This study uses computational fluid dynamics analysis to assess the effects of inferior turbinate resection techniques on nasal airflow in 5 patients.
Key Points
Question
Which portion of inferior turbinate reduction (ITR) is most effective to improve nasal airflow?
Findings
Based on computational fluid dynamics analysis of 5 patients, full-length ITR consistently had the best nasal airflow improvement. For partial-length ITR, the optimal locations varied for each patient.
Meaning
In a nasal airway surgery, reducing the full length of inferior turbinate likely provides the most consistent improvement in nasal airflow; for partial ITR, the site of reduction should be individualized to account for the wide variability in the inferior turbinate anatomy.
Abstract
Importance
Inferior turbinate reduction (ITR) is a commonly performed procedure for the treatment of nasal obstruction. Which portion of the inferior turbinates should be surgically addressed to improve nasal airflow has yet to be determined.
Objective
To use computational fluid dynamics (CFD) analysis to evaluate the airflow changes after reduction along different portions of the inferior turbinate.
Design, Setting, and Participants
Computed tomographic scans of 5 patients were selected. Seven CFD models were created for each patient: 1 unaltered and 6 various ITRs, including 3 one-third ITRs (anterior, middle, and posterior one-third); 2 two-thirds ITRs (anterior and posterior two-thirds); and 1 full-length ITR model. Total airflow rate and nasal resistance was obtained through CFD analysis, and regression analysis was performed on the increased nasal volume, locations, and nasal resistance for all 5 patients.
Main Outcomes and Measures
Total airflow rate and nasal resistance was obtained through CFD analysis, and regression analysis was performed on the increased nasal volume, locations, and nasal resistance for all 5 patients.
Results
Full ITR over the whole length was consistently most effective to improve nasal airflow and resistance for all 5 patients (2 men and 3 women), adjusted for the volume. Regression analysis showed a strong linear (R2≥0.79) relationship between nasal volume changes and nasal airflow. However, the most effective location of partial turbinate reduction was not consistent among patients. Surprisingly, for some patients, posterior ITRs were more effective than anterior ITRs. The site of most effective partial ITR differed from 1 side to the other even in the same individual.
Conclusions and Relevance
The effectiveness of partial ITR and target location likely depends on individual patient anatomy. The fact that full ITRs were consistently most effective and the linear regression between flow and nasal volume changes may indicate that the entire length of the IT has a functional impact on nasal airflow and resistance.
Level of Evidence
NA.
Introduction
Inferior turbinate reduction (ITR) is one of the most commonly performed procedures for the surgical treatment of nasal obstruction. A number of turbinate reduction techniques are available, and turbinate resection is often combined with other nasal airway procedures. Some authors even recommend addressing the inferior turbinates as a first-line procedure before considering other surgical treatment options when addressing nasal obstruction.1
The inferior turbinates (IT) extend the full length of the nasal cavity and contain bony and soft tissue components. The soft tissue components include an epithelial layer overlying venous sinusoids and seromucinous glands.2 The inferior turbinate is thought to play an important role in modulating nasal airflow, and the impact of inferior turbinate hypertrophy nasal airflow and nasal resistance has been well demonstrated in the literature. Using acoustic rhinometry and a subjective rating scale, Morris et al3 demonstrated a correlation between subjective nasal obstruction and the cross-sectional area of anterior aspect of the inferior turbinate. Several computational fluid dynamics (CFD) studies have demonstrated a clear relationship between increased inferior turbinate soft tissue volume and increased nasal resistance.4,5 Using CFD, Wexler et al6 simulated the effects of unilateral IT reduction (ITR) by performing a virtual soft tissue reduction of 2 mm on only 1 IT using a healthy individual’s magnetic resonance imaging. They noted a significant reduction in nasal resistance on the side with the IT volume reduction. Normal healthy nasal models have demonstrated that most nasal airflow occurs along the inferior and middle nasal cavity with only a small amount of nasal airflow occurring along the superior nasal cavity.5,7,8,9,10,11,12,13,14
There are little objective data examining the effectiveness of various degrees and locations of turbinate resection. Although turbinate reduction is a very commonly performed procedure, there is little consensus on how much and which portions of the turbinates should be resected to achieve optimal outcomes. Because the internal nasal valve is located in the anterior nasal cavity, a commonly held belief is that the anterior aspect of the IT contributes more significantly to nasal resistance than the posterior IT. Limited CFD and in vivo studies did suggest that narrowing in the anterior aspect of the nasal cavity had a larger impact on nasal resistance than narrowing the middle or posterior aspects of the nasal cavity.15,16,17 For these reasons, the anterior one-third or one-half of the inferior turbinate is commonly targeted for surgical reduction. Unfortunately, this belief has not yet been rigorously examined, and it is difficult to objectively predict changes in nasal airflow as a direct result of surgical manipulation of nasal airway architecture.
