Lay Summary
Male red facial skin is salient to both sexually mature males and females in rhesus macaques, but not to juveniles. It can be difficult to understand the function of visual sexual signals based on behavioral observations because signals can be both attractive and aversive. Here, we by-passed this issue by using an experimental approach based on modification of images of faces, gaining from the fact that monkeys gaze at salient stimuli, whether attractive or intimidating.
Keywords: color ornamentation, looking time paradigm, sexual selection, signaling.
Abstract
The effects of intrasexual and intersexual selection on male trait evolution can be difficult to disentangle, especially based on observational data. Male–male competition can limit an observer’s ability to identify the effect of female mate choice independently from sexual coercion. Here, we use an experimental approach to explore whether an ornament, the red facial skin exhibited by male rhesus macaques (Macaca mulatta), might be involved in both female mate choice and male–male competition. We used a noninvasive experimental approach based on the looking time paradigm in a free-ranging setting, showing images of differently colored male faces to both adult females (N = 91) and males (N = 77), as well as to juveniles (N = 94) as a control. Results show that both adult females and males looked longer at dark red faces compared with pale pink ones. However, when considering the proportion of subjects that looked longer at dark red faces regardless of preference strength, only females showed a significant dark red bias. In contrast, juveniles did not show any preferences between stimuli, suggesting that the adult bias is not a consequence of the experimental design or related to a general sensory bias for red coloration among all age–sex classes. Collectively, these results support the role the ornament plays in female mate choice in this species and provide the first evidence that this ornament may play a role in male–male competition as well, despite a general lack of observational evidence for the latter effect to date.
INTRODUCTION
Sexual selection explains the prevalence of conspicuous traits that influence lifetime reproductive success. Male ornaments are typically regarded as evolving in response to intersexual selection (female mate choice), and armaments, in response to intrasexual selection (male–male competition) (Andersson 1994). However, the 2 mechanisms are not independent and can both act on the same traits, either reinforcing or opposing each other simultaneously or sequentially (Hunt et al. 2009). For instance, female mate choice can secondarily act to increase the prevalence of traits that are involved in male–male competition if they are also informative about male condition and health (e.g., Berglund et al. 1996; Wiley and Poston 1996). Females can also act to increase competition between males, leading to indirect mate choice of the most competitive ones (e.g., Cox and LeBoeuf 1977; Semple 1998; Pizzari 2001). Despite these multiple well-known mechanisms of sexual selection, most studies investigate the effect of only 1 mechanism at a time (Hunt et al. 2009).
In comparison with research on other animal classes, only a few studies have addressed whether and how direct male–male competition and female mate choice influence evolution of the same trait in mammals (cf. Hunt et al. 2009). This may be because high levels of direct male–male competition and sexual coercion make it difficult to study the role that female mate choice plays in male trait evolution in mammals through behavioral observation (Clutton-Brock and McAuliffe 2009). In such contexts, mere correlations between trait expression and mating success provide limited information about female mating preferences and may only be a reflection of direct competition between males limiting the ability of females to mate with preferred partners. Furthermore, if trait expression is linked to male aggressiveness, females may actually solicit males exhibiting the most developed traits in order to reduce sexual coercion. An additional problem is that given potentially high costs associated with male–male competition, males might be intimidated by males exhibiting cues and signals of competitive superiority and so actively avoid them, limiting the ability of human observers to record meaningful agonistic interactions.
Uniquely among mammals, several species of catarrhine primate show bright red color ornaments (Bradley and Mundy 2008). Such signals are perceivable by conspecifics because catarrhines are trichromats, with visual systems enabling detection of ripe fruits, flowers, and young leaves against a background of dark green foliage (Dominy and Lucas 2001). Red ornaments develop their level of color expression due to blood flux in the epidermis that is under sex steroid hormone control. In males, this involves aromatization of testosterone to estrogen in the skin at estrogen receptors (Vandenbergh 1965; Rhodes et al. 1997; see also Dixson 2012). This trait may be potentially informative to conspecifics of both sexes in a sexual context, providing information about both the condition and the competitive ability of the signaler. Indeed, skin color is influenced by blood oxygenation and flow and is thus closely linked to underlying physiology and condition (Changizi et al. 2006; Bradley and Mundy 2008). Furthermore, according to the immunohandicap hypothesis (Folstad and Karter 1992), because the hormone testosterone is an immunosuppressant, only individuals in good condition may be able to exhibit the most intense coloration.
In several catarrhine species, skin color is strongly correlated with dominance rank and rapidly changes following dominance take-over, such that it is a signal of social status involved in male–male competition (mandrills, Mandrillus sphinx: Setchell and Wickings 2005; crested macaques, Macaca nigra: Engelhardt et al. 2008; drills, Mandrillus leucophaeus: Marty et al. 2009; geladas, Theropithecus gelada: Bergman et al. 2009). Evidence that the trait is involved in female mate choice is mixed, with some positive association reported in mandrills (Setchell 2005), but not in drills (Marty et al. 2009). The strong covariation between male dominance rank, skin brightness, and mating success makes it difficult to isolate the effect of female mate choice in these species, which exhibit pronounced levels of sexual dimorphism in body mass and canine length. The opposite holds true in rhesus macaques (Macaca mulatta). In this species, there is good behavioral evidence that the trait is not related to dominance rank (Higham et al. 2013; Dubuc, Allen, et al. 2014) but instead is attractive to females (Waitt et al. 2003; Dubuc, Allen, et al. 2014)—females more frequently sexually solicit darker red males for copulation (Dubuc, Allen, et al. 2014), which translates into a reproductive advantage, at least for high-ranking males (Dubuc, Winters, et al. 2014). In this species, females are frequently reported to resist mating with some males but solicit others and are thus able to express mate preference (Chapais 1983; Manson 1992; see also Dixson 2012).
