Abstract
Context:
It is a well-known fact that severe pregnancy-induced hypertension (PIH) can be disastrous at times as it can cause a lot of complications to both pregnant women and her baby. Hence, it is always desirable to know the extent of severity by a real-time and easily accessible modality like ultrasound.
Aims:
The aim of the study was to evaluate the incidence of raised intracranial pressure (ICP) in severe preeclampsia and eclampsia patients using ocular ultrasonography with optic nerve sheath diameter (ONSD) measurement.
Settings and Design:
This study design was a prospective and clinically controlled blinded observational study.
Materials and Methods:
After taking necessary permissions from the Institution Ethical Committee, 75 patients were enrolled for the study. However, finally, 25 patients in severe preeclampsia and 24 in eclampsia group were compared with 25 normal term antenatal women. Demographic profiles, hemodynamic parameters, laboratory markers for severity of PIH, and ultrasonographic OSND were measured.
Statistical Analysis Used:
They were statistically analyzed and compared using one-way ANOVA and Tukey's test. Value of P < 0.05 was considered statistically significant.
Results:
All the three groups were comparable in terms of age, body weight, gestation age, gestity, and the number of primigravida in each group. There was a significant difference (P < 0.05) in mean levels of hepatic aminotransferase levels and platelet counts between groups. Aspartate transaminase and alanine transaminase levels were much higher in Group II and III as compared to Group I, while platelet levels were lower in study groups indicating increase in severity of PIH. There was also a significant difference for systolic blood pressure and diastolic blood pressure (P < 0.001) as both were significantly higher in study groups. Among severe PIH groups (Group II and III), the difference was comparable.
Conclusions:
OSND is a surrogate marker for raised ICP in severe PIH patients. It is a rapid, bedside, noninvasive, and readily accessible tool and could be a part of a holistic approach for managing such patients.
Keywords: Eclampsia, optic nerve sheath diameter, severe preeclampsia, ultrasonography
INTRODUCTION
Pregnancy-induced hypertension (PIH) is ranked as the second leading cause of maternal mortality worldwide.[1] PIH in its severe form is a potentially severe disease. It could be associated with maternal complications such as pulmonary, cardiac, or renal complications, HELLP syndrome which comprises hemolysis, increased liver enzyme, low platelet count syndrome, and neurologic complications. Severe preeclampsia increases the risk of maternal mortality and perinatal morbidity and mortality.[2]
Central nervous system sequelae are attributed to its abnormal vascular calibration syndrome[3,4,5,6] affecting specific areas of brain. Clinical signs of raised intracranial pressure (ICP) are not specific and often present late. They are difficult to interpret during pregnancy and preeclampsia. The actual incidence of raised ICP in preeclampsia and eclampsia is unknown. The gold standard method for ICP measurement is based on the use of invasive devices.[7] However, such invasive procedure can result in complication such as infection and hemorrhage. Furthermore, these procedures are contraindicated in state like thrombocytopenia and coagulopathy.[8] A lot of studies had suggested that ultrasonographic measurements of the optic nerve sheath diameter (ONSD) can give a fair idea of raised ICP.[9,10] The optic nerve is surrounded by a dural sheath and a subarachnoid space containing cerebrospinal fluid. About 3 mm behind the point of retina and optic nerve confluence, the optic nerve is only covered by fat and its sheath is quite distensible in case of raised ICP. Majority of articles on OSND as a marker of raised ICP are in the settings of neurocritical care such as stroke, CNS trauma, meningitis, and epilepsy.[11,12,13,14,15,16,17,18,19] Assuming OSND as a surrogate marker for raised ICP, its measurement could be used as guide for assessing the clinical severity as well as it can help in deciding the right anesthesia modality for emergency cesarean section in such severe PIH cases.
METHODS
This prospective and clinically controlled blinded observational study was conducted after taking necessary permissions from the Institutional Ethical Committee and written informed consent from all participants. It was conducted from May 2016 to March 2017. Initially, a total 75 patients were enrolled for this study. Two study groups with 25 patients each for severe preeclampsia (Group II) and eclampsia (Group III) were compared with equal number of normal term pregnant (Group I) who were used as control. In eclampsia, only those patients with documented single episode of seizure were taken for the study. Out of them only 24 were able to complete the study phase. Patients were managed as per institutional treatment protocols.
Severe preeclampsia was defined as raised blood pressure (BP) >160/110 mmHg, proteinuria in excess of 5 g/day, oliguria (<500 mL/day), elevated serum creatinine, intrauterine growth retardation, pulmonary edema, central nervous system manifestations (headache, visual disturbances, seizures, and stroke), hepatic tenderness, or the HELLP syndrome.
