukemias’ were one of the first tumor types to be successfully treated with immunotherapy approaches as proven by the success of allogeneic stem cell transplantation (ASCT). In contrast to solid tumor malignancies, leukemic cells express several checkpoint inhibitor receptors as well as ligands making them potential direct targets for these therapies. For example, there is frequent expression of PD-L1 and PD-L2 ligands on various hematopoietic cells - activated and non-activated T-cells, B-cells and NK-cells. Similarly, markers typically associated with antigen presenting cells, such as CD80 and CD86, are commonly overexpressed in leukemias owing to a common lineage shared by leukemia cells and APCs [13–17]. Additionally, another immune tolerance mechanism specific to leukemias appears to be the selective depletion of leukemia-derived antigen specific T cells as a product of their interaction with immature host dendritic cells which cross present these antigens leading to unfavorable T cell activation and abortion of proliferation [18, 19].
T-cells in AML/MDS
The presence of a functional T cell population is crucial to the successful application of immune checkpoint based therapies for the treatment of malignancies. It has been demonstrated that, contrary to popular belief, T cell populations are preserved and may even be increased with CD4+CD8+ T cells, T regulatory cells, and CD4+ naïve and memory cell population distributions within normal ranges, both in the peripheral blood and bone marrow, in patients with AML [20, 21][28]. Wendelbo et al demonstrated that even in AML patients with chemotherapy induced leukopenia most circulating leukocytes in the AML patients were T lymphocytes, whereas B lymphocytes and monocytes usually constituted < 10% [30]. T cells in AML patients’ exhibit differences from T cells in healthy human controls, with gene expression profiling studies on T cells from patients with AML demonstrating aberrant T cell activation [22]. Similarly, in comparison to healthy individuals, flow cytometry immunophenotyping of peripheral blood lymphocytes in patients with AML showed expansion of the activated T-cell compartment, the pathophysiologic significance of which remains unclear [23].
Proliferation assays have not revealed any functional defects in the T cells of patients with AML with respect to proliferation or cytokine production, either at diagnosis or relapse [24]. Functional characterization further demonstrated that, while antiCD3 stimulated proliferation was significantly lower for peripheral blood T cells in AML patients receiving chemotherapy compared with healthy donors, proliferation in response to anti-CD3 + anti-CD28 was similar for AML patients on chemotherapy and healthy donor [25]. The authors concluded that peripheral blood T-cells in AML patients on chemotherapy had an increased responsiveness in the presence of optimal co-stimulation that could compensate for quantitative T-cell defects. Lamble et al demonstrated that in newly diagnosed AML patients the peripheral T-cells were able to proliferate and produce IFN-gamma in the presence of CD3 stimulation alone whereas the bone marrow T cells were only able to proliferate and produce IFN-gamma when PD-1 was concomitantly blocked [26]. This suggests the presence of co-inhibitory receptor mediated immune suppression in the AML bone marrow microenvironment that may be abrogated by checkpoint inhibition. In keeping with these findings, immune landscaping studies on larger number of patients have demonstrated overexpression of negative regulatory immune checkpoint PD-1 on T cells in bone marrow aspirates from patients with AML [21]. Expression of PD-1 on T cells increased significantly at the time of relapse, especially in post ASCT relapse [24].
These data have led to the conceptualization and initiation of several clinical trials evaluating checkpoint inhibitors in AML and MDS in varied clinical settings. Preliminary data from the ongoing trials demonstrates encouraging treatment efficacy and tolerability. In this review, we discuss the clinical and correlative data emerging from recently completed or ongoing clinical trials with checkpoint inhibitor based approaches and the future applications of these agents in the therapy of AML/MDS.
Immune check point therapies in Hematologic malignancies
Clinical trials with antibodies targeting immune check point pathways have demonstrated marked efficacy against a variety of solid tumors [5, 27, 28]. Following their therapeutic successes in solid tumor malignancies, particularly melanoma, the concept of immune check point therapy blockade has been adapted to hematological malignancies. One of the major successes of immune check point inhibitors has been their application in the treatment of Hodgkin’s lymphoma. Pathophysiologic features encountered in HL including the high frequency of chromosome 9 abnormalities (PD-L1 and PD-L2 gene loci) [29], dense immune infiltrates with Reed-Stenberg cells in the tumor microenvironment, and upregulation of PD-L1 and PD-L2 ligands in tumor cells by Epstein-Barr virus [30] infection have been cited as reasons for the success of immune check point inhibitor blockade in this entity. A phase I trial involving 23 HL heavily pre-treated patients, who were administered an anti-PD1 antibody nivolumab, reported high objective response rates of 87%, including 17% complete responses (CR) and 70% partial responses (PR) [31]. Comparable results were reported with another anti-PD-1 antibody pembrolizumab[32]. Similarly, immune checkpoint blockade has also been tested in non-Hodgkin’s Lymphoma, with favorable results in the setting of follicular lymphoma (FL) [33], with response rates of 66% (19/29 patients) including CR in 52% (15/29)and PR in 14% (4/29)_in a phase II trial evaluating rituximab and pidilizumab in relapse refractory follicular lymphoma [34]. Encouraging data has recently emerged with the use of PD-1 inhibitors in the high risk setting of diffuse large B-cell lymphoma (DLBCL) following auto-SCT with response rates of 51% and 16 month OS probability of 82% after pidilizumab treatment[35] While the results with single agent immune check point blockade therapy in multiple myeloma have been disappointing with 0% objective response rates among 27 patients treated with single agent nivolumab in R/R multiple myeloma in a phase Ib study [36], more recent data from trials evaluating these agents in combination with other standard anti-multiple myeloma agents (especially IMiDs) have demonstrated synergy with significantly higher objective response rates that would have been attained without the PD1 agents in patients with relapsed/refractory multiple myeloma[37]. Phase 3 clinical trials evaluating PD-1 in combination with standard therapy for MM are ongoing.
