Abstract
Background
Low levels of 1-25-dihydroxyvitamin D3 [1,25(OH)2D3] in serum may be a risk factor for several tumor types. Also, high cathelicidin antimicrobial peptide (CAMP) expression is regarded to be important against tumor progression. We evaluated the potential importance of 1,25(OH)2D3 in the diagnosis and treatment of papillary thyroid cancer (PTC).
Material/Methods
The preoperative serum level of 1,25(OH)2D3 was measured using a double-antibody sandwich enzyme-linked immunosorbent assay. Vitamin D3 receptor (VDR) expression was detected by streptavidin-peroxidase immunohistochemical staining in PTC specimens. Receiver operating characteristic (ROC) curves were created to assess the diagnostic value of 1,25(OH)2D3. The effect of 1,25(OH)2D3 on the proliferation and apoptosis of PTC cell lines were studied by Cell Counting Kit (CCK)-8 assay and Annexin V/propidium iodide staining, respectively. CAMP expression was measured by qRT-PCR and western blotting. Short interfering RNAs were used to reduce CAMP expression in PTC cell lines.
Results
The preoperative serum level of 1,25(OH)2D3 in PTC was obviously lower than that in nodular goiter (NG) (P<0.05). The ROC curve suggested that 1,25(OH)2D3 might serve as a potential diagnostic value at a cutoff of 20.13 pg/mL, The VDR showed higher expression in PTC than in paired adjacent non-cancerous tissue. 1,25(OH)2D3 inhibited the proliferation and induced the apoptosis of PTC cells, and increased CAMP expression significantly, whereas CAMP knockdown demonstrated opposite effects.
Conclusions
1,25(OH)2D3 may be a new, potential biomarker for the identification of PTC and NG. It may also become 1,25(OH)2D3 may a potential target for drug action to treat PTC through CAMP.
MeSH Keywords: Biological Markers; Calcitriol; Cathelicidins; Receptors, Calcitriol; Thyroid Neoplasms
Background
Papillary thyroid cancer (PTC) is the most common malignancy of the endocrine system [1]. Thyroid carcinogenesis involves multifactorial interplay between genetic and environmental factors [2]. However, the occurrence and development of PTC is incompletely understood. Recent advances in molecular endocrinology have led to considerable progress in understanding of the pathogenesis of thyroid cancers [3,4].
Some studies have indicated that low levels of 1–25-dihydroxyvitamin D3 [1,25(OH)2D3] in peripheral blood might be a risk factor for several types of cancer [5]. Furthermore, 1,25(OH)2D3 insufficiency has been reported to be associated with advanced cancer stage and an increased risk of metastasis and recurrence of colorectal, breast, and prostate cancer cells [6,7]. In addition to its role in calcium homeostasis, the activated form of 1,25(OH)2D3 is thought to have a vital role in the proliferation, differentiation, and apoptosis of cells as well as in angiogenesis; it is believed to be a crucial factor in the inhibition of tumor growth [8]. 1,25(OH)2D3 has several anti-proliferative effects through its non-classical roles in several malignancies [9,10]. Studies using animal models and in vitro investigations have shown 1,25(OH)2D3 to have anti-proliferative effects on thyroid cancers [11,12]. Those studies have highlighted the potential role of 1,25(OH)2D3-vitamin D3 receptor (1,25(OH)2D3-VDR) signaling in cancers. [13,14]. The VDR is a member of the nuclear hormone receptor superfamily that binds to DNA at vitamin D (VD) response elements to alter transcription of VD-responsive genes [15].
Recently, cathelicidin antimicrobial peptide (CAMP) has been shown to be associated with tumor surveillance and anti-tumor effects [16]. VDR-dependent mechanisms regulate CAMP in various cell types, and CAMP has been shown to have a role in modulation of the apoptosis of several types of cancer cell [17]. However, whether CAMP can inhibit the proliferation and induce the apoptosis of thyroid cancer cells is not known.
We investigated the preoperative serum level of 1,25(OH)2D3 and tissue expression of the VDR in patients with PTC or nodular goiter (NG) to explore its potential importance in the diagnosis and treatment of PTC. We hypothesized that 1,25(OH)2D3 could inhibit the proliferation and induce the apoptosis of PTC cells by increasing levels of CAMP.
Material and Methods
Ethical approval and consent to participate
The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of China Medical University (Shenyang, China). Written informed consent was obtained from all study participants.
Patients
Between December 2014 and March 2015, the First Affiliated Hospital of China Medical University recruited 158 patients with thyroid nodules who were undergoing thyroidectomy. Patients with hypothyroidism, hyperthyroidism, non-thyroid malignancies, or hepatic/renal disease (or taking multivitamins/vitamin-D supplements within the 12 months) were excluded.
Measurement of 1,25(OH)2D3 levels
The blood samples of 158 Chinese patients who resided in Northeast China (latitude 41ºN) were obtained during winter within 1 week before thyroidectomy, and the daily sun exposure time was estimated to be <1.5 hour for all participants. 1,25(OH)2D3 levels were measured in the sera of patients before surgery using a double-antibody sandwich enzyme-linked immunosorbent assay (ELISA) according to manufacturer (WKSU-BIO, Shanghai, China) instructions. The intra-assay precision and inter-assay precision were 15% and 9%, respectively.
