Abstract
Introduction:
Colonoscopy is a mildly painful procedure requiring conscious sedation. Though propofol is a widely used anesthetic agent in day-care procedures due to its rapid onset and quick recovery has a drawback of requiring resuscitation maneuvers more often than the conventional methods. Dexmedetomidine, a newly introduced, highly selective α2-adrenergic receptor agonist possessing hypnotic, sedative, anxiolytic, sympatholytic, and analgesic properties with impressive safety margin, needs to be explored for use in conscious sedation for colonoscopy procedure among South Indian population.
Materials and Methods:
A prospective randomized comparative study was conducted on patients aged between 25 and 60 years with the American Society of Anesthesiologist physical status classes I and II posted for colonoscopy under monitored anesthesia care. Study group was randomly divided into two groups and administered propofol and dexmedetomidine. The primary outcome variable was assessments of sedation scores between the two groups. Secondary outcome variables were pain score assessments, hemodynamic comparisons, and adverse events among the two groups. Appropriate statistical tests were applied to compare the findings.
Results:
After comparisons between the two groups, we found that patients on dexmedetomidine had similar sedation score as that of patients on propofol. However, there was a significantly higher incidence of systemic hypotension. Requirement of rescue analgesia and adverse events and other hemodynamic fluctuation were similar in both the groups.
Conclusion:
We conclude that dexmedetomidine has similar efficacy as propofol for conscious sedation required during colonoscopy. Occurrence of systolic hypotension was, however, significantly more among the group receiving dexmedetomidine.
Keywords: Colonoscopy, dexmedetomidine, propofol
INTRODUCTION
Colonoscopy is a mildly painful procedure, and it requires conscious sedation. Nowadays, colonoscopy is the standard procedure for diagnosis, screening, treatment, and follow-up for many colorectal diseases. Although some patients can tolerate colonoscopy procedure without any sedation and analgesic requirements, it is a distressful procedure for most patients. Earlier, various drugs such as midazolam, opioids, and ketamine have been used alone or in combination with propofol and are known to be associated with side effects such as respiratory depression.
Propofol is a widely used sedative hypnotic for day-care procedures as it is associated with faster onset and recovery of sedation.[1] It is known to cause dose-dependent respiratory depression, and this may be amplified in the presence of opioids requiring resuscitation maneuvers.[2,3]
Dexmedetomidine, a new drug, is highly selective α2-adrenergic receptor agonist. It possesses hypnotic, sedative, anxiolytic, sympatholytic, and analgesic properties without producing significant respiratory depression.[4] It also reduces both anesthetic and opioid analgesic requirements during the perioperative period. It has an impressive safety margin, and it may be suitable for conscious sedation during painful procedures.[5] It was reported that dexmedetomidine provides effective analgesia and reduces postoperative morphine requirements.[6] Furthermore, combination of dexmedetomidine with fentanyl provided good pain relief during shock wave lithotripsy procedure.[7] In laparoscopic bariatric surgery, the use of dexmedetomidine significantly reduced the pain and nausea.[8] Similarly, there are plenty of studies done on the use of dexmedetomidine usefulness during procedures such as ophthalmic surgeries, gynecologic surgeries, and fiber-optic intubation.[9,10,11,12,13] However, there are not many studies done on the effectiveness of dexmedetomidine use in conscious sedation during colonoscopy procedure in Indian hospital setup. Furthermore, as there is lack of literature on its effect on South Indian population, this study was conducted to compare the sedative efficacy of dexmedetomidine as compared to propofol.
MATERIALS AND METHODS
Sixty patients aged between 25 and 60 years, the American Society of Anesthesiologist physical status classes I and II posted for colonoscopy under monitored anesthesia care in a private hospital of South India, were included in this study. Study participants were included according to convenience sampling method. Patients allergic to α2-adrenergic agonist or sulfa drugs, history of alcohol or drug abuse, second- and third-degree heart block, cardiac, respiratory, renal, and liver diseases, pregnant women, lactating women, and patients with psychiatric disorders were excluded from this study. A written informed consent was taken from all the study participants after describing in full detail the nature and purpose of this study. Ethical clearance was obtained from the Institute's Ethics Committee.
