Abstract
Heritable mutations in the germ line lead to genetically heterogeneous, or mosaic, gonads. Many of the genes used in germ-line development also play roles in somatic development [Saffman, E. E. & Lasko, P. (1999) Cell. Mol. Life Sci. 55, 1141–1163]. Mutations in these genes may have cellular phenotypes throughout germ-line development leading to their differential elimination or survival, as has been observed in somatic cells [Morata, G. & Ripoll, P. (1975) Dev. Biol. 42, 211–221]. We investigate whether mutations in heterozygosis are subject to pregametic selection in the germ line. We initiated clones of wild-type homozygous cells at different stages of development in gonads heterozygous for eight different recessive chromosome deficiencies. Here we show that cell selection takes place in mosaic germ-line populations. This phenomenon represents a level of selection that precedes and conditions subsequent zygotic selection by affecting the genes available in the gametic population.
Germ-line development in animal species is a complex sequence of events involving many cellular processes and requiring large numbers of genes and gene functions (1). Often, in both invertebrates and vertebrates, only a fraction of the progeny of initially established germ-line precursor cells, or primordial germ cells (PGCs), actually contribute to the gametic population. The genetic requirements that determine which PGCs will contribute to the functional gonad could be affected by mutation in the germ line and subsequent gonial selection. Clearly, which germ cells finally give rise to gametes is of utmost importance with respect to the genetic makeup of the progeny.
Germ-line development (1), which involves processes such as specific proliferation programs, passive and active gonial migration, cell–cell contact, signaling among germ cells and with various somatic cell populations, cell rearrangement and intercalation, stem cell division, and gamete differentiation, along with basic cellular metabolism, is sure to require the expression of a significant fraction of the genome, also active in somatic development. Genetically heterogeneous, or mosaic, cell populations may arise as a result of mutation in the germ line. In mosaic populations in certain somatic tissues, proliferating cells have been shown to display differential developmental success according to genotype, a phenomenon known as cellular competition. A well known example of this is the effect of the haploinsufficient Minute mutations in cells of the wing of Drosophila melanogaster (2). Mutations, which may be recessive in adult organisms, may display dominant phenotypes caused by haploinsufficiency at the cellular level. In some somatic mosaic populations, this can lead to cell behavior phenotypes that differ between wild-type homozygous and heterozygous mutant cells, resulting in cell competition and selection (2, 3). Gametic (sperm) selection in Drosophila (4) and other organisms (5, 6) has been described, as well as germ-line and somatic negative selection of deleterious mutations in homozygosis (7). It is not known whether wild-type homozygous germ cells compete with heterozygous mutant cells in genetic mosaics. Mosaic analyses have shown that a larger fraction of zygotic lethal point mutations (67%) or deficiencies (88%) are homozygous lethal in germ cells than in somatic (tergite) cells (20% and 58%, respectively; ref. 8). Given the large number of genes likely to be required by the germ line, and the complexity of germ-line development, particularly the loss of 50% of germ-line cell precursors early in development (1) (see below), we undertook clonal and mosaic analyses to ask whether or not germ-line cells undergo cellular competition in genetic mosaics. Cell competition and selection in the germ line have important population and evolutionary implications (9, 10).
To date, only two mosaic and clonal analyses in the germ line, concerned with proliferative parameters of germ cells over developmental time, have been published (11, 12). Wieschaus and Szabad (11) performed a quantitative germ-line clonal analysis, initiating clones from blastoderm through pupal stages. By using the chorion phenotype of the mutation fs(1)K10 as a marker, they measured clone size in the number of eggs laid with the K10 phenotype. Perrimon (12) carried out mosaic analyses of the dominant female sterile mutations ovoD1, ovoD2, and ovoD3, initiating clones throughout larval development. Clones of recombinant wild-type homozygous cells initiated at early larval stages in ovoD1/+ females were larger (in number of ovarioles) than +/+ clones initiated at the same age in K10/+ females (11). This finding led Perrimon to suggest that recombinant +/+ gonia undergo more larval divisions than ovoD1/+ germ cells. At later larval stages, however, clones were measured in number of adult progeny produced by females containing germ-line clones. We have performed germ-line mosaic and clonal analyses measuring clone size in the number of ovarioles containing recombinant gonia, thus directly quantifying the germ cells contributing to the gametic population. Our results demonstrate cell competition leading to selection in mosaic gonads between recombinant +/+ germ cells and neighboring cells heterozygous for several different chromosome deficiencies.
Materials and Methods
Generation of Recombinant Chromosomes.