Tissue collection and immunohistochemistry
Tissues from 78 patients with PTC were collected. Tissue sections (4 μm) from paraffin-embedded tissue blocks were used for streptavidin-peroxidase immunohistochemical (IHC) staining. Sections were incubated with mouse polyclonal antibodies against the VDR (1: 200 dilution; Abcam, Cambridge, MA, USA) at 4°C overnight. Immunostaining was done using a permanent brown chromogenic substrate system (ZSGB-BIO, Beijing, China). Nuclei were counterstained with hematoxylin for 2 min and examined under a microscope (Olympus, Tokyo, Japan). Staining was quantified using a immunoreactive score [18]. Staining intensity was evaluated according to the following scale: negative (≤3 points) and positive (>3 points).
Histopathology
Final histological data were available for all patients in the study cohort. Two experienced pathologists assessed the presence or absence of PTC. For patients with PTC, tumors were classified using the tumor-node-metastasis stage according to the system established by the American Joint Committee on Cancer. Sample pathology type was confirmed by pathologist.
Cell culture
The human PTC cell lines K1 and IHH-4 were purchased from Health Science Research Resources Bank (Osaka, Japan). K1 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) mixed with MCDB105, Ham’s F12 medium, and 10% fetal bovine serum (FBS) at 2: 1: 1: 1. IHH-4 cells were cultured in RPMI 1640 mixed with DMEM and 10% FBS (2: 2: 1).
Cell proliferation
Cell proliferation was assessed using the Cell Counting Kit-8 (CCK-8) assay (Dojindo, Kumamoto, Japan). Cells (3000 per well) were planted onto a 96-well plate in a final volume of 100 μL. They were then treated with 1,25(OH)2D3 dissolved in dimethyl sulfoxide (Selleck Chemicals, Houston, TX, USA) at a final concentration of 0, 1, 10, and 100 μM for 24, 48, and 72 hours. Briefly, 10 μL of CCK-8 reagent was added to each well, and cells incubated for 3 hours. Cell viability was assessed using an ELISA plate reader by measuring the absorbance at 450 nm.
Apoptosis
Staining by Annexin V/propidium iodide (PI) was measured using fluorescein isothiocyanate (FITC) Annexin V Apoptosis Detection Kit I (Beckton Dickinson, Franklin Lakes, NJ, USA). K1 and IHH4 cells were seeded in 6-well plates. After overnight incubation, they were treated with 1,25(OH)2D3 (0, 1, 10, and 100 μM) and incubated for 48 hours at 37°C. Then, cells were harvested and washed twice with phosphate-buffered saline. Briefly, the samples were subjected to sequential staining with FITC Annexin V Apoptosis Detection Kit I according to manufacturer instructions. Finally, the samples were analyzed via flow cytometry (Beckton Dickinson) within 1 hour and images acquired. The experiment was repeated three times.
RNA extraction and quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
RNA was extracted from K1 and IHH4 cells using RNAiso Plus (TaKaRa Biotechnology, Dalian, China) according to manufacturer instructions. cDNA was synthesized using a reverse transcription kit (TaKaRa Biotechnology). SYBR Premix Ex Taq II (TaKaRa Biotechnology) was employed to carry out qRT-PCR with a LightCycler™ 480 system (Roche Diagnostics, Basel, Switzerland). The primer (Genscript Bio, Nanjing, China) sequences (forward and reverse, respectively) were: 5′-GGCTGGTGAAGCGGTGTAT-3′ and 5′-TGGGTACAAGATTCCGCAAAAA-3′ for CAMP; 5′-ACCACAGT CCATGCCATCAC-3 and 5′-TCCACCACCCTGTTGCTGTA-3′ for glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Relative expression of the VDR was calculated with the double-standard curves method.
Western blotting
Protein extracts from cell lysates were generated from 1,25(OH)2D3-treated PTC cells, which were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories, Hercules, CA, USA). Then, the blotted PVDF membranes were incubated with anti-CAMP (1: 1000 dilution; Abcam) and anti-beta-actin (1: 2000; Cell Signaling Technology, Danvers, MA, USA). Washed PVDF membranes were incubated for 1.5 hours with horseradish peroxidase-conjugated anti-rabbit IgG secondary antibodies (1: 5000; Cell Signaling Technology). Images of western blots were acquired by MicroChemi (DNR, Neve Yamin, Israel).
Cell transfection
The short interfering RNAs (siRNAs) used to reduce CAMP expression were purchased from TaKaRa Biotechnology. The sequence of the CAMP siRNA was 5′-GUGCUAUAGAUGGCAUCAATT-3′ (sense) and 5′-UUGAUGCCAUCUAUAGCACTT-3′ (antisense). The sequence of NC siRNA was 5′-UUCUCCGAACGUGUCACGUTT-3′ (sense) and 5′-TTAAGAGGCUUGCACAGUGCA-3′ (antisense). K1 and IHH4 cells were transfected using Lipofectamine® 2000 according to manufacturer (Invitrogen, Carlsbad, CA, USA) protocols. After 24 or 48 hours, cells were harvested for further experiments.