Study participants were divided into two groups (Group 1 and Group 2) on the basis of random sampling method. Group 1 had patients receiving propofol, and Group 2 consisted of those receiving dexmedetomidine. Patients from both the groups were kept nil per oral for solids for 6 and 2 h for clear fluids. All patients were given tablet ranitidine 150 mg overnight and on the morning of the procedure. In the procedure room, electrocardiography leads that noninvasive blood pressure (BP) cuff and pulse oximeter were connected to the patients. Baseline readings of heart rate, BP, and oxygen saturation percentage were noted. An intravenous (i.v.) access was secured using 18G or 20G cannula, and Ringer lactate/normal saline fluids were given based on the body weight of patients. All patients were premedicated with injection glycopyrrolate 0.2 mg i.v. and analgesic injection fentanyl 0.5 μg/kg i.v. over 5 min. Throughout the procedure, all patients were given a mixture of O2/N2O (4 L/4 L) as anesthetic agent using Bains circuit. A 50-ml syringe and an electronic infusion pump were used for the study drugs throughout the procedure. Group 1 received initial loading dose of propofol 2–3 mg/kg i.v. over 10 min, followed by a continuous i.v. infusion of 25–100 μg/kg/min till the end of colonoscopy[14] Group 2 received initial loading dose of dexmedetomidine 1 μg/kg i.v over 10 min, followed by a continuous infusion of 0.2–0.8 μg/kg/h, till the end of the procedure.[15]
Heart rate, BP, and oxygen saturation were recorded intraoperatively at every 5 min interval for the first 30 min and subsequently at 10 min intervals till the end of the procedure. Patient's sedation level was assessed using modified Observer's Assessment Alertness/Sedation scale, which states Scale 5 = responds readily to name spoken in normal tone, Scale 4 = responds lethargically to name spoken in normal tone, Scale 3 = responds after name spoken loudly, Scale 2 = responds after mild prodding or shaking, and Scale 1 = unarousable.[16]
During the procedure, if the patient had pain after the administration of study drugs, an additional bolus dose of injection fentanyl 0.5 μg/kg i.v. bolus was given as a rescue analgesia. Adverse events such as hypotension (defined as systolic BP <90 mmHg) were treated with fluid bolus and injection ephedrine 6 mg i.v. bolus. Bradycardia (heart rate <40/min) was treated with injection atropine 0.6 mg i.v. bolus. Apnea or bradypnea (respiratory rate of <10/min) was managed by manually ventilating the patient.
In the postoperative ward, all patients received oxygen using face mask at 5 L/min for 30 min. Heart rate, BP, and oxygen saturation percentage were observed. Postoperative analgesia was assessed using numerical pain intensity scale at baseline and later at 30 min interval for the next 2 h. A scale of 10 was considered as being worst possible pain and scale of 0 as no pain. Rating on the scale of >4 was treated with i.v. bolus of injection tramadol 1 mg/kg.
Statistical analysis was done using MedCalc® Version 14.8.4. 2014, MedCalc software, Microsoft Partner Company, VAT registration number: BE 0809 344 640. Address: Acacialaan 22,8400 Ostend, Belgium. The data were checked for normal distribution. Demographic data such as age, gender, and weight were matched using Mann–Whitney U-test. The primary outcome variable between the two groups was assessments of sedation scores. Secondary outcome variables were need for rescue analgesia, hemodynamic parameters, adverse events, and pain score assessments. Sedation score was analyzed using Fisher's exact test. Need for rescue analgesia among the two groups was analyzed by “Z-” test for proportions. All hemodynamic parameters were expressed as mean ± standard deviation. Adverse events were analyzed using Fischer's exact test and Chi-square test. Pain score assessment was done by Fisher's exact test. P < 0.05 was considered as statistically significant.
RESULTS
Table 1 shows demographic variables such as age, gender, and weight compared between the two groups. The baseline sedation scores among the study population depicted that >96% patients in Group 1 came under the scale of 4–5, and in Group 2, nearly 93% patients come under scale of 4–5 which was statistically not significant (P = 0.686). The baseline pain scale values for the patients in both the groups showed that majority in Group 1 came under the scale of 1–2 (93%) and in scale between 1 and 2 (96%) for Group 2 (P = 0.059).
Table 1.
