Abstract
Background and Aims:
The ideal dose of nalbuphine for brachial plexus block (BPB) is a matter of debate. This study was carried out to evaluate 5 mg or 10 mg of nalbuphine added to 0.375% levobupivacaine, with regard to the duration of analgesia. Our study also sought to assess the onset and duration of sensorimotor blockade, hemodynamic effects, sedation, and adverse effects.
Materials and Methods:
One hundred adult patients undergoing upper-limb surgeries under supraclavicular BPB were randomly allocated into two groups. Group LN5 received 29 ml of 0.375% levobupivacaine plus 5 mg of nalbuphine diluted in 1 ml of normal saline. Group LN10 received 29 ml of 0.375% levobupivacaine plus 10 mg of nalbuphine diluted in 1 ml of normal saline. Onset and duration of sensorimotor blockade, hemodynamic variables, duration of analgesia, and adverse effects were recorded. The data were analyzed with Students t-test and Chi-square test.
Results:
Onset of sensory block and motor block was 10.57 ± 3.5 and 17.16 ± 1.3 min, respectively, in Group LN5, while it was 8.64 ± 1.7 and 14.3 ± 1.2 min, respectively, in Group LN10. The duration of analgesia was significantly prolonged in Group LN10 compared to Group LN5 (833.55 ± 141.6 vs. 698.44 ± 138.6 min; P = 0.001). Postoperative visual analog scale value at 24 h was significantly lower in Group LN10 (P < 0.05).
Conclusion:
A higher dose of nalbuphine in BPB hastens the onset, and prolongs the duration of sensorimotor blockade and analgesia, without any significant side effects.
Keywords: Anesthesia, brachial plexus block, double-blind method, levobupivacaine, local, nalbuphine, prospective studies
INTRODUCTION
Brachial plexus block (BPB) is a routinely performing regional anesthesia technique for surgeries involving upper limb, especially below mid-arm orthopedic procedures. The BPB not only provides good intraoperative anesthesia but also produces very good postoperative analgesia, thereby reducing the incidence of complications and providing early mobilization.[1]
Local anesthetics alone for supraclavicular BPB provide good intraoperative conditions but produce a shorter duration of postoperative analgesia. Various adjuvants to local anesthetics were used to prolong postoperative analgesia with variable results and advantages.[2] Drugs such as epinephrine, morphine, pethidine, dexamethasone, clonidine, dexmedetomidine, butorphanol, and midazolam are used along with local anesthetics for this purpose.
Recently, nalbuphine was studied frequently as an adjuvant to local anesthetics in spinal, epidural, and intravenous (IV) block, and the results of all studies conclude that nalbuphine is effective when used as an adjuvant to local anesthetics in spinal, epidural, and IV block as it significantly prolongs the block duration.[3]
Nalbuphine is 14-hydroxymorphine derivative with a strong analgesic effect with mixed κ agonist and μ antagonist.[4] The analgesic effect of nalbuphine has been found to be equal to the analgesic effect of morphine but unlike it has a ceiling effect on respiration. Nalbuphine has the potential to maintain or even enhance μ-opioid-based analgesic effect while simultaneously mitigating the μ-opioid side effects.[5]
There is no study suggestive of any appropriate dose of nalbuphine as an adjuvant in supraclavicular BPB. Hence, the present study was conducted to evaluate the duration of analgesia of two different doses of nalbuphine, 5 and 10 mg added to 0.375% levobupivacaine, in patients posted for upper-limb surgeries under supraclavicular BPB. Our study also sought to assess the onset and duration of sensorimotor blockade, hemodynamic variables, and adverse effects in both the groups.