All deficiencies and hs:FLP chromosomes used (FLP12 and FLP22 on the first chromosome) were obtained from the Bloomington stock center (http://fly.ebi.ac.uk:7081/). 2L recombinants were created by standard meiotic recombination. 3L recombinants were created by inducing mitotic recombination in the male germ line with X rays. Male larvae at 70–80 h after egg-laying (h AEL) were irradiated with 1,500–1,700 rads (300 rads/min, 10 kV, 15 mA, with a 2-mm filter).
Immunohistochemistry.
For embryonic germ cell counts, embryos were collected on fruit juice plates at intervals of 2 h and allowed to develop at 25°C. Embryos were collected, fixed, and stained according to standard protocols. The anti-Vasa Ab was a gift from P. Lasko (McGill University, Montreal, Canada). Staining was visualized with the ABC HRP conjugate system (Vector Laboratories).
Generation of Mosaic Ovaries.
For clones marked as vas:lacZ−, we used a P{lacZ:ry+} = vas:lacZ insertion in the vasa locus [a gift from F. Laski (University of California, Los Angeles)] as a cellular marker (Fig. 1). Mitotic recombination was induced in a wild-type (vas:lacZ FRT39E/+) background. FRT39E has been described (13). FLP-induced recombination at the FRT sites results in loss of the vas:lacZ insertion and lack of β-galactosidase (β-gal) activity in recombinant cells, easily distinguishable from heterozygous cells after 5-bromo-4-chloro-3-indolylβ-d-galactoside (X-Gal) staining. The vas:lacZ transgenic line expresses β-gal constitutively only in germ-line cells, and in heterozygosis has no phenotype with respect to germ cell development or female fertility (unpublished observations). For clones marked as ovoD1−, we used the autosomal insert P{ovoD1; w+} and FRT79 (14). Female flies (n = 2,337) of the genotype FLP/+; ovoD1 FRT79/FRT79 were never observed to be fertile without heat shock.
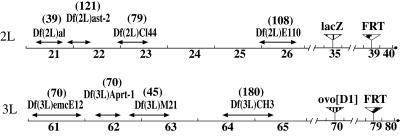
Figure 1.
Recombinant chromosomes created for clonal analysis. Arrows indicate extension of deficiencies based on cytological map breakpoints (http://fly.ebi.ac.uk:7081/). Numbers in parentheses indicate the predicted number of genes contained in the deficiency (http://fly.ebi.ac.uk:7081/annot/).
Statistical Tests.
All calculations are based on a pool of all experiment replicas. The average clone size 〈x〉 is calculated as 〈x〉 = (f(n) × n)/n, where f(n) is the number of ovaries observed to have n ovarioles containing recombinant cells; n ɛ (1, 2 … 19), with 19 being the maximum number of ovarioles observed for our wild-type strain Vallecas. Wilcoxon contrasts, t tests, and Poisson distribution calculations were carried out by using standard algorithms (15).
Results and Discussion
Germ cell precursors are specified very early in development (1). We counted the number of germ cells present at different stages of development by staining with α-Vasa Ab [exclusive to germ cells (1); Table 1]. Our embryonic germ cell counts were consistent with published accounts (refs. 1 and 16; see below). At stage 3 of embryogenesis (17), 1–1.5 h AEL, nuclei at the posterior end of the embryo cellularize to produce pole cells. After they undergo up to 2 rounds of mitosis, there are 30–40 pole cells at stage 6 (just under 3 h AEL). The pole cells are subsequently carried into the embryo with the invagination of the posterior hindgut primordium. They then migrate actively toward the anterior of the embryo within the primordial midgut (18). At stage 10 (≈4–5 h AEL), they come into close contact with the somatic midgut primordium cells (18, 19), which extend pseudopodia toward the pole cells (18) and undergo cell shape changes, allowing the pole cells to pass through the wall of endodermal cells. Up to and including stages 12 and 13 (until ≈10 h AEL), the pole cells contact and are surrounded by the mesodermal precursors of the somatic gonad in parasegments 10–13 (20), forming the primordial gonad in abdominal segment 5 by stage 14 (≈11 h AEL). The total number of germ cells present in the gonads at this stage is 9–12 per gonad, only ≈50% of the total number of pole cells that undertook this migration in stage 6 (1, 16). Some pole cells are occasionally observed at extragonadal positions in the embryo, and are assumed to degenerate (21). During larval stages the germ cells, now restricted to the gonad, undergo mitotic divisions until achieving the final number of gonia, ≈50 per ovary (11). During the third larval instar and the beginning of pupal development, the ovary is compartmentalized into ovarioles whose germaria probably contain two to three germ-line stem cells each (11).
Table 1.