Statistical analyses
Data analyses were carried out using SPSS v21.0 (IBM, Armonk, NY, USA) and Prism 6.0 (GraphPad, La Jolla, CA, USA). The data for 1,25(OH)2D3 did not adhere to a Gaussian distribution. Differences were assessed by non-parametric Mann-Whitney U-tests. Receiver operator characteristic (ROC) curves were established to evaluate the diagnostic value of 1,25(OH)2D3 to differentiate between PTC and NG. The chi-squared test was applied to assess the difference between VDR expression and clinicopathologic characteristics. P<0.05 was considered significant.
Results
The preoperative serum level of 1,25(OH)2D3 was lower in PTC than in NG
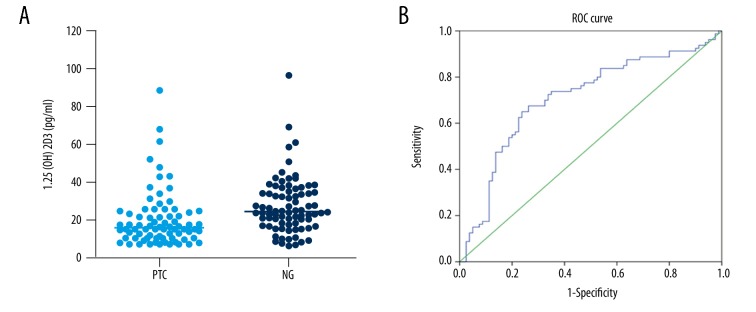
Preoperatively, we measured the 1,25(OH)2D3 level in the sera of 78 patients with PTC and 80 patients with NG during the same period. When evaluating the median (range) of 1,25(OH)2D3 for the entire group, the PTC group had a median 1,25(OH)2D3 level of 16.07 (7.17–88.67) pg/mL, and the NG group had a 1,25(OH)2D3 level of 24.94 (6.67–97.15) pg/mL (P=0.028), thereby showing a significant difference between the two groups (Figure 1A). However, the preoperative serum level of 1,25(OH)2D3 in PTC patients was not significantly different with regard to age, sex, tumor size, TNM stage, recurrence risk stratification (RRS), or lymph-node metastasis (LNM) (Table 1).
Figure 1.
(A) The preoperative serum level of 1,25(OH)2D3 in papillary thyroid cancer was lower than that in nodular goiter (P=0.028). (B) Diagnostic value of 1,25(OH)2D3. The area under the receiver operating characteristic curve was 0.728 (95% confidence interval=0.625–0.790, P<0.001).
Table 1.
Clinical and pathologic features of patients with the preoperative serum level of 1,25(OH)2D3 in PTC patients.
| Index | Median of 1,25(OH)2D3 | P(test) |
|---|---|---|
| Age | 0.648 | |
| ≥45 | 38.42 | |
| <45 | 40.76 | |
| Sex | 0.377 | |
| Male | 45.09 | |
| Female | 38.58 | |
| Tumor size | 0.402 | |
| ≤1 | 37.11 | |
| >1 | 41.44 | |
| TNM stage | 0.851 | |
| I+II | 39.75 | |
| III+IV | 38.59 | |
| RRS | 0.506 | |
| Low risk | 38.00 | |
| Middle and high risk | 41.44 | |
| LNM | 0.599 | |
| No | 38.40 | |
| Yes | 41.16 |
TNM stage – tumor node metastasis stage; RRS – recurrence risk stratification; LNM – lymph node metastasis.
Diagnostic value of 1,25(OH)2D3
We explored the potential diagnostic value of 1,25(OH)2D3 as a biomarker. A ROC curve was generated to ascertain if 1,25(OH)2D3 could predict malignancy.
The area under the ROC curve was 0.728 (95% confidence interval=0.625–0.790, P<0.001) (Figure 1B), which suggested that 1,25(OH)2D3 could be a has potential diagnostic value for PTC. When using a cutoff value of 20.13 pg/mL, the sensitivity and specificity were 67.5% and 73.7%, respectively, and the Youden Index was 0.413.
The VDR shows high expression in PTC
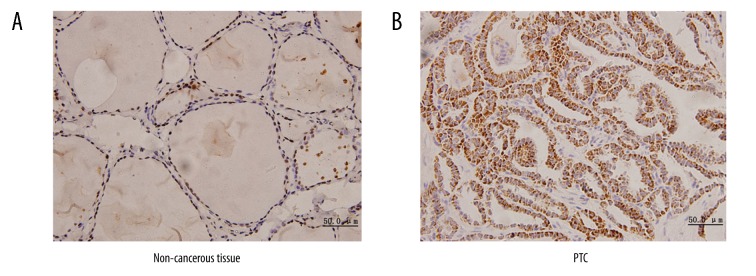
Expression of VDR protein in PTC was notably higher (51 out of 78, 65.4%) than that observed in paired adjacent non-cancerous tissue (21 out of 78, 26.9%), and IHC staining was localized in cytoplasm of PTC (Figure 2A), whereas IHC staining was localized in nuclei of paired adjacent non-cancerous tissue (Figure 2B). Expression of VDR protein was significantly different according to age (cutoff point at 45 years) (P=0.031). However, there were no significant differences in other clinicopathologic characteristics such as sex, tumor size, TNM stage, RRS, or LNM (Table 2).