MATERIALS AND METHODS
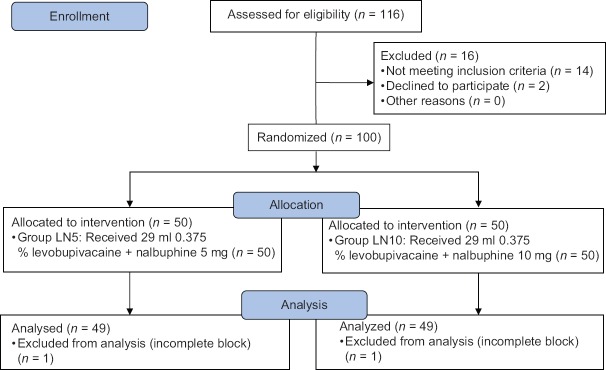
After obtaining the institutional ethics committee approval, one hundred adult patients with age between 20 and 60 years, undergoing elective orthopedic surgeries of fixation of fracture elbow and forearm under supraclavicular BPB in Rajiv Gandhi Institute of Medical Sciences College and Hospital, Kadapa, Andhra Pradesh, were randomized into two groups based on block randomization [Figure 1].
Figure 1.
Consort diagram showing the number of patients included and analyzed
The exclusion criteria included patient refusal; American Society of Anesthesiologists (ASA) physical status 3, 4, and 5; any known hypersensitivity or contraindication to levobupivacaine and nalbuphine hydrochloride; pregnancy; lactating mothers; uncontrolled diabetes and hypertension; hepatic, renal, or cardiopulmonary abnormality; alcoholism; long-term analgesic therapy; bleeding diathesis; and local skin site infections. Patients having a history of significant neurological, psychiatric, and neuromuscular disorders were also excluded from the study.
Written informed consent was taken from each individual willing to participate in the study. Preanesthetic checkup and routine investigations such as complete blood count, serum creatinine, and electrocardiogram (ECG) were done. Patients were kept nil by mouth for 6 h. All patients were clinically examined in the preoperative period, when the whole procedure was explained. A 10-cm visual analog scale (VAS) (0, no pain and 10, worst pain imaginable) was also explained during the preoperative visit. All patients received tablet clonazepam 0.5 mg orally on the night before surgery.
Patients were randomized using a computer-generated randomization list. Random group assigned was enclosed in a sealed envelope to ensure concealment of allocation sequence. The sealed envelope was opened by an anesthesiologist who was not involved in the study to prepare the drug solution according to randomization. The anesthesiologist performing the block and observing the patient was blinded to the treatment group. Data collection was done by the same anesthesiologist who was unaware of the group allocation. Patients were randomly assigned to one of the two equal groups to receive either of the following: Group LN5 –29 ml of 0.375% levobupivacaine plus 5 mg of nalbuphine diluted in 1 ml of normal saline (total 30 ml) and Group LN10 –29 ml of 0.375% levobupivacaine plus 10 mg of nalbuphine diluted in 1 ml of normal saline (total 30 ml).
After shifting the patient into operation theater, noninvasive monitors such as blood pressure (noninvasive blood pressure), oxygen saturation (SPO2), and ECG were applied and their baseline values were measured. IV access was established using 18 G cannula. Supplemental oxygen was provided via nasal cannula at 2 L/min to all patients. Sedation was provided by IV administration of midazolam 1 mg and fentanyl 40 μg before the block. A nerve stimulation technique with a Stimuplex© needle (B Braun 22G, 5 cm) and a stimulator was used. After desired motor response with stimulating current of <0.4 mA (2 Hz, 0.1 ms duration), the local anesthetic solution was injected in incremental 5 ml boluses with intermittent aspiration.
Sensory and motor blockades were assessed every 2 min after completion of injection till 30 min and then every 30 min after the end of surgery till first 12 h, thereafter hourly until the block had completely worn off. For sensory loss assessment, we used pinprick test with a 3-point scale: 0 – no block, 1 – analgesia (loss of sensation to pinprick), and 2 – loss of touch. Motor blockade was evaluated by the ability to flex the elbow and hand as: 0 – full flexion/extension movement in hand and arm against resistance, 1 – movement against gravity but not against resistance, 2 – flicker of movement in hand but not in arm, and 3 – no movement (complete motor block).
