Abstract
Carotid endarterectomy (CEA) has shown a significant benefit in preventing ipsilateral stroke when it is compared to conservative management. Surgical morbidity and mortality must be kept to a minimum to achieve this benefit. Neurological status of the CEA patients can be monitored easily during regional anesthesia depending on the awake testing (neurocognitive assessment) method of the CEA patients. In addition, specific parameters can help us to monitor and to predict the neurological status of the CEA patients during the procedures such as regional cerebral oxygen saturation (rSO2) and middle cerebral artery velocity (MCAv) changes. We conducted a computerized literature search involving humans, published in English until December 2017, and indexed through Medical Databases; MEDLINE/PubMed, EMBASE, and Web of Science. We reviewed articles performed for prospective and other types of studies related to CEA procedures and techniques which can predict patient's status during the procedure. Searching relevant articles and discussing the results to allow meaningful rate comparison, and to conclude a result view which benefits the CEA patients and the medical staff during the CEA procedures. In total, studies observed cerebral rSO2 and MCAv have significant value during CEA procedures. Patients with neurological symptoms during the procedures showed changes of cerebral rSO2 and MCAv more than the patients without neurological symptoms. Mentioned parameters (cerebral rSO2 and MCAv) showed significant increasing right after the procedure. Mostly, CEA surgeries under local anesthesia were observed, for monitoring patients’ consciousness status and comparing it to patients who undergo general anesthesia, to view the reliability of these techniques during CEA procedures, and to predict and avoid intraoperative neurological symptoms.
Keywords: Awake testing, carotid endarterectomy, cerebral monitoring, cerebral oximetry, transcranial Doppler
INTRODUCTION
Carotid endarterectomy (CEA) has shown a significant benefit in preventing ipsilateral stroke when it is compared to conservative management. Surgical morbidity and mortality must be kept to a minimum to achieve this benefit. Neurological status of the CEA patients can be monitored easily during regional anesthesia depending on the awake testing (neurocognitive assessment) method, and completely monitored, most medical centers consider awake testing as an essential method. In addition, specific parameters can help us to monitor and predict the neurological status of CEA patients during the procedures such as regional cerebral oxygen saturation (rSO2) and middle cerebral artery velocity (MCAv) changes, aiming to find a relationship between cerebral rSO2 and MCAv and the intraoperative neurological symptoms and evaluating the reliability of intraoperative cerebral monitoring techniques to expect the need for shunting during CEA procedures, including a comparison of near-infrared spectroscopy (NIRS) and transcranial-Doppler (TCD) to any other intraoperative cerebral monitoring systems; and on either symptomatic or asymptomatic patient to achieve better intraoperative controlling during CEA procedures.[1,2] Clinical studies considering the triple assessment method consisting of NIRS, TCD, and awake testing in detecting cerebral ischemic symptoms have the best outcome of controlling symptoms during CEA surgeries.
METHODOLOGY
We conducted a computerized literature search involving humans, published in English until December 2017, and indexed through Medical Databases; MEDLINE/PubMed, EMBASE, and Web of Science. We reviewed articles performed for prospective and other types of studies related to CEA procedures and techniques which can predict patient's status during the procedure. Searching relevant articles and discussing the results to allow meaningful rate comparison, and to conclude a result view which benefits the CEA patients and the medical staff during CEA procedures. The search strategy combined the following terms and keywords: “CEA or carotid endarterectomy,” “TCD or transcranial-Doppler,” “NIRS or near-infrared spectroscopy,” and “cerebral oximetry.” When more than one publication was identified referring to the same result data, the first publication was selected. Non-English articles excluded and if articles included animals they did not fulfill inclusion criteria, and they were, therefore, not considered eligible for inclusion in the study. Furthermore, we searched the reference lists of articles identified by this search.