Whether rhesus males perceive and react to coloration exhibited by other males remains unknown. An experimental study found that rhesus males were less likely to steal food from human experimenters wearing red compared with those wearing blue or green (Khan et al. 2011), suggesting that red skin coloration may nonetheless be intimidating to males (and thus involved in male–male competition), despite the lack of a relationship with dominance rank. This suggests that perhaps the intimidation of males by dark red males has led to avoidance behavior, limiting the ability of observers to detect a role of coloration in male–male interactions. If females prefer dark red males, it might be beneficial to males to pay attention to the color of their rivals and adjust their competitive behaviors accordingly.
In the present study, our objective was to examine whether the red skin coloration developed by rhesus macaque males during the mating season is salient to both sexually mature female and male rhesus macaques. To do so, we presented subjects with experimentally altered images of males to test female interest toward male facial coloration. By presenting images of males rather than modifying live animals, this experimental approach allows us to avoid the ethical issues involved with modifying a signal that is potentially involved in male fights and the logistical problem of altering the mating success of subjects that are involved in long-term studies (see Clutton-Brock and McAuliffe 2009). Moreover, this approach allows for a comparison of perception between age–sex categories that might otherwise react very differently to male coloration in normal behavioral contexts—for example, sexual solicitation by females vs. submissive retreats or attacks by males. The noninvasive experimental approach we used is based on the looking time paradigm first developed to study preverbal psychology and cognition in humans and subsequently used with success to study cognitive bias in primates, including macaques (reviewed in Winters et al. 2015). Macaques can recognize faces in pictures and react differently based on familiarity with subjects in the image (Schell et al. 2011; Deaner et al. 2005), facial expression (Bethell et al. 2012; Mandalaywala et al. 2014), and facial features (Waitt and Little 2006; Waitt et al. 2006; Pfefferle et al. 2014). There is evidence collected using this experimental paradigm that rhesus macaques pay attention to skin coloration in both captive (Waitt et al. 2003, 2006; Gerald et al. 2006, 2007) and free-ranging (Higham et al. 2011) settings but that red coloration alone is not sufficient to induce this preference (Hughes et al. 2014). The same approach has also shown that adults and immature rhesus macaques pay more attention to threatening facial stimuli (Bethell et al. 2012; Mandalaywala et al. 2014) and that therefore, both attraction and intimidation are expected to lead to increased attention. This allows us to use the same paradigm to examine and compare behavioral reaction with the signal by different sex–age categories.
Our study builds on the study of Waitt et al. (2003) on female attentional bias toward red male faces. However, to test specifically whether potential attentional bias was indeed linked to a sexual context, we went further than this previous study by: 1) documenting and then presenting intermale color variation as exhibited by males on Cayo Santiago during the mating season specifically; 2) testing subjects living in free-ranging conditions during the mating season, allowing us to test sexually active female subjects at the time of year where the signal is exhibited by males; and 3) testing not only adult females, but also subjects of other age–sex categories. In addition to male and female adults, we also tested juveniles as controls in order to investigate the possibility that interest toward images is related to a general sensory bias for red coloration found among all age–sex classes or is due to reaction to unusual or novel stimuli rather than to difference in sexual signal expression. Moreover, we used color-calibrated images of rhesus male faces that exhibited either pale or dark coloration as assessed by a model of rhesus macaque vision (see Methods for details) in order to prevent cognitive bias that is linked to reactions to novel stimuli. We set out to test 3 alternative hypotheses with corresponding predictions: 1) trait evolution has been driven solely by intersexual selection, so that only females show an attentional bias for dark red skin; 2) trait evolution has been driven by both intersexual and intrasexual selection, so that adults of both sexes show a bias; and finally, 3) there is a general sensory bias for red facial coloration in this species, so that juveniles as well as adults of both sexes pay attention to images of darker red males.
METHODS
Field site and population
Cayo Santiago Island is populated by a free-ranging population of rhesus macaques descended from individuals brought to the island in 1938. The population, now managed by the Caribbean Primate Research Center, is divided into 10 naturally formed groups that range freely across the island. The population shows no significant effect of inbreeding (Duggleby et al. 1986), and the variance in lifetime reproductive success between individuals is sufficient to create opportunities for selection (Dubuc, Ruiz-Lambides, et al. 2014). Animals are individually recognizable with tattoos providing a unique ID and ear notches both given as yearlings, and dates of birth are available from long-term records. The present study was approved by the IACUC of the University of Puerto Rico, Medical Sciences Campus (protocol no. A0100108).
Preparation of stimuli
We created a set of stimuli using photographs of male rhesus macaques collected in group R of the Cayo Santiago population that we manipulated to display either a dark red or pale pink color chosen from the distribution of colors naturally displayed. The 2 colors were selected on the basis of color measurements from photographs taken of 24 males in group R at the peak of the mating season (i.e., March 2012; see Dubuc, Allen, et al. 2014). For full details on image collection and measurements, see Dubuc, Allen, et al. (2014). Briefly, images of faces were captured in RAW format and a color standard was used to standardize images. Skin color and darkness were measured as the mean red (R), green (G), and blue (B) values of a fixed portion of the face. These values were then transformed from the camera’s color space to rhesus macaque color space using standard methods (Stevens et al. 2009) to provide estimates of the long-, medium-, and short-wavelength photoreceptor catches. Multiple photos of each male taken over several days were taken, and the average color of each male was calculated.