+/+ clones induced in vas:lacZ/+ and ovoD1/+ backgrounds
| Heterozygous background | Age of clone induction, h ± 1 AEL | Total no. of germ cells (n) | n experiment replicas | n females scored | n females with clone | Mitotic recombination frequency | n females dissected | n clones in one ovary (frequency) | n clones in both ovaries (frequency)† | Average no. ovarioles with clone, 〈x〉 ± σ |
|---|---|---|---|---|---|---|---|---|---|---|
| vas:lacZ/+ | 2 | 20.1 ± 8.6 (22) | 2 | 427 | 27 | 0.06 | 27 | 12 (0.44) | 15 (0.56)‡ | 4.28 ± 2.44 |
| 48 | 32.0 ± 4.7 (15) | 2 | 491 | 40 | 0.08 | 40 | 35 (0.87) | 5 (0.13) | 2.24 ± 1.78 | |
| ovoD1/+ | 2 | 20.1 ± 8.6 (22) | 3 | 2,302 | 85 | 0.04 | 34 | 24 (0.71) | 10 (0.29) | 9.45 ± 5.49 |
| 6 | 24.5 ± 5.2 (33) | 2 | 1,327 | 102 | 0.08 | 71 | 56 (0.79) | 15 (0.21) | 7.63 ± 4.11 | |
| 16 | 19.4 ± 3.8 (21) | 2 | 704 | 73 | 0.10 | 45 | 33 (0.73) | 12 (0.27) | 8.46 ± 4.77 | |
| 24 | 19.2 ± 2.0 (4) | 2 | 1,326 | 108 | 0.08 | 42 | 35 (0.83) | 7 (0.17) | 8.28 ± 4.83 | |
| 48 | 32.0 ± 4.7 (15) | 2 | 246 | 56 | 0.23 | 37 | 20 (0.54) | 17 (0.46) | 6.74 ± 4.43 | |
| 80 | 39.0 ± 7.2 (13) | 2 | 300 | 70 | 0.23 | 33 | 11 (0.33) | 22 (0.67) | 5.11 ± 4.08 | |
| 110 | 41.0 ± 7.6 (13) | 2 | 512 | 50 | 0.10 | 51 | 15 (0.29) | 36 (0.71)‡ | 2.46 ± 1.12 | |
| 174 | 50* | 2 | 1,008 | 190 | 0.19 | 23 | 10 (0.43) | 13 (0.57) | 1.5 ± 0.64 |
Inferred from Wieschaus and Szabad (11).
All frequencies of clones in both ovaries were only slightly higher than those predicted by the Poisson distribution, except for
, which were substantially higher (>10 times greater).
We first created clones of marked cells in the female germ line, using the FLP/FRT method of mitotic recombination (22) at 2 and 48 h AEL, for later comparison with clones in heterozygous mutant backgrounds. These ages of clone initiation sample germ cell populations before and after gonad formation. Before gonad formation, all germ cells undergo embryonic migration, and progeny of recombinant cells may contribute to one or both ovaries, whereas by 48 h, germ cells and their progeny are confined to a single ovary. Clones occupying both ovaries constituted 56% of clones when induced at 2 h AEL. At 48 h AEL, 13% of clones occupy both ovaries, the result of more than one recombination event per female, as supported by comparison with the Poisson distribution (Table 1). Clone size was measured as the fraction of ovarioles containing recombinant germ cells (Fig. 2 a–d). Distribution histograms of clone sizes show, as expected, that clones are smaller when induced at 48 h AEL than at 2 h AEL (Fig. 3a). The Student's t test of the average clone size 〈x〉 (P < 0.001) and the Wilcoxon contrast (α = 0.95) of the size distributions show significant differences between clone sizes at these stages of clone initiation.
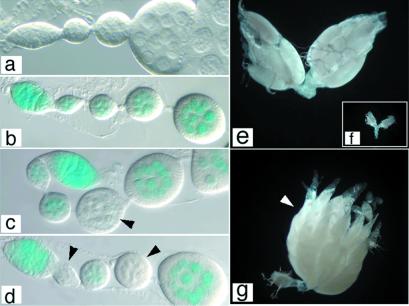
Figure 2.
Induction of mitotic recombination and identification of germ-line clones. (a–d) vasa:lacZ marker, 5-bromo-4-chloro-3-indolylβ-d-galactoside (X-Gal) stains were performed by using a standard protocol and were mounted in glycerol. (e–g) ovoD1 marker. (a) Wild-type adult female ovariole. (b) vas:lacZ/+ ovariole, X-Gal stain. All cells are positive for β-galactosidase activity. Control (n = 100) FLP/+; vas:lacZ FRT39E/FRT39E ovaries not heat shocked were identical to the one shown. (c and d) FLP/+; vas:lacZ FRT39E/FRT39E ovarioles containing +/+ recombinant germ cells. Arrowheads indicate egg chambers derived from recombinant cells. (e) Wild-type adult ovaries. (f) ovoD1/+ ovaries are atrophied and do not contain vitellogenic egg chambers. (g) FLP/+; ovoD1 FRT79/FRT79 ovary after heat shock, containing all recombinant germ cells that give rise to functional ovarioles containing all stages of oogenesis and mature eggs (arrowhead). (a–d) Anterior is to the right; (e–g) anterior is up. a–d and e–g were taken at the same magnification.