Demographic data and other characteristics. The values are expressed as mean±SD, numbers (N) or proportions
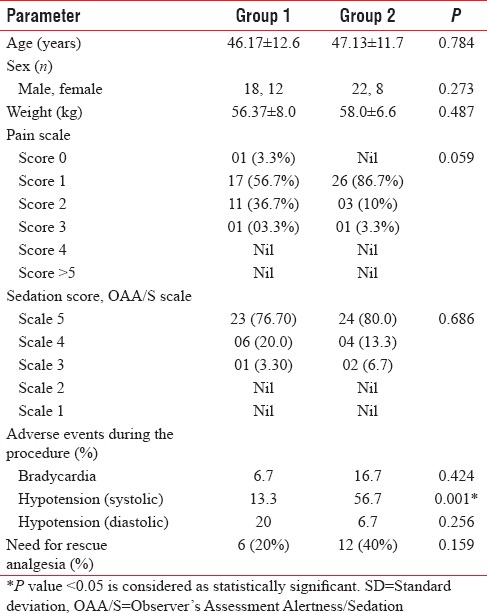
Adverse events were noted among the study population during the procedure [Table 1]. Bradycardia was seen in 6.7% in Group 1 and in 16.7% in Group 2 which was not significant (P = 0.424). Nearly 56.7% of patients in Group 2 experienced significant systolic hypotension as compared to 13.3% of patients in Group 1 (P = 0.001). No significant diastolic hypotension was noted between the two groups (P = 0.256). The need for rescue analgesia (pain scale >4) arose in 20% of patients in Group 1, while in Group 2, this increased to 40% (P = 0.159). Clinically, it seemed significant though it was not significant statistically [Table 1].
Figure 1 shows that when heart rate of Group 2 was compared with the baseline value, there was a decrease in the first 40 min. In Group 1, heart rate was maintained at baseline value till the 50th min, and then, there was a surge in heart rate. Thereafter, it persisted around the same value throughout the procedure. Heart rate was comparable between the two groups. Group 2 showed an additional 15%–20% decline from the baseline systolic BP as compared to Group 1 [Figure 2]. Figures 3 and 4 show diastolic BP and mean arterial pressure between the two groups. Both the groups showed similar pattern with an initial fall, and thereafter, it was maintained in the same pattern.
Figure 1.
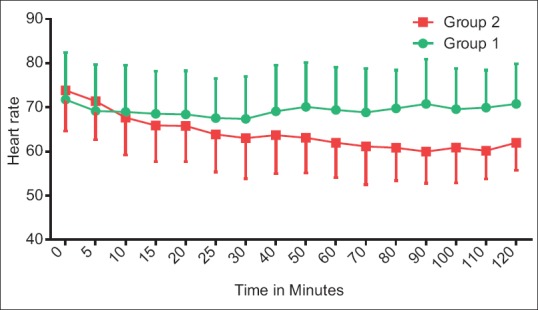
Hemodynamic comparisons between groups with respect to heart rate. The data are expressed as mean ± standard deviation
Figure 2.
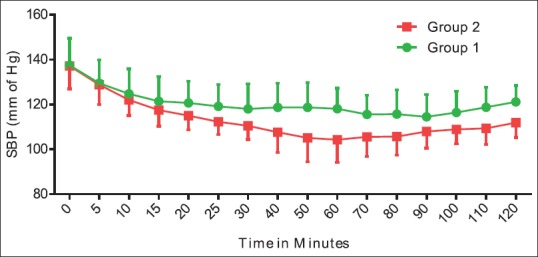
Hemodynamic comparisons between groups with respect to systolic blood pressure. The data are expressed as mean ± standard deviation
Figure 3.
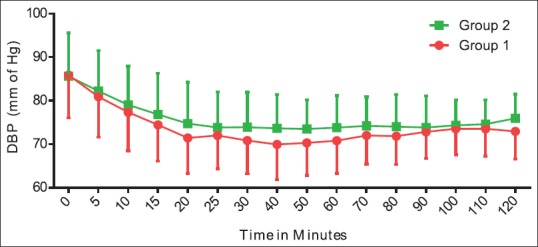
Hemodynamic comparisons between groups with respect to diastolic blood pressure. The data are expressed as mean ± standard deviation
Figure 4.