Eclampsia was defined as single or more episodes of seizure in a known case of preeclampsia. Healthy term pregnant women who present for emergency cesarean section due to some or other obstetric indications were used as a control for comparing OSND between groups.
Exclusion criteria were moderate-to-severe renal, hepatic, cardiovascular, or endocrinal dysfunction, coagulopathy, uncooperative patient or altered sensorium, patient refusal, multiple episodes of fits, history of ocular trauma or surgery, and blurring of vision.
OSND measurement was conducted in two axes of transverse and oblique sagittal. Value reported corresponds to the mean of the above two values for each patient. All measurements were taken by a single investigator who was blinded to groups and had enough experience of doing ocular sonography. All other observations were recorded by another coworker and he was not aware of OSND values for that patient. High-frequency linear probe of 7–12 MHz was used (Micromaxx, Sonosite, USA) while all patients were there in supine position. Using two-dimensional mode, depth of the optic nerve was localized and was marked at 3 mm behind the retinal and optic nerve junction with the help of machine caliper. At this point, transverse diameter of optic nerve was calculated. Scanning begins from lateral side of eye in coronal section and then in oblique sagittal plane measuring OSND in both the axes. Minimal pressure was insured on the eyeball, and ample amount of sterile-conducting medium was used. Power optimization for scanning in such cases was followed. Measurement in a normal antenatal patient is shown in Figure 1.
Figure 1.
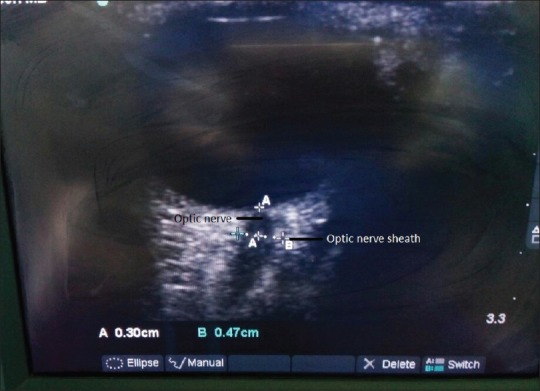
Measurements (in mm) optic nerve and optic nerve sheath diameter in normal term antenatal
Data were analyzed using SPSS version 16.0 (IBM, USA). One-way ANOVA was used to compare the three groups, and for analyzing variables between two different groups, post hoc test (Tukey's test) was used. Data are presented as mean ± standard deviation (SD) until specified and P < 0.05 and 0.001 were taken as significant and highly significant, respectively.
RESULTS
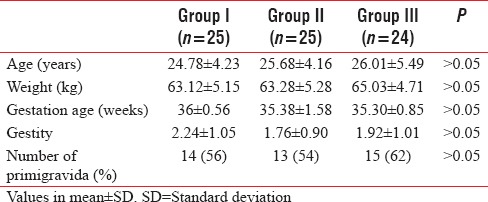
Initially, 25 patients were enrolled in each group. As one patient in each Group II and Group III complained of severe epigastric pain and visual disturbances, respectively, just before induction, so these were excluded. Table 1 demonstrates the demographic profile of three groups. All the three groups were comparable in terms of age, body weight, gestation age, gestity, and the number of primigravida in each group. Although incidence of PIH is more commonly associated with primigravida, in our study, there was an insignificant difference between three groups.
Table 1.
Demographic profiles
Various relevant laboratory markers for severity of PIH were measured and compared for the groups. These are presented in Table 2.
Table 2.
Laboratory investigations
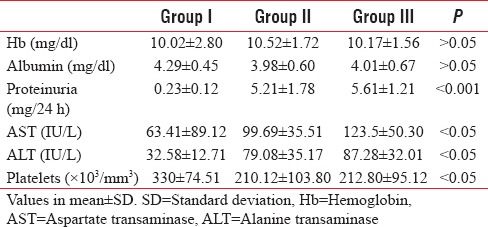
The mean hemoglobin and serum albumin levels were comparable between the groups, but there was a significant difference in mean levels of hepatic aminotransferase levels and platelet counts between groups. Aspartate transaminase and alanine transaminase levels were much higher in Group II and III as compared to Group I, while platelet levels were lower in study groups as compared to control group. None of the patients in all showed signs of HELLP syndrome.
