Abstract
Background
Fibrosis is the common pathological feature in most kinds of chronic kidney disease (CKD). TGF-β/Smads signaling is the master pathway regulating kidney fibrosis pathogenesis, in which Smad3 acts as the integrator of various pro-fibrosis signals. In this study, we analyzed the role of SIS3, a specific inhibitor of Smad3, in mouse unilateral ureteral obstruction (UUO) kidneys.
Material/Methods
UUO mice were intraperitoneally injected with 0.2 mg/kg/day or 2 mg/kg/day of SIS3 or control saline for 7 days, followed by analysis of structure injury, fibrosis status, inflammation, apoptosis, and TGF-β/Smads signaling activity.
Results
Our results indicated that SIS3 treatment dosage-dependently relieved the gross structure injury and tubular necrosis in UUO kidneys. Masson staining, immunohistochemistry, and real-time PCR showed significantly decreased extracellular matrix deposition, fibronectin staining intensity, and RNA levels of collagen I and collagen III in SIS3-treated UUO kidneys. SIS3 treatment also suppressed the activation of myofibroblasts, as evidenced by decreased expression levels of α-SMA and vimentin in UUO kidneys. The TGF-β/Smads signaling activity analysis showed that SIS3 inhibited the phosphorylation of Smad3 but not Smad2 and decreased the protein level of TGF-β1, suggesting specific inhibition of the TGF-β/Smad3 pathway in UUO kidneys. Furthermore, SIS3 treatment also ameliorated the increased pro-inflammatory TNF-α and COX2 in UUO kidneys and circulating IL-1β in UUO mice, and inhibited caspase-3 activity and the number of apoptotic cells.
Conclusions
SIS3 ameliorated fibrosis, apoptosis, and inflammation through inhibition of TGF-β/Smad3 signaling in UUO mouse kidneys.
MeSH Keywords: Fibrosis, Inflammation, Transforming Growth Factor beta, Apoptosis
Background
Kidney fibrosis is the common pathological feature for almost all kinds of chronic kidney diseases, including diabetic kidney and glomerulonephritis, and is strongly related with kidney function decline [1,2]. Kidney fibrosis is characterized by unbalanced extracellular matrix (ECM) metabolism resulting in excessive deposition of matrix component, which returns to impede the function of tubules and glomerulus. However, there is still no available drug to specifically target the fibrosis pathogenesis in clinical management of kidney disease.
It is commonly accepted that TGF-β/Smads signaling is the master pathway regulating kidney fibrosis [3]. In canonical TGF-β/Smads signaling, the active TGF-β ligand binds to receptor TβRII, which then recruits TβRI. The later then phosphorylates receptor-associated Smads protein, including Smad2 and Smad3. Phosphorylated Smad2 or 3 can bind with co-Smad (Smad4) and is translocated into the nucleus to exert transcription activity to regulate downstream gene expression [3]. It has been proven that Smad3 but not Smad2 has a pro-fibrosis role in kidney fibrosis [4]. Smad3 directly regulates expression of various fibrogenic genes like extracellular matrix protein and tissue inhibitor of metallopeptidases (TIMPs) [5]. Furthermore, Smad3 also can stimulate TGF-β1 expression to enhance TGF-β/Smads signaling in a positive feedback. On the other hand, Smad3 also induces the counteracting Smad7 to negatively regulate TGF-β signaling by degradation of TbRI and Smad3, thus maintaining balanced signaling activity [3,6]. In kidney fibrosis, ectopic activity of TGF-β/Smads signaling was observed. Genetics studies indicated that inhibition of TGF-β/Smads signaling, either by deletion of TβRII and Smad3, or by over-expression of Smad7, can suppress the fibrosis progression in different models of kidney injury [7–11]. These studies indicate that TGF-β/Smads signaling is an important therapeutic target in kidney fibrosis treatment.