Statistical analysis (data extraction)
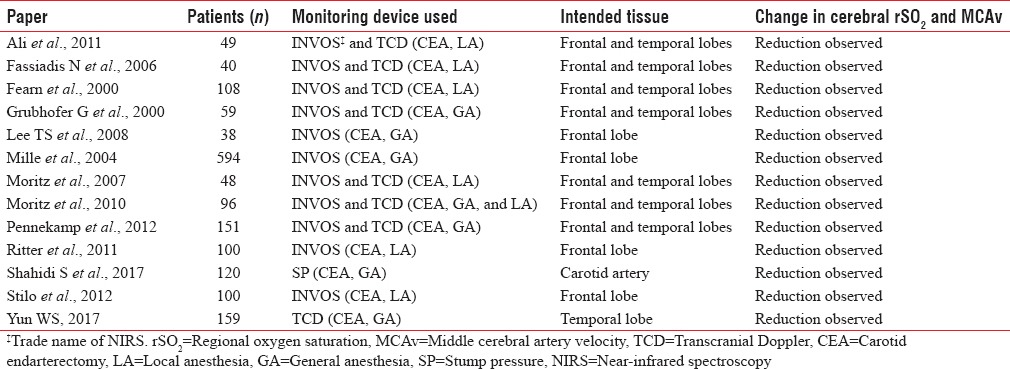
A type of systematic literature search in relating to NIRS and TCD monitoring during CEA surgeries was performed in MEDLINE/PubMed, EMBASE, and Web of Science databases, including prospective studies on NIRS and TCD for brain monitoring during CEA surgeries, plus, a comparison of NIRS and TCD to other intraoperative cerebral monitoring systems. We identified studies focused on the prediction of intraoperative cerebral ischemia and shunt indication. No threshold could be achieved for selective shunting to be determined since shunting criteria varied considerably across studies. The following data were extracted from all papers independently by the review authors: number of patients; type of anesthesia; usage of shunts; year of publication; and procedural complications. CEA papers (studies) included in this analysis are summarized in Table 1 with the inclusion of the number of patients included in each article, the monitoring device used, and whether cerebral rSO2 and MCAv were changed intraoperatively. The total number of the CEA studies was 13 papers, yielding a group of 1662 patient undergoing review.
Table 1.
In section “statistical analysis (data extraction)” as additional material
Carotid endarterectomy procedure
Carotid endarterectomy versus conservative therapy
CEA surgery has been maintained as the foundation treatment in carotid stenosis patients having a high percentage of occlusion. The North American Symptomatic Carotid Endarterectomy Trial, the European Carotid Surgery Trial, and VA Cooperative Study randomized studies showed a significant benefit of CEA in stroke prevention. At the point when stenosis of the internal carotid artery is over 70% or equal, CEA shows markedly reducing stroke rate in patients with symptomatic lesions. The CEA benefits achieved only if morbidity and mortality of the operation is <5%. Patients with nonsymptomatic carotid stenosis are operated if they are going to have major surgery (cardiac surgery) only and performed as prophylactic only if it goes with <3% of morbidity and mortality. Operating a stenosis <70% is not beneficial if it is compared to conservative therapy because it is needed to consider the factors affecting the surgery. Including morbidity and mortality of the procedure as it plays a significant role in CEA results. If those factors in CEA are <3%, the stroke risk will drop by 81%. Therefore, those factors need to be considered and cannot be neglected before performing the procedure. However, more studies are under investigation for that matter.[3] Asymptomatic patients need to be treated with intensive medical therapy rather than going under CEA procedure because intervention for asymptomatic stenosis patients without significant need has high clinical risks and because it has more risks than benefits which can be regarded as unethical. Stenting should be performed for asymptomatic carotid stenosis (ACS) with high-risk features for instant anatomical features that make endarterectomy more difficult. Among the few percent of patients with high-risk, for example, microemboli feature better to be treated by CEA than by stenting.[4] With conservative treatment applied, specialists have analyzed that stroke rates in patients with ACS have declined during 10 years of observation. Rates have tumbled to around 1% every year in patients conservatively treated. However, in this manner, we should question whether CEA has any beneficial role in patients with ACS.[5,6] Topakian et al. at his study in 2011 detailed in one investigation of 435 patients with ACS (>70% stenosis through ultrasound), just ten patients (2%) had strokes. Four of these strokes happened in patients with both echolucent plaques and only in those having embolic signs.[7] CEA has no beneficial results in acute attacks or stroke because of high bleeding risk related to the procedure. Only patients with unstable ischemic attacks situation which can turn into significant stroke may take the chance to perform CEA procedure.[8]