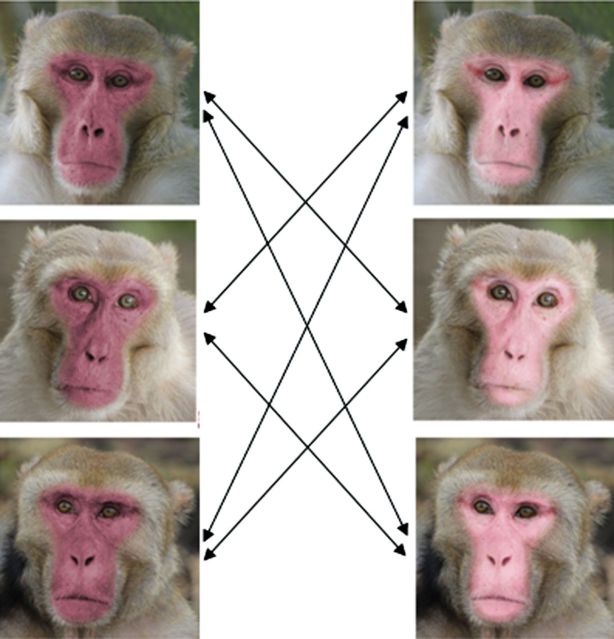
We chose 2 colors, one illustrating dark red males and the other pale pink males. We used Principal component analysis to find the most important dimension of variation in rhesus male color and selected the pale pink color 2 standard deviations above the mean color and the dark red color 2 standard deviations below the mean color. We then made stimuli by choosing 2 photographs each of 3 males where the subject had a neutral expression and was oriented toward the camera but not looking directly at it. We selected males of prime ages (9–12 years old) and intermediate ranks that did not exhibit distractive facial features (e.g., scars or wounds). For each of these 6 images, we created one pale pink and one dark red stimuli using image-editing tools in GIMP 2.0 (Figure 1). The face region was selected using the intelligent scissors tool, and the color of this was altered using the Hue Saturation tool. To improve the appearance, we used airbrush and eraser tools to blend the face with surrounding hair and features such as the eyes. We also altered the color of the background in images by selecting the region and using the hue saturation tool to give all images backgrounds with approximately equal appearances of blurred foliage. We printed stimuli onto matte photo paper (Staples Photo Supreme) using a color-calibrated printer (Canon Pixma Pro 100). We measured the printed face color using a Xrite ColorMunki spectrophotometer and used the V–O visual difference model (Vorobyev and Osorio 1998) to ensure that the printed color was very close (less than 3 just-noticeable differences) to the target color, as perceived by rhesus color vision. Pictures were printed on letter format paper (21.5cm × 28.9cm), with printed images of a dimension of 18.5cm × 18.5cm, in such a way that face length was 17cm.
Figure 1.
Stimuli used for the experiments. Each horizontal pair of images represents 2 versions of the same face, with its color manipulated to be either a dark red or pale pink rhesus color as exhibited by males at the peak of the mating season. During the experiments, faces of 2 different males were presented simultaneously to subjects as illustrated by the arrows; the relative position (right vs. left) of each coloration was randomized and balanced, for a total of 12 possible combinations.
Apparatus and experimental design
We simultaneously presented 2 pictures depicting 2 different males showing different colorations (one dark, one pale) inserted in frames attached to opposite ends of a black board at a distance of 1 m between the center of each image (78.5cm between the inside edges). These images could be hidden with blue sliding occluders. In all trials, we counterbalanced the pairing of the 3 males, the 2 color attributions, and the location on the apparatus across trials, with image position (left or right) randomly determined, with a total of 12 possibilities (Figure 1). Each test session required 2 experimenters—one managing the apparatus and one filming the subject’s looking behavior from behind the apparatus.
Experimenters would enter a social group for testing and would look for potential subjects—individuals slightly isolated from the rest of the group, resting or inactive, with open space in front of them. The apparatus was placed in front of a subject within 1–3 m, with the 2 images equidistant from the subjects. Precautions were taken to ensure both images were in similar lighting, at the same level, and that no objects were occluding visibility. The experimental procedure was as follows. The experimenter managing the apparatus positioned themself in front of the subject, and the recording started. The experimenter managing the apparatus would direct the subjects’ attention to the apparatus and then removed the occluders while facing down at the floor to avoid any interaction with the subject. At this point, the subject’s responses were recorded for 15 s. Following completion of each trial, the subject’s ID was recorded, regardless of whether they were successfully tested or not.
The cameraperson, blind to experimental condition, decided if and when to abort a session for any of the following reasons: the subject did not pay attention to the apparatus or did not see both images; the subject moved outside the camera range but still paid attention to the apparatus (preventing coding of looking time); the subject was distracted by an outside event taking place during the trial (e.g., a conspecific passing-by, a fight in the distance, etc.). We aimed to test subjects only once across all conditions, and any unintentional retests of subjects were discarded. A total of 629 trials were attempted, only 262 of which were used in the analyses.