SIS3 is a synthesized chemical that specifically inhibits Smad3 phosphorylation and its binding to Smad4. It was reported that SIS3 inhibits the myofibroblast differentiation and production of collagen production in TGF-β1-stimulated human dermal fibroblasts [12]. Pretreatment with SIS3 also can increase E-cadherin and decreased vimentin and α-SMA protein levels in RLE-6TN (rat lung epithelial-T-antigen negative) cells treated with TGF-β [13]. In human trabecular meshwork cells, SIS3 abolishes the extracellular matrix proteins deposition induced by gremlin [14] and TGF-βs [15]. In the kidneys, SIS3 treatment delays the early development of diabetic nephropathy in a type I diabetic mouse model through inhibition of endothelial-mesenchymal transition and fibrosis [16]. However, the role of SIS3 in UUO kidney fibrosis is not clear.
In this study, UUO mice were intraperitoneally injected with SIS3 and the kidney injury status was studied. We undertook the present study to investigate the role of SIS3 in UUO kidney in terms of fibrosis, apoptosis, and inflammation. These results highlight the potential therapeutic value of SIS3, which might be translated to a novel kidney-protective strategy in the future.
Material and Methods
UUO mouse model construction and SIS3 treatment
The UUO mouse model was established in BALB/c male mice (6 weeks old, 20±2g) by ligating the left ureter, as previously described [8]. Animals were randomly divided into 4 groups (n=8 for each group) including sham and UUO with SIS3 (Sigma, USA, Cat# S0447) of different dosages (0, 0.2, 2 mg/kg/day). One day after UUO surgery, mice of each group were intraperitoneally injected with different dosages of SIS3 (in 5% dimethyl sulfoxide) for 1 week. The sham group received vehicle injection (5% dimethyl sulfoxide) instead. Mice were maintained in a special pathogen-free (SPF) facility with 12-h light/12-h dark cycle and free access to food and water. On the 8th post-operation day, UUO or sham kidney was harvested for downstream analysis. For immunohistochemistry (IHC), kidneys were fixed in 4% paraformaldehyde and subjected to standard paraffin embedding. For Western blotting and RT-PCR, fresh kidney tissue was snap-frozen in liquid nitrogen and stored at −80°C. Plasma was isolated from anticoagulant (heparin)-treated blood and kept at −80°C. All animal experiments complied with corresponding regulations and were approved by the Animal Ethics Committee of Southwest Medical University.
Histological analysis
Paraffin sectioning was performed at 4 μM. After dewaxing in xylene and rehydration in gradient ethanol, hematoxylin-eosin staining (HE staining) was conducted with an HE staining kit (Beyotime, China, Cat# C0105). Periodic acid-Schiff staining (PAS) was performed with a periodic acid-Schiff staining kit (Solarbio, China, Cat# G1281). Masson’s trichrome staining was performed with a kit from Nanjing Jiancheng Bio., China (Cat# D026). The above staining procedure were done according to the manufacturer’s instructions. Tubular injury score was semiquantitatively calculated based on PAS staining according to the percentage of cortical tubular necrosis with an assigned value: 0, none; 1, 10%; 2, 10% to 25%; 3, 25% to 75%; and 4, >75% [16]. Immunohistochemical staining (IHC) was performed with Biotin-Streptavidin HRP-based SPlink Detection Kits (ZSGB-Bio, China, Cat# SP-9002) and all procedures followed the manufacturer’s instructions. Antibodies used in IHC were mouse anti-α-SMA (1: 100, Boster, China, Cat# BM0002) and mouse anti-fibronectin (1: 100, DHSB, USA, Cat# P1H11). Images were taken with a light microscope (Nikon Eclipse 80i, Japan) at 200× magnification.