Onset of sensory blockade was defined as the interval between the end of injection and sensory blockade and was demonstrated as loss of sensation to pinprick or by score of 1 on pinprick response. Onset of motor blockade was the interval between the end of injection and complete motor paralysis of wrist and hand. The duration of sensory blockade was defined as the time interval between sensory blockade and reappearance of pinprick response. The duration of motor blockade was defined as the time interval between maximum motor blockade and complete movement of wrist and fingers. Duration of analgesia was taken as the time interval between onset of sensory blockade and the first dose of rescue analgesic given to the patient. A complete block was defined as one associated with Grade 2 sensory anesthesia and Grade 3 motor block, and only these patients were included for further study. Patients with sensory block of Grade 0 and 1 and motor block of Grade 0, 1, and 2 were considered to have incomplete block and hence were excluded from further analysis and converted to general anesthesia.
Postoperative pain was assessed using VAS (0 – no pain to 10 − worst pain) every hour till the block lasted. Postoperative vitals (heart rate [HR], systolic blood pressure, diastolic blood pressure, mean arterial pressure, and SpO2) were recorded every 2 h for the first 6 h and thereafter every 4 h till the need for rescue analgesia. Rescue analgesia was provided with injection diclofenac sodium 75 mg intramuscularly when VAS ≥3 cm. The number of diclofenac injections given to each patient during first 24 h of the postoperative period was recorded. The time between complete sensory block and first analgesic request was recorded as a duration of analgesia.
Patients were observed for any incidence of hypotension, bradycardia, fall in peripheral SPO2, any discomfort, nausea, vomiting, shivering, pruritus, pain, or any other adverse effects and were managed according to clinical protocol.
Sample size calculation was done based on a pilot study of ten patients (5 in each group). The duration of analgesia in the pilot study in two groups was 672.9 ± 113.2 min and 796.2 ± 121.4 min, respectively. To detect an observed difference of 2 h in duration of analgesia between the groups, with a type 1 error of 5% and a power of 80%, the minimum sample size required was 43 in each group. We included 50 patients in each group for better validation of results. Data were checked, entered, and analyzed using SPSS version 19 for Windows (IBM Corp., Armonk, NY, USA). Quantitative data were represented as mean ± standard deviation, and for qualitative data, number and percentages were used. Student's t-test was used as test of significance to find an association for quantitative data. The Chi-square test was used as test of significance to find association for qualitative data. P < 0.05 was considered statistically significant.
RESULTS
Fifty patients in each group were enrolled for the study. One patient from Group LN5 and one patient from Group LN10 were excluded from the study due to incomplete/failed block. A total of 98 patients (49 in each group) were included in the study.
Patients of both groups were comparable with respect to the demographic profile for age, sex distribution, ASA physical status, body mass index, and the duration of surgery [Table 1]. There was no statistical significance in baseline hemodynamic parameters and type of fractures between the two groups (P > 0.05). Table 2 shows the type of fractures in the patients studied.
Table 1.
Demographic data
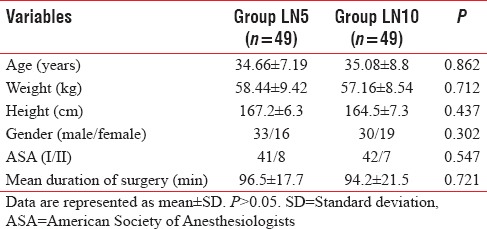
Table 2.
