Key Points
Drugs shown to enhance megakaryocyte ploidy and size variably effect terminal injury and apoptosis of in vitro–grown megakaryocytes.
The number of functional platelets released in vivo from infused megakaryocytes can be enhanced by these drug treatments.
Abstract
In vitro–grown megakaryocytes for generating platelets may have value in meeting the increasing demand for platelet transfusions. Remaining challenges have included the poor yield and quality of in vitro–generated platelets. We have shown that infusing megakaryocytes leads to intrapulmonary release of functional platelets. A Src kinase inhibitor (SU6656), a Rho-associated kinase inhibitor (Y27632), and an aurora B kinase inhibitor (AZD1152) have been shown to increase megakaryocyte ploidy and in vitro proplatelet release. We now tested whether megakaryocytes generated from CD34+ hematopoietic cells in the presence of these inhibitors could enhance functional platelet yield following megakaryocyte infusion. As expected, all inhibitors increased megakaryocyte ploidy, size, and granularity, but these inhibitors differed in whether they injured terminal megakaryocytes: SU6656 was protective, whereas Y27632 and AZD1152 increased injury. Upon infusion, inhibitor-treated megakaryocytes released threefold to ninefold more platelets per initial noninjured megakaryocyte relative to control, but only SU6656-treated megakaryocytes had a significant increase in platelet yield when calculated based on the number of initial CD34+ cells; this was fourfold over nontreated megakaryocytes. The released platelets from drug-treated, but healthy, megakaryocytes contained similar percentages of young, uninjured platelets that robustly responded to agonists and were well incorporated into a growing thrombus in vivo as controls. These studies suggest that drug screens that select megakaryocytes with enhanced ploidy, cell size, and granularity may include a subset of drugs that can enhance the yield and function of platelets, and may have clinical application for ex vivo–generated megakaryocytes and platelet transfusion.
Visual Abstract
Introduction
Platelet transfusions are commonly used for preventing and treating hemorrhage.1 Due to the increasing age of the US population and the increased use of invasive life-preserving procedures, platelet transfusion usage continues to grow in spite of a lowered platelet transfusion threshold.1 Coincident to the increased demands on the donor platelet pool, the megakaryocyte-promoting cytokine thrombopoietin was identified,2-4 enabling in vitro megakaryocyte growth with release of ex vivo–generated platelet-like particles (EV-PLPs).5 This advance suggested that EV-PLPs may be produced to complement or displace donor-derived platelets.6,7 Although considerable progress has been made in the generation of such particles from primary hematopoietic stem cells8,9 or from embryonic stem cells and induced pluripotent stem cells,10,11 there remain important challenges, including the low yield of EV-PLPs from such starting cells.12,13 Moreover, we have shown that EV-PLPs do not have the Gaussian-size distribution of donor-derived platelets, and >50% of these “platelets” are CD41−.14 Only ∼10% of EV-PLPs are uninjured and CD42a+CD42bHigh and Annexin V−.14 Not unexpectedly, these EV-PLPs are mostly preactivated, show little agonist response, survive poorly in vivo, and are weakly incorporated into in situ thrombi.14 Lefrançais et al recently published data identifying the lungs as a primary site of platelet release from megakaryocytes in mice.15 For both murine and human in vitro–grown megakaryocytes, we have shown that these cells are also entrapped in recipient mice lungs after being IV infused.14,16 These entrapped megakaryocytes shed platelets over the next minutes to few hours, and these have the size distribution, circulating half-life, and functionality of infused donor-derived platelets.14,16