Subject selection
Experiments were conducted at the peak of the 6-month mating season, over a 7-week period during which 67.7% of all conceptions occurred in the population (25 February through 16 April 2013). This allowed us to test subjects: 1) in a sexual context (cycling females and males with testosterone); and 2) at the time of year when the signal is actually exhibited by males, avoiding biases in reaction due to violation-of-expectation (i.e., when subjects are presented with visual scenes that either do or do not conform to the subjects’ expectations).
We tested subjects of 3 age–sex categories: 1) adult females (N = 91; which represents 23.7% of all adult females on Cayo Santiago) of ≥3 years old exhibiting visual signals or cues of sexual activity (see Dubuc, Allen, et al. 2014); 2) adult males (N = 77; 26.9% of all males) of ≥3 years old (minimum age at first reproduction; Bercovitch et al. 2003); and 3) juveniles (1–2 years) (N = 94; 25% of all juveniles). On average (± standard error of the mean [SEM]), adult female subjects were 6.30±0.40 years old (range: 3–18), adult males, 5.25±0.33 (range: 3–23), and juveniles, 1.41±0.05. There are several reasons why subjects of relatively young ages were the primary test subjects among the “adult” category. Firstly, because males disperse between groups several times in their lives, it is possible that adult males and females may be familiar with the males used in the images even if they did not live in the same group at the time of the study. Utilizing young adults reduces the likelihood of this potential confound. Next, young adults were also likely to be in a life history period where these signals may be of particular relevance: 1) young nulliparous females are less attractive to males and are thus actively looking for mating partners and 2) young adult males are in the process of natal dispersal involving the assessment of the strength of resident males. Therefore, we targeted sampling on young adults of 3–5 years (N = 45 females, 51 males), but also opportunistically sampled older adults (N = 46 females, 26 males). The “adult” category includes all subjects of 3 years old or older. A total of 47 subjects were tested in group R where the males used for stimuli preparation live, 16 adult females (17.6% of all adult females subjects), 17 adult males (22.1%), and 14 juveniles (14.9%).
Video coding
Each 15-s trial was later coded frame-by-frame (29 frames/s) by trained coders blind to condition using MPEG Streamclip (http://www.squared5.com/). Coders recorded the exact frame at which subject started and ended looking at the image on their right or left, which was summed for a total looking time at each image. A total of 35 trials not used in the analyses (e.g., discarded unintentional retests) were used for reliability assessment between the 3 coders. Coders agreed on which image subjects looked at more (± SEM) in 83.8±3.6% of the trials. We used Cohen’s Kappa to assess intercoder reliability: trained coders scored on average k = 0.71±0.08 agreement with each other (random: k = 0; perfect: k = 1). An untrained coder agreed on coding with trained coders in 75% of the trials used (N = 10 trials), showing an average agreement scores of k = 0.52±0.02, an acceptable level of agreement (Landis and Koch 1977; Fleiss 1981). This shows that agreement among coders was not a mere consequence of the training process creating a similar bias.
Statistical analyses
We first explored what factors influence general difference in looking time among age–sex. To do so, we ran a linear mixed model (LMM) setting looking time at each image as a response variable, stimuli type (dark red/pale pink), sex (female/male), and age class (adult/juvenile) as fixed factors, and ID as a random factor. Looking time was log-transformed to respect model assumptions. Next, we examined whether stimuli depicting faces of darker red males received more attention for each age-class separately. Firstly, we used Wilcoxon signed-rank tests to compare total looking time between the 2 stimuli. Secondly, we further explored patterns of attentional bias by examining whether the tendency to look longer at dark red faces differed between age–sex categories (female/male; adult/juvenile) by comparing an index of looking time ([time spent looking at dark red − time spent looking at pale pink]/total looking time) with a Kruskal–Wallis test, followed by post hoc Mann–Whitney tests. Finally, we used χ2 goodness-of-fit to test whether subjects were overall more likely to look at one face more than the other. In contrast to the previous approaches that are sensitive to strong preferences among a few individuals, the χ2 analysis tests whether a significant proportion of individuals prefer one image-type over the other. This is based on the number of individuals showing a preference, one way or another, and is particularly sensitive to small preferences among many individuals. Statistical analyses were performed in IBM SPSS Statistics 19.0.0. All analyses were 2 tailed with significance level set at α = 0.05.
RESULTS
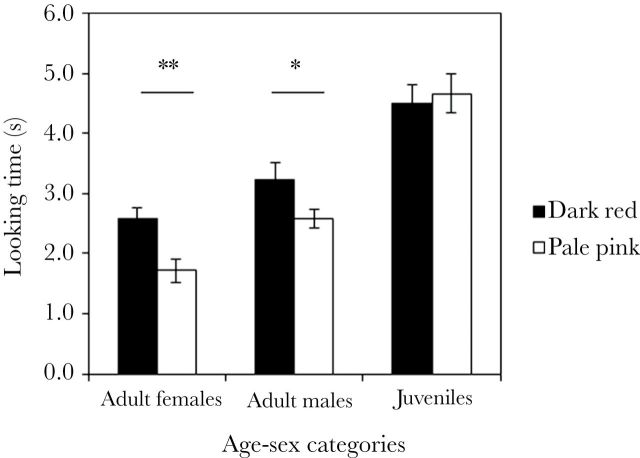
There was an overall significant effect of stimuli type (i.e., dark vs. pale) on looking time (LMM; F1,259 = 17.150, P < 0.001), with subjects looking longer at dark (3.46±0.18 s) than pale images (3.03±0.19 s). In addition, there was an effect of both age (F1,259 = 59.686, P < 0.001) and sex (F1,259 = 8.410, P = 0.004)—with juveniles (4.59±0.24 s) looking longer at each image than adults (2.49±0.14 s) and males (3.77±0.22 s) longer than females (2.75±0.15 s). When comparing total looking time exhibited by subjects, both adult females (Wilcoxon: Z = −3.280, N = 91, P = 0.001) and adult males (Z = −2.452, N = 77, P = 0.014) looked significantly longer at dark red faces but juveniles did not (Z = −0.845, N = 94, P = 0.400) (Figure 2). Similar results are reached if only young adults are considered (females: Z = −3.355, N = 45, P = 0.001; males: Z = −2.312, N = 51, P = 0.020). Finally, the extent of bias toward dark red faces differed between the 3 age–sex categories (Kruskal–Wallis: χ2 = 6.426, degrees of freedom [df] = 2, P = 0.040), with juveniles showing smaller differences in looking time between the 2 images than adult females (Mann–Whitney: U = 3427.5, N1 = 91, N2 = 94, P = 0.020) and adult males (U = 2979.0, N1 = 77, N2 = 94, P = 0.047). Adult females and males did not differ in the strength of their bias for dark red faces (U = 3484.5, N1 = 91, N2 = 77, Z = −0.060, P = 0.952).