SINGLE AGENT IMMUNE CHECKPOINT THERAPY APPROACHES
PD-1/PDL-1 inhibition in AML/MDS
The PD-1/PD-L1 pathway plays a role in immune evasion and cytotoxic T-cell exhaustion in hematologic malignancies including AML/MDS, and may be associated with progression of these diseases [38–43]. Zhang et al. noted that elevated PD-1/PD-L1 expression significantly blunted the anti-leukemic effects of CD8+ cytotoxic T-lymphocytes (CTLs) in murine AML models [38]. Anti-PD1 and/or anti-PD-L1 antibodies prevented CD8+ ‘exhaustion’, resulting in decreased AML burden in the blood and other organs, and increased murine survival. Zhou et al. demonstrated a unique phenotype i.e., co-expression of Tim-3 and PD-1 on CD8+ T cells, increased during AML progression and was associated with CD8+ exhaustion, similar to what has previously been described in melanoma patients [39, 44]. Combined PD-1/PD-L1 and Tim-3/galectin-9 blockade could reduce murine AML tumor burdens by preventing CD8+ CTL exhaustion. Chen et al found increased expression of PD-L1 at AML progression, which was an independent negative prognostic factor for French-American-British type M5 AML [43]. Additionally, PD-1 positive T-cells were shown to be significantly increased in the bone marrow aspirates of patients with relapsed AML as compared to healthy adult donors, with some groups describing the most significant increase in PD-1 expression on T-cells at relapse after ASCT [21, 24] In the presence of activated T cells, leukemic or stromal cells upregulate PD-L1/L2, resulting in the suppression of Thelper and promotion of Treg immune cell response, processes that may be reversed by anti-PD-1/PD-L1 therapy [45, 46].
Pidilizumab (MDV9300, formerly CT-011) is a humanized monoclonal IgG1 antibody to PD-1 [47]. This antibody was investigated in a phase I study in patients with relapsed/refractory solid and hematologic malignancies, including AML (8 patients) and MDS (1 patient) [33]. Results were clinically modest among the AML/MDS patients with a response in the form of a decrease in bone marrow blast percentage from 50% to 5% seen in one patient with AML. The patient eventually progressed 61 weeks after initiating pidilizumab therapy. Pidilizumab is currently undergoing investigation in a phase II study in combination with a dendritic cell vaccine in AML patients in complete remission (CR) (NCT01096602). Other relevant PD-1/PD-L1 antibodies under investigation in AML/MDS include nivolumab (anti-PD-1 monoclonal antibody), pembrolizumab (anti PD-1), durvalumab (anti PD-L1) and atezolizumab (anti PD-L1) (Table 1).
TABLE 1.
Interim results from ongoing Phase I/II studies of Immune checkpoint blockade in AML and MDS
| Reference | Agent | Immune check point pathway | Study design | Trial regimen | Study population (N) | ORRs (%) | 8-week mortality | Overall survival | Comments |
|---|---|---|---|---|---|---|---|---|---|
| [25] | Pidilizumab | PD-1/PD-L1 | Phase I | Single arm Monotherapy | AML, N = 8; MDS, N = 1 | 13%, CR in 1 AML patient | NR | NR | Clinically modest benefit as single agent. Safe and well tolerated at 0.2–6mg/kg dose. |
| [36] | Nivolumab | PD-1 | Phase 1/2 | Combined with azacitidine in R/R AML | AML, N=53 | 34% (CR/CRi=11, HI=7) | 8% | At 6 months follow up, OS was not reached in patients with CR, and 9.7 months in patients who had HI | Safe and tolerable. Durable CR rates, encouraging median OS in first salvage of 9.3 months. Grade 2–4 immune adverse events in 28% of patients and steroid responsive in all but 1 case. |
| [67] | Nivolumab | PD-1 | Phase 2 | Combined with azacitidine in frontline MDS | MDS, N=17 | 80% (CR=6, HI+mCR=6, HI=1) | NR | NR | Impressive response rates in the frontline setting. Immune mediated toxicities manageable with steroids |
| [67] | Nivolumab | PD-1 | Monotherapy in R/R MDS | MDS, N=15 | 0% | No benefit of single agent nivolumab in MDS with prior HMA failure | |||
| [67] | Ipilimumab | CTLA4 | Monotherapy in R/R MDS | MDS, N=16 | 30% (CR=1, mCR=2, HI=2) | Single-agent Ipilimumab is capable of inducing responses in previously treated MDS patients. | |||
| [48] | Pembrolizumab | PD-1/PD-L1 | Phase 1b | Single-arm in R/R intermediate ½ or high risk MDS after HMA failure (4 cycles) | N =28 | 15% (4 of 27 evaluable patients; 1 PR and 3 with mCR) | 0% | 49% at 6 months | Manageable safety profile and potential activity in patients with MDS after HMA failure. |
| [57] | Ipilimumab | CTLA4 | Phase 1 | Single arm in R/R MDS after HMA failure | N=11 | 0% | 0% | Median OS 12 months | 5 patients had stable disease, 4 of 5 had durable response>6 months. 3 patients underwent transplant post-ipilimumab and did not experience additional toxicities suggesting feasibility. Treatment with 3mg/kg is well tolerated and effective in disease stabilization. Immune mediated reactions responsive to steroids |
| [74] | Ipilimumab | CTLA4 | Phase I | Single arm in R/R AML after ASCT | N=12 | 42% | NR | With median follow up of 15 months, 12 month OS was 49% | Ipilimumab effective in post-transplant relapse setting. Response rates are higher in extramedullary AML. Effective dose is 10 mg/kg |
Abbreviations used: AML-acute myelogenous leukemia, MDS-myelodysplasia, CR-complete remission, NR-not reported, HMA-hypomethylating agent, ASCT-allogeneic stem cell transplant, OS-overall survival, HI-hematological improvement, N-number of patients. PR-partial remission, mCR-marrow complete response. Pts-patients
Pembrolizumab (MK-3475) is a humanized monoclonal that blocks the interaction between PD-1 and its PD-L1 ligand. Results from the MDS cohort in a phase Ib study of pembrolizumab in patients with hematologic malignancies (NCT01953692) were recently presented at the American Society of Hematology meeting, San Diego, December 2016 [48]. This study included 28 patients with intermediate 1/2- or high-risk MDS and failure to respond after at least 4 cycles of front-line therapy with a hypomethylating agent (HMA). 27 patients were evaluable for efficacy using the IWG 2006 response criteria for MDS[49]. Best overall responses included partial response (PR) in 1, marrow CR in 3 (11%), stable disease in 14 (52%), and progressive disease in 9 (33%) patients. Additionally, hematologic improvement (HI) was seen in 3 (11%) patients. Grade 3 and 4 treatment-related adverse events (AEs) occurred in 2 patients (7%), including grade 3 gastroenteritis and grade 4 tumor lysis syndrome in 1 patient, each. There were no treatment-related deaths. The median overall survival (OS) for all patients was 23 weeks and 4 of the 27 (15%) patients are alive > 2 years.