Anesthesia
Regional anesthesia versus general anesthesia
The awake patient's response test is a superior technique for monitoring patient's need for shunting during CEA. Because the awake assessment method used during regional anesthesia can avoid any shunts malfunction incidence (shunt-related stroke) that may occur in GA with routinely shunt using protocol during CEA procedures. Besides, CEA procedures using GA increases postoperative complications in those patients with myocardial issues.[9,10] Between 1968 and 1975, 290 patients were observed under CEA, divided into two groups one with general anesthesia and the second group with regional anesthesia; the first group consisting of 188 patients performed the CEA procedure with keeping shunt in all CEA patients. Three deaths were recorded in this category, plus some complications of motor impairment and weakness of extremities. In the second group, 102 patients were under regional anesthesia plus an intraoperative assessment of consciousness and stump pressure use, no deaths in this category neither motor or consciousness impairment were recorded. This study showed the absolute need for intraoperative evaluation in combination with stump pressure to avoid CEA complications.[11,12] Although regional anesthesia gives the assurance for shunting need, some medical centers prefer general anesthesia in combination to cervical plexus blockage, as it provides more controlled environment during surgeries, adding to that it allows rapid interfering just in case of sudden respiratory and blood pressure changing.[13,14]
Monitoring techniques
Vascular evaluations using stump pressure
Stump pressure is a technique used to evaluate the need for shunting during CEA surgeries; this method is depending on measuring the back pressure in the distal part of the internal carotid artery after clamping. As the recorded reading of the back pressure in the targeted artery, the need for shunting was decided.[15,16] Stump pressure values >25 mmHg shows the safe level as it was thought in the past years, later it was revised to mean of 40 mmHg as the safe level.[17,18] On the contrary, different studies recorded few patients had consciousness impairment (shunt requirement) even when stump pressure was giving records reach to 50 mmHg and greater, plus the technique showed some percentage of unnecessary need for shunt records in different clinical studies. Therefore, stump pressure considered as an inadequate technique and has poor sensitivity because of the noticed clinical situations opposite to the assuring readings by this technique. Stump pressure has faced dissatisfaction in some medical centers for the mentioned reason.[19,20]
Transcranial Doppler monitoring
TCD ultrasonography was introduced in clinics in the early eighties for the first time. TCD is a noninvasive monitoring technique involving the use of ≤2 MHz transducers, placed on the skull of the patient, through temporal bone windows (relatively thin bone) to measure the cerebral blood flow velocity and its alterations. The method is repeatable, allows continuous bedside monitoring of cerebral blood flow velocity, which is particularly useful in the intensive care setting. TCD has a significant role in the early phases of cerebral pathology.[21] Pulsed Doppler sectorial probe is the most used transducer in practice. Using a 2.0–3.5 MHz frequency waves, an adjustment of the probe to the scalp through a headband to keep the angle of insonation for maintaining continuous recording. Middle cerebral artery (MCA) is the most intracranial artery examined by TCD method because it is easily detected through the temporal window. MCA has vital importance in this technique because it collects about 60%–70% of the internal carotid artery blood flow. About 45–60 mm of depth needed to detect MCA. Moreover, about 50 ± 20 s of time required to achieve MCA echographic image on TCD monitor.[22]