In this study, CFD analysis was used to assess the effects of inferior turbinate resection on nasal airflow. The goal was to assess airflow after simulating turbinate reduction along different portions of the inferior turbinate in an attempt to better understand the functional role of the different regions of the IT.
Methods
Institutional review board approval was obtained for the study from Upstate Medical University and Thomas Jefferson Hospital. Participants provided written informed consent and they were not compensated. Five maxillofacial computed tomography scans (CT) belonging to 5 separate individuals were selected for this study. All patients’ CT scans demonstrated no sign of septal perforation and had a varying degree of inferior turbinate hypertrophy and septal deviation. The CT scans were obtained at a range of 0.33-mm to 0.625-mm thickness, with pixel sizes ranging from 0.39 mm to 0.47 mm.
The scans were used to create 3-dimensional nasal models in Amira (Amira 5.0, Visage Imaging Inc). Nasal airways were segmented from surrounding sinonasal soft tissue. Maxillary, ethmoid, sphenoid, and frontal sinuses were not included as part of the nasal airway model. The nasal airway inlets were bilateral nostril openings and the nasal airway outlet was located in the nasopharynx below the level of palate.
Using Amira, 6 additional geometric models were created to simulate various locations and degrees of ITR for each CT scan. The nasal airway segment boundary was virtually expanded to simulate submucosal removal of IT soft tissue without disturbance of IT bone boundary seen on CT scans. On average 1.5- to 2.5-mm thickness of IT soft tissue volume was removed along the medial and inferior aspects of the inferior turbinates. The axial length of inferior turbinates were determined based on CT scan—the distance between the first to the last slice showing the IT. The lengths of IT were then divided into 3 equal anterior- to posterior-length segments to represent partial one-third ITR. Using this information, the anterior one-third model had just the anterior one-third of bilateral IT addressed. Similarly, the middle one-third and posterior one-third models had only the middle and posterior one-third reduced, respectively (Figure 1). By combining the boundaries of the one-third models, two-thirds IT reduction models were created. The anterior two-thirds model consisted of combining the anterior one-third and the middle one-third boundaries with unaltered posterior one-third IT. The posterior two-thirds model was created by combining the middle one-third model and the posterior one-third model boundaries with unaltered anterior one-third of IT. For all one-third and two-thirds models, to avoid unnatural abrupt contour changes in IT, IT contour was smoothed out to create a natural transition zone immediately anterior and posterior to the experimental region of interest. Finally, full-length IT reduction models were created by combining the anterior one-third, the middle one-third, and the posterior one-third boundaries.
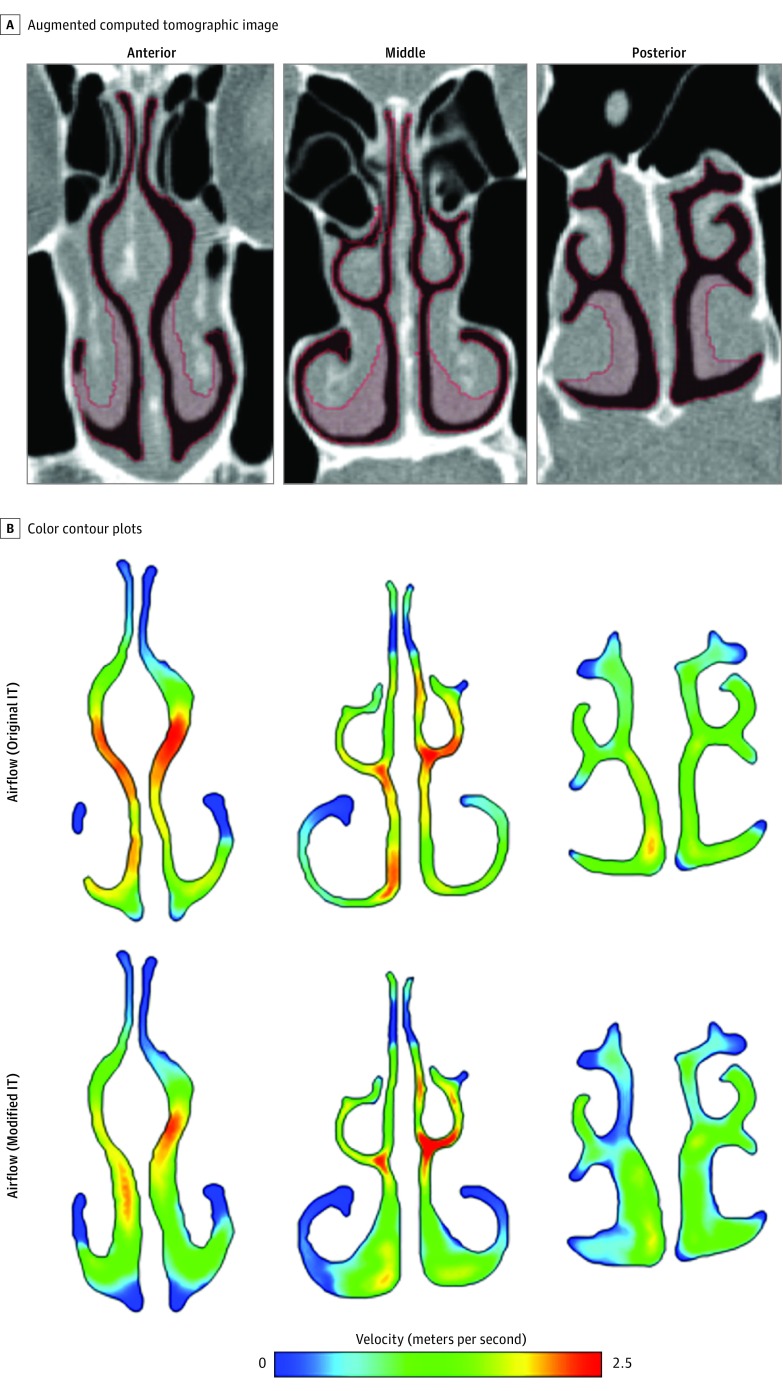
Figure 1. Virtual Inferior Turbinate Reduction.