Figure 2.
Expression of the vitamin D3 receptor (VDR). (A) Positive staining of the VDR in the nuclei of paired adjacent non-cancerous tissue. (B) Positive staining of the VDR in the cytoplasm of papillary thyroid cancer. Original magnification 400×.
Table 2.
Clinical and pathologic features of patients with or without positive VDR expression in PTC patients.
| Index | VDR | P(test) | |
|---|---|---|---|
| Positive | Negative | ||
| Age | 0.031* | ||
| ≥45 | 32 | 10 | |
| <45 | 19 | 17 | |
| Sex | 0.583 | ||
| Male | 8 | 3 | |
| Female | 43 | 24 | |
| Tumor size | 0.370 | ||
| ≤1 | 21 | 14 | |
| >1 | 30 | 13 | |
| TNM stage | 0.280 | ||
| I+II | 38 | 23 | |
| III+IV | 13 | 4 | |
| RRS | 0.912 | ||
| Low risk | 29 | 15 | |
| Middle and high risk | 22 | 12 | |
| LNM | 0.541 | ||
| No | 32 | 15 | |
| Yes | 19 | 12 | |
<0.05 vs. VDR positive expression <45 years old.
1,25(OH)2D3 inhibits the proliferation and induces the apoptosis of PTC cell lines
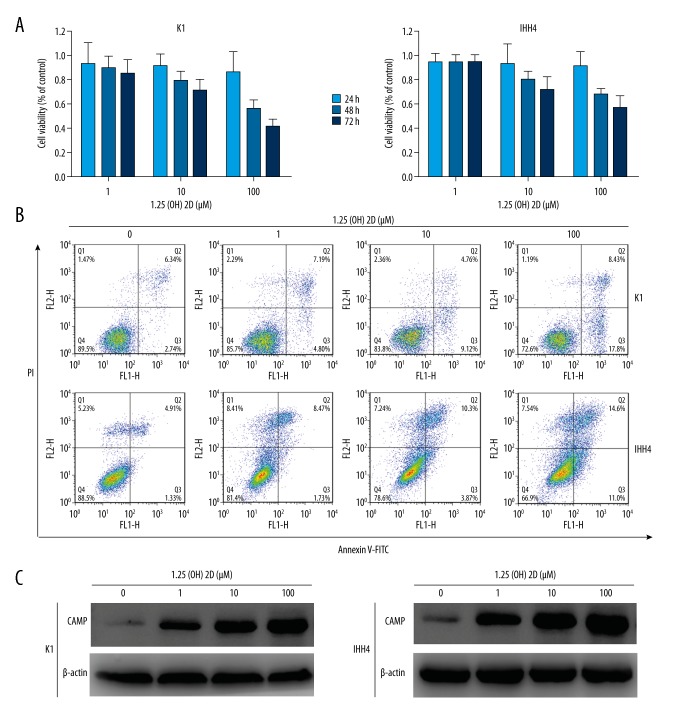
The viability of PTC cells was reduced by 1,25(OH)2D3 in a dose- and time-dependent fashion. The half-maximal inhibitory concentration of 1,25(OH)2D3 was 112.5 μM in K1 cells and 157.1 μM in K1 cells (Figure 3A). Moreover, 1,25(OH)2D3 induced the apoptosis of K1 and IHH4 cells as assessed by flow cytometry (Annexin V-FITC/PI double-staining), which was increased significantly in a dose-dependent manner. 1,25(OH)2D3 (100 μM) increased the proportion of PTC cells undergoing apoptosis (Figure 3B). These results suggested that 1,25(OH)2D3 exhibited potent effects against PTC cell lines.
Figure 3.
1,25(OH)2D3 inhibited the proliferation and induced the apoptosis of K1 and IHH4 cells. (A) Cell proliferation was measured by the CCK-8 assay after treatment with different concentrations of 1,25(OH)2D3 for 24, 48, and 72 hours. (B) Apoptosis was measured using a FITC Annexin V Apoptosis Detection Kit I after treatment with different concentrations of 1,25(OH)2D3 for 48 hours. (C) Western blotting of expression of CAMP (cathelicidin antimicrobial peptide protein) after treatment with different concentrations of 1,25(OH)2D3 for 48 h in K1 and IHH4 cells.
1,25(OH)2D3 upregulates CAMP expression in PTC cell lines
We measured the endogenous CAMP expression in K1 and IHH4 cells. Western blotting revealed that 1,25(OH)2D3 upregulated expression of CAMP protein in a dose-dependent manner (Figure 3C). These results suggested that CAMP may participate in the proliferation and apoptosis induced by 1,25(OH)2D3 in PTC cell lines.