Western blotting analysis
Total protein of kidney tissue was extracted with RIPA lysis buffer and protein concentration was quantified with Coomassie brilliant blue method. We resolved 40 μg of protein in 12% SDS-PAGE gel. After transfer to PVDF membrane, the membrane was blocked with 5% fat-free milk for 1 h at room temperature (RT). Primary antibody incubation was performed at 4°C overnight. The next day, the membrane was washed 3 times with TBST for 5 min each time. Corresponding HRP-conjugated secondary antibody was applied for 1 h at RT. After washing with TBST, signals were developed with Immobilon Western Chemiluminescent HRP Substrate (Minipore, USA, Cat# WBKLS0500) and checked with the ChemiDoc XRS+ system (Bio-Rad, USA). Quantification of expression was performed with Image J 1.47V software (NIH, USA). Primary antibodies used were mouse anti-TGF-β1 (R&D Systems, USA, Cat# MAB240), rabbit anti-Smad2 (CST, USA, Cat# 5339), rabbit anti-p-Smad2 (CST, USA, Cat# 3108), rabbit anti-samd3 (CST, USA, Cat# 9523), rabbit anti-p-smad3 (CST, USA, Cat# 9520), mouse anti-α-SMA (Boster, China. Cat#, BM0002), mouse anti-TNF-α (Proteintech, USA, Cat# 60291-1-Ig), rabbit anti-COX2 (CST, USA, Cat# 12282), rabbit anti-vimentin (CST, USA, Cat#5741), rabbit anti-caspase-3 (CST, USA, Cat# 9665), and mouse anti-GAPDH (Minipore, USA, Cat# MAB374MI). Secondary antibodies used were HRP-conjugated goat anti-mouse polyclonal antibody (ZSGB-Bio, China, Cat# ZB2305) and HRP-conjugated goat anti-rabbit polyclonal antibody (ZSGB-Bio, China, Cat# ZB2301).
Real-time PCR
Total RNA was extracted from kidney tissues using Trizol reagent (Thermo Scientific, USA, Cat# 15596018) following the manufacturer’s instructions. We reverse-transcribed 1 μg of total RNA into cDNA with the RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, USA, Cat# 1622). Real-time PCR was performed with UltraSYBR Mixture (Cwbio., China, Cat# CW2061) on the Mastercycler ep Realplex real-time PCR system (Eppendorf, Germany). Primers used in this study included: α-SMA forward 5′-GTGGCTATTCCTTCGTGACTAC-3′ and reverse 5′-CAGGCAGTTCGTAGC TCTTC-3′, Collagen I forward 5′-CAGAGAGGAGAAAGAGGCTTC-3′ and reverse 5′-CTCACGTCCA GATTCACCAG-3′, Collagen III forward 5′-GGACAGATTCTGGTGCAGAG-3′ and reverse 5′-GGTA TGTAATGTTCTGGGAGGC-3′, GAPDH forward 5′-AAGGTCGGTGTGAACGGATTTG-3′ and reverse 5′-TGGCAACAATCTCCACTTTGCC-3′. All primers were purchased from Sangon Biotech Co., Ltd. (Shanghai, China). The relative expression level of the genes of interest was normalized with GAPDH.
ELISA
Plasma IL-1β concentration was determined with a mouse IL-1β ELISA kit (Neobioscience, China, Cat# EMC001b) according to the manufacturer’s instructions.
TUNEL assay
Paraffin sectioning was performed at 3.5 μM. After dewaxing in xylene and rehydration in gradient ethanol, terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate (dUTP) nick-end labeling staining (TUNEL staining) was conducted with an in situ cell apoptosis detection kit (Boster, China, Cat# MK1020). The above staining procedures were done according to the manufacturer’s instructions. The number of TUNEL-positive cells was counted in 10 random high-power fields.
Statistical analysis
Data are presented as mean ±SEM. SPSS 13.0 software was used for one-way ANOVA and comparison between groups. GraphPad Prism 6.0 software was used to arrange data and generate statistical graphs. A P value <0.05 was considered statistically significant.
Results
SIS3 treatment ameliorated the pathological lesions induced by UUO
To explore the protective role of SIS3, the UUO model mice were intraperitoneally injected with saline or SIS3 (0.2 mg/kg/day or 2 mg/kg/day) from the second day to seventh day after model construction. At the 7th day, HE and PAS staining analysis showed that the UUO kidneys exhibited remarkable structural damage, including tubular dilation and atrophy, desquamation of epithelial cells, and expansion of interstitium. In contrast, SIS3 treatment relieved the above structural damage in UUO kidneys in a dose-dependent way (Figure 1A, 1B). The tubular injury scoring analysis, which judged the tubular necrosis, also revealed recovered tubule structure in SIS3-treated UUO kidneys (Figure 1C).