A, Red shading highlights the virtual inferior turbinate reduction (ITR) that is being performed along anterior (left), middle (middle), and posterior ITs (right). B, The color contour plots compare the velocity magnitude between previrtual and postvirtual anterior third, middle third, and posterior third ITR.
Once all the nasal models were created, tetrahedral prism meshes were created in ICEM (ANSYS) following the technique previously reported.18,19,20 Each nasal model consisted of 110 000 to 250 000 nodes and approximately 610 000 to 800 000 mesh elements (Figure 1, Figure 2, and Figure 3). Although mesh independence was not directly examined in this study, the current study followed the exact same meshing protocol of our previous studies where mesh independence checks were shown to be sufficient to accurately capture the flow phenomena of interest.18,19,20 Fluent was then used to complete CFD analysis with following conditions: laminar, steady, pressure inlet at nostril plane of 15 Pascal (Pa), pressure outlet at pharynx of 0 Pa, nonslip boundary.18,19,20 This pressure drop of 15 Pa was chosen to simulate restful breathing, a state that is most relevant to patients’ symptoms during routine daily life. Previous experimental study also confirmed that nasal airflow at restful breathing flow rate is mostly laminar.8 Once CFD analysis was completed, total flow rate was obtained for each nasal model (Supplement). because the total flow rate was simulated under a fixed pressure drop, it reflects an inverse relationship to nasal resistance. Therefore, high flow rate indicates low nasal resistance, and vice versa.
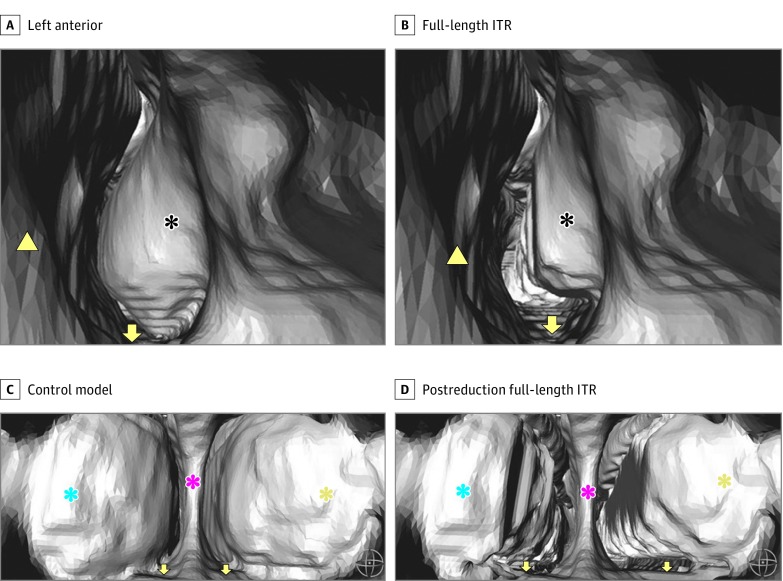
Figure 2. Three-Dimensional Reconstruction of Nasal Airway.
IT indicates inferior turbinate; ITR, inferior turbinate reduction.Three-dimensional reconstruction of left nasal airway from an anterior view. A, Control model’s left anterior head of the IT can be seen. B, Postreduction view of the full-length ITR. Three-dimensional reconstruction of posterior nasal airway immediately behind the posterior septal margin is seen from the nasopharynx. C, Posterior heads of bilateral IT can be seen in the control model. D, Postreduction full-length ITR. The black asterisks indicate anterior head of left IT. The yellow arrowheads mark anterior left side septum. Yellow arrows mark the nasal floor. Blue asterisks mark the posterior head of left IT. Pink asterisks mark the posterior septum. Yellow asterisks mark the posterior head of right IT.
