Abstract
Background:
Magnesium sulfate and dexmedetomidine were used as adjuvants to local anesthesia to improve the quality of regional anesthesia.
Aims:
The aim of this study is to evaluate and compare the effects of magnesium sulfate and dexmedetomidine when added to ropivacaine on the quality of infraclavicular brachial plexus block (BPB).
Settings and Design:
This was a prospective randomized double-blinded controlled study.
Patients and Methods:
A total of 105 adult patients undergoing surgery in hands, wrist, and forearm using infraclavicular BPB were randomly assigned into three groups. Ultrasound-guided infraclavicular BPB was performed using 35 ml ropivacaine 0.5% diluted with 4 ml normal saline 0.9%. Magnesium sulfate 150 mg and dexmedetomidine 100 μg were added in the magnesium sulfate group and dexmedetomidine group, respectively. Duration of analgesia, onset times and durations of sensory block (SB) and motor block (MB), patient's satisfaction, and complications were recorded.
Statistical Analysis:
Statistical software SPSS 16 was used for statistical analysis.
Results:
Dexmedetomidine and magnesium sulfate provided longer duration of analgesia and lesser consumption of postoperative rescue analgesia than the control group (P = 0. 000); dexmedetomidine provided the longest duration of analgesia versus control and magnesium sulfate groups (P = 0.000). Dexmedetomidine provided the quickest onset times and the longest durations of both SB and MB than control and magnesium sulfate groups (P = 0.000). Dexmedetomidine group had higher incidences of bradycardia and hypotension.
Conclusions:
Magnesium sulfate or dexmedetomidine is a useful adjuvant to ropivacaine for infraclavicular BPB in lengthening the duration of analgesia. Dexmedetomidine provided quicker onset and longer duration of both SB and MB and longer duration of analgesia with lesser consumption of postoperative rescue analgesia; however, it showed a higher incidence of intraoperative hypotension and bradycardia than magnesium sulfate.
Keywords: Brachial plexus, dexmedetomidine, infraclavicular, magnesium sulfate
INTRODUCTION
The infraclavicular block is a safe and effective approach for brachial plexus block (BPB) that can provide anesthesia for hands, wrist, and forearm.[1] Ropivacaine is a propyl analog of bupivacaine[2] that has the same anesthetic potency with longer duration of action and lesser cardiac and central nervous system toxicity of bupivacaine.[3] Several adjuvants can be used to lengthen the duration of the infraclavicular BPB such as clonidine,[4] opioids,[5] neostigmine,[6] midazolam,[7] and dexamethasone.[6]
Magnesium is a physiological calcium channel blocker and has also N-methyl-D-aspartate (NMDA) receptor antagonist effect.[8] Since magnesium sulfate can prevent central sensitization by the peripheral nociceptive stimulation, it can be used as an adjuvant to local anesthetic (LA) solution for different kinds of regional anesthesia and analgesia to improve the quality and prolong the duration of the block.[9]
Dexmedetomidine is highly selective α2-adrenergic agonist more potent and faster than clonidine.[10] It has analgesic, sedative, antihypertensive, and anesthetic-sparing effects;[11] when added to local anesthesia for regional or peripheral nerve block (PNB), it prolongs the duration of the block and duration of analgesia.[12,13]
The aim of our study was to evaluate and compare the effect of magnesium sulfate and dexmedetomidine when added to ropivacaine 0.5% for infraclavicular BPB on the duration of analgesia, as a primary goal, and the onset times and durations of sensory block (SB) and motor block (MB) and patient's satisfaction, as the secondary goals.
PATIENTS AND METHODS
After obtaining approval from the Institutional Review Board (code number: 30898/04/16), registration in the Pan African Clinical Trials Registry (PACTR201605001631245), and patients informed consent, a prospective double-blinded randomized study was carried out on adult patients with ASA physical status classes I and II undergoing elective surgery in hands, wrist, and forearm. The study was done at Tanta University Hospital, Egypt, between February and July 2016.
Patients with the following conditions were excluded from the study: coagulopathy, renal or hepatic dysfunction, patients receiving α adrenergic agonist or antagonist, pregnant women, and those with a psychiatric or neurological deficit.
The patients were randomized through a computer-generated randomization sequence into three groups using sealed opaque envelope and each patient chose the envelope which determined his/her group.