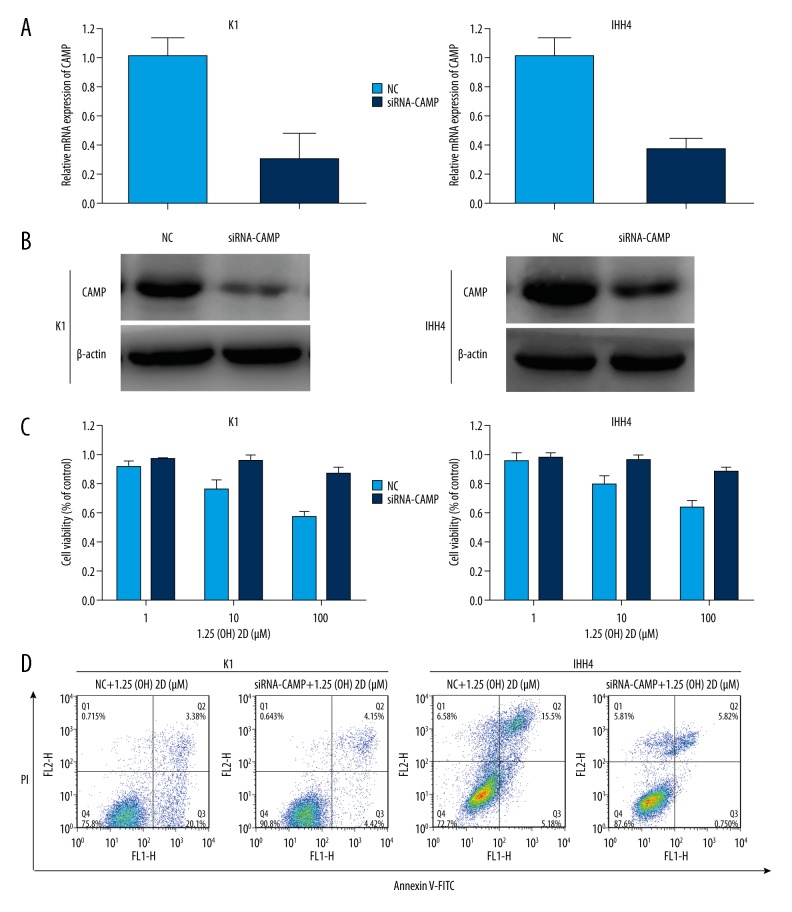
CAMP knockdown can reverse the proliferation and apoptosis caused by 1,25(OH)2D3
To ascertain the role of CAMP in 1,25(OH)2D3-directed inhibition of the proliferation and increased apoptosis of PTC cells, we first knocked down CAMP using a specific siRNA in PTC cell lines (Figure 4A). The CCK-8 assay and Annexin V-FITC/PI double-staining showed that knockdown of CAMP expression attenuated the proliferation and reduced the apoptosis caused by 1,25(OH)2D3 in PTC cell lines significantly (Figure 4B–4D). These results demonstrated the important role of CAMP in the proliferation and apoptosis in PTC cell lines induced by 1,25(OH)2D3.
Figure 4.
Knockdown of cathelicidin antimicrobial peptide (CAMP) expression can reverse the proliferation and apoptosis caused by 1,25(OH)2D3. (A) Relative expression of CAMP after transfecting CAMP siRNA into K1 and IHH4 cells was analyzed by qRT-PCR and normalized to GAPDH expression. (B) Expression of CAMP protein was measured, and beta-actin protein was used for normalization after treatment with only 1,25(OH)2D3 or CAMP siRNA plus 1,25(OH)2D3 for 48 hours. (C) CAMP siRNA reduces the inhibition of cell proliferation caused by 1,25(OH)2D3. (D) CAMP siRNA reverses the apoptosis caused by 1,25(OH)2D3. The 1,25(OH)2D3 concentration in these experiments was 100 μM.
Discussion
Many PTC patients have a good prognosis and slow course of disease, but this can be complicated by distant metastasis or inoperable disease [19]. Therefore, finding a good biomarker to aid the diagnosis of PTC and to aid treatment of those with inoperable disease or who are refractory to radioiodine treatment is very important.
It is believed that 3% of the human genome is under the influence of 1,25(OH)2D3 and that many of these genes are involved in essential cell-regulatory mechanisms, the disruption of which may result in carcinogenesis [20]. 25-hydroxyvitamin D3 [25(OH)D3] is used commonly to evaluate nutritional status in clinical and basic research because of its relatively high level and long half-life in peripheral blood, and its ease of detection [21]. Low levels of 1,25(OH)2D3, but not 25(OH)D3, have been shown to have a significant association with cancer [22,23].
Some risk factors for thyroid cancer have been established (age, sex, previous exposure to radiation, family history) but some would argue that a modifiable risk factor is lacking. Our knowledge regarding the 1,25(OH)2D3 level in malignant thyroid tissue is limited. The preoperative 1,25(OH)2D3 concentration in serum is particularly important to assess the biologic behavior of malignant tumors. Few studies have examined the association between preoperative serum levels of 1,25(OH)2D3 and the prevalence of malignant tumors, and fewer still have addressed this association with thyroid cancer. In addition, the results in thyroid-cancer studies have been inconsistent [24–26]. Lee et al. reported that 1,25(OH)2D3 levels are normal in patients with medullary thyroid cancer. Penna et al. found that 1,25(OH)2D3 was lacking or insufficient (<20 pmol/L) in 25.4% and 33.3% of cases with PTC and follicular thyroid carcinoma, respectively, but only 1.8% of cases in the healthy control group had low 1,25(OH)2D3 levels. Stepien et al. suggested that the 1,25(OH)2D3 level is associated negatively with PTC, follicular thyroid cancer, and anaplastic thyroid cancer, and is correlated with clinical staging.