CTLA-4 blockade
The CTLA-4 on T cells competes with CD28 for binding to CD80 and CD86, two ligands expressed on APCs, and down-regulates T-cell receptor activation [4, 50–53]. Saudemont et al demonstrated that overexpression of B7-H1 (PD-L1) or B7.1 (CD80) may contribute to escape of leukemia cells from antitumor immunity and promote long-term persistence of disease in AML murine models [54]. Blocking B7-H1 or B7.1/CTLA4 enhanced CTL killing of such long-term persistent cells and prolonged survival of mice injected with the persistent leukemia cells. Laurent et al demonstrated that CTLA-4 is constitutively expressed on the surface of leukemic blasts in AML patients at diagnosis. The expression of CTLA-4 on AML blasts was similar in untreated and chemo-resistant AML marrow samples. CTLA-4 blockade was able to induce killing of leukemic cells even in cases resistant to traditional antileukemic chemotherapy [55].
Ipilimumab is a human IgG1 isotype monoclonal antibody that binds CTLA-4, blocking the inhibitory signal, thereby allowing cytotoxic T cell mediated antitumor immune response [56]. Two studies are actively investigating the role of single agent ipilimumab in R/R MDS (NCT02530463, NCT01757639). In a phase I study, ipilimumab showed disease stabilization in 45% (5 out of 11) patients with high-risk MDS. Four of the 5 patients had stable disease lasting >6 months [57]. Grade ≥ 3 immune related adverse events were noted in 7 patients, and were rapidly responsive to steroids.
In order to improve the response rate and the durability of response in patients with AML/MDS treated with checkpoint inhibitors, combinations of these agents with standard anti-leukemic therapy are needed. The most widely applied strategy exploits the ability of epigenetic therapies to modulate immune checkpoint expression on tumor infiltrating lymphocytes and leukemic cells [58]. Azacitidine is an epigenetic drug approved by FDA for the treatment of MDS and frequently used in the treatment of AML in older patients [59–61]. Azacitidine has a number of favorable effects on anti-tumor immune regulation including up-regulation and sensitization to tumor antigens [including MAGE-1, cancer testes antigen (CTA), NY-ESO] [62–64], increased expression or restoration of HLA class-1 expression in tumors [65, 66], and up-regulation of co-stimulatory molecules [65]. However, in addition to the favorable impact on anti-tumor immunity, azacitidine up-regulates inhibitory checkpoints PD1/PDL1.
Garcia-Manero et al reported preliminary results from a phase II study (NCT02530463) that evaluated multiple cohorts including nivolumab alone (cohort 1), ipilimumab alone (cohort 2), nivolumab + ipilimumab (cohort 3) in MDS patients who had failed first-line therapy with a HMA agent and combination cohorts of azacitidine with nivolumab (cohort 4), azacitidine with ipilimumab (cohort 5), and azacitidine with nivolumab + ipilimumab (cohort 6) in previously untreated intermediate 2/high-risk MDS [67] Efficacy data from cohorts 1, 2, and 4 were reported at ASH 2016. Intriguingly, single agent ipilimumab showed an overall response rate (ORR) of 30% (5 of 16 including CR in 1, mCR in 2, HI in 2) in intermediate/high-risk MDS patients failing HMA, 9 patients had no response and 2 progressed on therapy with ipilimumab. Three of the 16 (18%) patients had drug related ≥grade 3 non-hematologic toxicity, including one episode each of acute kidney injury, maculo-papular rash, and generalized muscle weakness. One patient was taken off study due to side effects from ipilimumab [67]. Notably, the same study included 15 intermediate/high-risk MDS patients failing HMA who received single agent nivolumab in a parallel cohort with no responses noted..
IMMUNE CHECKPOINT BASED COMBINATION APPROACHES IN AML/MDS
Combinations of immune checkpoints with hypomethylating agents in AML
The immunomodulatory priming effect for subsequent response to PD-L1 blockade after treatment with azacitidine was first described by Wrangle et al when they noticed high response rates in lung cancer patients who had failed on a clinical trial with azacitidine and then happened to be up enrolled on a subsequent trial with a PD-1/PD-L1 inhibitor [58]. Similarly, azacitidine up-regulated PD-1, PD-L1, and PD-L2 (>/= 2-fold) in >50% of 61 evaluable patients with MDS/AML during their first course of therapy [68]. There was a trend toward increased expression of all three genes in azacitidine resistant patients compared with sensitive patients, suggesting up-regulation of inhibitory immune checkpoints as a potential mechanism of resistance to azacitidine and that concomitant inhibition of the PD-1/PD-L1 axis may be a potential mechanism to prevent or overcome resistance to azacitidine [68]. A number of trials combining epigenetic agents with PD1/PDL1 based therapies have recently started enrollment for AML and/or MDS including azacitidine with PD1 inhibitor nivolumab (NCT02397720), azacitidine with or without PDL1 Inhibitor durvalumab (NCT02775903), azacitidine with PDL1 inhibitor atezolizumab (NCT02508870).
The combination of nivolumab (Opdivo, BMS-936558, Bristol-Myers Squibb) and azacitidine is currently being evaluated in a phase 1/2 trial (NCT02397720). Preliminary results reported by Daver et al (ASH, 2016) are encouraging [69]. Among 53 relapsed refractory (R/R) AML patients, median age 68 years, poor risk cytogenetics (43%), median 2 (range, 1–7) prior therapies the combination of azacitidine and nivolumab produced an overall response rate (ORR) of 34%, including 11 complete remissions/complete remission with insufficient count recovery (CR/CRi) (21%) and 7 hematologic improvements (HI) including three HI patients who had concomitant >50% blast reduction (14%). The 8-week mortality was 8%. This compares favorably to a historic response rate of 15–20% in a similar patient population treated at the same institution with single-agent HMA (azacitidine or decitabine) therapy. The response rates were significantly higher in patients with diploid cytogenetics. At a median follow up of 6 months, only one of 11 patients who achieved CR had lost response and two patients had died: one from relapse and another due to cardiac failure while in remission. Grade 2–4 immune adverse events (iAE) were observed in 15 (28%) patients with a time to onset of 4 days to 3.5 months. The iAEs profile differed from that seen in solid tumors with most common iAEs of pneumonitis, nephritis, colitis, and dermatitis as opposed to endocrine, skin, and liver iAEs that have been typically described in solid tumor trials. Patients who achieved CR/CRi had higher pre-therapy total CD3 and CD8+ T-cells infiltrate in the bone marrow aspirates. Responders demonstrated progressive increase in BM CD8+ and CD4+ T-cell infiltrate on longitudinally assessed bone marrow aspirates on therapy. Both responders and non-responders had an increase in CTLA4+ CD8+ cells in the bone marrow aspirates done longitudinally on therapy suggesting that CTLA4 up-regulation may drive resistance. The efficacy of combination blockade of these two major co-inhibitory pathways is being evaluated in ongoing clinical trials (ClinicalTrials.gov Identifier: NCT02397720). These associations were not seen when peripheral blood T-cells were evaluated suggesting that evaluation of the bone marrow was critical to identify T-cell and checkpoint expression related changes and biomarkers in AML/MDS trials.[69].