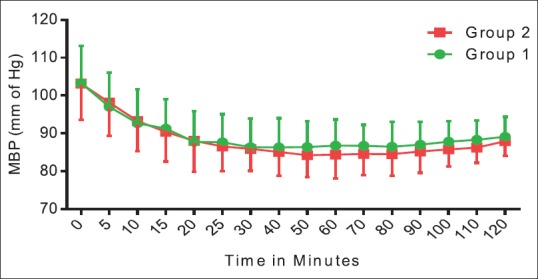
Hemodynamic comparisons between groups with respect to mean blood pressure. The data are expressed as mean ± standard deviation
DISCUSSION
Colonoscopy being a standard procedure for diagnosis, screening, treatment, and follow-up for many colorectal diseases requires conscious sedation for outpatients. Colonoscopy is often a painful and embarrassing procedure requiring a suitable drug for maximizing patient comfort with adequate sedation, good analgesia, and minimal adverse events.[17] The results of our study indicate that dexmedetomidine has similar sedation efficacy as that of propofol, a standard drug used during colonoscopy. However, patients receiving dexmedetomidine experienced a significant fall in systolic BP, but it did not amount to any end-organ damage. This finding was supported by study where they used dexmedetomidine solely for sedation.[17] Dexmedetomidine is a potent α2-agonist. It decreases the central sympathetic outflow and circulating catecholamines, thus resulting in systolic hypotension. Hypotension could be further exacerbated by dehydration due to bowel preparations and overnight fasting which is a routine protocol for colonoscopy. Studies have shown that dexmedetomidine can be used in hypertensives and coronary heart disease patients due to its hypotensive effect.[18,19]
Other hemodynamic fluctuations such as bradycardia and diastolic hypotension were comparable to that experienced using propofol. The need for rescue analgesia was also comparable to that of propofol. The use of fentanyl 0.5 μg/kg i.v with dexmedetomidine achieved sufficient analgesia during the procedure. In contrast, other studies show that dexmedetomidine was combined with fentanyl 1 μg/kg to achieve the required analgesia. Lower dose of fentanyl was found to be sufficient among the Indian population probably due to it synergistic action with that of dexmedetomidine for sedation. Indian population is known to have a higher body fat percentage, thus increasing the sensitivity to fentanyl.[20] Furthermore, by minimizing the dose of fentanyl, opioid-induced respiratory depression can be reduced which would have otherwise negated the respiratory sparing effects of dexmedetomidine. Our findings are similar to a study which used dexmedetomidine as the sole agent and found that its use was associated with prolonged recovery, bradycardia, and hypotension.[17] We found no significant bradycardia and this can be attributed to the use of glycopyrrolate as premedication which is a vagolytic. Glycopyrrolate could have prophylactically prevented severe bradycardia or ventricular ectopics. Studies have shown that dexmedetomidine does not produce significant respiratory depression during the procedure, thus favoring its usefulness in remote locations where facilities available are limited.[17,21]
Not documenting the recovery time and postoperative discharge time are the limitations of this study. Studying the efficacy of conscious sedation of dexmedetomidine on a larger sample and exploring the feasibility of use of this drug in procedures done in remote location form the future scope of this study.
CONCLUSION
In our study, colonoscopy patients receiving dexmedetomidine experienced similar conscious sedation as that of propofol. Need for rescue analgesia and adverse events were same as that of propofol. However, dexmedetomidine caused significant systolic hypotension as compared to propofol. Hence, its usefulness on patients undergoing various procedures needs further exploration.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ulmer BJ, Hansen JJ, Overley CA, Symms MR, Chadalawada V, Liangpunsakul S, et al. Propofol versus midazolam/fentanyl for outpatient colonoscopy: Administration by nurses supervised by endoscopists. Clin Gastroenterol Hepatol. 2003;1:425–32. doi: 10.1016/s1542-3565(03)00226-x. [DOI] [PubMed] [Google Scholar]
- 2.Rex DK, Overley C, Kinser K, Coates M, Lee A, Goodwine BW, et al. Safety of propofol administered by registered nurses with gastroenterologist supervision in 2000 endoscopic cases. Am J Gastroenterol. 2002;97:1159–63. doi: 10.1111/j.1572-0241.2002.05683.x. [DOI] [PubMed] [Google Scholar]
- 3.Muller S, Borowics SM, Fortis EA, Stefani LC, Soares G, Maguilnik I, et al. Clinical efficacy of dexmedetomidine alone is less than propofol for conscious sedation during ERCP. Gastrointest Endosc. 2008;67:651–9. doi: 10.1016/j.gie.2007.09.041. [DOI] [PubMed] [Google Scholar]