Patients were randomized into three groups:
Group I (ropivacaine group): Patients received ultrasound-guided infraclavicular BPB with 35 ml ropivacaine 0.5% and 4 ml normal saline 0.9% (total volume 39 ml)
Group II (magnesium sulfate group): Patients received ultrasound-guided infraclavicular BPB with 35 ml ropivacaine 0.5% and magnesium sulfate (150 mg) (magnesium sulfate, Sedico) with 2.5 ml normal saline 0.9% (total volume 39 ml)
Group III (dexmedetomidine group): Patients received ultrasound-guided infraclavicular BPB with 35 ml ropivacaine 0.5% and dexmedetomidine (100 μg) (Precedex, Hospira) with 3 ml normal saline 0.9% (total volume 39 ml).
The study drugs were prepared by an anesthesiologist who had no role in the research.
On arrival to the operating room, an intravenous (i.v.) line 20-gauge was inserted in the nonoperating hand. Lactate Ringer's solution was started at a rate of 5 ml/kg/h and patients were monitored with standards monitoring including electrocardiography, pulse oximetry, and noninvasive blood pressure. O2 was administered through nasal sponges at a rate of 5 L/min.
Infraclavicular BPB was done while the patient in supine position and his head slightly turned to the other side with the upper extremity abducted 90°. The entry site was identified at 2 cm medially and 2 cm caudal to the coracoid process. After skin preparation, 3 ml of lidocaine 1% was injected subcutaneously at the site of injection. The probe of ultrasound (8–12 MHz, SonoScape SSI 6600, China) was placed medial to the coracoids process in the parasagittal plane to identify the axillary artery and the three cords of the brachial plexus. A 100-mm 20-gauge insulated needle (Visioplex, Vygon, France) attached to nerve stimulator was advanced in-plane to anesthetize each cord. Once the optimal motor response in the range of 0.3–0.5 mA was obtained, the LA solution was injected around each cord. Injection of LA solution was slowly with frequent aspiration every 3 ml to avoid unintentional intravascular injection. The SB and MB were assessed every 3 min in the first 30 min after injection of LA and every 30 min postoperatively till the infraclavicular block is worn off.
The SB was assessed using cold test by alcohol swab and by pinprick test. All dermatomes supplied by radial, ulnar, median, and musculocutaneous nerves were assessed. The SB was graded: 0 = normal sensation; 1 = loss of sensation to pinprick; and 2 = loss of touch sensation.
The onset time of the SB is the time interval from injection of LA till the complete SB achieved. The duration of the SB is the time interval between the onset of the complete SB and complete resolution of the SB. Duration of analgesia is the time interval between the onset of the complete SB and the first dose of postoperative analgesia.
The MB was graded according to modified Bromage scale: 0 = no movement in fingers, wrist, and elbow; 1 = finger movement only; 2 = flexion of the wrist against gravity; and 3 = flexion of elbow against gravity.
The onset time of the MB is the time interval between injection of LA and time of the complete MB. The duration of MB is the time interval between the onset of the complete MB and complete resolution of the MB.
The block was considered successful when the SB is 2 and MB is 0 within 30 min after injection of the local analgesia (LA). Otherwise, the block was considered as failed or inadequate block and the patients would receive general anesthesia or analgesia to complete the surgical intervention. These patients were excluded from the study. Intraoperative mean arterial blood pressure (MAP) and heart rate (HR) were recorded preoperatively and every 15 min after the administration of LA solution till the end of surgery. Postoperative pain was assessed using a 10-cm visual analog scale (VAS) (0: no pain to 10: worst pain imaginable) and recorded at admission to postoperative care unit and 1, 2, 4, 6, 8, 12, 18, and 24 h postoperative. Patients received postoperative analgesia in the form of diclofenac sodium (75 mg intramuscular) every 12 h, and if the patient still complained of pain, pethidine 1 mg/kg was given i.v. as rescue analgesia. The first dose of diclofenac sodium was given when VAS was >3. Total consumption of rescue analgesia was recorded. Patient's satisfaction was assessed by direct asking the patients regarding the degree of their satisfaction about the block using a four-point scale (1 = very dissatisfied, 2 = dissatisfied, 3 = satisfied, and 4 = very satisfied). Any intraoperative or postoperative complications were recorded such as pneumothorax, vascular puncture, Horner syndrome, somnolence, local anesthetic toxicity, bradycardia (HR <50 beats/min and managed by atropine 0.5 mg), and hypotension (defined as a decreased of blood pressure >25% of the baseline and managed by i.v. fluids and ephedrine 10 mg bolus if no response to fluid administration).