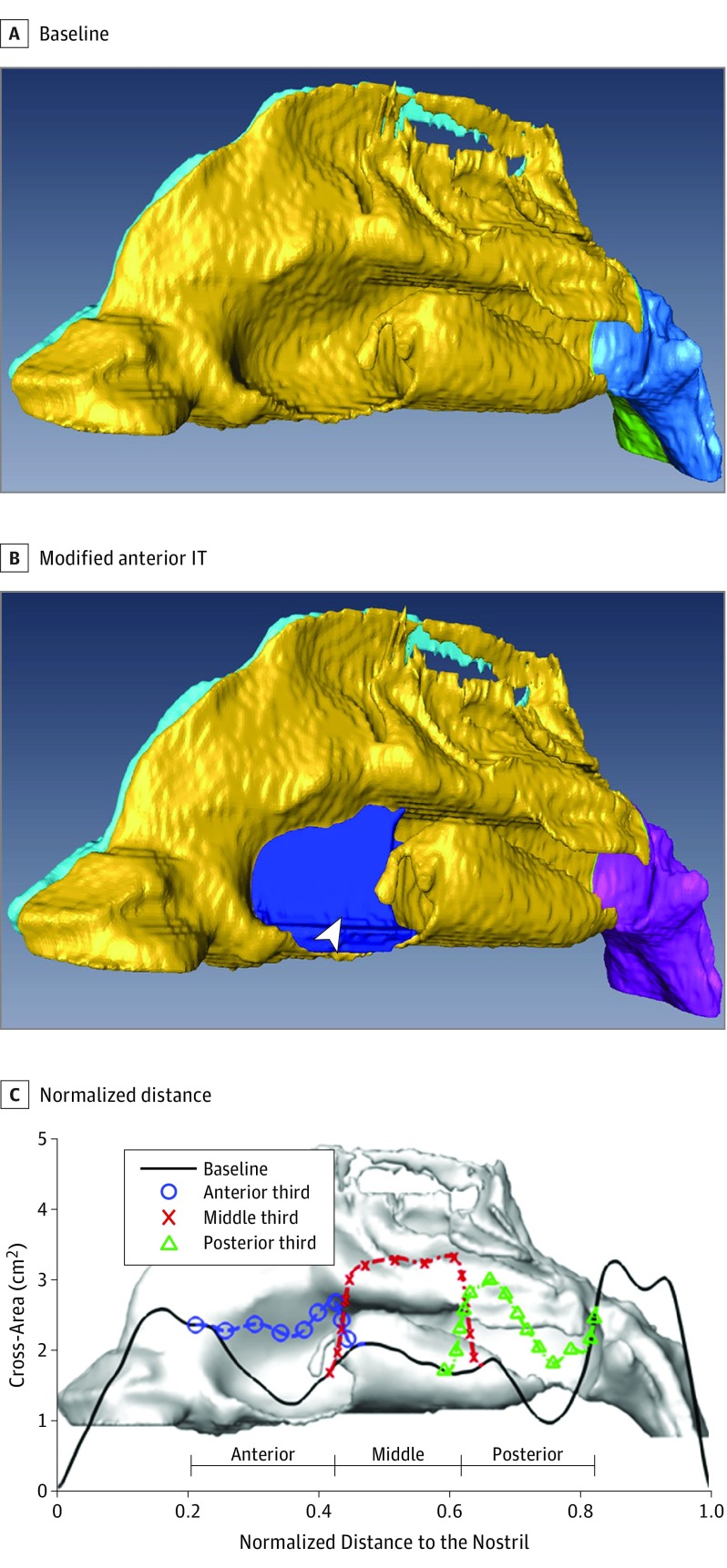
Figure 3. Airway Model.
Abbreviations: CFD, computational fluid dynamics; IT, inferior turbinate; ITR, inferior turbinate reduction. An example of a nasal airway model. Patient 4’s CFD airway models of control (A) and partial anterior third ITR (B) are shown. Additional nasal cavity volume is highlighted in blue along the anterior third IT. C, Nasal airway cross-sectional area is plotted as a function of the axial length for control model and the 3 virtual partial third ITR models.
For each turbinate reduction model, the volume of turbinate tissue reduced was calculated. Although the equal-length turbinate segments were selected in each model, the amount of volume reduction was slightly different in each model. For example, the volume of turbinate reduction in the anterior one-third model was different from the middle one-third model and the posterior one-third model. This was owing to variations in the amount of soft tissue present along the different IT segments. To control for differences in volume reduction in the different models, we calculated flow change per unit volume change. The goal was to better assess for the location-specific effects of partial turbinate reduction by accounting for the volume change differences in the various models. This value, flow change per unit volume change (FCPUVC) was calculated using the following equation for each model: (altered model flow rate − unaltered model flow rate)/(altered model nasal volume − unaltered model nasal volume).