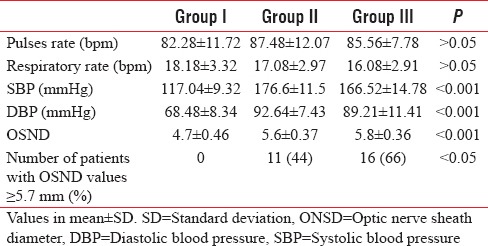
Hemodynamic variables along with OSND were measured at preoperative phase. Trends measured and comparison done among groups is given in Table 3. As obvious from table, while heart rate and respiratory rate were comparable, but there was a significant difference in systolic BP and diastolic BP (P < 0.001) as both were significantly higher in Group II and III. Among PIH groups (Group II and III), the difference was comparable. The ONSD values were comparable for study groups, but there was a highly significant difference (P < 0.001) when compared to control group of normal term antenatal. The mean OSND values for Group II and Group III were 5.6 and 5.8 mm, respectively, while it was 4.7 mm for Group I.
Table 3.
Hemodynamic parameters and optic nerve sheath diameter values
DISCUSSION
Raised ICP could lead to disastrous complications and adverse outcomes. Invasive measurement by intraventricular or intraparenchymal leads is the gold standard and frequently used for point-of-care management in trauma patients. However, its applications may not be feasible in other complex scenarios such as severe PIH patients scheduled for emergency cesarean section.
A clinical sign of raised ICP is often quite late and unreliable. It may lead to an unwanted delay in treatment. Kimberly and Noble used magnetic resonance imaging (MRI) for detecting raised ICP using ONSD.[20] MRI use may be limited in emergency and other complex situations such as our study group patients. Increase in cerebral edema is considered to be the main factor which results in further deterioration of severe preeclampsia patients to eclampsia. Researchers like Zeeman et al. showed cerebral edema to be present in the majority of such patients.[21] In two of our patients of eclampsia group, there was only a borderline increase in their BP (raised BP is otherwise considered as a hallmark of PIH). This further signifies the role of ultrasound-guided ONSD values for their diagnosis and management along with other severity markers. There is a wide variation reported when it comes to determining the optimal cutoff values. Rajajee et al.[22] and Amini et al.[23] use invasive techniques to know cutoff value and values ranged from 4.8 to 5.9 mm. Values ≥5.8 mm have been associated with ICP levels above 20 mmHg. Most of the articles about OSND as a surrogate marker for raised ICP came from studies done on trauma victims in Intensive Care Unit (Geeraerts et al.).[9,11,17] There seem to be considerable variations on the cutoff values for OSND for raised ICP. In our study, the mean ONSD in control group was 4.36 (0.47 SD) mm, which was quite different from Group II and Group III. OSND values were significantly higher as they were 5.67 mm and 5.74 mm in Group II and Group III, respectively [Figures 2 and 3]. Similar significant difference was reported in a study conducted by Dubost et al.[24] They compared preeclampsia to normal term antenatal cases, and they found a significant difference between these two groups (5.4 mm vs. 4.5 mm). From our small study, although we could not suggest a definite cutoff valve for OSND at what value the severe preeclampsia patient will further deteriorate to eclampsia, but our study suggests that such a tipping-off value for OSND could be close to 5.7 mm (as it lies in middle for mean values found in both severe PIH groups). In our study, 11 (44%) in Group II and 16 (66%) in Group III had ONSD values ≥ 5.7 mm. Hence, these 11 patients in severe preeclampsia group seem to be at greater risk for further deteriorating into eclampsia and need immediate emergency management. Analysis of a big cohort is warranted to get a more acceptable cut-off value. Although we have not reported the serum magnesium levels in Group III, its correlation with OSND is desirable as it can be used to trace and correlate well with the clinical status of the patients. It is also further warranted to conduct larger studies to explore whether subarachnoid block should be avoided in patients with significantly higher OSND values (indicating raised ICP) given the risk of brainstem herniation.[25]
Figure 2.
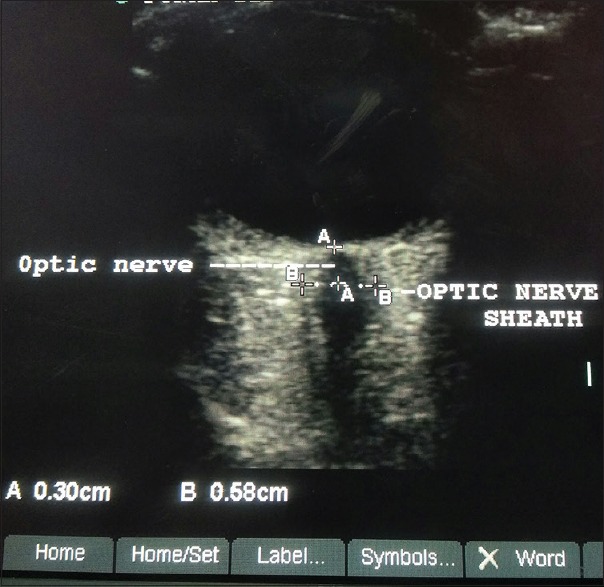
Measurements (in mm) optic nerve and optic nerve sheath diameter in an severe preeclampsia
Figure 3.