Statistical analysis
Calculation of the sample size was based on the effect of adding magnesium sulfate or dexmedetomidine to local anesthetic solutions on the duration of analgesia. Based on the results of previous studies,[14,15] 30 patients were required to detect a significant difference in the duration of analgesia of 140 min at α error of 0.05 and study power of 90%. The sample size was calculated using the statistical software STATA-9 (StataCorp LP, College Station, Texas, USA). Quantitative data were described as mean ± standard deviation and were analyzed using one-way ANOVA with post hoc Tukey honestly significant difference test. Categorical data were presented as number (n) or percentage (%) and were analyzed by Chi-square test. Comparison of the continuous data within each group was performed using repeated measures analysis of variance. We used SPSS 16 (SPSS Inc., Chicago, IL, USA) for statistical analysis. P <0.05 was considered statistically significant.
RESULTS
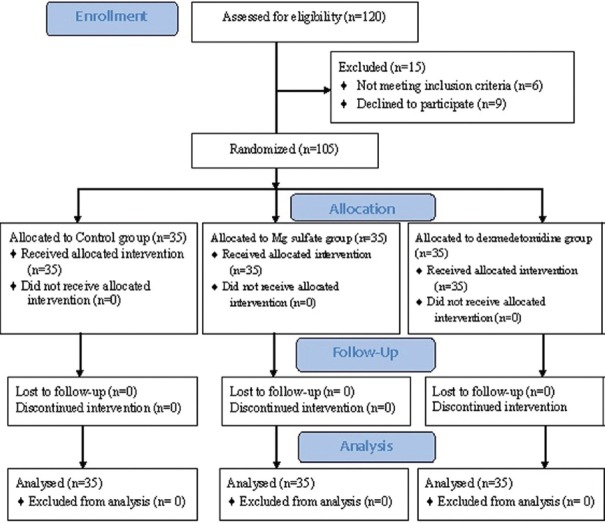
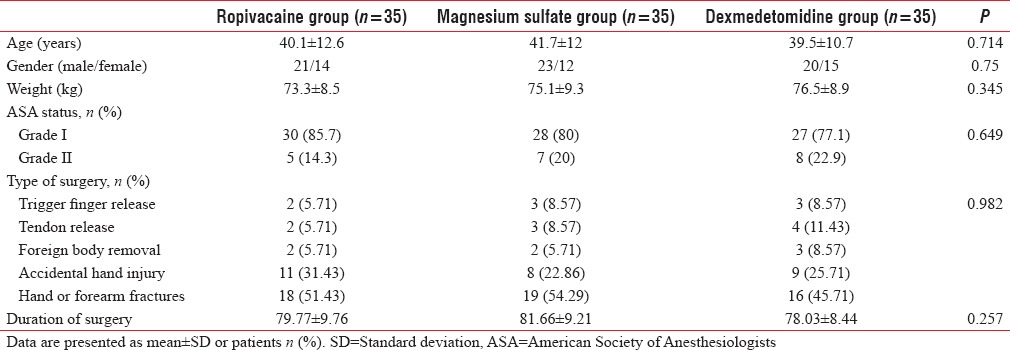
In each group, 35 patients were enrolled [Figure 1], and there was no statistical difference between the group's gender, age, weight, ASA physical status classification, or type of the surgery [Table 1].
Figure 1.
Consort flow diagram of participants through each stage of the randomized trial
Table 1.