Figure 2.
Comparison of looking time (average ± SEM seconds) toward dark red (gray) and pale pink (white) faces. Significant differences are indicated with asterisks (**P < 0.01, *P < 0.05).
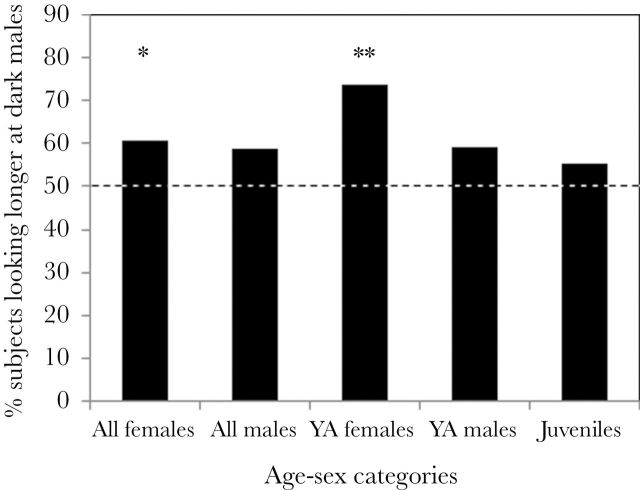
A higher proportion of subjects looked longer at stimuli depicting dark red faces than expected by chance (χ2 = 7.84, df = 1, P = 0.005), although only adult females drove the pattern (χ2 = 4.02, df = 1, P = 0.045), with no significant difference for adult males (χ2 = 2.22, df = 1, P = 0.136) and juveniles (χ2 = 1.32, df = 1, P = 0.251) (Figure 3). The same results are obtained if only young adults are considered (3–5 years old): females (χ2 = 10.02, df = 1, P = 0.002), but not males (χ2 = 1.62, df = 1, P = 0.203), were more likely to pay attention to dark red faces.
Figure 3.
Proportion of tested subjects that looked longer at dark red male faces during a trial among different age–sex categories. For adult subjects, results are depicted according to whether all adult subjects (N = 91 females, 77 males) or only young adults (YA) (3–5 years old; N = 45 females, 51 males) are considered. Significant differences are indicated with asterisks (**P < 0.01, *P < 0.05).
DISCUSSION
Using an experimental approach based on the looking time paradigm, we showed that adults of both sexes pay attention to facial skin coloration exhibited by males during the mating season, suggesting that the trait is salient to both sexes. However, only a significant proportion of adult females preferred to look at dark red faces, suggesting a particularly important effect for this sex (see below). In contrast, although juveniles showed more interest toward the facial images than adults, they did not show a preference for faces of one color over the other. The lack of attentional bias by juveniles allows us to discard the possibility that adult bias toward dark red faces was generated solely by a general sensory bias or by the experiment design itself, and shows that red skin coloration is relevant only to sexually mature subjects. These results further support the view that females are attracted to dark red males (Waitt et al. 2003; Dubuc, Allen, et al. 2014) and provide the first evidence for male responses to the skin coloration of other males. When combined with evidence that rhesus males are intimidated by humans wearing red coloration (Khan et al. 2011), and given that red coloration is a signal of status in closely related primate species (e.g., Setchell and Wickings 2005; Bergman et al. 2009; Marty et al. 2009), this suggest that facial red skin color exhibited by rhesus males may be communicative to males in this species, despite a lack of any relationship to male dominance rank previously reported (see Higham et al. 2013; Dubuc, Allen, et al. 2014).
That said, the chi-square results also show that only a significant proportion of females (not males) show a bias toward dark red females. This result is consistent with the signed-rank test results and is due to the fact that those males that showed a preference, did so with a more pronounced bias than females did, such that overall looking time differences were similar. The differences between males and females in the proportion preferring the dark red faces might be a mere consequence of the smaller sample size (77 males against 91 females). However, because a similar phenomenon was detected for young adults and that we had a similar sample size between the sexes for the age category (51 males against 46 females), this explanation seems unlikely to be sufficient. A possible explanation for the difference in bias is that males evaluated a wider range of cues and signals when examining male faces depicted in the images than females did. For instance, male faces might contain other information about competitive ability, such as the development of the temporalis muscles. This idea is supported by the fact that males in general spent more time looking at the images than females, which could have been due to inspection of multiple traits. An experimental approach modifying several facial traits at once might be needed to explore these ideas.