Type of fractures in the study participants
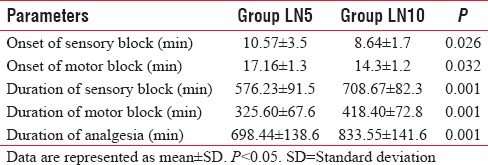
The sensory and motor block onset was significantly faster in Group LN10 than in Group LN5. The mean sensory block onset time was 10.57 ± 3.5 min in Group LN5 as compared to 8.64 ± 1.7 min in Group LN10 (P = 0.026). The mean motor block onset time was 17.16 ± 1.3 min in Group LN5 as compared to 14.3 ± 1.2 min in Group LN10 (P = 0.032) [Table 3]. The duration of sensory block was prolonged in Group LN10 (708.67 ± 82.3 min) when compared to Group LN5 (576.23 ± 91.5 min) (P = 0.001). The duration of motor block was also prolonged in Group LN10 (418.40 ± 72.8 min) when compared to Group LN5 (325.60 ± 67.6 min) (P = 0.001) [Table 3]. The duration of analgesia was significantly prolonged in Group LN10 (833.55 ± 141.6 min) when compared with Group LN5 (698.44 ± 138.6 min) (P = 0.001) [Table 3].
Table 3.
Characteristics of block in each group
Perioperative hemodynamic parameters of blood pressure, HR, and ECG were stable. The respiratory rate and peripheral SPO2 were comparable between the groups. There was no complaint of difficulty in breathing or any clinical evidence of diaphragmatic palsy or pneumothorax in any patient.
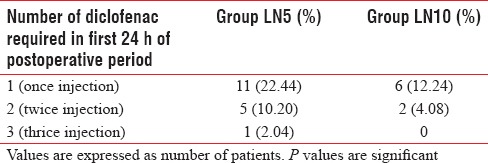
Table 4 shows the side effects encountered throughout our study, which indicates that Group LN10 suffered from slightly more incidence of nausea, vomiting, sedation, and pruritus, but it was statistically insignificant (P > 0.05) when compared with Group LN5. 17/49 patients (34.69%) in Group LN5 required diclofenac sodium injection as rescue analgesia, whereas 8/49 patients (16.32%) in Group LN10 required rescue analgesia in the first 24 h of postoperative period [P = 0.037; Table 5].
Table 4.
Comparison of side effects in each group
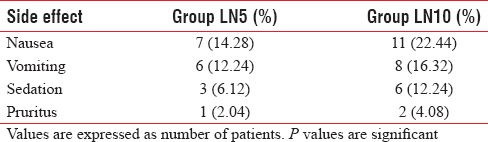
Table 5.
Rescue analgesic requirement in postoperative period
DISCUSSION
Results of this randomized, double-blinded study shows that addition of 10 mg nalbuphine to 0.375% levobupivacaine produces a longer duration of analgesia compared to 5 mg nalbuphine in supraclavicular BPB. The higher dose of nalbuphine also hastens the onset and prolongs the duration of sensory and motor block. Fewer patients (16.32%) in Group LN10 required diclofenac sodium injection as rescue analgesic than patients (34.69%) in Group LN5.
Nalbuphine is a semisynthetic opioid with mixed κ agonist and μ antagonist properties. Nalbuphine has been proven to prevent hemodynamic stress response associated with endotracheal intubation. Like fentanyl and propofol, nalbuphine is also popular in producing analgesia during monitored anesthesia care. The drug is also very effective in subarachnoid as well as epidural route for prolonging sensory and motor block duration and also postoperative analgesia. Success and nontoxicity of the drug in subarachnoid and epidural route ensure that the drug can safely be used perineurally in any peripheral nerve block.[6]
Dose of levobupivacaine was selected from previous studies.[7] We had selected a slightly lesser dose of drug to be on a safer side. Ambi et al. had chosen 36 ml of 0.5% levobupivacaine for perineural ultrasound-guided axillary BPB and here we have taken 30 ml of 0.375% levobupivacaine.[8] Chatrath et al., in their studies, used 10 mg nalbuphine in the lumbar epidural route safely without any significant complication. Again, the blood concentration of anesthetic drugs after various routes of administration reveals that drug concentration is highest after intercostal nerve blockade, followed in order of decreasing concentration by injection into caudal epidural space, lumbar epidural space, brachial plexus, and subcutaneous tissue. Thus, any drug used in brachial plexus blockade is associated with a lower risk of absorption and side effects than administered epidurally. Hence, we had chosen 10 mg nalbuphine for the use in BPB. We have found the reference of much higher dose (20 mg) of nalbuphine used for BPB for the patients undergoing elective forearm and hand surgery.