INVOS™ cerebral oximeter monitoring
INVOS™ monitoring is an adjunctive noninvasive method which is intended for regional rSO2 monitoring in the brain or other targeted tissue through a sensor. The INVOS monitoring can be considered as an initial diagnostic method only but not used for confirming diagnostic purposes. INVOS monitoring has a significant role in improving patient management and outcomes after surgeries.[23] Intracranial rSO2 can be measured by NIRS as the human tissues are translucent to infrared light emitted through NIRS system.[24] Two disposable sensors (photodetector) with a near-infrared light source in each patch that can be applied on the forehead for monitoring purpose. Proprietary method called spatial resolution used to avoid the effect of extracerebral reflected photons. Regional rSO2 readings are machine-specific and are not variable among distinct brands of oximeter devices.[25]
Etiology
Smoking: Association of decreased blood vessels compliance, HDL levels, high-platelet aggregation, hematocrit, and fibrinogen levels is noticed in smokers. About 18% of strokes are associated with nicotine smoking. Atherosclerosis was higher in progression in both active and passive smokers. Risk of using oral contraceptives by smokers should be considered as it increases the risk of strokes.[26,27] Hypertension and high cholesterol levels: Hypertension is the most prominent modifiable risk factor for ischemic stroke. Association of high total cholesterol, triglycerides, low LDL cholesterol is considered, especially atherosclerotic and lacunar stroke subtypes are noticed in ischemic stroke patients.[26,28] Diabetes: Diabetes increases the risk of ischemic stroke in different races at different rates. 22% of prevalence in elderly “African” people, and 20% of older Hispanics, with attributable stroke risks of 13% and 20%, correspondingly. Moreover, this is related to insulin-resistance mechanism as healthy individuals with high insulin levels have a higher relative risk of stroke.[29,30] Buerger's disease: Stroke and Buerger's disease has an infrequent connection, but the pathological base is associated.[26] Vasculitis: Due to the inflammation of cerebral vessels, a stroke may arise with higher incidence. Transient ischemic attack is also frequent in these patients.[26] Radiotherapy Complication: Head-and-neck tumor radiotherapy is associated relatively by two folds to ischemic stroke risk. Different size cranial arteries are prone to significant stenosis in patients with chronic radiotherapy treatments. Radiation affects directly intima media and intima adventitia which in turn leads to vasa vasorum defects and atherosclerosis results.[31]
Pathomechanism of atherosclerosis
Atherosclerosis starts as disruption of endothelial integrity due to physical injury or metabolic stress. Alteration in expression of cellular adhesion and surface molecules vascular cell adhesion molecule 1 and intercellular adhesion molecule which result in dysregulation of blood cells adhesion. Changes in cell membrane permeability will appear which results in adherence and degranulation of platelet cells at the adluminal surface of blood vessels. Platelets begin releasing growth factors targeting smooth muscles leading them to proliferate at vascular surface making a new layer (neointima). Subsequent recruitment occurs leads to atherosclerosis due to platelets adhesiveness and degranulation.[32] Endothelial and smooth muscle cells start to alter their physiologic functions and start to secrete pro-inflammatory signals and cytokines. So-called lipid-laden macrophages will show in tunica media because of their high demand function at the site, and the repeated damage will result in atherosclerotic plaque and fatty streaks formation.[33,34]