- 4.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 5.Dyck JB, Maze M, Haack C, Vuorilehto L, Shafer SL. The pharmacokinetics and hemodynamic effects of intravenous and intramuscular dexmedetomidine hydrochloride in adult human volunteers. Anesthesiology. 1993;78:813–20. doi: 10.1097/00000542-199305000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Gurbet A, Basagan-Mogol E, Turker G, Ugun F, Kaya FN, Ozcan B, et al. Intraoperative infusion of dexmedetomidine reduces perioperative analgesic requirements. Can J Anaesth. 2006;53:646–52. doi: 10.1007/BF03021622. [DOI] [PubMed] [Google Scholar]
- 7.Kaygusuz K, Gokce G, Gursoy S, Ayan S, Mimaroglu C, Gultekin Y, et al. A comparison of sedation with dexmedetomidine or propofol during shockwave lithotripsy: A randomized controlled trial. Anesth Analg. 2008;106:114–9. doi: 10.1213/01.ane.0000296453.75494.64. [DOI] [PubMed] [Google Scholar]
- 8.Tufanogullari B, White PF, Peixoto MP, Kianpour D, Lacour T, Griffin J, et al. Dexmedetomidine infusion during laparoscopic bariatric surgery: The effect on recovery outcome variables. Anesth Analg. 2008;106:1741–8. doi: 10.1213/ane.0b013e318172c47c. [DOI] [PubMed] [Google Scholar]
- 9.Jaakola ML, Ali-Melkkilä T, Kanto J, Kallio A, Scheinin H, Scheinin M, et al. Dexmedetomidine reduces intraocular pressure, intubation responses and anaesthetic requirements in patients undergoing ophthalmic surgery. Br J Anaesth. 1992;68:570–5. doi: 10.1093/bja/68.6.570. [DOI] [PubMed] [Google Scholar]
- 10.Ghali A, Mahfouz AK, Ihanamäki T, El Btarny AM. Dexmedetomidine versus propofol for sedation in patients undergoing vitreoretinal surgery under sub-tenon's anesthesia. Saudi J Anaesth. 2011;5:36–41. doi: 10.4103/1658-354X.76506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aho M, Erkola O, Kallio A, Scheinin H, Korttila K. Dexmedetomidine infusion for maintenance of anesthesia in patients undergoing abdominal hysterectomy. Anesth Analg. 1992;75:940–6. [PubMed] [Google Scholar]
- 12.Aantaa R, Kanto J, Scheinin M, Kallio A, Scheinin H. Dexmedetomidine, an alpha 2-adrenoceptor agonist, reduces anesthetic requirements for patients undergoing minor gynecologic surgery. Anesthesiology. 1990;73:230–5. doi: 10.1097/00000542-199008000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Bergese SD, Patrick Bender S, McSweeney TD, Fernandez S, Dzwonczyk R, Sage K, et al. A comparative study of dexmedetomidine with midazolam and midazolam alone for sedation during elective awake fiberoptic intubation. J Clin Anesth. 2010;22:35–40. doi: 10.1016/j.jclinane.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Hassan NE, Betz BW, Cole MR, Wincek J, Reischman D, Sanfilippo DJ, et al. Randomized controlled trial for intermittent versus continuous propofol sedation for pediatric brain and spine magnetic resonance imaging studies. Pediatr Crit Care Med. 2011;12:e262–5. doi: 10.1097/PCC.0b013e31820ab881. [DOI] [PubMed] [Google Scholar]
- 15.Candiotti KA, Bergese SD, Bokesch PM, Feldman MA, Wisemandle W, Bekker AY, et al. Monitored anesthesia care with dexmedetomidine: A prospective, randomized, double-blind, multicenter trial. Anesth Analg. 2010;110:47–56. doi: 10.1213/ane.0b013e3181ae0856. [DOI] [PubMed] [Google Scholar]
- 16.Glass PS, Bloom M, Kearse L, Rosow C, Sebel P, Manberg P. Bispectral analysis measures sedation and memory effects of propofol, midazolam, isoflurane, and alfentanil in healthy volunteers. Anesthesiology. 1997;86:836–47. doi: 10.1097/00000542-199704000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Jalowiecki P, Rudner R, Gonciarz M, Kawecki P, Petelenz M, Dziurdzik P, et al. Sole use of dexmedetomidine has limited utility for conscious sedation during outpatient colonoscopy. Anesthesiology. 2005;103:269–73. doi: 10.1097/00000542-200508000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Kamibayashi T, Maze M. Clinical uses of alpha2 -adrenergic agonists. Anesthesiology. 2000;93:1345–9. doi: 10.1097/00000542-200011000-00030. [DOI] [PubMed] [Google Scholar]
- 19.Taittonen MT, Kirvelä OA, Aantaa R, Kanto JH. Effect of clonidine and dexmedetomidine premedication on perioperative oxygen consumption and haemodynamic state. Br J Anaesth. 1997;78:400–6. doi: 10.1093/bja/78.4.400. [DOI] [PubMed] [Google Scholar]
- 20.Deurenberg-Yap M, Schmidt G, van Staveren WA, Deurenberg P. The paradox of low body mass index and high body fat percentage among Chinese, Malays and Indians in Singapore. Int J Obes Relat Metab Disord. 2000;24:1011–7. doi: 10.1038/sj.ijo.0801353. [DOI] [PubMed] [Google Scholar]
- 21.Venn RM, Hell J, Grounds RM. Respiratory effects of dexmedetomidine in the surgical patient requiring intensive care. Crit Care. 2000;4:302–8. doi: 10.1186/cc712. [DOI] [PMC free article] [PubMed] [Google Scholar]