We explored the relationship between preoperative serum levels of 1,25(OH)2D3 and various clinicopathologic features in PTC patients. Our results revealed that the preoperative serum level of 1,25(OH)2D3 in PTC was obviously lower than that in NG. The ROC curve revealed that 1,25(OH)2D3 had potential diagnostic value as a biomarker. Thus, combined use of this serum marker with other molecular markers could optimize PTC diagnosis [27].
Recent results from qRT-PCR analyses for the VDR showed obvious differences between PTC and normal thyroid tissues, and IHC analyses for the VDR revealed positive staining in PTC, but low, scarce, or even absent VDR staining in adjacent normal thyroid tissues. The authors suggested that a high cytochrome P450 (CYP)27B1/low CYP24A1 profile in PTC might enhance VDR expression and reduce VDR inactivation, thereby increasing the local availability of 1,25(OH)2D3 [28,29]. We found that VDR expression was increased significantly in PTC tissue compared with paired adjacent non-cancerous tissue. However, VDR expression was significantly different only according to age (cutoff at 45 years) in PTC (P<0.05), and not different with regard to sex, tumor size, TNM stage, RRS, or LNM. The results of our study differ from those of certain other reports and the Youden index is low, possibly because of its limitations (patients were from a single center selected in a narrow time range). Therefore, Further studies are needed to establish the best method to determine cutoff value and evaluate its validity in future, such as season; BMI; eating habits; use of cigarettes, alcohol, and education.
Several studies have reported that high VDR expression may be an important response against tumor progression [30]. We hypothesized that the transcription or translation of the VDR may be activated in PTC pathogenesis, or that increased VDR expression might promote the occurrence of PTC. Therefore, the specific mechanism and discrepancy between VDR expression between PTC and NG require further study.
1,25(OH)2D3 binds to the nuclear VDR in target organs to form heterodimers with the retinoid X receptor. This action results in the recruitment of other transcriptional cofactors that inhibit the growth of thyroid, breast, pancreatic, colorectal, ovarian, and prostate cancer cells [31]. When the VDR binds to 1,25(OH)2D3, it changes its conformation, induces the transcription of target genes, and adjusts its biologic effects through a non-classical or classical pathway [32].
Use of animal models and in vitro studies have shown 1,25(OH)2D3 to have anti-proliferative effects on thyroid cancer [11,12]. Studies have suggested that several cytokines have important roles in the proliferation and apoptosis of thyroid cancer cells [33,34]. Some studies have provided evidenced that CAMP is a direct or indirect target of the VDR [35,36]. As a vital member of the cationic antimicrobial peptides family, the particular mechanism by which CAMP inhibits proliferation and induces apoptosis is not known, but it has been assumed that CAMP could induce phosphatidylserine externalization and DNA fragmentation in a manner independent of caspase activation [37]. Some studies have found that CAMP can enhance the expression of Bcl-2 and reduce the expression of Bax [38]. Other research has suggested an increase in caspase-3 activity caused by CAMP (which plays a vital part in apoptosis) in direct and indirect ways. Microscopy studies have suggested that CAMP causes cell shrinkage, membrane “blebbing”, nuclear condensation, DNA fragmentation and an increase in caspase-3 activity [39].
In the present study, we discovered that 1,25(OH)2D3 inhibited proliferation and induced apoptosis in PTC cell lines. Moreover, 1,25(OH)2D3 increased CAMP expression significantly, whereas CAMP knockdown demonstrated opposite effects. Our data revealed a novel mechanism for the 1,25(OH)2D3-VDR/CAMP axis in PTC cell lines. Hence, 1,25(OH)2D3, if used in PTC cell lines, can inhibit proliferation and induce apoptosis by upregulating CAMP expression through activation of the VDR.
Conclusions
The preoperative serum level of 1,25(OH)2D3 in patients with PTC was obviously lower than that in patients with NG. 1,25(OH)2D3 could be a new, potential biomarker to identify PTC and NG. 1,25(OH)2D3 can inhibit the proliferation and induce the apoptosis of PTC cells and may become a target for drug action to treat PTC through CAMP.
Footnotes
Conflict of interests
None.