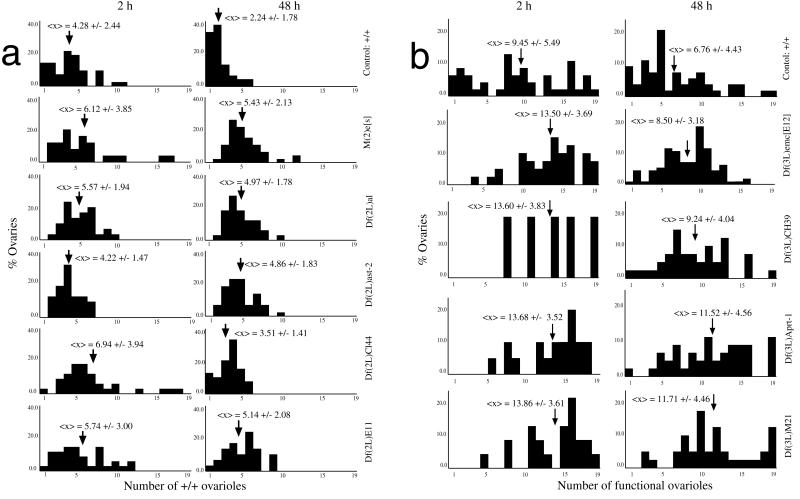
Figure 3.
Size of +/+ clones in wild-type and mutant backgrounds. Histograms indicate the relative contributions (percentage of all ovaries scored) to the 19 (maximum) classes of numbers of ovarioles with recombinant germ cells, at a given time of clone initiation (2 and 48 h AEL). Arrows indicate <x>, the average clone size in number of ovarioles containing +/+ recombinant cells. (a) All genetic backgrounds are heterozygous for both vas:lacZ, the deficiencies indicated (Right). (b) All genetic backgrounds are heterozygous for both ovoD1, the deficiencies indicated (Right).
Wieschaus and Szabad (11) counted the number of K10 eggs laid by females with recombinant cells and calculated the fraction of total egg production represented by these eggs. In their study, clones initiated at blastoderm stages (3 ± 0.5 h AEL) occupied 0.02–0.50 of mosaic ovaries, and clones initiated at 48–72 h AEL occupied 0.02–0.06 of mosaic ovaries (11). These ranges are comparable to those observed in our experiments: 0.05–0.58 at 2 h AEL and 0.05–0.32 at 48 h AEL.
ovoD1 is a dominant female sterile mutation that histologically affects mid to late stages of oogenesis (23). Heterozygous adult females have atrophied ovaries containing some germ cells but lacking vitellogenic egg chambers (data not shown). At earlier stages, we found ovoD1/+ germ cells to be as wild type, behaviorally (with respect to cellularization, migration, and morphogenetic processes), cytologically (with respect to cell morphology and size), and numerically [numbers of germ cells at different developmental times (Table 1)]. We carried out a cell lineage analysis of +/+ germ cells in an ovoD1/+ background at eight different developmental stages to determine whether and when germ cell selection of +/+ vs. ovoD1/+ might be occurring in development. We measured clone size by isolating egg-laying ovoD1/+ females after heat shock-induced mitotic recombination and counting the number of functional ovarioles (containing vitellogenic egg chambers; Fig. 2 e–g), which are easily identified in the ovoD1/+ mosaic ovary. Recombination frequency was approximately correlated with the number of germ cells at the age of clone initiation (Table 1). A notable exception was at 110 h AEL, when despite an increase in target size with respect to the previous age of clone induction, we observed a decrease in recombination frequency. This drop may indicate a cell cycle synchrony (possible G1 arrest) among germ cells at that stage of development.