Figure 1.
The specific inhibitor of Smad3(SIS3) treatment ameliorated kidney structural injury after unilateral ureteral obstruction (UUO). (A) Representative images of hematoxylin-eosin (HE) staining of kidneys in different treatment groups. (B) Representative images of periodic acid-Schiff staining (PAS) staining of kidneys of different treatment groups. (C) Statistical analysis of tubular injury score. Data represented as mean ±SEM of 8 mice for each group. * P<0.05 versus sham-DMSO group. # P<0.05 versus UUO-DMSO group. The magnification is ×200 in A and B.
SIS3 treatment suppressed interstitial fibrosis in UUO kidney
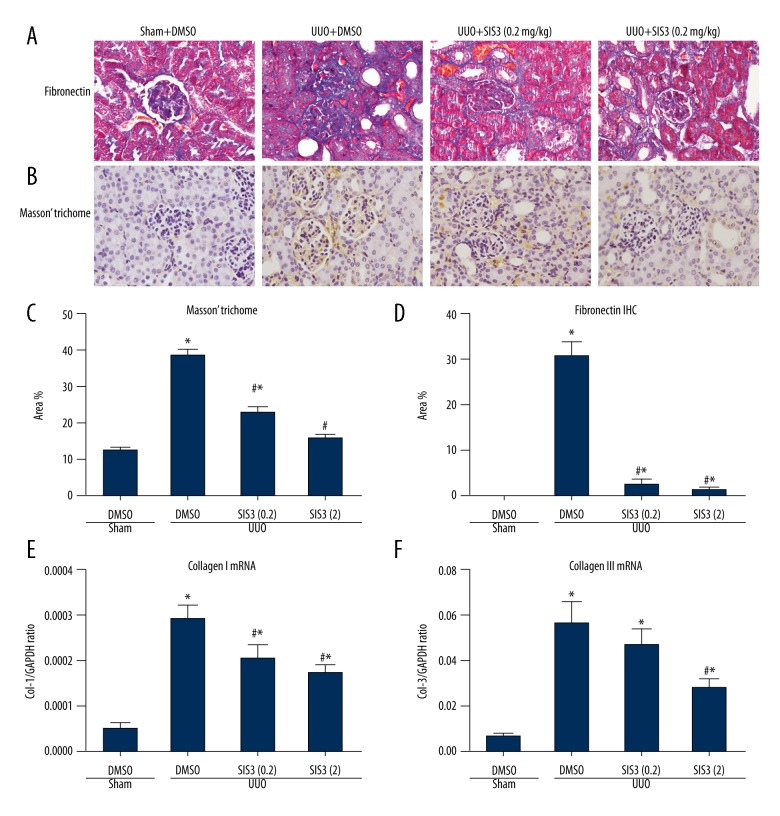
We next examined whether SIS3 treatment suppressed the kidney fibrosis, which is the primary injury of UUO model. As shown in Figure 2A, 2C, Masson trichrome staining revealed abundant extracellular matrix deposition in UUO kidneys. In contrast, SIS3 treatment significantly decreased the intensity of Masson staining. In line with the Masson staining, the expression level of extracellular matrix protein fibronectin was also decreased upon SIS3 treatment as detected by IHC (Figure 2B, 2D). We also used RT-PCR to detected the expression level of matrix protein collagen I and III. Compared with the sham kidney, UUO significantly promoted the transcription of collagen I and III, but SIS3 treatment attenuated the collagen I and III RNA expression in a dose-dependent way (Figure 2E, 2F).
Figure 2.
The specific inhibitor of Smad3 (SIS3) treatment suppressed interstitial fibrosis in unilateral ureteral obstruction (UUO) kidneys. (A) Masson trichrome staining to show the extracellular matrix (ECM) deposition. (B) Immunohistochemistry (IHC) staining to assess the expression of fibronectin. (C) Quantitative analysis of Masson trichrome staining. (D) Quantitative analysis of fibronectin staining. Real-time PCR was used to detect the expression of collagen I and III mRNA in E and F, respectively. * P<0.05 versus sham-DMSO group. # P<0.05 versus UUO-DMSO group. The view magnification is ×200 in A and B.