Comparisons between methods reliability
There are many techniques for cerebral monitoring during CEA procedures, for example, TCD, NIRS, electroencephalography, somatosensory-evoked potential, carotid artery stump pressure, and awake test (neurocognitive assessment) that can help us predicting the need for a shunt during CEA surgeries. However, the awake testing is the gold standard method among these techniques of cerebral monitoring.[35,36,37,38] Former studies confirmed a strong correlation between MCAv, cerebral rSO2, and neurological status during CEA.[39,40,41] Stilo et al. at his study in 2012 showed a significant correlation between cerebral rSO2 changes and the loss of cortical functions right after clamping stage in CEA procedures.[42,43] A decrease in rSO2 of more than 20% may indicate the need for shunt during CEA.[44] Shahidi et al. at his study in 2017 indicated stump pressures role in a study of 120 patients, stump pressure readings of mean 40 mmHg were predicted and considered to be safe.[18,45,46] For demonstrating the role of TCD in CEA, Navarro et al. at his systematic review in 2007 showed a great relation between TCD and shunt need during CEA procedures reviewing articles between (1982 and 2005).[47] TCD is a safe cerebral monitoring during CEA and it can predict the need to use of carotid shunt.[48,49]
CONCLUSION
Data in the studies observed gave us a general idea of how the NIRS, TCD, and other intraoperative monitoring methods could be sensitive and have a high value during CEA procedures. Studies observed were intended to compare intraoperative monitoring methods to help in detecting cerebral ischemia, especially cerebral oximetry/NIRS, and TCD contrasted with alert testing (awake neurocognitive assessment) in recognizing the requirement for shunting in CEA procedures. Cerebral oximetry/NIRS showed high changes and can be used for all patients which it can be counted to its pros. TCD showed high changes as well, but screening availability is limited to patients with temporal window only, which consider as a usage limitation. Clinical studies considering the triple assessment method as best (combination of NIRS, TCD, and awake testing) in detecting cerebral ischemic symptoms and it has best outcome of controlling symptoms during CEA surgeries.
Financial support and sponsorship
This study was financially supported by University of Debrecen.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
I acknowledge and offer the sincerest gratitude to Dr. Gyöngyösi Zoltán and Prof. Dr. Fülesdi Béla for supporting and allowing contribution to writing this review with the Department of Anesthesiology and Intensive Care.
REFERENCES
- 1.Pennekamp CW, Immink RV, den Ruijter HM, Kappelle LJ, Ferrier CM, Bots ML, et al. Near-infrared spectroscopy can predict the onset of cerebral hyperperfusion syndrome after carotid endarterectomy. Cerebrovasc Dis. 2012;34:314–21. doi: 10.1159/000343229. [DOI] [PubMed] [Google Scholar]
- 2.Gyöngyösi Z, Molnár L, Fülesdi B. Cerebral oxigen saturation and middle cerebral artery flow velocity changes during carotid endarterectomy. J Cardiothorac Vasc Anesth. 2016;30:19–20. [Google Scholar]
- 3.Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, et al. Guidelines for the primary prevention of stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:517–84. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- 4.Spence JD. Management of patients with an asymptomatic carotid stenosis – Medical management, endovascular treatment, or carotid endarterectomy? Curr Neurol Neurosci Rep. 2016;16:3. doi: 10.1007/s11910-015-0605-6. [DOI] [PubMed] [Google Scholar]