Clones initiated at 2 h AEL occupy both ovaries much more frequently than would be predicted by the Poisson distribution (Table 1). This observation is not surprising at this age because progeny of recombinant cells may migrate to populate both ovaries. The frequencies of clones in both ovaries initiated after gonad formation (≥16 h AEL) differ only slightly from those predicted by the Poisson distribution, again with the exception of those initiated at 110 h AEL (Table 1). The observation that multiple recombination events at this age do not seem to be independent is consistent with the possibility of cell cycle synchrony among germ cells at this stage. Clones initiated up until 80 h AEL were able to occupy an entire ovary (19 ovarioles), but after that stage were found to be limited to few ovarioles (data not shown). This clone size is consistent with the timing, in late third larval instar, of compartmentalization of the ovary into ovarioles, because progeny of +/+ recombinant cells will be restricted to the ovariole(s) where the clone initiated and can no longer become incorporated into many ovarioles during ovary morphogenesis (Table 1). No significant difference in sizes of clones from left and right ovaries was observed in clones occupying both ovaries (data not shown).
The number of eggs laid per unit time per ovariole was observed to be independent of the number of functional ovarioles (data not shown); that is, egg production rate is ovariole-autonomous. This result is surprising given earlier data suggesting that the metabolic resources of the female abdomen are rate limiting with respect to developmental processes (24). No mosaic ovary was ever observed to contain more than 19 functional ovarioles, which is the maximum number observed for our wild-type strain Vallecas, even in ovaries that seemed to be completely populated by recombinant cells.
Comparison of +/+ clone size distributions and average clone size 〈x〉 at 2 and 48 h AEL for vas:lacZ/+ and ovoD1/+ backgrounds shows that clones are significantly larger in ovoD1/+ backgrounds (Table 1 and Fig. 3), indicating competition between +/+ and ovoD1/+ germ cells in mosaic ovaries.
To determine whether or not +/+ germ cells display proliferation advantages similar to those of wing cells in an M/+ background (2), we induced clones of +/+ cells marked with vas:lacZ+ in a background heterozygous for M(2)es. t tests of 〈x〉 (P < 0.025) and Wilcoxon contrast (α = 0.95) revealed that clones in this background are significantly larger than those in a wild-type (vas:lacZ/+) background (Tables 1 and 2; Fig. 3a).
Table 2.
+/+ clones induced in backgrounds heterozygous for Minute and deficiencies and vas:lacZ+ clone marker
| Heterozygous background | Age of clone induction, h ± 1 AEL | n experiment replicas | n females examined | n females with clone | Mitotic recombination frequency | n clones in one ovary (frequency) | n clones in both ovaries (frequency) | Average no. ovarioles with clone, 〈x〉 ± σ |
|---|---|---|---|---|---|---|---|---|
| +/Df(2L)al | 2 | 1 | 225 | 20 | 0.09 | 10 (0.50) | 10 (0.50) | 5.57 ± 1.94 |
| 48 | 1 | 237 | 28 | 0.12 | 23 (0.82) | 5 (0.18) | 4.97 ± 1.78 | |
| +/Df(2L)ast-2 | 2 | 1 | 222 | 19 | 0.09 | 7 (0.37) | 12 (0.63) | 4.22 ± 1.47 |
| 48 | 1 | 254 | 35 | 0.14 | 26 (0.74) | 9 (0.26) | 4.86 ± 1.83 | |
| +/Df(2L)C144 | 2 | 2 | 196 | 26 | 0.13 | 19 (0.73) | 7 (0.27) | 6.94 ± 3.94 |
| 48 | 2 | 338 | 34 | 0.10 | 29 (0.85) | 5 (0.15) | 3.51 ± 1.41 | |
| +/Df(2L)E110 | 2 | 1 | 199 | 21 | 0.11 | 8 (0.38) | 13 (0.62) | 5.74 ± 3.00 |
| 48 | 1 | 158 | 22 | 0.14 | 15 (0.68) | 7 (0.32) | 5.14 ± 2.08 | |
| +/M(2)es | 2 | 2 | 165 | 19 | 0.12 | 12 (0.63) | 7 (0.37) | 6.12 ± 3.85 |
| 48 | 2 | 375 | 40 | 0.11 | 35 (0.88) | 5 (0.13) | 5.43 ± 2.13 |
We also studied clones of wild-type homozygous cells in backgrounds heterozygous for chromosome deficiencies in the 2L arm of the second chromosome by using vas:lacZ and in the 3L arm by using ovoD1 to identify clones as described. The deficiencies were chosen to be nonoverlapping, as large as possible, recessive in phenotype [with the exception of Df(3L)M21], and easy to recombine onto the chromosome containing the clone marker and the FRT site (Fig. 1). Mitotic recombination results in clones of cells that are wild-type, both with respect to the clonal analysis marker and with respect to the deficiency, in a heterozygous gonad.
Clones of +/+ recombinant cells initiated at the two ages indicated (2 and 48 h AEL) marked as vas:lacZ+ in heterozygous deficiency backgrounds were significantly larger than controls (t test, P < 0.025–0.001; Wilcoxon contrast, α = 0.95; Tables 1 and 2; Fig. 3a). The only exception is the deficiency Df(2L)ast-2, for clones induced at 2 h AEL, which does not differ from controls at this age, albeit significantly different at 48 h AEL. The estimated number of genes included in the deficiencies was not correlated with the “strength” of selection (as measured by comparing the difference in functional ovariole distributions between controls and deficiency backgrounds; data not shown).