Patient's characteristics in the three groups
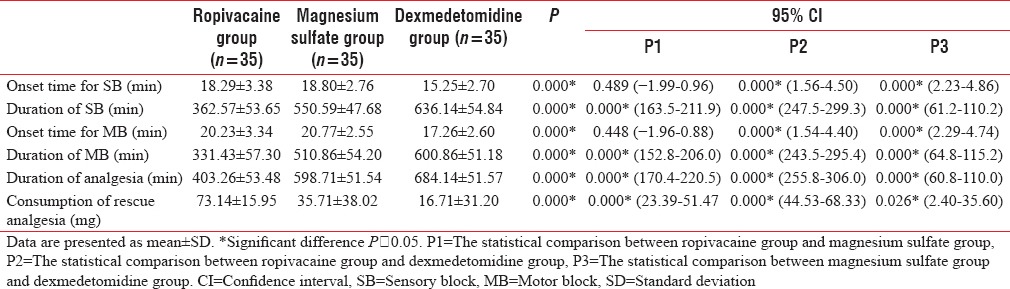
The mean duration of analgesia with ropivacaine was 403.26 ± 53.48 min, with magnesium sulfate was 598.71 ± 51.54 min, and with dexmedetomidine was 684.14 ± 51.57 min (P = 0.000). Dexmedetomidine group provided the longest duration of analgesia as compared to ropivacaine group (P = 0.000, confidence interval [CI]; 255.8–306.0) and magnesium sulfate group (P = 0.000, CI; 60.8–110.0). Dexmedetomidine group provided the quickest onset time (15.25 ± 2.70 min) and the longest duration of SB (636.14 ± 54.84 min) as compared to ropivacaine group (P = 0.000) and magnesium sulfate group (P < 0.05). The mean onset time for the complete MB was 20.23 ± 3.34 min with ropivacaine group, 20.77 ± 2.55 min with magnesium sulfate group, and 17.26 ± 2.60 min with dexmedetomidine group (P = 0.000). The MB lasted for 331.43 ± 57.30 min with ropivacaine group, 510.86 ± 54.20 min with magnesium sulfate group, and 600.86 ± 51.18 min with dexmedetomidine group (P = 0.000). Dexmedetomidine group provided a quicker onset and the longest duration of MB as compared to ropivacaine group (P < 0.05) and magnesium sulfate group (P = 0.000). Consumption of postoperative rescue analgesia was significantly lower in magnesium sulfate and dexmedetomidine groups than ropivacaine group (P = 0.000, CI; 23.39–51.47 and 44.53–68.33, respectively) [Table 2].
Table 2.
The block characteristics in the three groups
Preoperative values of MAP and HR were non-significantly different (P = 0.455, 0.536, respectively) among the three groups. Intraoperative MAP and HR significantly decreased in dexmedetomidine group as compared to its preoperative value and to the other two studied groups [Figures 2 and 3].
Figure 2.
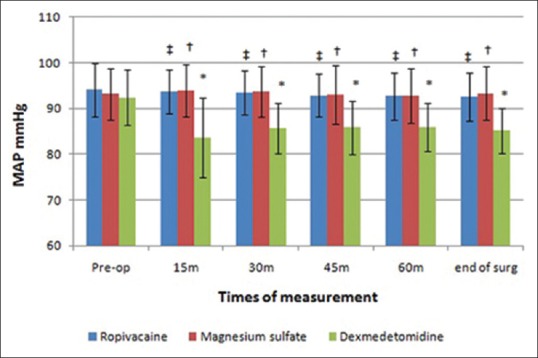
Mean arterial blood pressure (mmHg) changes in the three groups. *Significant difference as compared to the preoperative value in dexmedetomidine group. †Significant difference between magnesium sulfate group and dexmedetomidine group. ‡Significant difference between ropivacaine group and dexmedetomidine group
Figure 3.
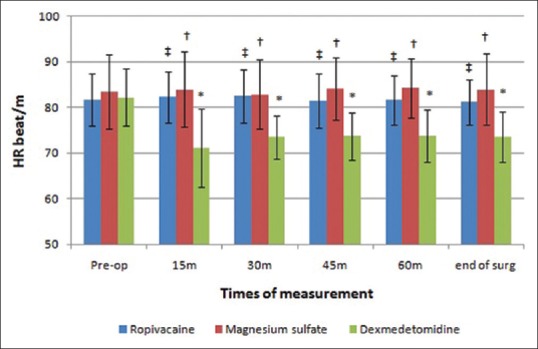
Heart rate (beat/min) changes in the three groups. *Significant difference as compared to the preoperative value in dexmedetomidine group. †Significant difference between magnesium sulfate group and dexmedetomidine group. ‡Significant difference between ropivacaine group and dexmedetomidine group
Postoperative VAS was lower in magnesium sulfate and dexmedetomidine groups than that in ropivacaine group. At 12-h postoperative, the VAS was significantly lower in dexmedetomidine group than magnesium sulfate group (P = 0.005) [Figure 4].
Figure 4.
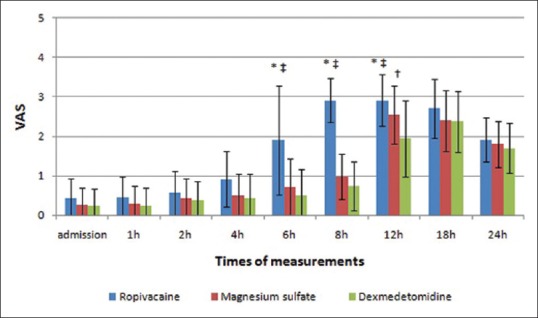
Visual analog score changes in the three groups. *Significant difference between ropivacaine group and magnesium sulfate group. †Significant difference between magnesium sulfate group and dexmedetomidine group. ‡Significant difference between ropivacaine group and dexmedetomidine group
Dexmedetomidine group had higher incidences of bradycardia (7 patients) and hypotension (5 patients) than the other two groups [Table 3].