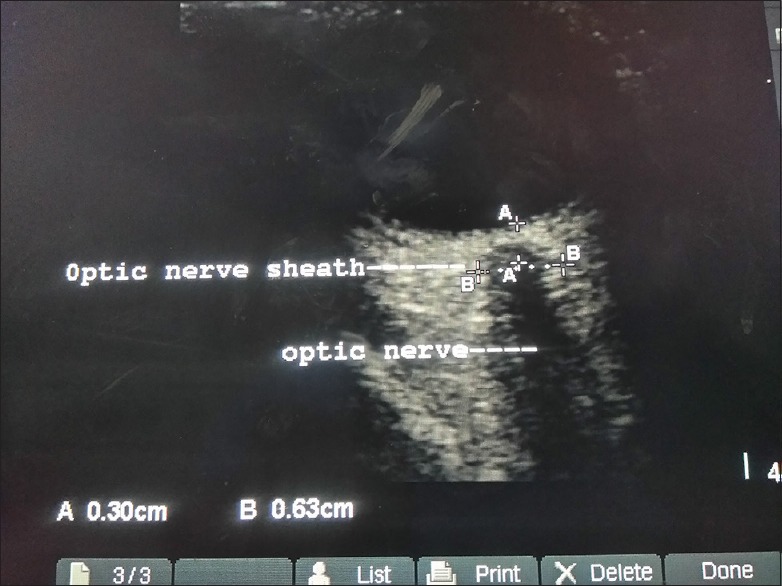
Measurements (in mm) optic nerve and optic nerve sheath diameter in an eclampsia patient
CONCLUSIONS
The measurement of OSND by ultrasonography provides a quick, noninvasive, and accessible potential tool to evaluate severe PIH patients. This modality can be used in managing such patients who are at risk of severe neurological outcomes of eclampsia. At the same time, it should be emphasized that transorbital OSND should be a part of holistic approach for the management of such patients and not as a sole-driving factor for decision-making for a better outcome.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hariharan N, Shoemaker A, Wagner S. Pathophysiology of hypertension in preeclampsia. Microvasc Res. 2017;109:34–7. doi: 10.1016/j.mvr.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Vest AR, Cho LS. Hypertension in pregnancy. Curr Atheroscler Rep. 2014;16:395. doi: 10.1007/s11883-013-0395-8. [DOI] [PubMed] [Google Scholar]
- 3.Ducros A, Bousser MG. Reversible cerebral vasoconstriction syndrome. Pract Neurol. 2009;9:256–67. doi: 10.1136/jnnp.2009.187856. [DOI] [PubMed] [Google Scholar]
- 4.Sattar A, Manousakis G, Jensen MB. Systematic review of reversible cerebral vasoconstriction syndrome. Expert Rev Cardiovasc Ther. 2010;8:1417–21. doi: 10.1586/erc.10.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494–500. doi: 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- 6.Fugate JE, Claassen DO, Cloft HJ, Kallmes DF, Kozak OS, Rabinstein AA, et al. Posterior reversible encephalopathy syndrome: Associated clinical and radiologic findings. Mayo Clin Proc. 2010;85:427–32. doi: 10.4065/mcp.2009.0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steiner LA, Andrews PJ. Monitoring the injured brain: ICP and CBF. Br J Anaesth. 2006;97:26–38. doi: 10.1093/bja/ael110. [DOI] [PubMed] [Google Scholar]
- 8.Raboel PH, Bartek J, Jr, Andresen M, Bellander BM, Romner B. Intracranial pressure monitoring: Invasive versus non-invasive methods – A review. Crit Care Res Pract. 2012;2012:950393. doi: 10.1155/2012/950393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geeraerts T, Newcombe VF, Coles JP, Abate MG, Perkes IE, Hutchinson PJ, et al. Use of T2-weighted magnetic resonance imaging of the optic nerve sheath to detect raised intracranial pressure. Crit Care. 2008;12:R114. doi: 10.1186/cc7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tayal VS, Neulander M, Norton HJ, Foster T, Saunders T, Blaivas M, et al. Emergency department sonographic measurement of optic nerve sheath diameter to detect findings of increased intracranial pressure in adult head injury patients. Ann Emerg Med. 2007;49:508–14. doi: 10.1016/j.annemergmed.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 11.Geeraerts T, Merceron S, Benhamou D, Vigué B, Duranteau J. Non-invasive assessment of intracranial pressure using ocular sonography in neurocritical care patients. Intensive Care Med. 2008;34:2062–7. doi: 10.1007/s00134-008-1149-x. [DOI] [PubMed] [Google Scholar]