In UUO-induced kidney fibrosis, the excessive extracellular matrix deposition is mainly from activated fibroblasts that differentiated into myofibroblasts [18]. As shown in Figure 3, UUO kidneys presented robust α-SMA positive myofibroblasts, which was accompanied by elevated expression of α-SMA mRNA and protein. In contrast, SIS3 remarkably decreased the number of α-SMA-positive myofibroblast cells in treated UUO kidneys. Consistently, compared with the UUO kidneys, the expression of α-SMA was suppressed by SIS3 treatment both at RNA and protein levels in a dose-dependent manner. Furthermore, SIS3 treatment also significantly decreased the expression of vimentin in UUO kidneys, which was abundantly enriched in activated myofibroblasts (Figure 3B, 3D). These data suggest that SIS3 can inhibit the activation of myofibroblast cells and thus reduce fibrosis.
Figure 3.
The specific inhibitor of Smad3 (SIS3) treatment inhibited myofibroblast activation in unilateral ureteral obstruction (UUO) kidneys. (A) Immunohistochemistry (IHC) analysis of α-SMA expression in kidneys from different groups. (B) Western blotting analysis of α-SMA and vimentin expression. (C) Quantitative analysis of α-SMA in B. (D) Quantitative analysis of vimentin in B. (E) mRNA expression analysis of α-SMA by real-time PCR. * P<0.05 versus sham-DMSO group. # P<0.05 versus UUO-DMSO group. The view magnification is ×200 in A.
SIS3 inhibited the activation of pro-fibrotic TGF-β/Smad3 signaling in UUO
TGF-β/Smads signal is the master pathway that regulates fibrosis in many kinds of disease, including kidney fibrosis [6]. As SIS3 is a chemical inhibitor of Smad3 [12], we next analyzed the activity of TGF-β/Smads signaling by Western blotting. As shown in Figure 4, in UUO kidneys, the phosphorylation levels of Smad2 and Smad3 were remarkably increased when compared with the sham kidneys and the signal ligand TGF-β1 also showed increased expression. However, the levels of phosphorylated Smad3 and TGF-β1 were suppressed in SIS3-treated UUO kidneys in a dose-dependent manner. Notably, the phosphorylation level of Smad2 did not change upon SIS3 treatment in UUO kidneys, suggesting the specificity of SIS3 in Smad3 activity inhibition. These data indicate that SIS3 causes specific inhibition of TGF-β/Smad3 signaling in UUO kidneys.
Figure 4.
The specific inhibitor of Smad3 (SIS3) treatment decreased pro-fibrotic TGF-β/Smad3 signaling in unilateral ureteral obstruction (UUO) kidneys. (A) Western blotting analysis of TGF-β1, total Smad2 (t-Smad2), total Smad3 (t-Smad3), phosphorylated Smad2 (p-Smad2), and phosphorylated Smad3 (p-Smad3) in kidneys of different groups. GAPDH was used as loading control. (B–D) Show quantitative analysis of p-Smad3, p-Smad2, and TGF-β1 in A. * P<0.05 versus sham-DMSO group. # P<0.05 versus UUO-DMSO group.
SIS3 treatment reduces inflammation following UUO
Ectopic inflammation is another feature during kidney fibrosis [6]. We next investigated whether the reduced fibrosis in SIS3-treated UUO kidneys was accompanied with decreased inflammation reaction. As shown in Figure 5, inflammation-related proteins TNF-α and COX2 were robustly activated in UUO kidneys. However, SIS3 treatment significantly suppressed the level of TNF-α at a dose of 2 mg/kg and COX2 at both 0.2 and 2 mg/kg in UUO kidneys. Furthermore, ELISA analysis showed that SIS3 treatment also ameliorated the increased plasma IL-1β level, indicating that SIS3 recovered the ectopic circulatory inflammation level in UUO condition (Figure 5D).
Figure 5.