Table 3.
Patient's satisfaction and complications in the three groups
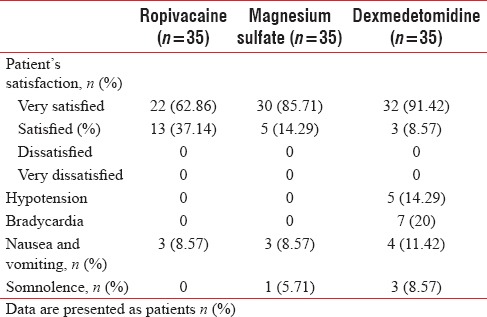
Finally, all patients from all groups were either very satisfied or satisfied with the anesthesia. Patient's satisfaction was higher in dexmedetomidine group and magnesium sulfate group as compared to ropivacaine group (P = 0.004 and 0.029, respectively) [Table 3].
DISCUSSION
The results of our research showed that the addition of magnesium sulfate (150 mg) or dexmedetomidine (100 μg) to ropivacaine 0.5% for infraclavicular BPB resulted in lengthening the duration of SB and MB, prolonged duration of analgesia, and reduction of postoperative rescue analgesia consumption with better patient's satisfaction than those of the control group. Dexmedetomidine hastened the onset of SB and MB with longer durations of SB, MB, and analgesia, as well as lesser consumption of postoperative rescue analgesia; however, the incidence of intraoperative hypotension and bradycardia was higher than those of magnesium sulfate.
Previous studies had been investigated the use of magnesium sulfate as an adjuvant to LA solutions for PNB.[14,16,17,18] Analgesic effects of magnesium sulfate on the peripheral nerve (PN) may be explained by the NMDA receptors antagonist effect that causes prevention of central sensitization from peripheral nociceptive stimulation,[14] as well as magnesium reduced release of acetylcholine through the competitive block of the calcium entry in presynaptic endings.[19] Another possible mechanism for the action of magnesium sulfate on the PN is the surface charge theory.[14] The modulation of the external magnesium concentration bathing a nerve bundle can enhance the PNB caused by LA, as well as the high concentration of magnesium attracted by the negative charges of the outer membrane surface affected Na+ channel gating and could cause hyperpolarization which results in inhibition of nerve conduction.[20]
Mukherjee et al.[16] studied the effects of using 150 mg magnesium sulfate as an adjuvant to ropivacaine 0.5% for supraclavicular BPB in 100 patients undergoing forearm and hand surgeries. They concluded that the addition of magnesium sulfate to ropivacaine 0.5% resulted in prolongation of the SB and MB durations and the time for the first analgesic request as well as decreased total analgesic consumption without side effects. Haghighi et al.[17] in their study on 60 patients undergoing orthopedic surgery of the upper extremities concluded that the addition of 3 mL of 20% magnesium sulfate to lidocaine (5 mg/kg) lengthened the duration of MB and SB of the axillary BPB. Lee et al.[14] proved that the use of 2 ml of magnesium sulfate (10%) as an adjuvant to bupivacaine 0.5% with epinephrine (1:200,000) for the interscalene nerve block in 66 patients underwent arthroscopic rotator cuff repair increased the duration of analgesia and reduced the postoperative pain. The favorable effects of magnesium sulfate when added to the LA solution on the improvement of the quality of the regional anesthetic technique, such as i.v. regional anesthesia and intrathecal and epidural block, had been demonstrated in the previous studies.[21,22,23] On the other hand, Choi et al.[24] demonstrated that magnesium sulfate (200 mg) added to ropivacaine 0.2% for axillary BPB in 38 patients undergoing upper extremity surgery reduced neither the level of postoperative pain nor the need for the postoperative opioid.
The results of our research showed that dexmedetomidine provided the quickest onset of action and the longest duration of SB, MB, and analgesia when combined with ropivacaine. However, the incidence of hypotension and bradycardia was higher than other two groups.