Results
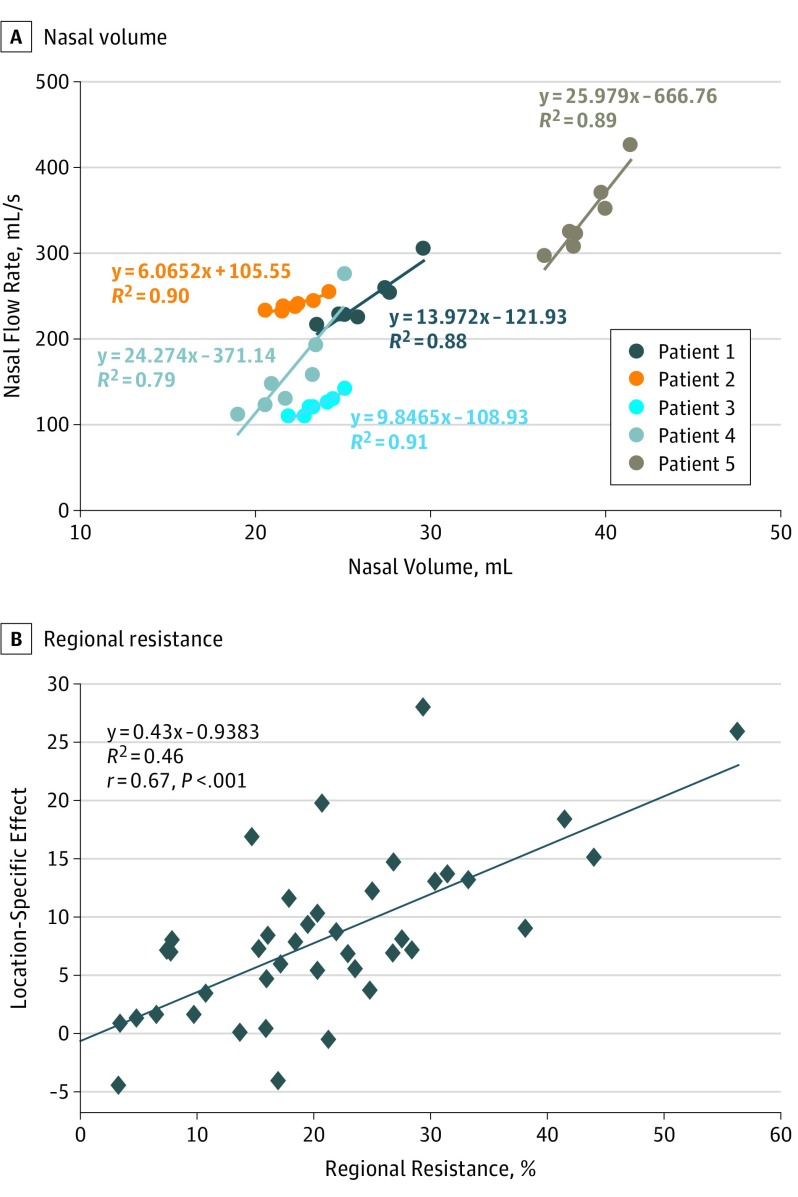
The Supplement summarizes the overall values for nasal airway volume separated by left and right nasal cavity and the total nasal cavity volume, which combines the left and right nasal cavity with the nasopharynx volume. In regression analysis, a strong relationship (R2≥0.79) was identified in all patients (Figure 4). This suggests that volume reduction of IT was generally associated with an increase in nasal airflow and corresponding decrease in nasal resistance. This can be seen as an increase in total airflow and the positive percent change of airflow compared with the control model in the Supplement.
Figure 4. Regression Analysis .
A, Regression analysis of 5 patients. For every 1 mL of inferior turbinate volume reduced, total nasal airflow increased by the following factor: in patient 1 by 13.97 mL/s; in patient 2 by 6.07 mL/s; in patient 3 by 9.84 mL/s; in patient 4 by 25.9 mL/s; in patient 5 by 24.27 mL/s. B, regional flow change per unit volume change (FCPUVC) significantly correlated with regional resistance percentage (r = 0.67, P < .001).
We analyzed the location-specific effect of ITR by compensating for the differences in the IT volume being reduced using FCPUVC (Supplement). This value demonstrated how much the flow rate changed per unit volume of IT that was reduced specific to that location. When looking specifically at only partial one-third ITR models, different portions of the IT had varying degrees of efficacy in improving airflow. As such, anterior one-third ITR was not consistently the location of the highest efficacy as is commonly believed by clinicians. For patients 1 and 4, posterior one-third ITR was the most effective, whereas for patient 2, it was the middle one-third ITR. For patients 3 and 5, the anterior one-third ITR was most effective.