The specific inhibitor of Smad3 (SIS3) treatment reduced inflammation in unilateral ureteral obstruction(UUO) kidneys and plasma. (A) Western blotting analysis to detect the expression of TNF-α and COX2 in kidneys. (B, C) Show quantitative analysis of TNF-α and COX2 in A. (D) ELSIA analysis to detect the plasma level of IL-1β. * P<0.05 versus sham-DMSO group. # P<0.05 versus UUO-DMSO group.
SIS3 treatment attenuated apoptosis following in UUO kidneys
Renal tubular apoptosis has a close relationship with renal fibrosis. Finally, we examined whether SIS3 treatment suppressed the renal tubular apoptosis after UUO. The TUNEL assay results showed that renal tubular apoptosis was rarely observed in the sham group, while apoptotic cells in the UUO group significantly increased. However, compared with the UUO group, the number of TUNEL+ cells in the SIS3 treatment group significantly decreased (Figure 6A, 6B). Furthermore, SIS3 treatment also significantly decreased the expression of cleaved-caspase 3 in UUO kidneys, which was abundantly expressed in activated apoptotic cells (Figure 6C, 6D).
Figure 6.
The specific inhibitor of Smad3 (SIS3) treatment attenuated apoptosis in unilateral ureteral obstruction(UUO) kidneys. (A) Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate (dUTP) nick-end labeling staining (TUNEL staining) analysis of apoptosis in kidneys from different groups. (B) Quantitative analysis of TUNEL+ cells/HPF (high-power field) in A. (C). Western blotting analysis of cleaved-caspase 3 expression. (D) Quantitative analysis of cleaved-caspase 3 in C. * P<0.05 versus sham-DMSO group. # P<0.05 versus UUO-DMSO group. The view magnification is ×400 in A.
Discussion
Fibrosis is the common pathological change in almost all kinds of chronic kidney diseases, and targeting the fibrosis is one of the options in CKD treatment [6]. In this paper, we first analyzed the anti-fibrosis role of SIS3 in a UUO kidney fibrosis model. We found that SIS3 treatment reversed the tubular injury, ECM gene protein and RNA expression, and myofibroblast activation in UUO kidneys in a dosage-dependent manner. The pro-fibrotic TGF-β/Smad3 pathway was suppressed upon SIS3 treatment. Furthermore, SIS3 also ameliorated the inflammation injury and reduced the renal tubular apoptosis in UUO kidneys.
Although it has been well accepted that TGF-β/Smads signaling is the master pathway for various fibrosis diseases, including fibrotic kidney disease, TGF-β/Smads signaling can be activated in TGF-β ligand-dependent and -independent ways. For example, the downstream Smad2 and Smad3 can be activated (phosphorylated) by advanced glycation end-products (AGEs) in diabetic nephropathy and angiotensin II in hypertensive nephropathy through ERK/P38-Smad cross-talk [19,20]. This indicates that targeting the upstream signal transduction of canonical TGF-β/Smads pathway may not completely block the pro-fibrotic signaling. However, Smad3 is believed to act as the final integrator of various pro-fibrotic signals and can directly regulate ECM-involved gene expression. Thus, Lan proposed that Smad3 is an ideal therapeutic target for fibrosis treatment [6], and this is supported by the fact that Smad3 knock-out mice have a more robust anti-fibrosis effect than TβRII knock-out mice in UUO kidneys [7,8].
Furthermore, blockade of the upstream signal of TGF-β/Smads signaling may have additional adverse effects. For example, although deletion of TβRII suppresses kidney fibrosis in UUO mice, it also promotes inflammation injury [7]. In contrast, Smad3 knock-out inhibits fibrosis, apoptosis, and inflammation in various mouse kidney disease models [8,9,21]. In our study, the blockade of Smad3 by the chemical inhibitor SIS3 not only inhibited fibrosis as evidenced by decreased ECM gene expression and myofibroblast activation (Figures 2, 3), but also suppressed the inflammation reaction and the renal tubular apoptosis in UUO kidneys (Figures 5, 6). This is in line with the previous genetic studies in a mouse model and further strengthens the consensus that Smad3 is a perfect target for anti-fibrosis treatment [8,9,21].