Besides its central-mediated analgesia,[25] the mechanism by which dexmedetomidine enhances the quality of regional anesthesia when used as an adjuvant to LA can be explained by two peripheral mechanisms. The first is the vasoconstrictor effect around the site of injection which leads to delay of the absorption of the LA and prolong the duration of the LA effect.[26,27] The second mechanism is the direct action of dexmedetomidine on the activity of PN. Dexmedetomidine may inhibit the compound action potentials that results in direct inhibition of the on nerve transmission.[28] Another mechanism of the direct suppression of impulse propagation along neurons by dexmedetomidine may be due to blocking the hyperpolarization-activated cation current (Ih current).[29,30] Thus, blocking the Ih current will result in prolonged hyperpolarization of the nerve, which in turn will cause an analgesic action. Blocking the Ih current may also have the potential to produce a selective sensory effect as this effect appears to be more pronounced in C-fibers than in Aα fibers.[29]
In agreement with our results, Ammar and Mahmoud[13] concluded that the addition of dexmedetomidine (0.75 μg/kg) to bupivacaine (0.33%) for infraclavicular BPB in 60 patients undergoing upper extremity surgery hastened the onset of SB and MB, prolonged the duration of postoperative analgesia, and decreased opioid requirements with lower pain assessment scale, but there were no side effects documented in their study. Esmaoglu et al.[31] reported that the addition of dexmedetomidine (100 μg) to levobupivacaine 0.5% for axillary BPB in 60 patients undergoing hand and forearm surgery resulted in fast onset time with long duration of the axillary block with prolonged duration of analgesia. Bradycardia was reported as a side effect in their study. Das et al.[15] concluded that the use of dexmedetomidine (100 μg) as an adjuvant to ropivacaine 0.5% for supraclavicular BPB prolonged the SB and MB duration and the duration of postoperative analgesia and decreased total analgesic need with no adverse effects.
As regards the effect of dexmedetomidine on the onset of the block, Gandhi et al.[32] found that the onset time of the SB and MB was faster in the control group than dexmedetomidine. Dexmedetomidine did not affect SB and MB onset time in some studies.[15,33] It shortened the SB onset time without affecting the MB onset time in another study.[34]
Mirkheshti et al.[35] and Marhofer et al.[36] noticed that dexmedetomidine shortened the MB onset time.
Our study showed that dexmedetomidine induced bradycardia and hypotension in some patients during the procedures, which was evident in other studies as well.[31,33,37]
There are some limitations in our study; first is the limited number of patients enrolled in our research. The second, we did not assess the level of sedation. We only reported the incidence of somnolence.
CONCLUSIONS
Magnesium sulfate or dexmedetomidine is a useful adjuvant to ropivacaine for infraclavicular BPB in lengthening the duration of analgesia. Dexmedetomidine provided quicker onset time and longer durations of SB and MB and longer duration of analgesia with lesser consumption of postoperative rescue analgesia, but the incidence of intraoperative hypotension and bradycardia was higher than magnesium sulfate.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Borgeat A, Ekatodramis G, Dumont C. An evaluation of the infraclavicular block via a modified approach of the raj technique. Anesth Analg. 2001;93:436–41. doi: 10.1097/00000539-200108000-00040. [DOI] [PubMed] [Google Scholar]
- 2.Chatrath V, Sharan R, Kheterpal R, Kaur G, Ahuja J, Attri JP, et al. Comparative evaluation of 0.75% ropivacaine with clonidine and 0.75% ropivacaine with clonidine and 0.5% bupivacaine with clonidine in infraclavicular brachial plexus block. Anesth Essays Res. 2015;9:189–94. doi: 10.4103/0259-1162.153758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vainionpää VA, Haavisto ET, Huha TM, Korpi KJ, Nuutinen LS, Hollmén AI, et al. A clinical and pharmacokinetic comparison of ropivacaine and bupivacaine in axillary plexus block. Anesth Analg. 1995;81:534–8. doi: 10.1097/00000539-199509000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Kohli S, Kaur M, Sahoo S, Vajifdar H, Kohli P. Brachial plexus block: Comparison of two different doses of clonidine added to bupivacaine. J Anaesthesiol Clin Pharmacol. 2013;29:491–5. doi: 10.4103/0970-9185.119147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bazin JE, Massoni C, Groslier D, Fenies V, Bittar M, Schoeffler P, et al. Brachial plexus block: Effect of the addition of sufentanil to local anesthetic mixture on postoperative analgesia duration. Ann Fr Anesth Reanim. 1997;16:9–13. doi: 10.1016/s0750-7658(97)84271-2. [DOI] [PubMed] [Google Scholar]