Deficiencies coupled to the ovoD1 mutation in cis may show an even stronger selection effect than that seen with ovoD1 alone, the difference between the two effects being attributable to the deficiency. For all deficiencies tested, and at both ages of clone induction, clones of +/+ recombinant cells were significantly larger than ovoD1/+ controls (t test, P < 0.025–0.001; Wilcoxon contrast, α = 0.95; Table 3 and Fig. 3b). As in the vas:lacZ experiments, there is no clear correlation between the number of genes contained in the deficiency and the selection effect (data not shown). In fact, the largest deficiency in terms of both estimated numbers of genes included and cytology, Df(3L)CH39, was observed to have the lowest strength of selection at both ages of clone initiation. The smallest deficiency (containing the least number of predicted genes, although not the smallest cytologically), Df(3L)M21, was observed to have a relatively large effect, which could be attributed to this deficiency including the Minute M(3)63B. For two of the deficiencies tested in the ovoD1/+ background [Df(3L)CH39 and Df(3L)M21], clones initiated at 48 h AEL populated both ovaries much more frequently than predicted by the Poisson distribution (Table 1). This clone behavior reinforces the notion of positive selection for +/+ recombinant germ cells.
Table 3.
+/+ clones induced in backgrounds heterozygous for Minute and deficiencies and ovoD1+ clone marker
| Heterozygous background | Age of clone induction, h ± ln AEL | n experiment replicas | n females examined | n females with clone | Mitotic recombination frequency | n females dissected | n clones in one ovary (frequency) | n clones in both ovaries (frequency)† | Average no. ovarioles with clone, 〈x〉 ± σ |
|---|---|---|---|---|---|---|---|---|---|
| +/Df(3L)emcE12 | 2 | 3 | 1890 | 44 | 0.02 | 25 | 7 (0.28) | 18 (0.72) | 13.50 ± 3.69 |
| 48 | 1 | 660 | 85 | 0.13 | 84 | 59 (0.70) | 25 (0.30) | 8.50 ± 3.18 | |
| +/Df(3L)CH39 | 2 | 1 | 563 | 4* | 0.01 | 4 | 3 (0.75) | 1 (0.25)‡ | 13.60 ± 3.83 |
| 48 | 1 | 694 | 28 | 0.04 | 28 | 18 (0.64) | 10 (0.36) | 9.24 ± 4.04 | |
| +/Df(3L)Aprt-1 | 2 | 1 | 201 | 10 | 0.05 | 10 | 1 (0.10) | 9 (0.90) | 13.68 ± 3.52 |
| 48 | 1 | 133 | 23 | 0.17 | 23 | 2 (0.09) | 21 (0.91) | 11.54 ± 4.56 | |
| +/Df(3L)M21 | 2 | 2 | 649 | 9 | 0.01 | 9 | 4 (0.44) | 5 (0.56)‡ | 13.86 ± 3.61 |
| 48 | 2 | 501 | 22 | 0.04 | 22 | 10 (0.45) | 12 (0.54) | 11.71 ± 4.46 |
The recombinant chromosome was lost before more data could be gathered.
All frequencies of clones in both ovaries were only slightly higher than those predicted by the Poisson distribution, except for ‡, which were substantially higher (>10 times greater).
Recombinant wild-type homozygous clones in vas:lacZ/+ heterozygous females are similar in size to clones observed in previous studies (11), allowing us to consider this experiment as a cell lineage control for studies of +/+ cells in mutant heterozygous backgrounds. Comparison of clone sizes in the cell lineage experiments, with sizes of +/+ clones initiated at the same ages in ovoD1/+ females, reveals positive selection of +/+ cells over ovoD1/+ cells. This selection is observed at all ages of development studied, despite the lack of detectable mutant phenotype of heterozygous cells with respect to cell number, cytology, and behavior at previtellogenic stages, indicating that some other parameter of cell proliferation or survival is affected earlier by the ovoD1 mutation. The selection observed explains the large clone sizes seen in previous mosaic analyses of ovoD1 (12).