Combinations of immune checkpoints with HMAs in MDS
A phase II study is evaluating azacitidine in combination with nivolumab (N=17), azacitidine with ipilimumab (N=20), and azacitidine with nivolumab + ipilimumab (N=20) in frontline intermediate 2/high-risk MDS (NCT02530463). The azacitidine in combination with nivolumab was the first cohort to complete enrollment (N=17) and was reported at ASH 2016 [67]. The median number of treatment cycles was 4 (range, 2–11), and a response was noted in 13 of 17 (80%) patients (CR in 6, mCR + HI in 6, HI-P in 1). Three patients were too early to evaluate response and two patients have progressed. In contrast, as previously discussed, nivolumab as a single agent in intermediate/high risk MDS after HMA failure in the same protocol showed no response (0 of 15 responses) suggesting that similar to AML the combination of azacitidine + nivolumab may be the approach to pursue and build on [67]. Grade ≥ 3 toxicities included lung infections, elevated transaminases, and hyperglycemia in 2 patients, each and colitis, chest pain, hyponatremia, hypotension, and muscle weakness in 1 patient, each.
Other PD-L1 inhibitor therapies undergoing clinical investigation in combination with azacitidine include azacitidine with or without durvalumab (PD-L1 inhibitor). Patients are randomized on this study to receive azacitidine alone or azacyitidine with durvalumab in two independent cohorts: (1) frontline MDS, IPSS-R Intermediate – high risk and (2) frontline AML >/= 65 years of age who are not candidates for induction therapy (NCT02775903). Atezolizumab (PD-L1 inhibitor) is being evaluated in second line therapy in MDS as either single agent atezolizumab or azacitidine + atezolizumab in patients with MDS failing HMA therapy, and in frontline therapy with azacitidine + atezolizumab in untreated intermediate-high risk MDS (NCT02508870).
Similar to solid tumors combined blockade of major co-inhibitory pathways may result in improved responses, albeit with the caveat that toxicities may also be increased. Dual checkpoint blockade with CTLA-4 and PD-L1 inhibitors with azacitidine are currently undergoing investigation in patients with MDS and AML, including a phase II study of nivolumab and ipilimumab + azacitidine in frontline AML ≥65 years of age or salvage 1/2 AML (NCT02397720), a phase II study of nivolumab and ipilimumab + azacitidine in frontline and R/R MDS (NCT02530463), a phase I study evaluating durvalumab + azacitidine +/− tremelimumab (IgG2 CTLA4 inhibitor) in R/R MDS (NCT02117219). Data from these studies is not yet available.
Combinations with cytotoxic chemotherapy
In vivo experiments in mouse models have shown that injection of cytosine arabinoside induced expression of CD80 and CD86 and reduced expression of PD-1 in leukemic cells, making them more susceptible to CTL mediated killing [70]. Furthermore, the release of antigens (including potential neoantigens) post-cytotoxic chemotherapy may help prime the CTLs enhancing anti-tumor efficacy. A phase II study evaluating nivolumab in combination with idarubicin and cytarabine induction therapy for patients <60 years of age with newly diagnosed AML is exploring this concept (NCT02464657).
Immune checkpoint inhibitors in the transplant setting
In a phase I trial in hematologic malignancies reported by Berger et al 4 of 17 patients treated with pidilizumab had prior ASCT [33], including one patient with AML treated with pidilizumab 8 weeks following transplant, who subsequently experienced grade 4 gastrointestinal GVHD, and died of persistent AML and GVHD. Per the investigators it was not entirely clear whether the gastrointestinal GVHD was spontaneous or attributable to pidilizumab. Although post-ASCT enhancement of immune surveillance by application of immune checkpoint inhibitors appears to be an attractive area for investigation, a pertinent concern with the use of these agents in the post-ASCT setting is the risk of inciting GVHD due to a non-antigen-specific T cell stimulation [71].
Murine models have shown that while PD-1 blockade may lead to increased GVHD [72], CTLA-4 blockade had a more selective graft versus tumor effect [73]. Davids et al demonstrated ipilimumab to be efficacious as a single agent in treating post-ASCT AML relapses [74]. In the study, twenty-eight patients were treated, including 12 with relapsed AML (3 of these had leukemia cutis). All patients were >3 months post-ASCT, median time post-ASCT was 19.3 months, all were off immunosuppression, and had no history of grade 3/4 GVHD. The patients received ipilimumab at dose of 3 (n=6) or 10 (n=22) mg/kg every 3 weeks. Complete responses were observed in 5 (42%) patients, including 3 leukemia cutis patients. The 5 responders had received a median 3 prior salvages (range, 1–14) prior to ASCT. Four of the 5 responses were durable, lasting for more than a year. All responses were seen at the 10mg/kg dose. All 5 responders had >99% donor T-cells suggesting donor T-cells could be activated to mount anti-leukemia responses. The median number of cycles to response was 4 (range, 1–8). Immune-related adverse events were noticed in 6 (21%) patients, including 1 death and 4 GVHD flares (chronic GVHD of liver, n=3 and acute GVHD of gut, n=1) precluding further administration of ipilimumab. Intriguingly, responses were particularly high in patients who relapsed with extramedullary AML. This may be related to the fact that the systemic presence of AML cells depletes leukemia antigen specific T cells, thereby promoting peripheral T cell tolerance [18].
PD-1 inhibition has also been shown to be safe and effective post-SCT in patients with other malignancies such as hodgkins lymphoma (HL)[75]. A phase I trial involving ipilimumab or nivolumab in treating patients with AML who relapse post-transplant is currently recruiting patients (NCT01822509). Overall, it may be that immune check point inhibitors prove to be active at treating MRD and relapses post-ASCT, but the risks of inciting GVHD flares must be carefully considered and patients monitored closely by experienced leukemia and stem cell physicians for early signs of GVHD flare or irAEs.
Immune checkpoint inhibitors and minimal residual disease
Another area of active investigation in therapy of AML/MDS includes approaches to eradicate minimal residual disease setting. Pre-clinical data suggests that immune checkpoint pathways may contribute to tumor dormancy or long-term persistence of disease in AML [54]. Vaccination with leukemic cells transduced with CD154, IL2 vaccine led to the generation of tumor specific CD8 T cells, with improved survival in mouse models [76]. Saudemont et al demonstrated that long-term murine survivors continued to harbor minimal residual disease, and that the tumor cells became increasingly insensitive to CTL-mediated lysis over time, which was associated with overexpression of PD-L1 and CTLA-4 [54]. Anti-CTLA-4 and Anti-PD-L1 blockade was effective in increasing effector cytokine production, and enhancing CTL mediated killing of the residual tumor cells. A phase II trial is evaluating single agent nivolumab as maintenance in high risk AML in complete remission (NCT02532231), and another phase II study is assessing its efficacy in eliminating MRD in MRD-positive AML in complete remission (NCT02275533).