- 5.Marquardt L, Geraghty OC, Mehta Z, Rothwell PM. Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment: A prospective, population-based study. Stroke. 2010;41:e11–7. doi: 10.1161/STROKEAHA.109.561837. [DOI] [PubMed] [Google Scholar]
- 6.Abbott AL. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: Results of a systematic review and analysis. Stroke. 2009;40:e573–83. doi: 10.1161/STROKEAHA.109.556068. [DOI] [PubMed] [Google Scholar]
- 7.Topakian R, King A, Kwon SU, Schaafsma A, Shipley M, Markus HS, et al. Ultrasonic plaque echolucency and emboli signals predict stroke in asymptomatic carotid stenosis. Neurology. 2011;77:751–8. doi: 10.1212/WNL.0b013e31822b00a6. [DOI] [PubMed] [Google Scholar]
- 8.European Stroke Organisation (ESO) Executive Committee; ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25:457–507. doi: 10.1159/000131083. [DOI] [PubMed] [Google Scholar]
- 9.Pasin L, Nardelli P, Landoni G, Cornero G, Magrin S, Tshomba Y, et al. Examination of regional anesthesia for carotid endarterectomy. J Vasc Surg. 2015;62:631–40. doi: 10.1016/j.jvs.2015.03.074. [DOI] [PubMed] [Google Scholar]
- 10.Leichtle SW, Mouawad NJ, Welch K, Lampman R, Whitehouse WM, Jr, Heidenreich M, et al. Outcomes of carotid endarterectomy under general and regional anesthesia from the American College of Surgeons’ National Surgical Quality Improvement Program. J Vasc Surg. 2012;56:81–8. doi: 10.1016/j.jvs.2012.01.005. e3. [DOI] [PubMed] [Google Scholar]
- 11.Connolly JE, Kwaan JH, Stemmer EA. Improved results with carotid endarterectomy. Ann Surg. 1977;186:334–42. doi: 10.1097/00000658-197709000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erwin D, Pick MJ, Taylor GW. Anaesthesia for carotid artery surgery. Anaesthesia. 1980;35:246–9. doi: 10.1111/j.1365-2044.1980.tb05091.x. [DOI] [PubMed] [Google Scholar]
- 13.Marcucci G, Siani A, Accrocca F, Gabrielli R, Giordano A, Antonelli R, et al. Preserved consciousness in general anesthesia during carotid endarterectomy: A six-year experience. Interact Cardiovasc Thorac Surg. 2011;13:601–5. doi: 10.1510/icvts.2011.280321. [DOI] [PubMed] [Google Scholar]
- 14.Ebner FH, Trenti E, Baldinelli F, Natto M, Ebner H. Carotid endarterectomy: Comparing anesthesia in awakened and intubated patients with general anesthesia. Minerva Cardioangiol. 2008;56:29–34. [PubMed] [Google Scholar]
- 15.Tambakis CL, Papadopoulos G, Sergentanis TN, Lagos N, Arnaoutoglou E, Labropoulos N, et al. Cerebral oximetry and stump pressure as indicators for shunting during carotid endarterectomy: Comparative evaluation. Vascular. 2011;19:187–94. doi: 10.1258/vasc.2010.oa0277. [DOI] [PubMed] [Google Scholar]
- 16.Calligaro KD, Dougherty MJ. Correlation of carotid artery stump pressure and neurologic changes during 474 carotid endarterectomies performed in awake patients. J Vasc Surg. 2005;42:684–9. doi: 10.1016/j.jvs.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Mulaudzi TV, Biccard BM, Robbs JV, Paruk N, Pillay B, Rajaruthnam P, et al. Carotid artery stump pressure and associated neurological changes in predominantly symptomatic carotid artery disease patients undergoing awake carotid endarterectomy. Cardiovasc J Afr. 2009;20:116–8. [PMC free article] [PubMed] [Google Scholar]
- 18.Shahidi S, Owen-Falkenberg A, Gottschalksen B. Clinical validation of 40-mmHg carotid stump pressure for patients undergoing carotid endarterectomy under general anesthesia. J Cardiovasc Surg (Torino) 2017;58:431–8. doi: 10.23736/S0021-9509.16.08173-8. [DOI] [PubMed] [Google Scholar]
- 19.Botes K, Le Roux DA, Van Marle J. Cerebral monitoring during carotid endarterectomy – A comparison between electroencephalography, transcranial cerebral oximetry and carotid stump pressure. S Afr J Surg. 2007;45:43–6. [PubMed] [Google Scholar]