Mosaic analysis in other heterozygous mutant backgrounds consistently indicates positive selection of +/+ germ cells. Clones of +/+ cells in Minute heterozygous backgrounds [M(2)es and Df(3L)M21, which includes M(3)63B] are larger than controls, indicating that wild-type recombinant cells compete with the Minute heterozygous cells, proliferate more and/or contribute more successfully to gamete production. This selective advantage is similar to that observed for such clones in imaginal disk cells, but not in histoblast nests in tergites (2). Developmental requirements are different for these cell populations: disk cells undergo intercalar proliferation to create a finite morphogenetic space, whereas histoblasts fill the space defined for them by the larval substratum (2). Germ cells display cell competition, and are thus more similar in their clonal behavior in genetic mosaics to imaginal disk cells. This observation suggests that heterogeneities in the somatic cells of the gonad may act as limiting factors for gonial spatial organization and interaction with somatic cells in morphogenesis, as does positional information, based on gene expression and cell–cell signaling, in imaginal disk development. In support of this idea, we observed the maximum clone size for all experiments to be 19 ovarioles, which is the wild-type maximum for our strain Vallecas, even in ovaries that seemed to be completely populated by recombinant cells. This observation suggests that somatic cells, and not (or at least not exclusively) germ cells, limit the morphogenesis and number of ovarioles formed, consistent with observations that ovariole morphogenesis proceeds normally in the absence of germ cells (25).
Clones of +/+ cells in backgrounds heterozygous for recessive deficiencies are significantly larger than controls. The removal of one copy of the genes included in the deficiencies, therefore, has a dominant phenotype at the cellular level and results in the selection of +/+ cells over Df/+ cells in genetic mosaics. The inference (see above) that a large fraction of the genome is used in germ-line development might predict that the larger the deficiency (and hence the greater the number of genes included), the greater the selection effect. However, no correlation was observed between the predicted number of genes contained in the deficiency and the strength of selection, indicating that the effect of having only one dosis of a set of genes depends on which particular genes are involved, and not simply on the number of genes.
The difference between clone size distributions in controls and in heterozygous Minute or deficiency backgrounds is greater for clones initiated at 48 h AEL than at 2 h AEL, for all Minutes and deficiencies tested. In other words, the rate of selection seems to be higher in germ-line mosaics initiated after gonad formation than before. This finding is at first surprising, because the behavior of clones induced at a given developmental time should include all behaviors of clones induced later in development. However, as in imaginal discs that create their own compartment boundaries (2), wild-type homozygous germ cells created in mosaic backgrounds may suffer proliferative or somatic restrictions early in development, but they become more competitive when reaching the gonads, or later in their development.
The possibility of germ-line selection as a mechanism contributing to natural selection and organismal evolution was raised as early as the 1930s (26, 27). Population genetics calculations have predicted that such selection, if existent, would have significant effects on the frequencies and types of mutations and alleles in a population (9, 10). Selection in proliferating cells has been observed at the cellular level in certain somatic cell populations (28, 29). Studies such as those by Sâpiro (26) and Abrahamson et al. (30) showed that premeiotic male germ cells undergo selection for X-linked lethals and suggested that such mutations were subject to selection because of their haploinsufficiency. What we show here, however, is that autosomal deletions in the germ line can be selected against in heterozygosis, even in the presence of a wild-type allele.
By definition, heritable mutations arise only in the germ line. In germ-line genetic mosaics, cell selection may affect the number of gametes of a given germ cell that appears in the next generation. Because of the dominant or dosis-dependent effects on cell behavior shown here, second-site mutations, arising in the germ line and acting as suppressors or enhancers of preexisting inherited mutations, may be selected for in heterozygosis. Once these mutations (or combination of mutations) have been passed on to the gametes, they are subject to further selection during embryonic somatic morphogenesis in the progeny. This germ-line selection will affect a noticeable fraction of gene functions in both somatic and germ-line development, thus becoming a mechanism of selection for somatic development. This process may be involved in concerted evolution and molecular coevolution (31). It represents a cellular level of selection that operates independently of and before zygotic selection based on morphogenetic and/or cell physiological phenotypes.
Acknowledgments
We thank Paul Lasko for the anti-Vasa Ab; Frank Laski for the vas:lacZ flies; Juan Gabriel Rodríguez and Juan García-Bellido for help with the statistical analysis; James Crow, Gabby Dover, Michael Akam, and Juan Modolell for constructive criticism of the manuscript; and all members of the laboratory for helpful discussions and suggestions. This work was supported by grants from the Dirección General de Investigación Científica y Técnica and the Comunidad de Madrid, and an institutional grant from the Fundación Ramón Areces. C.E. was supported by a fellowship from the Consejo Superior de Investigaciones Científicas (Spain), program PB92-0036, Grant FP95X2091819.