For patients 1, 2, and 3, when each nasal cavity side was analyzed separately, certain partial ITR locations led to a decrease in nasal airflow, suggesting that the partial ITR worsened airflow on that particular side (Supplement). In fact, the site of most effective partial ITR in the same individual differed from 1 side to the other.
In further analysis of the location-specific effect using FCPUVC, simply removing more volume did not always lead to greater efficacy in improving airflow when comparing the one-third length ITR models to two-thirds lengths ITR models. For patient 2 and patient 4, middle one-third ITR was more effective per unit volume reduced in improving the airflow than the respective two-thirds ITR models (Table). Importantly, in all patients, full length ITR models consistently had the highest FCPUVC values, suggesting full length ITR had the highest efficacy in improving nasal airflow compared with all partial ITR models.
Table. Top 3 ITR Models With Highest Location-Specific Effect Among All Models.
| Patient | Top 3 ITR Models With Highest Location-Specific Effect Ratio |
|---|---|
| 1 | Full length > Posterior 2/3 > Posterior 1/3 |
| 2 | Full length > Middle 1/3 > Posterior 2/3 |
| 3 | Full length > Anterior 2/3 > Anterior 1/3 |
| 4 | Full length > Posterior 1/3 > Posterior 2/3 |
| 5 | Full length > Anterior 2/3 > Anterior 1/3 |
Abbreviation: ITR, inferior turbinate reduction.
The contribution of the anterior, middle, or posterior one-third of the nasal airway to the total nasal resistance prior to ITR was calculated based on regional pressure drop and can be predictive of which section of partial ITR is more effective (eTable 2 in the Supplement). For example, patient 4 had greater resistance from the posterior one-third of nasal airway, and posterior one-third ITR reduction was most effective. In contrast, patient 5 had greater nasal resistance from the anterior one-third of the nasal airway, and anterior one-third ITR was most effective. As summarized in the Supplement, 10 of 15 most effective partial ITR locations were successfully predicted by analyzing the baseline regional resistance in the control model. Figure 4B shows regional FCPUVC significantly correlated with regional resistance (r = 0.67, P < .001).
Discussion
In recent years, there have been many advances in the instrumentation and techniques available for inferior turbinate surgery. Despite these advances, a clinical challenge is deciding the extent and location of ITR. An often used rule of thumb is that ITR should focus on the anterior aspects of the inferior turbinate because this is near the internal valve region, although this concept has not yet been rigorously validated. Virtual nasal airway modeling affords the ability to simulate virtual surgery from imaging studies, and CFD analysis allows the ability to mathematically predict nasal airflow.
Results from this study suggest that there is not a consistent relationship between the region of turbinate reduction and the degree of airflow improvement. There appears to be variability among different individuals in terms of the portions of the inferior turbinates that have the largest impact on nasal airflow. Therefore, consistently focusing on only one portion of the inferior turbinate during ITR may not lead to the most effective improvement in airflow. For example, as seen in patient 4, the more severe posterior obstruction of the nasal airway related to severe hypertrophy of the posterior inferior turbinate seemed to outweigh the rule of thumb (Figure 3). In this particular patient, the models demonstrated that reduction of the posterior portion of the IT had the greatest impact on airflow. This finding shows that each patient may have a specific location along the IT that is contributing to a greater or lesser degree to the airflow. In some patients, anterior IT can be the greatest contributing location to nasal obstruction, whereas in other patients, it may be the posterior IT or the middle IT.
The results of this study suggest that the changes in airflow in response to surgery are variable and depend on patients' individual anatomic factors. This corresponds to the principle of conservation of mass in that all air molecules that enter the nose must exit the nose. Thus, if a specific location along the IT is more hypertrophic and causes that particular section of the nasal cavity to be further narrowed, this will lead to a greater decrease in nasal airflow in that specific location. As such, universally treating only 1 specific location along the IT in all patients may result in suboptimal results because other portions of the IT that were left untreated may be contributing to nasal airflow obstruction. These factors should be thoroughly examined preoperatively in an attempt to gain a more complete understanding of each patient's individual anatomy. Such anatomic factors may not always be obvious on preoperative evaluation, but assessing for and finding these factors may allow surgeons to plan more effectively.
The data from this study also suggest that full-length ITR results in the most consistent, significant increase in airflow, as suggested by the highest location-specific effect value, FCPUVC, which allowed compensating for the differences in the ITR volume reduced. This seems logical because full-length ITR would ensure that all potentially obstructing segments of inferior turbinates are addressed.