- 20.Hans SS, Jareunpoon O. Prospective evaluation of electroencephalography, carotid artery stump pressure, and neurologic changes during 314 consecutive carotid endarterectomies performed in awake patients. J Vasc Surg. 2007;45:511–5. doi: 10.1016/j.jvs.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 21.Chang JJ, Tsivgoulis G, Katsanos AH, Malkoff MD, Alexandrov AV. Diagnostic accuracy of transcranial Doppler for brain death confirmation: Systematic review and meta-analysis. AJNR Am J Neuroradiol. 2016;37:408–14. doi: 10.3174/ajnr.A4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Andrea A, Conte M, Scarafile R, Riegler L, Cocchia R, Pezzullo E, et al. Transcranial Doppler ultrasound: Physical principles and principal applications in neurocritical care unit. J Cardiovasc Echogr. 2016;26:28–41. doi: 10.4103/2211-4122.183746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denault A, Deschamps A, Murkin JM. A proposed algorithm for the intraoperative use of cerebral near-infrared spectroscopy. Semin Cardiothorac Vasc Anesth. 2007;11:274–81. doi: 10.1177/1089253207311685. [DOI] [PubMed] [Google Scholar]
- 24.Damian MS, Schlosser R. Bilateral near infrared spectroscopy in space-occupying middle cerebral artery stroke. Neurocrit Care. 2007;6:165–73. doi: 10.1007/s12028-007-0010-3. [DOI] [PubMed] [Google Scholar]
- 25.Edmonds HL. Detection and correction of Brain Oxygen Imbalance. Surgical and Critical Care Applications of the INVOSTM Cerebral Oximeter. 1st ed. Louisville (USA): Covidien; 2013. [Google Scholar]
- 26.Chong JY, Sacco RL. Risk factors for stroke, assessing risk, and the mass and high-risk approaches for stroke prevention. Continuum Lifelong Learn Neurol. 2005;11:18–34. [Google Scholar]
- 27.Khawaja O, Maziarz M, Biggs ML, Longstreth WT, Jr, Ix JH, Kizer JR, et al. Plasma free fatty acids and risk of stroke in the cardiovascular health study. Int J Stroke. 2014;9:917–20. doi: 10.1111/ijs.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): A case-control study. Lancet. 2010;376:112–23. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- 29.Rundek T, Gardener H, Xu Q, Goldberg RB, Wright CB, Boden-Albala B, et al. Insulin resistance and risk of ischemic stroke among nondiabetic individuals from the Northern Manhattan study. Arch Neurol. 2010;67:1195–200. doi: 10.1001/archneurol.2010.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li M, Li Y, Liu J. Metabolic syndrome with hyperglycemia and the risk of ischemic stroke. Yonsei Med J. 2013;54:283–7. doi: 10.3349/ymj.2013.54.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plummer C, Henderson RD, O’Sullivan JD, Read SJ. Ischemic stroke and transient ischemic attack after head and neck radiotherapy: A review. Stroke. 2011;42:2410–8. doi: 10.1161/STROKEAHA.111.615203. [DOI] [PubMed] [Google Scholar]
- 32.Wahlgren CM, Zheng W, Shaalan W, Tang J, Bassiouny HS. Human carotid plaque calcification and vulnerability. Relationship between degree of plaque calcification, fibrous cap inflammatory gene expression and symptomatology. Cerebrovasc Dis. 2009;27:193–200. doi: 10.1159/000189204. [DOI] [PubMed] [Google Scholar]
- 33.Mathiesen EB, Bønaa KH, Joakimsen O. Echolucent plaques are associated with high risk of ischemic cerebrovascular events in carotid stenosis: The Tromsø study. Circulation. 2001;103:2171–5. doi: 10.1161/01.cir.103.17.2171. [DOI] [PubMed] [Google Scholar]
- 34.Singh AS, Atam V, Jain N, Yathish BE, Patil MR, Das L, et al. Association of carotid plaque echogenicity with recurrence of ischemic stroke. N Am J Med Sci. 2013;5:371–6. doi: 10.4103/1947-2714.114170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moritz S, Kasprzak P, Arlt M, Taeger K, Metz C. Accuracy of cerebral monitoring in detecting cerebral ischemia during carotid endarterectomy: A comparison of transcranial Doppler sonography, near-infrared spectroscopy, stump pressure, and somatosensory evoked potentials. Anesthesiology. 2007;107:563–9. doi: 10.1097/01.anes.0000281894.69422.ff. [DOI] [PubMed] [Google Scholar]