Platelet yield depends on megakaryocyte maturity as indicated by megakaryocyte ploidy, size, and granularity.17,18 Also, 8 and 16N megakaryocytes show higher expression of genes involved in proplatelet formation, platelet functions, signal transduction and protein transport in comparison with 2 and 4N cells.19,20 Unfortunately, cultured megakaryocytes have lower ploidy and are smaller and less granular than primary megakaryocytes.18,21 Different strategies have been tested to increase ploidy of in vitro–grown megakaryocytes. Among these approaches have been studies of small compound inhibitors with a potential to increase ploidy of in vitro–grown megakaryocytes like the Src kinase inhibitor SU6656,22,23 the Rock inhibitor Y27632,24,25 and the aurora B kinase inhibitor AZD1152.24,26,27 The compounds were called by the main protein kinases affected, but, as illustrated for SU6656, which also targets aurora B kinase,28 can have additional targets. All 3 drugs increase EV-PLP formation; however, the biological function of these final EV-PLP products or released platelets after infusing the megakaryocytes compared with donor-derived platelets has yet to be studied. We, therefore, evaluate the usefulness of small molecule inhibitors to increase functional EV-PLPs, but more importantly, platelets released in vivo after infusing in vitro–grown megakaryocytes beginning from hematopoietic progenitor cells (HPCs). We believe that measurements of ploidy and formation of EV-PLPs in vitro may not reflect the actual ability to release functional platelets from megakaryocytes as we have previously shown that although both G1ME and G1ME2 cells differentiate into megakaryocytes in vitro and shed EV-PLPs, after reexpression of GATA1, only the G1ME2 megakaryocytes were capable of shedding platelets when infused into recipient mice.29
Here, we investigated the inhibitors SU6656, Y27632, and AZD1152, which significantly increase megakaryocyte ploidy and EV-PLP formation whether they increase in vivo release of functional platelets. We demonstrate that the inhibitors influence in vitro megakaryopoiesis differently. Although SU6656 was protective against loss of CD42b surface expression, Y27632 and AZD1152 increased CD42b cleavage and megakaryocyte apoptosis. Infusion of all 3 inhibitor-treated megakaryocytes increased platelet yield per megakaryocyte rather than control, but only SU6656 increased platelet yield per initial CD34+ HPC. Released platelets, from the control and all 3 drug-treated megakaryocytes, had similar half-lives and functionality, comparable to human donor-derived platelets. These studies support that these drugs had variable effects on megakaryocyte yield, but all 3 enhanced platelet yield per megakaryocyte, mainly because of the increased number of large, final megakaryocytes. Importantly, platelets shed in vivo from these treated cells had no loss in functionality and circulating half-life. Thus, these studies should encourage additional drug discovery focused on enhancing yield of high ploidy, well-mature megakaryocytes that can give rise to functional platelets for clinical transfusional needs.
Materials and methods
In vitro culture of CD34+ HPCs into megakaryocytes