Other potential targetable immune checkpoint pathways
Beyond PD-1 and CTLA-4, clinically exploitable co-inhibitory receptors include lymphocyte activation gene-3 (LAG-3) and T cell immunoglobulin domain and mucin domain 3(TIM-3). LAG-3 is a CD4-like molecule expressed on NK cells, γδ T cells, Treg cells, and activated αβ T cells [77]. LAG-3 engages MHC-II on APCs and inhibits their ability to elicit effector T cell responses [78]. Signaling through the PD-1 and LAG-3 pathways has distinct functional consequences, and dual pathway inhibition may prove synergistic [78, 79]. LAG-3 blockade is yet to be clinically evaluated in AML and MDS [80]. TIM-3 is a co-inhibitory receptor that negatively regulates T cell function by binding to Galectin-9 on APCs [81]. The combined expression of Tim-3 and PD-1 identified a CD8+ T-cell exhaustion phenotype in mice with disseminated AML [39]. Zhou et al demonstrated marked survival benefit in mice with AML following combined TIM-3 and PD-1 blockade. A clinical trial to evaluate TIM-3 blockade and dual TIM-3 and PD-1 blockade in AML/MDS will open to enrollment in the near future (Clinicaltrials.gov NCT03066648).
Converse to blocking co-inhibitory checkpoint pathways, activation of co-stimulatory pathways such as OX40 and ICOS represents a rational strategy to effectuate anti-tumor cytotoxicity and may prove synergistic when administered in combination with checkpoint inhibitors. OX40 (CD134), 4-1BB (CD137) and ICOS are co-stimulatory molecules that are up-regulated on APCs, B-cells, macrophages, and T-cells following their activation, and play a major role in the functional maturation of T-cells [82–84]. Studies evaluating the immune checkpoint landscape have shown OX40 and ICOS to be overexpressed in bone marrow aspirates of patients with AML, especially in relapsed AML [21]. Agonist antibodies targeting these stimulatory checkpoint receptors are currently undergoing evaluation in patients with advanced solid malignancies or lymphomas as single agents or in combinations [NCT02554812, NCT02559024, NCT02315066, and NCT02520791]. Clinical trials to evaluate these agonist antibodies in AML and MDS are being planned.
In conclusion, it is becoming increasingly clear that immune evasion mechanisms are active in patients with AML and MDS. Furthermore, T-cells expressing a variety of immune checkpoints are present in the tumor microenvironment (bone marrow) in AML/MDS, paving a rationale for combination immune checkpoint therapies in these diseases. It appears that a heavy systemic tumor burden may abrogate responses to immune checkpoint monotherapy, suggesting the need to combine these agents with standard cytotoxic, epigenetic, and targeted approaches when attempting to treat patients with active disease. Preliminary data emerging from clinical trials are promising, particularly for the combination of checkpoint inhibitors and epigenetic agents in AML/MDS [85]. These agents may have a role in a wide range of therapeutic settings, including as frontline therapy in older AML or frontline high-risk MDS, as salvage therapies in patients who are chemo-resistant and lack targetable molecular mutations, as maintenance therapy in the post remission phase of AML, and to eliminate MRD or treat frank post-transplant relapses. A crucial factor that must be taken into consideration while applying such immune therapies is the optimal timing of therapy, both to improve efficacy and to reduce the incidence of immune related side effects. Biomarkers of response are being identified and are extremely important to allow us to select patients with highest likelihood of benefit from immune checkpoint based therapies. Exploiting the true potential of these agents and their specific role in AML/MDS requires rationally designed clinical trials with robust correlative assays. A number of such trials are ongoing and will guide further development of these agents.
Supplementary Material
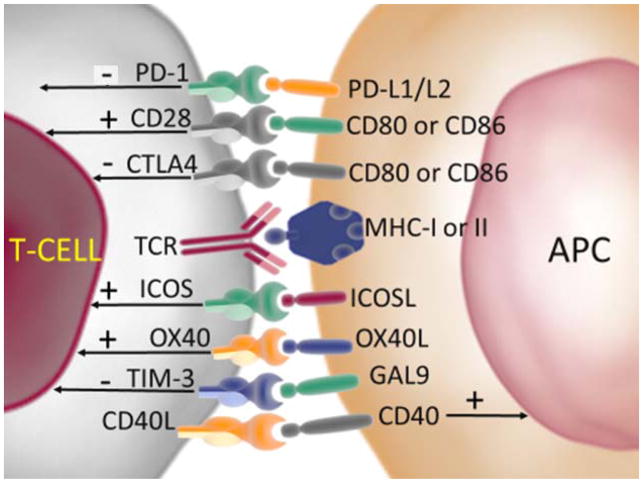
Figure 1.
Immunological pathways of check point inhibitors and stimulators
Antigen peptide presentation on MHC leads to MHC/TCR engagement leading to T cell activation [1]. T cell activation is modulated by several pathways involving co-stimulatory and co-inhibitory signals. Co-inhibitory signals include CTLA4, PD-1/PD-L1, and TIM-3. Co stimulatory signals are mediated by CD28, ICOS, and OX40 among others on the T cell. T-cell indicates cytotoxic T lymphocyte, APC indicates antigen presenting cell, TCR indicates T cell receptor, MHC indicates major histocompatibility complex.
Figure 2.
Current PD-1/PD-L1 and CTLA4 check point inhibitors in AML and MDS
CTLA-4 indicates cytotoxic T- lymphocyte-associated-protein 4; MHC indicates major histocompatibility complex; PD-1/PD-L1/2, programmed cell-death protein 1 receptor/ligand; T-cell indicates cytotoxic T lymphocytes
TABLE 2.