In our study, the onset of sensory and motor block was earlier with the higher dose of nalbuphine (10 mg). Tiwari et al. reported that the addition of nalbuphine to local anesthetic in intrathecal route produces earlier onset of sensory and motor blocks.[9] They also found that higher dose of nalbuphine also hastens the onset of sensory and motor block compared with lower dose. Similar results were also observed by Mukherjee et al.[10] They found that with higher dose of nalbuphine, earlier onset of sensory and motor blocks occurs, but these values have no statistical significance (P > 0.05).
In our study, the duration of sensory block (708.67 ± 82.3 min in Group LN10 vs. 576.23 ± 91.5 min in Group LN5) was significantly increased in LN10 group than in the LN5 group (P = 0.0001). The duration of motor block (418.40 ± 72.80 min in LN10 Group vs. 325.60 ± 67.6 min in LN5 Group) was also significantly prolonged in LN10 group than in LN5 group (P = 0.001). These results were very similar with Ahluwalia et al. who reported that, in subarachnoid route, sensory and motor blocks were significantly prolonged in nalbuphine-treated group while compared with control.[11] However, on the contrary, Mukherjee et al. found that though sensory block was significantly prolonged along with increasing concentration of nalbuphine, motor was quite comparable between the two groups.
In our study, duration of analgesia was 833.55 ± 141.6 min and 698.44 ± 138.6 min in LN10 and LN5 groups, respectively. The duration of analgesia was significantly (P = 0.001) prolonged in higher-dose nalbuphine group. However, Chatrath et al. observed that epidurally administered nalbuphine does not prolong the duration of analgesia in a statistically significant manner while compared with the equipotent dose of tramadol in the same route. Again, Mukherjee et al. reported that the duration of analgesia in subarachnoid block was increased proportionally with the increased dose of nalbuphine when administered intrathecally and the difference was statistically significant.
In our study, patients of LN10 Group required significantly less number of diclofenac sodium injection as rescue analgesia in first 24 h of the postoperative period than the patients of LN5 Group (P < 0.05). Mukherjee et al. also observed that rescue analgesic requirement was significantly decreased with a higher dose of nalbuphine when administered intrathecally.
In our study, we have observed nausea, vomiting, sedation, and pruritus as side effects in both groups, but the incidence was quite comparable between the two groups (P > 0.05). Nausea does not require any active management except increasing the fluid transfusion rate. Two patients in LN10 group and one patient in LN5 group suffered from vomiting. All the three patients were managed with slow IV ondansetron 4 mg. Although the incidence of sedation was higher in LN10 group, it was quite arousable and did not cause any respiratory depression. Similarly, pruritus was also higher in LN10 group, but it was self-limiting. Jyothi et al., in their study, with three different doses of nalbuphine (0.8, 1.6, and 2.5 mg intrathecally) observed complications such as nausea, vomiting, urinary retention, shivering, pruritus, hypotension, and respiratory depression.[12] Shivering, urinary retention, and hypotension were probably due to spinal anesthesia-related complications. Again, Ahluwalia et al. reported that only nausea and vomiting (5 vs. 2) were associated with intrathecal nalbuphine group while compared with tramadol in the same route. Mukherjee et al. administered nalbuphine intrathecally (placebo, 0.2, 0.4, and 0.8 mg) for orthopedic surgery and observed that few side effects such as pruritus, nausea, vomiting, respiratory depression, and bradycardia were exclusively associated with the highest dose of nalbuphine, and hypotension was evident in all the groups which was probably due to spinal anesthesia.
The major drawback of our study was that we had not taken any standardized dose of nalbuphine due to nonavailability of proper pharmaceutical reference relating to dose equivalence with other well-known opioids. Furthermore, the unavailability of ultrasound in our institute is another great drawback of this study.