Data from the models in this study indicate that in all patients, there exists a strong linear relationship (R2≥0.79) between flow rate and nasal volume. Increase in nasal airway volume through IT soft tissue volume reduction was associated with increase in total airflow rate, regardless of the location of the reduction. However, in patients 1, 2, and 3, when each individual side in the same patient was analyzed, certain partial ITR led to worse airflow, suggesting that partial ITR in certain anatomical configurations of the IT may lead to worsening of airflow despite the improvement in nasal volume. Although there appears to be a general relationship between overall volume and flow, it is important to emphasize that each portion of the turbinate may have drastically different effects in terms of nasal resistance (Table) (Supplement). In addition, the region of the turbinate with the most significant effect on airflow is variable from 1 person to the next.
The contribution of regional nasal airway anatomy to the total nasal resistance prior to ITR may be predictive of which section of ITR is more effective. The surgical approach should be personalized for the unique nasal anatomy of each patient to maximize the effect. The fact that full ITR was consistently most effective after adjusting for the volume of turbinate tissue resected seems to indicate that the entire length of the IT has an important functional impact on nasal airflow.
It is also important to keep in mind that there is much variation in the absolute amount of volume increase that can be achieved with different surgical procedures. The inferior turbinates occupy a large portion of the nasal airway, and the amount of volume increase that can be obtained by ITR may be much larger than the volume increase that can be achieved with other surgical procedures. Therefore, ITR could have a larger effect on nasal airflow than other surgical procedures. For example, nasal valve surgery might provide a large increase in airflow per unit volume change, but the absolute amount by which the nasal airway volume might be increased with valve surgery is small. On the other hand, turbinate reduction may achieve a much larger volume change, resulting in a larger overall increase in nasal airflow. Future studies analyzing the delicate interactions between different components of internal nasal valve, nasal septum, and other portions of the nasal airway may be of benefit to expand our understanding of resultant regional airflow pattern changes from nasal airway surgery maneuvers. In addition, it is important to remember that overall nasal flow rate and resistance do not provide a complete picture of nasal airflow physiology. Similar flow or resistance numbers can result in different regional air flow patterns. These functional implications are not yet fully understood.
Limitations
Limitations of this study include the small data set and the lack of clinical correlation. Although definitive conclusions cannot be drawn from an analysis of 5 patients’ CT scans, the data obtained in this study suggest that there is variability in terms of which segment of the inferior turbinate has the greatest effect on nasal resistance and airflow. Future studies with more patients will help to further clarify this relationship. It is important to also note that the models created in this study focused on fairly conservative resection of the soft tissue portion of the turbinates. More aggressive resections may result in unpredictable changes in airflow and were not the focus of this study.21,22,23,24 The data presented are objective flow and volume values without corresponding subjective findings. This is a limitation, but the main goal of this study was to provide some objective data that may help guide surgical decision making. Surgeons will need to use a combination of objective and subjective assessment to decide on the optimal treatment plan for patients with nasal airway obstruction.
Conclusions
Full-length inferior turbinate procedures result in a significantly greater increase in flow rate change per unit volume reduced, compared with partial length ITR. This appears to be a consistent relationship even in patients with significantly different inferior turbinate sizes.
It is problematic to use a rule of thumb to know which partial length procedure is likely to have a larger impact. The impact of partial-length procedures likely depends on individual anatomy. If a certain portion of the inferior turbinate is severely hypertrophic and has a higher regional resistance, that portion may be the rate-limiting area in terms of nasal obstruction and limiting airflow. It may not matter that this portion of the turbinate is along the anterior or posterior portion of the turbinate. These individual anatomic factors need to be closely evaluated for patients undergoing inferior turbinate surgery. These findings seem to indicate that the full length of inferior turbinate (not just the anterior head) is functionally important in treating nasal obstruction.