The role male dark red coloration might play in male–male interaction still remains unknown. The lack of social status correlates of male red skin color in rhesus macaques could be related to the fact that males of this population reach dominance through queuing rather than dyadic fights (Berard 1999). The absence of rapid takeovers of the alpha position (especially by new immigrant males) leads to stable hierarchies in which high-ranking males are socially familiar to all group members, potentially making signals of social status obsolete (Bergman and Sheehan 2013). Yet, a signal advertising the motivation of males to defend access to their mating partner regardless of their rank might nonetheless still be useful, which could explain the results of the present study, as well as those obtained by Khan et al. (2011) related to human clothing color. The need to fend off other males from females is particularly likely in rhesus macaques because several females are often sexually active on a given day in this seasonally breeding species (e.g., Dubuc et al. 2011). In this context, males may benefit by avoiding investing mating effort toward females who are being mate-guarded by highly motivated males willing to defend access to their female mating partners aggressively. Another non-mutually exclusive possibility is that male perception of dark red males might have evolved secondarily as a response to female attraction to this trait. For instance, high-ranking males may gain by preventing males that are attractive to females from immigrating into the social group, especially given high rates of sneak copulations and fertilizations in this species (e.g., Berard et al. 1994; Dubuc et al. 2012). Finally, it remains possible that the attentional bias shown by adult males is not adaptive in rhesus macaques, but related to an ancestral function of male coloration in signaling social status or as a secondary by-product of attraction toward opposite-sex conspecifics. However, if such an ancestral bias leads to males avoiding conflicts and fights with dark red males as suggested by Khan et al.’s (2011) study, this could nonetheless still reinforce trait selection by increasing the access to mating partners of those avoided males. Detailed examination of whether male skin coloration influences the outcome of male–male interactions at the behavioral level will be needed to test these ideas.
Evidence that both intrasexual and intersexual selection can simultaneously influence the selection of skin ornamentation has been shown for many bird species (e.g., rock sparrow, Petronia petronia: Griggio et al. 2007; red jungle fowl, Gallus gallus: Ligon et al. 1990; Zuk et al. 1990; yellowthroat, Geothlypis trichas: Tarof et al. 2005; reviewed in Hunt et al. 2009). There is much less evidence for such a phenomenon in mammals (Clutton-Brock and McAuliffe 2009). To our knowledge, evidence for effects of both intrasexual and intersexual selection on mammalian traits have been reported for only one other ornament, male mane color in lions (Panthera leo) (West and Packer 2002), but none for weapons primarily involved in direct male–male competition, such as horn or antler size in ungulates. It is possible that intersexual selection typically only acts on ornaments and not on sexually selected traits that have a direct utilitarian function in sexual coercion. Alternatively, a lack of evidence of intersexual selection on armaments might be due to the fact that behavioral observations limit the opportunity to disentangle the effects of intrasexual versus intersexual selection. Although experiments might allow us to avoid such issues, the experimental modification of male weapon size is rarely undertaken (Clutton-Brock and McAuliffe 2009), potentially because such experiments are invasive, logistically difficult and unethical. Although the looking time paradigm is based on a basic behavior that is likely to be shared by mammals in general, an approach similar to the one used here could be useful in exploring whether weapons are also used as cues or signals by females in their mate choice decisions.
Our results provide new insights into the function of red sexual ornaments in catarrhines more generally, and suggests new lines of behavioral studies that might help us to understand the evolution of red skin color in male rhesus macaques and catarrhine primates in general. Unlike in rhesus macaques, it may be more difficult to detect effects of intersexual selection in other catarrhine species where females are more commonly mate-guarded by alpha males, who also have the most conspicuous coloration. Females might prefer high-ranking males (who in many other species have demonstrated their competitiveness by fighting for dominance) and facilitate mate-guarding (Clutton-Brock and McAuliffe 2009). Interestingly, in an anecdotal event in which a mandrill male unusually kept his intense red coloration after being overthrown from the alpha position, this male remained sexually attractive to females (Setchell 2005). This intriguing observation combined with the present results suggests that red skin ornaments might be under both intrasexual and intersexual selections in catarrhines more generally, with different mechanisms playing a more important relative role depending on the importance of direct male–male competition. Fortunately, the looking time paradigm employed here allows such questions to be addressed experimentally, while facilitating interspecific comparison.
FUNDING
This project was made possible by intramural funds from NYU, FQRSC, as well as NCRR and ORIP of NIH (grant number 2P40RR03640-25) to CPRC.
Acknowledgments
The study is dedicated to the late Dr Corri Waitt. We thank the CPRC for permission to conduct this study. For help in the field, we thank N. Rivera Barreto, G. Caraballo Cruz, and particularly B. Aure and J. Glick for their help identifying subjects and locating sexually active females. We thank K. Hughes, T. Mandalaywala, and L. Santos for advice on setting the experimental design, and 4 anonymous reviewers for their fruitful comments on a previous version of the manuscript. The content of this publication does not represent the official views of NCRR, ORIP, or NIH.