- 6.Yadav RK, Sah BP, Kumar P, Singh SN. Effectiveness of addition of neostigmine or dexamethasone to local anaesthetic in providing perioperative analgesia for brachial plexus block: A prospective, randomized, double blinded, controlled study. Kathmandu Univ Med J (KUMJ) 2008;6:302–9. doi: 10.3126/kumj.v6i3.1704. [DOI] [PubMed] [Google Scholar]
- 7.Jarbo K, Batra YK, Panda NB. Brachial plexus block with midazolam and bupivacaine improves analgesia. Can J Anaesth. 2005;52:822–6. doi: 10.1007/BF03021776. [DOI] [PubMed] [Google Scholar]
- 8.Do SH. Magnesium: A versatile drug for anesthesiologists. Korean J Anesthesiol. 2013;65:4–8. doi: 10.4097/kjae.2013.65.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malleeswaran S, Panda N, Mathew P, Bagga R. A randomised study of magnesium sulphate as an adjuvant to intrathecal bupivacaine in patients with mild preeclampsia undergoing caesarean section. Int J Obstet Anesth. 2010;19:161–6. doi: 10.1016/j.ijoa.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Gerlach AT, Dasta JF. Dexmedetomidine: An updated review. Ann Pharmacother. 2007;41:245–52. doi: 10.1345/aph.1H314. [DOI] [PubMed] [Google Scholar]
- 11.Huang R, Hertz L. Receptor subtype and dose dependence of dexmedetomidine-induced accumulation of [14C] glutamine in astrocytes suggests glial involvement in its hypnotic-sedative and anesthetic-sparing effects. Brain Res. 2000;873:297–301. doi: 10.1016/s0006-8993(00)02525-7. [DOI] [PubMed] [Google Scholar]
- 12.Kettner SC. Dexmedetomidine as adjuvant for peripheral nerve blocks. Br J Anaesth. 2013;111:123. doi: 10.1093/bja/aet179. [DOI] [PubMed] [Google Scholar]
- 13.Ammar AS, Mahmoud KM. Ultrasound-guided single injection infraclavicular brachial plexus block using bupivacaine alone or combined with dexmedetomidine for pain control in upper limb surgery: A prospective randomized controlled trial. Saudi J Anaesth. 2012;6:109–14. doi: 10.4103/1658-354X.97021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee AR, Yi HW, Chung IS, Ko JS, Ahn HJ, Gwak MS, et al. Magnesium added to bupivacaine prolongs the duration of analgesia after interscalene nerve block. Can J Anaesth. 2012;59:21–7. doi: 10.1007/s12630-011-9604-5. [DOI] [PubMed] [Google Scholar]
- 15.Das A, Majumdar S, Halder S, Chattopadhyay S, Pal S, Kundu R, et al. Effect of dexmedetomidine as adjuvant in ropivacaine-induced supraclavicular brachial plexus block: A prospective, double-blinded and randomized controlled study. Saudi J Anaesth. 2014;8:S72–7. doi: 10.4103/1658-354X.144082. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Mukherjee K, Das A, Basunia SR, Dutta S, Mandal P, Mukherjee A, et al. Evaluation of magnesium as an adjuvant in ropivacaine-induced supraclavicular brachial plexus block: A prospective, double-blinded randomized controlled study. J Res Pharm Pract. 2014;3:123–9. doi: 10.4103/2279-042X.145387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haghighi M, Soleymanha M, Sedighinejad A, Mirbolook A, Naderi Nabi B, Rahmati M, et al. The effect of magnesium sulfate on motor and sensory axillary plexus blockade. Anesth Pain Med. 2015;5:e21943. doi: 10.5812/aapm.21943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ekmekci P, Bengisun ZK, Akan B, Kazbek BK, Ozkan KS, Suer AH, et al. The effect of magnesium added to levobupivacaine for femoral nerve block on postoperative analgesia in patients undergoing ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2013;21:1119–24. doi: 10.1007/s00167-012-2093-4. [DOI] [PubMed] [Google Scholar]
- 19.Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–9. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- 20.Akutagawa T, Kitahata LM, Saito H, Collins JG, Katz JD. Magnesium enhances local anesthetic nerve block of frog sciatic nerve. Anesth Analg. 1984;63:111–6. [PubMed] [Google Scholar]