Source of support: This work was supported by the Liaoning BaiQianWan Talents Program [grant number 2014921033], the Science and Technology Project of Shenyang City [grant number F16-205-1-41], and the Natural Science Foundation of Liaoning Province [grant number 2015020536]
References
- 1.Costa R, Carneiro BA, Chandra S, et al. Spotlight on lenvatinib in the treatment of thyroid cancer: Patient selection and perspectives. Drug Des Dev Ther. 2016;10:873–84. doi: 10.2147/DDDT.S93459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin HY, Chin YT, Yang YC, et al. Thyroid hormone, cancer, and apoptosis. Compr Physiol. 2016;6:1221–37. doi: 10.1002/cphy.c150035. [DOI] [PubMed] [Google Scholar]
- 3.Fukahori M, Yoshida A, Hayashi H, et al. The associations between ras mutations and clinical characteristics in follicular thyroid tumors: New insights from a single center and a large patient cohort. Thyroid. 2012;22:683–89. doi: 10.1089/thy.2011.0261. [DOI] [PubMed] [Google Scholar]
- 4.Evangelisti C, de Biase D, Kurelac I, et al. A mutation screening of oncogenes, tumor suppressor gene TP53 and nuclear encoded mitochondrial complex i genes in oncocytic thyroid tumors. BMC Cancer. 2015;15:157. doi: 10.1186/s12885-015-1122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldman D, Krishnan AV, Swami S, et al. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14:342–57. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 6.Colston KW, Lowe LC, Mansi JL, Campbell MJ. Vitamin D status and breast cancer risk. Anticancer Res. 2006;26:2573–80. [PubMed] [Google Scholar]
- 7.Wang WL, Tenniswood M. Vitamin D, intermediary metabolism and prostate cancer tumor progression. Front Physiol. 2014;5:183. doi: 10.3389/fphys.2014.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ness RA, Miller DD, Li W. The role of vitamin D in cancer prevention. Chin J Nat Med. 2015;13:481–97. doi: 10.1016/S1875-5364(15)30043-1. [DOI] [PubMed] [Google Scholar]
- 9.Guyton KZ, Kensler TW, Posner GH. Vitamin D and vitamin D analogs as cancer chemopreventive agents. Nutr Rev. 2003;61:227–38. doi: 10.1301/nr.2003.jul.227-238. [DOI] [PubMed] [Google Scholar]
- 10.Peng W, Wang K, Zheng R, Derwahl M. 1,25 dihydroxyvitamin d31,25 dihydroxyvitamin D3 inhibits the proliferation of thyroid cancer stem-like cells via cell cycle arrest. Endocr Res. 2016;41:71–80. doi: 10.3109/07435800.2015.1037048. [DOI] [PubMed] [Google Scholar]
- 11.Clinckspoor I, Verlinden L, Overbergh L, et al. 1,25-dihydroxyvitamin D-3 and a superagonistic analog in combination with paclitaxel or suberoylanilide hydroxamic acid have potent antiproliferative effects on anaplastic thyroid cancer. J Steroid Biochem Mol Biol. 2011;124:1–9. doi: 10.1016/j.jsbmb.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Dackiw AP, Ezzat S, Huang P, et al. Vitamin D3 administration induces nuclear p27 accumulation, restores differentiation, and reduces tumor burden in a mouse model of metastatic follicular thyroid cancer. Endocrinology. 2004;145:5840–46. doi: 10.1210/en.2004-0785. [DOI] [PubMed] [Google Scholar]
- 13.Guy M, Lowe LC, Bretherton-Watt D, et al. Vitamin D receptor gene polymorphisms and breast cancer risk. Clin Cancer Res. 2004;10:5472–81. doi: 10.1158/1078-0432.CCR-04-0206. [DOI] [PubMed] [Google Scholar]
- 14.Bikle D, Teichert A, Hawker N, et al. Sequential regulation of keratinocyte differentiation by 1,25(OH)2D3, VDR, and its coregulators. J Steroid Biochem Mol Biol. 2007;103:396–404. doi: 10.1016/j.jsbmb.2006.12.063. [DOI] [PubMed] [Google Scholar]
- 15.MacDonald PN, Baudino TA, Tokumaru H, et al. Vitamin D receptor and nuclear receptor coactivators: Crucial interactions in vitamin d-mediated transcription. Steroids. 2001;66:171–76. doi: 10.1016/s0039-128x(00)00200-2. [DOI] [PubMed] [Google Scholar]
- 16.Deng X, Qiu QQ, Ma K, et al. Aliphatic acid-conjugated antimicrobial peptides – potential agents with anti-tumor, multidrug resistance-reversing activity and enhanced stability. Org Biomol Chem. 2015;13:7673–80. doi: 10.1039/c5ob00752f. [DOI] [PubMed] [Google Scholar]
- 17.Park K, Elias PM, Oda Y, et al. Regulation of cathelicidin antimicrobial peptide expression by an endoplasmic reticulum (ER) stress signaling, vitamin D receptor-independent pathway. J Biol Chem. 2011;286:34121–30. doi: 10.1074/jbc.M111.250431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaiman M, Olevson Y, Habler L, et al. Diagnostic value of estrogen receptors in thyroid lesions. Med Sci Monit. 2010;16:BR203–7. [PubMed] [Google Scholar]
- 19.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 20.Welsh J. Vitamin D and cancer: Integration of cellular biology, molecular mechanisms and animal models. Scand J Clin Lab Inv. 2012;72:103–11. doi: 10.3109/00365513.2012.682870. [DOI] [PubMed] [Google Scholar]