REFERENCES
- Andersson M. 1994. Sexual selection. Princeton (NJ): Princeton University Press. [Google Scholar]
- Berard J. 1999. A four-year study of the association between male dominance rank, residency status, and reproductive activity in rhesus macaques (Macaca mulatta). Primates. 40:159–175. [DOI] [PubMed] [Google Scholar]
- Berard JD, Nuernberg P, Epplen JT, Schmidtke J. 1994. Alternative reproductive tactics and reproductive success in male rhesus in male rhesus macaques. Behaviour. 129:177–201. [Google Scholar]
- Bercovitch FB, Widdig A, Trefilov A, Kessler MJ, Berard JD, Schmidtke J, Nürnberg P, Krawczak M. 2003. A longitudinal study of age-specific reproductive output and body condition among male rhesus macaques, Macaca mulatta. Naturwissenschaften. 90:309–312. [DOI] [PubMed] [Google Scholar]
- Berglund A, Bisazza A, Pilastro A. 1996. Armaments and ornaments. Biol J Linn Soc. 58:385–399. [Google Scholar]
- Bergman TJ, Ho L, Beehner JC. 2009. Chest color and social status in male geladas (Theropithecus gelada). Int J Primatol. 30:791–806. [Google Scholar]
- Bergman TJ, Sheehan MJ. 2013. Social knowledge and signals in primates. Am J Primatol. 75:683–694. [DOI] [PubMed] [Google Scholar]
- Bethell EJ, Holmes A, MacLarnon A, Semple S. 2012. Emotion mediates social attention in a non-human primate. PLoS One. 7:e44387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley BJ, Mundy NI. 2008. The primate palette: the evolution of primate coloration. Evol Anthropol. 17:97–111. [Google Scholar]
- Changizi MA, Zhang Q, Shimojo S. 2006. Bare skin, blood and the evolution of primate colour vision. Biol Lett. 2:217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapais B. 1983. Reproductive activity in relation to male dominance and the likelihood of ovulation in rhesus monkeys. Behav Ecol Sociobiol. 12:215–228. [Google Scholar]
- Clutton-Brock T, McAuliffe K. 2009. Female mate choice in mammals. Q Rev Biol. 84:3–27. [DOI] [PubMed] [Google Scholar]
- Cox CR, LeBoeuf BJ. 1977. Female incitation of male-male competition: a mechanism in sexual selection. Am Nat. 111:317–335. [Google Scholar]
- Deaner RO, Khera AV, Platt ML. 2005. Monkeys pay per view: adaptive valuation of social images by rhesus macaques. Curr Biol. 15:543–548. [DOI] [PubMed] [Google Scholar]
- Dixson AF. 2012. Primate sexuality. 2nd ed. Oxford: Oxford University Press. [Google Scholar]
- Dominy NJ, Lucas PW. 2001. Ecological importance of trichromatic vision to primates. Nature. 410:363–366. [DOI] [PubMed] [Google Scholar]
- Dubuc C, Allen WL, Maestripieri D, Higham JP. 2014. Is male rhesus macaque red color ornamentation attractive to females? Behav Ecol Sociobiol. 68:1215–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuc C, Muniz L, Heistermann M, Engelhardt A, Widdig A. 2011. Testing the priority-of-access model in a seasonally breeding primate species. Behav Ecol Sociobiol. 65:1615–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuc C, Muniz L, Heistermann M, Widdig A, Engelhardt A. 2012. Do males time their mate-guarding effort with the fertile phase in order to secure fertilisation in Cayo Santiago rhesus macaques? Horm Behav. 61:696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuc C, Ruiz-Lambides A, Widdig A. 2014. Variance in male lifetime reproductive success and estimation of the degree of polygyny in a primate. Behav Ecol. 25:878–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuc C, Winters S, Allen WL, Brent LJN, Cascio J, Maestripieri D, Ruiz-Lambides AV, Widdig A, Higham JP. 2014. Sexually-selected skin colour is heritable and linked to fecundity in a non-human primates. Proc R Soc Lond B. 281:20141602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggleby CR, Haseley PA, Rawlins RG, Kessler MJ. 1986. An overview of blood group genetic studies on the Cayo Santiago rhesus monkeys. In: Rawlins RG, Kessler MJ, editors. The Cayo Santiago macaques. Albany: State University of New York Press, p. 269–282. [Google Scholar]
- Engelhardt A, Neumann C, Heistermann M, Perwitasari-Farajallah D. 2008. Sex skin coloration in male Sulawesi crested black macaques (Macaca nigra). Primate Eye. 96:337. [Google Scholar]
- Fleiss JL. 1981. Statistical methods for rates and proportions. 2nd ed. New York: John Wiley. [Google Scholar]
- Folstad I, Karter AJ. 1992. Parasites, bright males and the immunocompetence handicap. Am Nat. 2:603–622. [Google Scholar]
- Gerald MS, Waitt C, Little AC, Kraiselburd E. 2007. Females pay attention to female secondary sexual color: an experimental study in Macaca mulatta. Int J Primatol. 28:1–7. [Google Scholar]
- Gerald MS, Waitt C, Maestripieri D. 2006. An experimental examination of female responses to infant face coloration in rhesus macaques. Behav Processes. 73:253–256. [DOI] [PubMed] [Google Scholar]
- Griggio M, Serra L, Licheri D, Monti A, Pilastro A. 2007. Armaments and ornaments in the rock sparrow: a possible dual utility of a carotenoid-based feather signal. Behav Ecol Sociobiol. 61:423–433. [Google Scholar]