Ongoing trials of Immune checkpoint blockade in AML and MDS
| Immune check point inhibitor | Mechanism of action | Study population | Therapy regimen | Primary objectives | Phase of study and | Clinical trials gov identifier |
|---|---|---|---|---|---|---|
| Ipilimumab | CTLA4 | R/R AML, High Risk MDS | Ipilimumab | Toxicity, | 1, ongoing | NCT01757639 |
| Ipilimumab or Nivolumab | CTLA4/PD-1 | R/R AML after ASCT >18 yrs | Ipilimumab/Nivolumab | Toxicity, Maximum tolerated dose | 1/1b, recruiting | NCT01822509 |
| Ipilimumab and Nivolumab | CTLA4 and PD-1 | R/R MDS, after hypomethylating agent failure | Ipilimumab and Nivolumab | Efficacy, ORR | 2, ongoing | NCT02530463 |
| Ipilimumab | CTLA4 | Ipilimumab | ||||
| Nivolumab | PD-1 | Nivolumab | ||||
| Ipilimumab and Nivolumab | CTLA4 and PD-1 | Frontline MDS | Ipilimumab and Nivolumab + azacitidine | |||
| Ipilimumab | CTLA4 | Ipilimumab + azacitidine | ||||
| Nivolumab | PD-1 | Nivolumab + azacitidine | ||||
| Pidilizumab | PD-1 | AML in CR >18 yrs | Pidilizumab + DC vaccine | Toxicity | 2, ongoing | NCT01096602 |
| Nivolumab | PD-1 | R/R AML >18 yrs, denovo AML > 65 years | Nivolumab + AZA | Maximum tolerated dose | 2, recruiting | NCT02397720 |
| Nivolumab | PD-1 | Denovo high risk AML >18 yrs | Nivolumab + Ida/cytarabine | Maximum tolerated dose | 2, recruiting | NCT02464657 |
| Nivolumab | PD-1 | AML in CR at high risk for relapse >18 yrs | Nivolumab | EFS | 2, recruiting | NCT02532231 |
| Nivolumab | PD-1 | AML in CR, MRD positive | Nivolumab | EFS, elimination of MRD | 2, recruiting | NCT02275533 |
| Nivolumab and Lirilumab | CTLA4 and anti-KIR | R/R MDS >18 yrs | Nivolumab and Lirilumab + azacitidine | Efficacy, ORR | 2, recruiting | NCT02599649 |
| Pembrolizumab | PD-1 | R/R MDS >18 yrs | Pembrolizumab | Toxicity, efficacy | 1, recruiting | NCT01953692 |
| Pembrolizumab | PD-1 | R/R AML >18 yrs | Pembrolizumab + HDAC | EFS | 2, recruiting | NCT02768792 |
| Pembrolizumab | PD-1 | Frontline AML, >65 years R/R AML | Pembrolizumab + azacitidine | Efficacy, ORR | 2, recruiting | NCT02845297 |
| Durvalumab | PD-L1 | MDS frontline, AML, frontline>65 years | Durvalumab + azacitidine | Efficacy, ORR | 2, recruiting | NCT02775903 |
| Durvalumab | PD-L1 | R/R MDS | Durvalumab + azacitidine +/− tremelimumab | Toxicity | 1, recruiting | NCT02117219 |
| Atezolizumab | PD-L1 | R/R MDS >18 yrs | Atezolizumab-HMA R/R MDS, Atezolizumab + azacitidine-HMA R/R MDS, atezolizumab + azacitidine-HMA naive | Adverse events | 1, recruiting | NCT02508870 |
Abbreviations used: R/R- relapsed refractory; ASCT-allogeneic stem cell transplant; MDS-myelodysplasia; AML-acute myelogenous leukemia; CR-complete remission; MRD-minimal residual disease; DC vaccine-dendritic cell vaccine; AZA-azacitidine; Ida-idarubicin; ORR-overall response rates; EFS-event free survival; yrs-years
Acknowledgments
Funding source: This manuscript was supported in part by the MD Anderson Cancer Centre Leukaemia Support Grant (CCSG) CA016672 and generous philanthropic contributions to the MD Anderson Moon Shots Program. This research is supported in part by the MD Anderson Cancer Center Leukemia SPORE CA100632 and by the Charif Souki Cancer Research Fund.
Footnotes
Conflicts of Interest Disclosure: The authors have no relevant conflicts of interest.
References
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8(6):467–77. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13(4):227–42. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 5.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Day SJ, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21(8):1712–7. doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 7.Sui X, et al. The anticancer immune response of anti-PD-1/PD-L1 and the genetic determinants of response to anti-PD-1/PD-L1 antibodies in cancer patients. Oncotarget. 2015;6(23):19393–404. doi: 10.18632/oncotarget.5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishimura H, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291(5502):319–22. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 9.Nishimura H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141–51. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 10.Freeman GJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yokosuka T, et al. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med. 2012;209(6):1201–17. doi: 10.1084/jem.20112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alatrash G, Daver N, Mittendorf EA. Targeting Immune Checkpoints in Hematologic Malignancies. Pharmacol Rev. 2016;68(4):1014–1025. doi: 10.1124/pr.116.012682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costello RT, et al. Regulation of CD80/B7-1 and CD86/B7-2 molecule expression in human primary acute myeloid leukemia and their role in allogenic immune recognition. Eur J Immunol. 1998;28(1):90–103. doi: 10.1002/(SICI)1521-4141(199801)28:01<90::AID-IMMU90>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Re F, et al. Expression of CD86 in acute myelogenous leukemia is a marker of dendritic/monocytic lineage. Exp Hematol. 2002;30(2):126–34. doi: 10.1016/s0301-472x(01)00768-8. [DOI] [PubMed] [Google Scholar]
- 15.Vollmer M, et al. Expression of human leucocyte antigens and co-stimulatory molecules on blasts of patients with acute myeloid leukaemia. Br J Haematol. 2003;120(6):1000–8. doi: 10.1046/j.1365-2141.2003.04212.x. [DOI] [PubMed] [Google Scholar]
- 16.Whiteway A, et al. Expression of co-stimulatory molecules on acute myeloid leukaemia blasts may effect duration of first remission. Br J Haematol. 2003;120(3):442–51. doi: 10.1046/j.1365-2141.2003.04085.x. [DOI] [PubMed] [Google Scholar]
- 17.Graf M, et al. High expression of costimulatory molecules correlates with low relapse-free survival probability in acute myeloid leukemia (AML) Ann Hematol. 2005;84(5):287–97. doi: 10.1007/s00277-004-0978-0. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L, et al. CD40 ligation reverses T cell tolerance in acute myeloid leukemia. J Clin Invest. 2013;123(5):1999–2010. doi: 10.1172/JCI63980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teague RM, Kline J. Immune evasion in acute myeloid leukemia: current concepts and future directions. J Immunother Cancer. 2013;1(13) doi: 10.1186/2051-1426-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vidriales MB, et al. Lymphoid subsets in acute myeloid leukemias: increased number of cells with NK phenotype and normal T-cell distribution. Ann Hematol. 1993;67(5):217–22. doi: 10.1007/BF01715050. [DOI] [PubMed] [Google Scholar]
- 21.Naval Daver SB, Garcia-Manero Guillermo, Cortes Jorge E, Ravandi Farhad, Ning Jing, et al. Defining the Immune Checkpoint Landscape in Patients (pts) with Acute Myeloid Leukemia. ASH Session. 2016;617 [Google Scholar]