CONCLUSION
Finally, we do conclude that, during daycare forearm and hand surgery, addition of 10 mg nalbuphine hydrochloride to levobupivacaine 0.375% solution in supraclavicular BPB hastens the onset time of sensory and motor block, prolongs the duration of sensory and motor blockades, and reduces the requirement of rescue analgesic in postoperative period without any appreciable side effect.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bruce BG, Green A, Blaine TA, Wesner LV. Brachial plexus blocks for upper extremity orthopaedic surgery. J Am Acad Orthop Surg. 2012;20:38–47. doi: 10.5435/JAAOS-20-01-038. [DOI] [PubMed] [Google Scholar]
- 2.Murphy DB, McCartney CJ, Chan VW. Novel analgesic adjuncts for brachial plexus block: A systematic review. Anesth Analg. 2000;90:1122–8. doi: 10.1097/00000539-200005000-00023. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed F, Narula H, Khandelwal M, Dutta D. A comparative study of three different doses of nalbuphine as an adjuvant to intrathecal bupivacaine for postoperative analgesia in abdominal hysterectomy. Indian J Pain. 2016;30:23–8. [Google Scholar]
- 4.Pick CG, Paul D, Pasternak GW. Nalbuphine, a mixed kappa 1 and kappa 3 analgesic in mice. J Pharmacol Exp Ther. 1992;262:1044–50. [PubMed] [Google Scholar]
- 5.Gunion MW, Marchionne AM, Anderson TM. Use of the mixed agonist-antagonist nalbuphine in opioid based analgesia. Acute Pain. 2004;6:29–39. [Google Scholar]
- 6.Chatrath V, Attri JP, Bala A, Khetarpal R, Ahuja D, Kaur S, et al. Epidural nalbuphine for postoperative analgesia in orthopedic surgery. Anesth Essays Res. 2015;9:326–30. doi: 10.4103/0259-1162.158004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das A, RoyBasunia S, Mukherjee A, Biswas H, Biswas R, Mitra T, et al. Perineural nalbuphine in ambulatory upper limb surgery: A comparison of effects of levobupivacaine with and without nalbuphine as adjuvant in supraclavicular brachial plexus block – A prospective, double-blinded, randomized controlled study. Anesth Essays Res. 2017;11:40–6. doi: 10.4103/0259-1162.200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambi U, Bhanupriya P, Hulkund SY, Prakashappa DS. Comparison between perivascular and perineural ultrasound-guided axillary brachial plexus block using levobupivacaine: A prospective, randomised clinical study. Indian J Anaesth. 2015;59:658–63. doi: 10.4103/0019-5049.167476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tiwari AK, Tomar GS, Agrawal J. Intrathecal bupivacaine in comparison with a combination of nalbuphine and bupivacaine for subarachnoid block: A randomized prospective double-blind clinical study. Am J Ther. 2013;20:592–5. doi: 10.1097/MJT.0b013e31822048db. [DOI] [PubMed] [Google Scholar]
- 10.Mukherjee A, Pal A, Agrawal J, Mehrotra A, Dawar N. Intrathecal nalbuphine as an adjuvant to subarachnoid block: What is the most effective dose? Anesth Essays Res. 2011;5:171–5. doi: 10.4103/0259-1162.94759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahluwalia P, Ahluwalia A, Varshney R, Thakur S, Bhandari S. A prospective randomized double blind study to evaluate the effects of intrathecal nalbuphine in patients of lower abdominal surgeries under spinal anaesthesia. Int J Sci Stud. 2015;3:19–23. [Google Scholar]
- 12.Jyothi B, Gowda S, Shaikh SI. A comparison of analgesic effect of different doses of intrathecal nalbuphine hydrochloride with bupivacaine and bupivacaine alone for lower abdominal and orthopedic surgeries. Indian J Pain. 2014;28:18–23. [Google Scholar]