- Higham JP, Hughes KD, Brent LJ, Dubuc C, Engelhardt A, Heistermann M, Maestriperi D, Santos LR, Stevens M. 2011. Familiarity affects the assessment of female facial signals of fertility by free-ranging male rhesus macaques. Proc R Soc Lond B. 278:3452–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higham JP, Pfefferle D, Heistermann M, Maestripieri D, Stevens M. 2013. Signaling in multiple modalities in male rhesus macaques: sex skin coloration and barks in relation to androgen levels, social status, and mating behavior. Behav Ecol Sociobiol. 67:1457–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes KD, Higham JP, Allen WL, Elliot AJ, Hayden BY. 2014. Extraneous color affects female macaques’ gaze preference for photographs of male conspecifics. Evol Hum Behav. 36:25– 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J, Breuker CJ, Sadowski JA, Moore AJ. 2009. Male-male competition, female mate choice and their interaction: determining total sexual selection. J Evol Biol. 22:13–26. [DOI] [PubMed] [Google Scholar]
- Khan SA, Levine WJ, Dobson SD, Kralik JD. 2011. Red signals dominance in male rhesus macaques. Psychol Sci. 22:1001–1003. [DOI] [PubMed] [Google Scholar]
- Landis JR, Koch GG. 1977. The measurement of observer agreement for categorical data. Biometrics. 33:159–174. [PubMed] [Google Scholar]
- Ligon JD, Thornhill R, Zuk M, Johnson K. 1990. Male–male competition, ornamentation and the role of testosterone in sexual selection in red jungle fowl. Anim Behav. 40:367–373. [Google Scholar]
- Mandalaywala TM, Parker KJ, Maestripieri D. 2014. Early experience affects the strength of vigilance for threat in rhesus monkey infants. Psychol Sci. 25:1893–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson JH. 1992. Measuring female mate choice in Cayo Santiago rhesus macaques. Anim Behav. 44:405–416. [Google Scholar]
- Marty JS, Higham JP, Gadsby EL, Ross C. 2009. Dominance, coloration and social and sexual behavior in male drills (Mandrillus leucophaeus). Int J Primatol. 30:807–823. [Google Scholar]
- Pizzari T. 2001. Indirect partner choice through manipulation of male behaviour by female fowl, Gallus gallus domesticus. Proc R Soc Lond B. 268:181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferle D, Kazem AJ, Brockhausen RR, Ruiz-Lambides AV, Widdig A. 2014. Monkeys spontaneously discriminate their unfamiliar paternal kin under natural conditions using facial cues. Curr Biol. 24:1806–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes L, Argersinger ME, Gantert LT, Friscino BH, Hom G, Pikounis B, Hess DL, Rhodes WL. 1997. Effects of administration of testosterone, dihydrotestosterone, oestrogen and fadrozole, an aromatase inhibitor, on sex skin colour in intact male rhesus macaques. J Reprod Fertil. 111:51–57. [DOI] [PubMed] [Google Scholar]
- Schell A, Rieck K, Schell K, Hammerschmidt K, Fischer J. 2011. Adult but not juvenile Barbary macaques spontaneously recognize group members from pictures. Anim Cogn. 14:503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple S. 1998. The function of Barbary macaque copulation calls. Proc R Soc Lond B. 265:287–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setchell JM. 2005. Do female mandrills prefer brightly colored males? Int J Primatol. 26:715–735. [Google Scholar]
- Setchell JM, Wickings EJ. 2005. Dominance, status signals and coloration in male mandrills (Mandrillus sphinx). Ethology. 111:25–50. [Google Scholar]
- Stevens M, Stoddard MC, Higham JP. 2009. Studying primate color: towards visual system-dependent methods. Int J Primatol. 30:893–917. [Google Scholar]
- Tarof SA, Dunn PO, Whittingham LA. 2005. Dual functions of a melanin-based ornament in the common yellowthroat. Proc R Soc Lond B. 272:1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbergh JG. 1965. Hormonal basis of sex skin in male rhesus monkeys. Gen Comp Endocrinol. 5:31–34. [DOI] [PubMed] [Google Scholar]
- Vorobyev M, Osorio D. 1998. Receptor noise as a determinant of colour thresholds. Proc R Soc Lond B. 265:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waitt C, Gerald MS, Little AC, Kraiselburd E. 2006. Selective attention toward female secondary sexual color in male rhesus macaques. Am J Primatol. 68:738–744. [DOI] [PubMed] [Google Scholar]
- Waitt C, Little AC. 2006. Preferences for symmetry in conspecific facial shape among Macaca mulatta. Int J Primatol. 27:133–145. [Google Scholar]
- Waitt C, Little AC, Wolfensohn S, Honess P, Brown AP, Buchanan-Smith HM, Perrett DI. 2003. Evidence from rhesus macaques suggests that male coloration plays a role in female primate mate choice. Proc R Soc Lond B. 270:144–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West PM, Packer C. 2002. Sexual selection, temperature, and the lion’s mane. Science. 297:1339–1343. [DOI] [PubMed] [Google Scholar]
- Wiley RH, Poston J. 1996. Perspective: indirect mate choice, competition for mates, and coevolution of the sexes. Evolution. 50:1371–1381. [DOI] [PubMed] [Google Scholar]
- Winters S, Dubuc C, Higham JP. 2015. The looking time experimental paradigm in studies of non-human perception and cognition. Ethology. 121:1–16. [Google Scholar]
- Zuk M, Thornhill R, Ligon JD, Johnson K, Austad S, Ligon SH, Thornhill NW, Costin C. 1990. The role of male ornaments and courtship behavior in female mate choice of red jungle fowl. Am Nat. 136:459–473. [Google Scholar]