- 22.Le Dieu R, et al. Peripheral blood T cells in acute myeloid leukemia (AML) patients at diagnosis have abnormal phenotype and genotype and form defective immune synapses with AML blasts. Blood. 2009;114(18):3909–16. doi: 10.1182/blood-2009-02-206946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van den Hove LE, et al. Peripheral blood lymphocyte subset shifts in patients with untreated hematological tumors: evidence for systemic activation of the T cell compartment. Leuk Res. 1998;22(2):175–84. doi: 10.1016/s0145-2126(97)00152-5. [DOI] [PubMed] [Google Scholar]
- 24.Schnorfeil FM, et al. T cells are functionally not impaired in AML: increased PD-1 expression is only seen at time of relapse and correlates with a shift towards the memory T cell compartment. J Hematol Oncol. 2015;8:93. doi: 10.1186/s13045-015-0189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wendelbo O, et al. Functional characterization of T lymphocytes derived from patients with acute myelogenous leukemia and chemotherapy-induced leukopenia. Cancer Immunol Immunother. 2004;53(8):740–7. doi: 10.1007/s00262-004-0505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adam Lamble YK, Huang Fei, Sasser Kate, Adams Homer, Tognon Cristina, et al. Mass Cytometry As a Modality to Identify Candidates for Immune Checkpoint Inhibitor Therapy within Acute Myeloid Leukemia. ASH abstract#2829. 2016 [Google Scholar]
- 27.Page DB, et al. Immune modulation in cancer with antibodies. Annu Rev Med. 2014;65:185–202. doi: 10.1146/annurev-med-092012-112807. [DOI] [PubMed] [Google Scholar]
- 28.Robert C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 29.Green MR, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116(17):3268–77. doi: 10.1182/blood-2010-05-282780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Green MR, et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res. 2012;18(6):1611–8. doi: 10.1158/1078-0432.CCR-11-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ansell SM, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–9. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moskowitz C, et al. PD-1 Blockade with the Monoclonal Antibody Pembrolizumab (MK-3475) in Patients with Classical Hodgkin Lymphoma after Brentuximab Vedotin Failure: Preliminary Results from a Phase 1b Study (KEYNOTE-013). 56th ASH Annual Meeting and Exposition; 2014; San Franciso, CA. [Google Scholar]
- 33.Berger R, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14(10):3044–51. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 34.Westin JR, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol. 2014;15(1):69–77. doi: 10.1016/S1470-2045(13)70551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Armand P, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol. 2013;31(33):4199–206. doi: 10.1200/JCO.2012.48.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lesokhin AM, et al. Nivolumab in Patients With Relapsed or Refractory Hematologic Malignancy: Preliminary Results of a Phase Ib Study. J Clin Oncol. 2016;34(23):2698–704. doi: 10.1200/JCO.2015.65.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.San Miguel J, et al. Pembrolizumab in Combination with Lenalidomide and Low-Dose Dexamethasone for Relapsed/Refractory Multiple Myeloma (RRMM): Keynote-023. American Society of Hematology Annual Meeting; 2015. [Google Scholar]
- 38.Zhang L, Gajewski TF, Kline J. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood. 2009;114(8):1545–52. doi: 10.1182/blood-2009-03-206672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Q, et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117(17):4501–10. doi: 10.1182/blood-2010-10-310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daver N, et al. Defining the Immune Checkpoint Landscape in Patients (pts) with Acute Myeloid Leukemia (AML) Blood. 2016;128(22) [Google Scholar]
- 41.Mumprecht S, et al. Programmed death 1 signaling on chronic myeloid leukemia-specific T cells results in T-cell exhaustion and disease progression. Blood. 2009;114(8):1528–36. doi: 10.1182/blood-2008-09-179697. [DOI] [PubMed] [Google Scholar]
- 42.Shi L, et al. The role of PD-1 and PD-L1 in T-cell immune suppression in patients with hematological malignancies. J Hematol Oncol. 2013;6(1):74. doi: 10.1186/1756-8722-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen X, et al. Clinical significance of B7-H1 (PD-L1) expression in human acute leukemia. Cancer Biol Ther. 2008;7(5):622–7. doi: 10.4161/cbt.7.5.5689. [DOI] [PubMed] [Google Scholar]
- 44.Fourcade J, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207(10):2175–86. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou Q, et al. Program death-1 signaling and regulatory T cells collaborate to resist the function of adoptively transferred cytotoxic T lymphocytes in advanced acute myeloid leukemia. Blood. 2010;116(14):2484–93. doi: 10.1182/blood-2010-03-275446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dolen Y, Esendagli G. Myeloid leukemia cells with a B7-2(+) subpopulation provoke Th-cell responses and become immuno-suppressive through the modulation of B7 ligands. Eur J Immunol. 2013;43(3):747–57. doi: 10.1002/eji.201242814. [DOI] [PubMed] [Google Scholar]
- 47.Marketwired. U.S. FDA Lifts Partial Clinical Hold on Medivation’s Pidilizumab. Mar 9, 2016. [Google Scholar]
- 48.Guillermo Garcia-Manero MST, Martinelli Giovanni, Ribrag Vincent, Yang Hui, Balakumaran Arun, et al. Pembrolizumab, a PD-1 Inhibitor, in Patients with Myelodysplastic Syndrome (MDS) after Failure of Hypomethylating Agent Treatment. American Society of Hematology. 2016 Dec; abstract. [Google Scholar]
- 49.Cheson BD, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–25. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 50.Riley JL, et al. Modulation of TCR-induced transcriptional profiles by ligation of CD28, ICOS, and CTLA-4 receptors. Proc Natl Acad Sci U S A. 2002;99(18):11790–5. doi: 10.1073/pnas.162359999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Linsley PS, et al. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1(9):793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 52.Schneider H, et al. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313(5795):1972–5. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- 53.Egen JG, Allison JP. Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity. 2002;16(1):23–35. doi: 10.1016/s1074-7613(01)00259-x. [DOI] [PubMed] [Google Scholar]
- 54.Saudemont A, Quesnel B. In a model of tumor dormancy, long-term persistent leukemic cells have increased B7-H1 and B7.1 expression and resist CTL-mediated lysis. Blood. 2004;104(7):2124–33. doi: 10.1182/blood-2004-01-0064. [DOI] [PubMed] [Google Scholar]