Abbreviation
- h AEL
h after egg laying
References
- 1.Saffman E E, Lasko P. Cell Mol Life Sci. 1999;55:1141–1163. doi: 10.1007/s000180050363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morata G, Ripoll P. Dev Biol. 1975;42:211–221. doi: 10.1016/0012-1606(75)90330-9. [DOI] [PubMed] [Google Scholar]
- 3.García-Bellido A, Cortés F, Milán M. Proc Natl Acad Sci USA. 1994;91:10222–10226. doi: 10.1073/pnas.91.21.10222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Price C S C, Dyer K A, Coyne J A. Nature (London) 1999;400:449–452. doi: 10.1038/22755. [DOI] [PubMed] [Google Scholar]
- 5.Birkhead T R. Rev Reprod. 1998;3:123–129. doi: 10.1530/ror.0.0030123. [DOI] [PubMed] [Google Scholar]
- 6.LaMunyon C W, Wood S. Proc Natl Acad Sci USA. 1997;94:185–189. doi: 10.1073/pnas.94.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charlesworth B. Genet Res Camb. 1994;63:213–227. doi: 10.1017/s0016672300032365. [DOI] [PubMed] [Google Scholar]
- 8.García-Bellido A, Robbins L G. Genetics. 1983;103:235–247. doi: 10.1093/genetics/103.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hastings I M. Genetics. 1989;123:191–197. doi: 10.1093/genetics/123.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otto S P, Hastings I M. Genetica (The Hague) 1998;102/103:507–524. [PubMed] [Google Scholar]
- 11.Wieschaus E, Szabad J. Dev Biol. 1979;68:29–46. doi: 10.1016/0012-1606(79)90241-0. [DOI] [PubMed] [Google Scholar]
- 12.Perrimon N. Genetics. 1984;108:927–939. doi: 10.1093/genetics/108.4.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Struhl G, Barbash D A, Lawrence P A. Development (Cambridge, UK) 1997;124:2155–2165. doi: 10.1242/dev.124.11.2155. [DOI] [PubMed] [Google Scholar]
- 14.Chou T, Perrimon N. Genetics. 1996;144:1673–1679. doi: 10.1093/genetics/144.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peña Sánchez de Rivera D. Statistics: Models and Methods. Madrid: Aliaza; 1994. [Google Scholar]
- 16.Sonnenblick B P. Proc Natl Acad Sci USA. 1941;27:484–489. doi: 10.1073/pnas.27.10.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campos-Ortega J, Hartenstein V. The Embryonic Development of Drosophila melanogaster. Berlin: Springer; 1985. [Google Scholar]
- 18.Jaglarz M K, Howard K R. Development (Cambridge, UK) 1995;121:3495–3503. doi: 10.1242/dev.121.11.3495. [DOI] [PubMed] [Google Scholar]
- 19.Callaini G, Riparbelli M G, Dallai R. Dev Biol. 1995;170:365–375. doi: 10.1006/dbio.1995.1222. [DOI] [PubMed] [Google Scholar]
- 20.Warrior R. Dev Biol. 1994;166:180–194. doi: 10.1006/dbio.1994.1306. [DOI] [PubMed] [Google Scholar]
- 21.Technau G M, Campos-Ortega J A. Roux's Arch Dev Biol. 1986;195:489–498. doi: 10.1007/BF00375889. [DOI] [PubMed] [Google Scholar]
- 22.Golic K G, Lindquist S. Cell. 1989;59:499–509. doi: 10.1016/0092-8674(89)90033-0. [DOI] [PubMed] [Google Scholar]
- 23.Andrews J, García-Estefania D, Delon I, Lu J, Mevel-Ninio M, Spierer A, Payre F, Pauli D, Oliver B. Development (Cambridge, UK) 2000;127:881–892. doi: 10.1242/dev.127.4.881. [DOI] [PubMed] [Google Scholar]
- 24.García-Bellido A. J Insect Physiol. 1965;11:1701–1708. [Google Scholar]
- 25.Aboïm A N. Rev Suisse Zool. 1945;52:53–154. [Google Scholar]
- 26.Sâpiro N I. Compt Rend (Doklady) Acad Sci URSS. 1936;3:119–122. [Google Scholar]
- 27.Haldane J B S. Am Nat. 1937;71:337–349. [Google Scholar]
- 28.Buss L. Proc Natl Acad Sci USA. 1982;79:5337–5341. doi: 10.1073/pnas.79.17.5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoner D S, Rinkevich B, Weissman I L. Proc Natl Acad Sci USA. 1999;96:9148–9153. doi: 10.1073/pnas.96.16.9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abrahamson S, Meyer H U, Himoe E, Daniel G. Genetics. 1966;54:687–696. doi: 10.1093/genetics/54.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dover G A, Flavell R B. Cell. 1984;38:622–623. doi: 10.1016/0092-8674(84)90255-1. [DOI] [PubMed] [Google Scholar]