Ectopic inflammation is an important feature in kidney fibrosis. NF-κB signaling is the key pathway that regulates inflammation, and increased NF-κB activity and downstream pro-inflammatory cytokines expression are regularly accompanied by fibrotic kidney disease [6]. TGF-β/Smads signaling cross-talks with NF-κB signaling through promoting expression of IκBα by Smad7 [22], and Smad3 knock-out can block the inflammation in addition to inhibition of fibrosis in kidney fibrosis. Our results also showed ameliorated inflammation in UUO kidneys treated with SIS3, resulting in decreased inflammation insults.
Renal tubular apoptosis is another important feature of kidney fibrosis. Research has shown that inhibition of tubular cell apoptosis protects against renal damage and development of fibrosis following UUO [23]. It is clear that cross-talks between the MAP kinase and Smad signaling pathways stimulated by upstream TGF-β1 receptor. TGF-β1 promoted the cleavage and activation of pro-caspase-9, and then activation of caspase-3. Our findings clearly show that SIS3 treatment reduced tubular apoptosis in the UUO kidneys in a dose-dependent manner, and confirm that Smad3 is directly or indirectly involved in renal tubular apoptosis in vivo, as described by Inazaki et al. [21].
Conclusions
In conclusion, SIS3 protected UUO kidneys against fibrosis, apoptosis, and inflammation injury through inhibition of TGF-β/Smad3 signaling. Our findings suggest that SIS3 and other drugs targeting Smad3 are promising in further anti-fibrosis treatment of kidney disease.
Footnotes
Conflict of interest
None.
Source of support: This work was supported by the Scientific Research Innovation Team grant of the Education Department of Sichuan province (17TD0046), the Young Scientist Innovation Workshop grant of Luzhou city (2016LZXNYD-T05), the Core Laboratory Construction Project of the Education Department of Sichuan province (2017/794), and the Joint Natural Science grant of Luzhou government and Southwest Medical University (2017LZXNY D-Z03)
References
- 1.Meng XM, Zhang Y, Huang XR, et al. Treatment of renal fibrosis by rebalancing TGF-β/Smad signaling with the combination of asiatic acid and naringenin. Oncotarget. 2015;6(35):36984–97. doi: 10.18632/oncotarget.6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeng F, Miyazawa T, Kloepfer LA, Harris RC. ErbB4 deletion accelerates renal fibrosis following renal injury. Am J Physiol. 2017 doi: 10.1152/ajprenal.00260.2017. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meng XM, Tang PM, Li J, Lan HY. TGF-β/Smad signaling in renal fibrosis. Front Physiol. 2015;6:82. doi: 10.3389/fphys.2015.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meng XM, Huang XR, Chung AC, et al. Smad2 protects against TGF-beta/Smad3-mediated renal fibrosis. J Am Soc Nephrol. 2010;21(9):1477–87. doi: 10.1681/ASN.2009121244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verrecchia F, Chu ML, Mauviel A. Identification of novel TGF-beta/Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J Biol Chem. 2001;276(20):17058–62. doi: 10.1074/jbc.M100754200. [DOI] [PubMed] [Google Scholar]
- 6.HYL Diverse roles of TGF-β/Smads in renal fibrosis and inflammation. Int J Biol Sci. 2011;7(7):1056–67. doi: 10.7150/ijbs.7.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meng XM, Huang XR, Xiao J, et al. Diverse roles of TGF-beta receptor II in renal fibrosis and inflammation in vivo and in vitro. J Pathol. 2012;227(2):175–88. doi: 10.1002/path.3976. [DOI] [PubMed] [Google Scholar]
- 8.Sato M, Muragaki Y, Saika S, et al. Targeted disruption of TGF-β1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest. 2003;112(10):1486–94. doi: 10.1172/JCI19270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Z, Huang XR, Lan HY. Smad3 mediates ANG II-induced hypertensive kidney disease in mice. Am J Physiol. 2012;302(8):F986–97. doi: 10.1152/ajprenal.00595.2011. [DOI] [PubMed] [Google Scholar]