- 55.Laurent S, et al. CTLA-4 expressed by chemoresistant, as well as untreated, myeloid leukaemia cells can be targeted with ligands to induce apoptosis. Br J Haematol. 2007;136(4):597–608. doi: 10.1111/j.1365-2141.2006.06472.x. [DOI] [PubMed] [Google Scholar]
- 56.Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med. 2012;366(26):2517–9. doi: 10.1056/NEJMe1205943. [DOI] [PubMed] [Google Scholar]
- 57.Zeidan AM, et al. Stabilization of Myelodysplastic Syndromes (MDS) Following Hypomethylating Agent (HMAs) Failure Using the Immune Checkpoint Inhibitor Ipilimumab: A Phase I Trial. Blood. 2015;126(23):1666–1666. [Google Scholar]
- 58.Wrangle J, et al. Alterations of immune response of Non-Small Cell Lung Cancer with Azacytidine. Oncotarget. 2013;4(11):2067–79. doi: 10.18632/oncotarget.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fenaux P, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10(3):223–32. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dombret H, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126(3):291–9. doi: 10.1182/blood-2015-01-621664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fenaux P, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28(4):562–9. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- 62.Guo H, et al. Disruption of EphA2 receptor tyrosine kinase leads to increased susceptibility to carcinogenesis in mouse skin. Cancer Res. 2006;66(14):7050–8. doi: 10.1158/0008-5472.CAN-06-0004. [DOI] [PubMed] [Google Scholar]
- 63.Srivastava P, et al. Induction of cancer testis antigen expression in circulating acute myeloid leukemia blasts following hypomethylating agent monotherapy. Oncotarget. 2016;7(11):12840–56. doi: 10.18632/oncotarget.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coral S, et al. 5-aza-2′-deoxycytidine-induced expression of functional cancer testis antigens in human renal cell carcinoma: immunotherapeutic implications. Clin Cancer Res. 2002;8(8):2690–5. [PubMed] [Google Scholar]
- 65.Coral S, et al. Prolonged upregulation of the expression of HLA class I antigens and costimulatory molecules on melanoma cells treated with 5-aza-2′-deoxycytidine (5-AZA-CdR) J Immunother. 1999;22(1):16–24. doi: 10.1097/00002371-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 66.Serrano A, et al. Rexpression of HLA class I antigens and restoration of antigen-specific CTL response in melanoma cells following 5-aza-2′-deoxycytidine treatment. Int J Cancer. 2001;94(2):243–51. doi: 10.1002/ijc.1452. [DOI] [PubMed] [Google Scholar]
- 67.Guillermo Garcia-Manero NGD, Montalban-Bravo Guillermo, Jabbour Elias J, DiNardo Courtney D, Kornblau Steven M, et al. A Phase II Study Evaluating the Combination of Nivolumab or Ipilimumab with Azacitidine in Patients with Previously Treated or Untreated Myelodysplastic Syndromes. American Society of Hematology. 2016 Dec; (oral presentation) [Google Scholar]
- 68.Yang H, et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia. 2014;28(6):1280–8. doi: 10.1038/leu.2013.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Naval Daver SB, Garcia-Manero Guillermo, Cortes Jorge E, Ravandi Farhad, Jabbour Elias J, et al. Phase IB/II Study of Nivolumab in Combination with Azacytidine in Patients with Relapsed Acute Myeloid Leukemia. American Society of Hematology. 2016 Dec; abstract. [Google Scholar]
- 70.Vereecque R, Saudemont A, Quesnel B. Cytosine arabinoside induces costimulatory molecule expression in acute myeloid leukemia cells. Leukemia. 2004;18(7):1223–30. doi: 10.1038/sj.leu.2403391. [DOI] [PubMed] [Google Scholar]
- 71.Saha A, et al. Host programmed death ligand 1 is dominant over programmed death ligand 2 expression in regulating graft-versus-host disease lethality. Blood. 2013;122(17):3062–73. doi: 10.1182/blood-2013-05-500801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blazar BR, et al. Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma-dependent mechanism. J Immunol. 2003;171(3):1272–7. doi: 10.4049/jimmunol.171.3.1272. [DOI] [PubMed] [Google Scholar]
- 73.Blazar BR, et al. Opposing roles of CD28:B7 and CTLA-4:B7 pathways in regulating in vivo alloresponses in murine recipients of MHC disparate T cells. J Immunol. 1999;162(11):6368–77. [PubMed] [Google Scholar]
- 74.Davids MS, et al. Ipilimumab for Patients with Relapse after Allogeneic Transplantation. N Engl J Med. 2016;375(2):143–53. doi: 10.1056/NEJMoa1601202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Herbaux C, et al. Efficacy and tolerability of nivolumab after allogeneic transplantation for relapsed Hodgkin’s lymphoma. Blood. 2017 doi: 10.1182/blood-2016-11-749556. [DOI] [PubMed] [Google Scholar]
- 76.Saudemont A, et al. Gene transfer of CD154 and IL12 cDNA induces an anti-leukemic immunity in a murine model of acute leukemia. Leukemia. 2002;16(9):1637–44. doi: 10.1038/sj.leu.2402590. [DOI] [PubMed] [Google Scholar]
- 77.Workman CJ, et al. Phenotypic analysis of the murine CD4-related glycoprotein, CD223 (LAG-3) Eur J Immunol. 2002;32(8):2255–63. doi: 10.1002/1521-4141(200208)32:8<2255::AID-IMMU2255>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 78.Huang CT, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21(4):503–13. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 79.Grosso JF, et al. Functionally distinct LAG-3 and PD-1 subsets on activated and chronically stimulated CD8 T cells. J Immunol. 2009;182(11):6659–69. doi: 10.4049/jimmunol.0804211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Berrien-Elliott MM, et al. Durable adoptive immunotherapy for leukemia produced by manipulation of multiple regulatory pathways of CD8+ T-cell tolerance. Cancer Res. 2013;73(2):605–16. doi: 10.1158/0008-5472.CAN-12-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu C, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6(12):1245–52. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 82.Wang C, et al. Immune regulation by 4-1BB and 4-1BBL: complexities and challenges. Immunol Rev. 2009;229(1):192–215. doi: 10.1111/j.1600-065X.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 83.Richter G, et al. Tumor necrosis factor-alpha regulates the expression of inducible costimulator receptor ligand on CD34(+) progenitor cells during differentiation into antigen presenting cells. J Biol Chem. 2001;276(49):45686–93. doi: 10.1074/jbc.M108509200. [DOI] [PubMed] [Google Scholar]
- 84.Richter G, Burdach S. ICOS: a new costimulatory ligand/receptor pair and its role in T-cell activion. Onkologie. 2004;27(1):91–5. doi: 10.1159/000075612. [DOI] [PubMed] [Google Scholar]
- 85.Lucia Masarova HK, Daver Naval. Immune Checkpoint Approaches in AML and MDS: A Next Frontier? Targeted Oncology. 2016 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.