eTable. CFD Data
References
- 1.Harrill WC, Pillsbury HC III, McGuirt WF, Stewart MG. Radiofrequency turbinate reduction: a NOSE evaluation. Laryngoscope. 2007;117(11):1912-1919. [DOI] [PubMed] [Google Scholar]
- 2.Goyal P, Hwang P. Surgery of the septum and turbinates. In: David Kennedy PH, ed. Rhinology: Diseases of the Nose, Sinuses, and Skull Base. 2012. Thieme; New York, New York. [Google Scholar]
- 3.Morris LG, Burschtin O, Lebowitz RA, Jacobs JB, Lee KC. Nasal obstruction and sleep-disordered breathing: a study using acoustic rhinometry. Am J Rhinol. 2005;19(1):33-39. [PubMed] [Google Scholar]
- 4.Chen XB, Lee HP, Chong VF, Wang Y. Impact of inferior turbinate hypertrophy on the aerodynamic pattern and physiological functions of the turbulent airflow - a CFD simulation model. Rhinology. 2010;48(2):163-168. [DOI] [PubMed] [Google Scholar]
- 5.Lee HP, Poh HJ, Chong FH, Wang Y. Changes of airflow pattern in inferior turbinate hypertrophy: a computational fluid dynamics model. Am J Rhinol Allergy. 2009;23(2):153-158. [DOI] [PubMed] [Google Scholar]
- 6.Wexler D, Segal R, Kimbell J. Aerodynamic effects of inferior turbinate reduction: computational fluid dynamics simulation. Arch Otolaryngol Head Neck Surg. 2005;131(12):1102-1107. [DOI] [PubMed] [Google Scholar]
- 7.Grant O, Bailie N, Watterson J, Cole J, Gallagher G, Hanna B. Numerical model of a nasal septal perforation. Stud Health Technol Inform. 2004;107(Pt 2):1352-1356. [PubMed] [Google Scholar]
- 8.Hahn I, Scherer PW, Mozell MM. Velocity profiles measured for airflow through a large-scale model of the human nasal cavity. J Appl Physiol (1985). 1993;75(5):2273-2287. [DOI] [PubMed] [Google Scholar]
- 9.Hornung DE, Leopold DA, Youngentob SL, et al. Airflow patterns in a human nasal model. Arch Otolaryngol Head Neck Surg. 1987;113(2):169-172. [DOI] [PubMed] [Google Scholar]
- 10.Kelly JT, Prasad AK, Wexler AS. Detailed flow patterns in the nasal cavity. J Appl Physiol (1985). 2000;89(1):323-337. [DOI] [PubMed] [Google Scholar]
- 11.Keyhani K, Scherer PW, Mozell MM. Numerical simulation of airflow in the human nasal cavity. J Biomech Eng. 1995;117(4):429-441. [DOI] [PubMed] [Google Scholar]
- 12.Proetz AW. Air currents in the upper respiratory tract and their clinical importance. Ann Otol Rhinol Laryngol. 1951;60(2):439-467. [DOI] [PubMed] [Google Scholar]
- 13.Subramaniam RPRR, Morgan KT, Kimbell JS. Computational fluid dynamics simulations of inspiratory airflow in the human nose and nasopharynx. Inhal Toxicol. 1998;10:473-502. [Google Scholar]
- 14.Xiong GX, Zhan JM, Jiang HY, Li JF, Rong LW, Xu G. Computational fluid dynamics simulation of airflow in the normal nasal cavity and paranasal sinuses. Am J Rhinol. 2008;22(5):477-482. [DOI] [PubMed] [Google Scholar]
- 15.Cole P. The four components of the nasal valve. Am J Rhinol. 2003;17(2):107-110. [PubMed] [Google Scholar]
- 16.Cole P, Chaban R, Naito K, Oprysk D. The obstructive nasal septum. effect of simulated deviations on nasal airflow resistance. Arch Otolaryngol Head Neck Surg. 1988;114(4):410-412. [DOI] [PubMed] [Google Scholar]
- 17.Garcia GJ, Rhee JS, Senior BA, Kimbell JS. Septal deviation and nasal resistance: an investigation using virtual surgery and computational fluid dynamics. Am J Rhinol Allergy. 2010;24(1):e46-e53. [DOI] [PubMed] [Google Scholar]
- 18.Zhao K, Dalton P, Yang GC, Scherer PW. Numerical modeling of turbulent and laminar airflow and odorant transport during sniffing in the human and rat nose. Chem Senses. 2006;31(2):107-118. [DOI] [PubMed] [Google Scholar]
- 19.Zhao K, Pribitkin EA, Cowart BJ, Rosen D, Scherer PW, Dalton P. Numerical modeling of nasal obstruction and endoscopic surgical intervention: outcome to airflow and olfaction. Am J Rhinol. 2006;20(3):308-316. [DOI] [PubMed] [Google Scholar]
- 20.Zhao K, Scherer PW, Hajiloo SA, Dalton P. Effect of anatomy on human nasal air flow and odorant transport patterns: implications for olfaction. Chem Senses. 2004;29(5):365-379. [DOI] [PubMed] [Google Scholar]
- 21.Garcia GJ, Bailie N, Martins DA, Kimbell JS. Atrophic rhinitis: a CFD study of air conditioning in the nasal cavity. J Appl Physiol (1985). 2007;103(3):1082-1092. [DOI] [PubMed] [Google Scholar]
- 22.Moore EJ, Kern EB. Atrophic rhinitis: a review of 242 cases. Am J Rhinol. 2001;15(6):355-361. [PubMed] [Google Scholar]
- 23.Dayal A, Rhee JS, Garcia GJ. Impact of middle versus inferior total turbinectomy on nasal aerodynamics. Otolaryngol Head Neck Surg. 2016;155(3):518-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hariri BM, Rhee JS, Garcia GJ. Identifying patients who may benefit from inferior turbinate reduction using computer simulations. Laryngoscope. 2015;125(12):2635-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. CFD Data