- 10.Zhou L, Fu P, Huang XR, et al. Mechanism of chronic aristolochic acid nephropathy: Role of Smad3. Am J Physiol. 2010;298(4):F1006–17. doi: 10.1152/ajprenal.00675.2009. [DOI] [PubMed] [Google Scholar]
- 11.Chen HY, Huang XR, Wang W, et al. The protective role of Smad7 in diabetic kidney disease: mechanism and therapeutic potential. Diabetes. 2011;60(2):590–601. doi: 10.2337/db10-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jinnin M, Ihn H, Tamaki K. Characterization of SIS3, a novel specific inhibitor of Smad3, and its effect on transforming growth factor-beta1-induced extracellular matrix expression. Mol Pharmacol. 2006;69(2):597–607. doi: 10.1124/mol.105.017483. [DOI] [PubMed] [Google Scholar]
- 13.Cao Y, Liu Y, Ping F, et al. miR-200b/c attenuates lipopolysaccharide -induced early pulmonary fibrosis by targeting ZEB1/2 via p38 MAPK and TGF-β/smad3 signaling pathways. Lab Invest. 2017 doi: 10.1038/labinvest.2017.123. [DOI] [PubMed] [Google Scholar]
- 14.Sethi A, Jain A, Zode GS, et al. Role of TGFbeta/Smad signaling in gremlin induction of human trabecular meshwork extracellular matrix proteins. Invest Ophthalmol Vis Sci. 2011;52(8):5251–59. doi: 10.1167/iovs.11-7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sethi A, Mao W, Wordinger RJ, Clark AF. Transforming growth factor-beta induces extracellular matrix protein cross-linking lysyl oxidase (LOX) genes in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011;52(8):5240–50. doi: 10.1167/iovs.11-7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Qu X, Yao J, et al. Blockade of endothelial-mesenchymal transition by a Smad3 inhibitor delays the early development of streptozotocin-induced diabetic nephropathy. Diabetes. 2010;59(10):2612–24. doi: 10.2337/db09-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lv LL, Tang PM, Li CJ, et al. The pattern recognition receptor, Mincle, is essential for maintaining the M1 macrophage phenotype in acute renal inflammation. Kidney Int. 2017;91(3):587–602. doi: 10.1016/j.kint.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 18.Kim D, Lee AS, Jung YJ, et al. Tamoxifen ameliorates renal tubulointerstitial fibrosis by modulation of estrogen receptor alpha-mediated transforming growth factor-beta1/Smad signaling pathway. Nephrol Dial Transplant. 2014;29(11):2043–53. doi: 10.1093/ndt/gfu240. [DOI] [PubMed] [Google Scholar]
- 19.Chung AC, Zhang H, Kong YZ, et al. Advanced glycation end-products induce tubular CTGF via TGF-beta-independent Smad3 signaling. J Am Soc Nephrol. 2010;21(2):249–60. doi: 10.1681/ASN.2009010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang F, Chung AC, Huang XR, Lan HY. Angiotensin II induces connective tissue growth factor and collagen I expression via transforming growth factor-beta-dependent and -independent Smad pathways: the role of Smad3. Hypertension. 2009;54(4):877–84. doi: 10.1161/HYPERTENSIONAHA.109.136531. [DOI] [PubMed] [Google Scholar]
- 21.Inazaki K, Kanamaru Y, Kojima Y, et al. Smad3 deficiency attenuates renal fibrosis, inflammation, apoptosis after unilateral ureteral obstruction. Kidney Int. 2004;66(2):597–604. doi: 10.1111/j.1523-1755.2004.00779.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, Huang XR, Li AG, et al. Signaling mechanism of TGF-beta1 in prevention of renal inflammation: role of Smad7. J Am Soc Nephrol. 2005;16(5):1371–83. doi: 10.1681/ASN.2004121070. [DOI] [PubMed] [Google Scholar]
- 23.Docherty NG, O’Sullivan OE, Healy DA, et al. Evidence that inhibition of tubular cell apoptosis protects against renal damage and development of fibrosis following ureteric obstruction. Am J Physiol Renal Physiol. 2006;290(1):F4–13. doi: 10.1152/ajprenal.00045.2005. [DOI] [PubMed] [Google Scholar]