- 36.Moritz S, Schmidt C, Bucher M, Wiesenack C, Zimmermann M, Schebesch KM, et al. Neuromonitoring in carotid surgery: Are the results obtained in awake patients transferable to patients under sevoflurane/fentanyl anesthesia? J Neurosurg Anesthesiol. 2010;22:288–95. doi: 10.1097/ANA.0b013e3181e16e14. [DOI] [PubMed] [Google Scholar]
- 37.Ali AM, Green D, Zayed H, Halawa M, El-Sakka K, Rashid HI, et al. Cerebral monitoring in patients undergoing carotid endarterectomy using a triple assessment technique. Interact Cardiovasc Thorac Surg. 2011;12:454–7. doi: 10.1510/icvts.2010.235598. [DOI] [PubMed] [Google Scholar]
- 38.Ritter JC, Green D, Slim H, Tiwari A, Brown J, Rashid H, et al. The role of cerebral oximetry in combination with awake testing in patients undergoing carotid endarterectomy under local anaesthesia. Eur J Vasc Endovasc Surg. 2011;41:599–605. doi: 10.1016/j.ejvs.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 39.Fassiadis N, Zayed H, Rashid H, Green DW. Invos cerebral oximeter compared with the transcranial Doppler for monitoring adequacy of cerebral perfusion in patients undergoing carotid endarterectomy. Int Angiol. 2006;25:401–6. [PubMed] [Google Scholar]
- 40.Grubhofer G, Plöchl W, Skolka M, Czerny M, Ehrlich M, Lassnigg A, et al. Comparing Doppler ultrasonography and cerebral oximetry as indicators for shunting in carotid endarterectomy. Anesth Analg. 2000;91:1339–44. doi: 10.1097/00000539-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Steppan J, Hogue CW., Jr Cerebral and tissue oximetry. Best Pract Res Clin Anaesthesiol. 2014;28:429–39. doi: 10.1016/j.bpa.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stilo F, Spinelli F, Martelli E, Pipitó N, Barillà D, De Caridi G, et al. The sensibility and specificity of cerebral oximetry, measured by INVOS-4100, in patients undergoing carotid endarterectomy compared with awake testing. Minerva Anestesiol. 2012;78:1126–35. [PubMed] [Google Scholar]
- 43.Nielsen HB. Systematic review of near-infrared spectroscopy determined cerebral oxygenation during non-cardiac surgery. Front Physiol. 2014;5:93. doi: 10.3389/fphys.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mille T, Tachimiri ME, Klersy C, Ticozzelli G, Bellinzona G, Blangetti I, et al. Near infrared spectroscopy monitoring during carotid endarterectomy: Which threshold value is critical? Eur J Vasc Endovasc Surg. 2004;27:646–50. doi: 10.1016/j.ejvs.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 45.Lee TS, Hines GL, Feuerman M. Significant correlation between cerebral oximetry and carotid stump pressure during carotid endarterectomy. Ann Vasc Surg. 2008;22:58–62. doi: 10.1016/j.avsg.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 46.Bond R, Rerkasem K, Rothwell PM. Routine or selective carotid artery shunting for carotid endarterectomy (and different methods of monitoring in selective shunting) Stroke. 2003;34:824–5. doi: 10.1161/01.STR.0000059381.17983.77. [DOI] [PubMed] [Google Scholar]
- 47.Navarro JC, Lao AY, Sharma VK, Tsivgoulis G, Alexandrov AV. The accuracy of transcranial Doppler in the diagnosis of middle cerebral artery stenosis. Cerebrovasc Dis. 2007;23:325–30. doi: 10.1159/000099130. [DOI] [PubMed] [Google Scholar]
- 48.Yun WS. Cerebral monitoring during carotid endarterectomy by transcranial Doppler ultrasonography. Ann Surg Treat Res. 2017;92:105–9. doi: 10.4174/astr.2017.92.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fearn SJ, Picton AJ, Mortimer AJ, Parry AD, McCollum CN. The contribution of the external carotid artery to cerebral perfusion in carotid disease. J Vasc Surg. 2000;31:989–93. doi: 10.1067/mva.2000.104598. [DOI] [PubMed] [Google Scholar]