- 21.Buvanendran A, McCarthy RJ, Kroin JS, Leong W, Perry P, Tuman KJ, et al. Intrathecal magnesium prolongs fentanyl analgesia: A prospective, randomized, controlled trial. Anesth Analg. 2002;95:661–6. doi: 10.1097/00000539-200209000-00031. [DOI] [PubMed] [Google Scholar]
- 22.Ghatak T, Chandra G, Malik A, Singh D, Bhatia VK. Evaluation of the effect of magnesium sulphate vs. clonidine as adjunct to epidural bupivacaine. Indian J Anaesth. 2010;54:308–13. doi: 10.4103/0019-5049.68373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narang S, Dali JS, Agarwal M, Garg R. Evaluation of the efficacy of magnesium sulphate as an adjuvant to lignocaine for intravenous regional anaesthesia for upper limb surgery. Anaesth Intensive Care. 2008;36:840–4. doi: 10.1177/0310057X0803600614. [DOI] [PubMed] [Google Scholar]
- 24.Choi IG, Choi YS, Kim YH, Min JH, Chae YK, Lee YK, et al. The effects of postoperative brachial plexus block using mgSO(4) on the postoperative pain after upper extremity surgery. Korean J Pain. 2011;24:158–63. doi: 10.3344/kjp.2011.24.3.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo TZ, Jiang JY, Buttermann AE, Maze M. Dexmedetomidine injection into the locus ceruleus produces antinociception. Anesthesiology. 1996;84:873–81. doi: 10.1097/00000542-199604000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Talke P, Lobo E, Brown R. Systemically administered alpha2-agonist-induced peripheral vasoconstriction in humans. Anesthesiology. 2003;99:65–70. doi: 10.1097/00000542-200307000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Ruffolo RR., Jr Distribution and function of peripheral alpha-adrenoceptors in the cardiovascular system. Pharmacol Biochem Behav. 1985;22:827–33. doi: 10.1016/0091-3057(85)90535-0. [DOI] [PubMed] [Google Scholar]
- 28.Kosugi T, Mizuta K, Fujita T, Nakashima M, Kumamoto E. High concentrations of dexmedetomidine inhibit compound action potentials in frog sciatic nerves without alpha(2) adrenoceptor activation. Br J Pharmacol. 2010;160:1662–76. doi: 10.1111/j.1476-5381.2010.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaumann DM, Brunet PC, Jirounek P. Hyperpolarizing afterpotentials in C fibers and local anesthetic effects of clonidine and lidocaine. Pharmacology. 1994;48:21–9. doi: 10.1159/000139158. [DOI] [PubMed] [Google Scholar]
- 30.Brummett CM, Hong EK, Janda AM, Amodeo FS, Lydic R. Perineural dexmedetomidine added to ropivacaine for sciatic nerve block in rats prolongs the duration of analgesia by blocking the hyperpolarization-activated cation current. Anesthesiology. 2011;115:836–43. doi: 10.1097/ALN.0b013e318221fcc9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esmaoglu A, Yegenoglu F, Akin A, Turk CY. Dexmedetomidine added to levobupivacaine prolongs axillary brachial plexus block. Anesth Analg. 2010;111:1548–51. doi: 10.1213/ANE.0b013e3181fa3095. [DOI] [PubMed] [Google Scholar]
- 32.Gandhi R, Shah A, Patel I. Use of dexmedetomidine along with bupivacaine for brachial plexus block. Natl J Med Res. 2012;2:67–9. [Google Scholar]
- 33.Song JH, Shim HY, Lee TJ, Jung JK, Cha YD, Lee DI, et al. Comparison of dexmedetomidine and epinephrine as an adjuvant to 1% mepivacaine in brachial plexus block. Korean J Anesthesiol. 2014;66:283–9. doi: 10.4097/kjae.2014.66.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaygusuz K, Kol IO, Duger C, Gursoy S, Ozturk H, Kayacan U, et al. Effects of adding dexmedetomidine to levobupivacaine in axillary brachial plexus block. Curr Ther Res Clin Exp. 2012;73:103–11. doi: 10.1016/j.curtheres.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mirkheshti A, Saadatniaki A, Salimi A, Manafi Rasi A, Memary E, Yahyaei H, et al. Effects of dexmedetomidine versus ketorolac as local anesthetic adjuvants on the onset and duration of infraclavicular brachial plexus block. Anesth Pain Med. 2014;4:e17620. doi: 10.5812/aapm.17620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marhofer D, Kettner SC, Marhofer P, Pils S, Weber M, Zeitlinger M, et al. Dexmedetomidine as an adjuvant to ropivacaine prolongs peripheral nerve block: A volunteer study. Br J Anaesth. 2013;110:438–42. doi: 10.1093/bja/aes400. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Wang CS, Shi JH, Sun B, Liu SJ, Li P, et al. Perineural administration of dexmedetomidine in combination with ropivacaine prolongs axillary brachial plexus block. Int J Clin Exp Med. 2014;7:680–5. [PMC free article] [PubMed] [Google Scholar]