- 12.Nabeta HW, Bahr NC, Rhein J, Fossland N, Kiragga AN, Meya DB, et al. Accuracy of noninvasive intraocular pressure or optic nerve sheath diameter measurements for predicting elevated intracranial pressure in cryptococcal meningitis. Open Forum Infect Dis. 2014;1(3) doi: 10.1093/ofid/ofu093. ofu093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shirodkar CG, Rao SM, Mutkule DP, Harde YR, Venkategowda PM, Mahesh MU, et al. Optic nerve sheath diameter as a marker for evaluation and prognostication of intracranial pressure in Indian patients: An observational study. Indian J Crit Care Med. 2014;18:728–34. doi: 10.4103/0972-5229.144015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim YK, Seo H, Yu J, Hwang GS. Noninvasive estimation of raised intracranial pressure using ocular ultrasonography in liver transplant recipients with acute liver failure – A report of two cases. Korean J Anesthesiol. 2013;64:451–5. doi: 10.4097/kjae.2013.64.5.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newman WD, Hollman AS, Dutton GN, Carachi R. Measurement of optic nerve sheath diameter by ultrasound: A means of detecting acute raised intracranial pressure in hydrocephalus. Br J Ophthalmol. 2002;86:1109–13. doi: 10.1136/bjo.86.10.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moretti R, Pizzi B, Cassini F, Vivaldi N. Reliability of optic nerve ultrasound for the evaluation of patients with spontaneous intracranial hemorrhage. Neurocrit Care. 2009;11:406–10. doi: 10.1007/s12028-009-9250-8. [DOI] [PubMed] [Google Scholar]
- 17.Geeraerts T, Launey Y, Martin L, Pottecher J, Vigué B, Duranteau J, et al. Ultrasonography of the optic nerve sheath may be useful for detecting raised intracranial pressure after severe brain injury. Intensive Care Med. 2007;33:1704–11. doi: 10.1007/s00134-007-0797-6. [DOI] [PubMed] [Google Scholar]
- 18.Moretti R, Pizzi B. Ultrasonography of the optic nerve in neurocritically ill patients. Acta Anaesthesiol Scand. 2011;55:644–52. doi: 10.1111/j.1399-6576.2011.02432.x. [DOI] [PubMed] [Google Scholar]
- 19.Dubourg J, Javouhey E, Geeraerts T, Messerer M, Kassai B. Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: A systematic review and meta-analysis. Intensive Care Med. 2011;37:1059–68. doi: 10.1007/s00134-011-2224-2. [DOI] [PubMed] [Google Scholar]
- 20.Kimberly HH, Noble VE. Using MRI of the optic nerve sheath to detect elevated intracranial pressure. Crit Care. 2008;12:181. doi: 10.1186/cc7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeeman GG, Fleckenstein JL, Twickler DM, Cunningham FG. Cerebral infarction in eclampsia. Am J Obstet Gynecol. 2004;190:714–20. doi: 10.1016/j.ajog.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Rajajee V, Vanaman M, Fletcher JJ, Jacobs TL. Optic nerve ultrasound for the detection of raised intracranial pressure. Neurocrit Care. 2011;15:506–15. doi: 10.1007/s12028-011-9606-8. [DOI] [PubMed] [Google Scholar]
- 23.Amini A, Kariman H, Arhami Dolatabadi A, Hatamabadi HR, Derakhshanfar H, Mansouri B, et al. Use of the sonographic diameter of optic nerve sheath to estimate intracranial pressure. Am J Emerg Med. 2013;31:236–9. doi: 10.1016/j.ajem.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 24.Dubost C, Le Gouez A, Jouffroy V, Roger-Christoph S, Benhamou D, Mercier FJ, et al. Optic nerve sheath diameter used as ultrasonographic assessment of the incidence of raised intracranial pressure in preeclampsia: A pilot study. Anesthesiology. 2012;116:1066–71. doi: 10.1097/ALN.0b013e318246ea1a. [DOI] [PubMed] [Google Scholar]
- 25.Bloch J, Regli L. Brain stem and cerebellar dysfunction after lumbar spinal fluid drainage: Case report. J Neurol Neurosurg Psychiatry. 2003;74:992–4. doi: 10.1136/jnnp.74.7.992. [DOI] [PMC free article] [PubMed] [Google Scholar]