- 21.Mocanu V, Vieth R. Three-year follow-up of serum 25-hydroxyvitamin D, parathyroid hormone, and bone mineral density in nursing home residents who had received 12 months of daily bread fortification with 125 mu g of vitamin D-3. Nutr J. 2013;12:137. doi: 10.1186/1475-2891-12-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Lyra EC, da Silva IA, Hirata Katayama ML, et al. 25(OH)D-3 and 1,25(OH)(2)D-3 serum concentration and breast tissue expression of l alpha-hydroxylase, 24-hydroxylase and vitamin d receptor in women with and without breast cancer. J Steroid Biochem Mol Biol. 2006;100:184–92. doi: 10.1016/j.jsbmb.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Charalampopoulos A, Charalabopoulos A, Batistatou A, et al. Parathormone and 1,25(OH)2D3 but not 25(OH)D3 serum levels, in an inverse correlation, reveal an association with advanced stages of colorectal cancer. Clin Exp Med. 2010;10:69–72. doi: 10.1007/s10238-009-0069-6. [DOI] [PubMed] [Google Scholar]
- 24.Stepien T, Krupinski R, Sopinski J, et al. Decreased 1–25 dihydroxyvitamin D3 concentration in peripheral blood serum of patients with thyroid cancer. Arch Med Res. 2010;41:190–94. doi: 10.1016/j.arcmed.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Lee S, Morimoto S, Onishi T, et al. Normal serum 1,25-dihydroxyvitamin D in patients with medullary carcinoma of the thyroid. J Clin Endocrinol Metab. 1982;55:361–63. doi: 10.1210/jcem-55-2-361. [DOI] [PubMed] [Google Scholar]
- 26.Penna-Martinez M, Ramos-Lopez E, Stern J, et al. Vitamin D receptor polymorphisms in differentiated thyroid carcinoma. Thyroid. 2009;19:623–28. doi: 10.1089/thy.2008.0388. [DOI] [PubMed] [Google Scholar]
- 27.Zhu XC, Zhou K, Xu SQ, et al. Diagnostic value of semiquantitative analysis of 99mtechnetium-methoxyisobutylisonitrile (99mTc-MIBI) imaging in predicting early-stage cervical lymph node metastasis of thyroid carcinoma. Med Sci Monit. 2017;23:1552–58. doi: 10.12659/MSM.899966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khadzkou K, Buchwald P, Westin G, et al. 25-hydroxyvitamin D3 1alpha-hydroxylase and vitamin D receptor expression in papillary thyroid carcinoma. J Histochem Cytochem. 2006;54:355–61. doi: 10.1369/jhc.5A6734.2005. [DOI] [PubMed] [Google Scholar]
- 29.Clinckspoor I, Hauben E, Verlinden L, et al. Altered expression of key players in vitamin D metabolism and signaling in malignant and benign thyroid tumors. J Histochem Cytochem. 2012;60:502–11. doi: 10.1369/0022155412447296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu XY, Zhou T, Cao N, et al. Role of vitamin D metabolism and activity on carcinogenesis. Oncol Res. 2015;22:129–37. doi: 10.3727/096504015X14267282610894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vanoirbeek E, Krishnan A, Eelen G, et al. The anti-cancer and anti-inflammatory actions of 1,25(OH)(2)D(3) Best Pract Res Clin Endocrinol Metab. 2011;25:593–604. doi: 10.1016/j.beem.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osborne JE, Hutchinson PE. Vitamin D and systemic cancer: Is this relevant to malignant melanoma? Brit J Dermatol. 2002;147:197–213. doi: 10.1046/j.1365-2133.2002.04960.x. [DOI] [PubMed] [Google Scholar]
- 33.Liu W, Asa SL, Fantus IG, et al. Vitamin d arrests thyroid carcinoma cell growth and induces p27 dephosphorylation and accumulation through pten/akt-dependent and -independent pathways. Am J Pathol. 2002;160:511–19. doi: 10.1016/S0002-9440(10)64870-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang SH, Phelps E, Utsugi S, Baker JR., Jr Susceptibility of thyroid cancer cells to 7-hydroxystaurosporine-induced apoptosis correlates with Bcl-2 protein level. Thyroid. 2001;11:725–31. doi: 10.1089/10507250152484556. [DOI] [PubMed] [Google Scholar]
- 35.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin d receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. Faseb J. 2005;19:1067–77. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 36.White JH. Vitamin d as an inducer of cathelicidin antimicrobial peptide expression: Past, present and future. J Steroid Biochem Mol Biol. 2010;121:234–38. doi: 10.1016/j.jsbmb.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 37.Ren SX, Cheng AS, To KF, et al. Host immune defense peptide ll-37 activates caspase-independent apoptosis and suppresses colon cancer. Cancer Res. 2012;72:6512–23. doi: 10.1158/0008-5472.CAN-12-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim HJ, Cho DH, Lee KJ, et al. Ll-37 suppresses sodium nitroprusside-induced apoptosis of systemic sclerosis dermal fibroblasts. Exp Dermatol. 2011;20:843–45. doi: 10.1111/j.1600-0625.2011.01327.x. [DOI] [PubMed] [Google Scholar]
- 39.Ciornei CD, Tapper H, Bjartell A, et al. Human antimicrobial peptide ll-37 is present in atherosclerotic plaques and induces death of vascular smooth muscle cells: A laboratory study. BMC Cardiovasc Disord. 2006;6:49. doi: 10.1186/1471-2261-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]