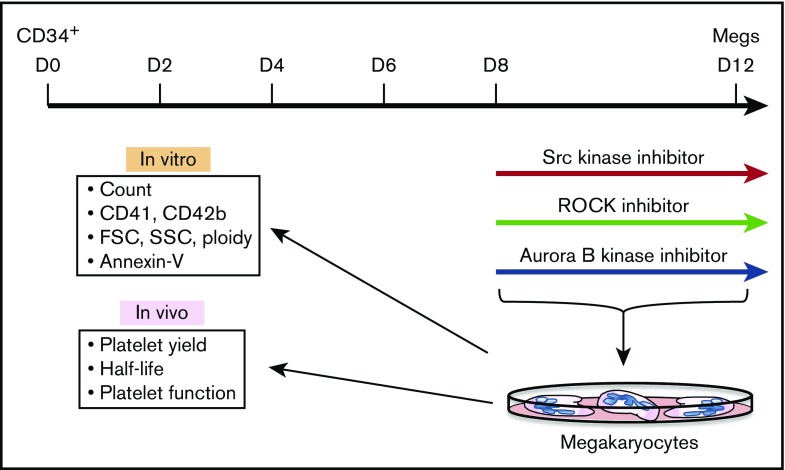
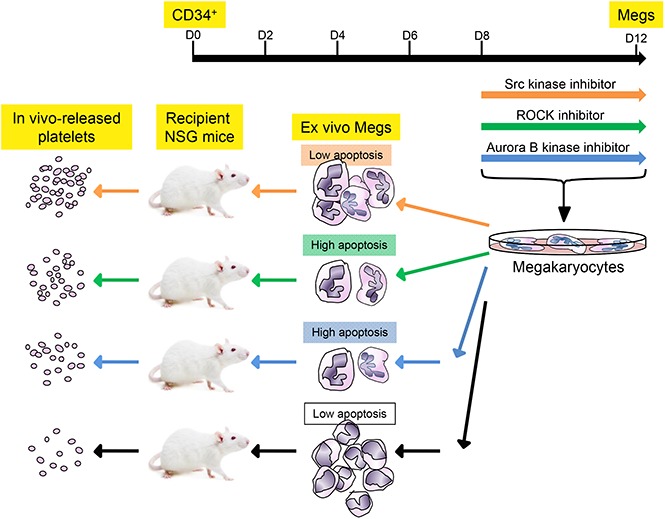
Granulocyte colony-stimulating factor–mobilized human CD34+ HPCs, from the Fred Hutchinson Cancer Research Center Hematopoietic Cell Processing and Repository Core, were differentiated into megakaryocytes in medium composed of 80% Iscove modified Dulbecco medium (Invitrogen), 20% bovine serum albumin/insulin/transferrin serum substitute (Stem Cell Technologies), 20 μg/mL low-density lipoproteins (CalBiochem), 100 μM 2-mercaptoethanol (Sigma-Aldrich) with the following recombinant human cytokines added: 1 ng/mL stem cell factor, 100 ng/mL thrombopoietin, 13.5 ng/mL interleukin 9 and 7.5 ng/mL interleukin 6. To determine the effect of SU6656, Y27632, and AZD1152 inhibitors on megakaryocyte quality and subsequent platelet production and functionality, megakaryocytes were treated with 2.5 μM SU6656,22,23 10 μM Y27632,24,25 0.1 μM AZD1152 or dimethylsulfoxide (Sigma-Aldrich) continuously from days 8 to 12 of culture prior to study (Figure 1).
Figure 1.
Schematic of the megakaryocyte/platelet studies. Schematic of studies beginning with CD34+ HPCs to in vitro megakaryocyte differentiation in the presence of inhibitors to then in vitro and in vivo studies of the resulting day 12 megakaryocytes and EV-PLPs. Megs, megakaryocytes.
Flow cytometry
Flow cytometry evaluation was performed using a Canto flow cytometer (Becton Dickinson) and analyzed using FlowJo software (TreeStar). For the determination of cell surface markers, conjugated mouse antibodies to human (h) CD41, CD42b, CD42a, PAC-1, CD62P, and Annexin V were used (supplemental Table 1). For ploidy analysis, megakaryocytes were stained with Vybrant DyeCycle Violet according to the manufacturer’s instructions. Mean ploidy of human megakaryocytes was calculated as 2N × the number of cells at 2N ploidy level/100 + 4N × the number of cells at 4N ploidy level/100 +…+64N × the number of cells at 64N ploidy level/100.30
Infusion of MKs into NSG-immunodeficient mice
NOD-SCID interleukin receptor 2 γ (NSG) mice were produced at the Children's Hospital of Philadelphia using breeders from The Jackson Laboratory. Human megakaryocytes, collected at day 12, spun at 1200 rpm for 3 minutes, and resuspended in 200-μL phosphate-buffered saline (Invitrogen), or human donor-derived platelets (hdPlts) isolated as previously described16 from healthy volunteers were infused through the tail vein of 8- to 12-week-old NSG mice. To detect the presence of human platelets in the mouse circulation, retro-orbital blood collection was performed post–megakaryocyte infusion using heparinized microcapillaries (Fisherbrand). The blood was then transferred into EDTA DI potassium salt–containing blood-collection tubes (Kabe Labortechnik GMBH). Whole mice blood was stained using anti-hCD41 and anti-hCD42b antibodies, and in some studies, with Annexin V or thiazole orange as described.31 Number of human platelets released from 1 CD42b+ megakaryocyte was calculated at peak release time after infusion. Calculation was performed using the number of CD42b+ human platelets per milliliter of blood in a 30-g mouse divided by the number of infused megakaryocyte (see supplemental Materials and methods description of hematocytometer studies). Based on that value, 2 other calculations were performed: number of human platelets released per large CD42b+ megakaryocyte based on the gate shown in Figure 3A, and number of human platelets per CD34+ HPC using the calculation in Figure 4B.
Figure 3.
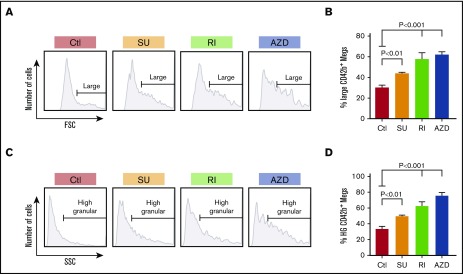
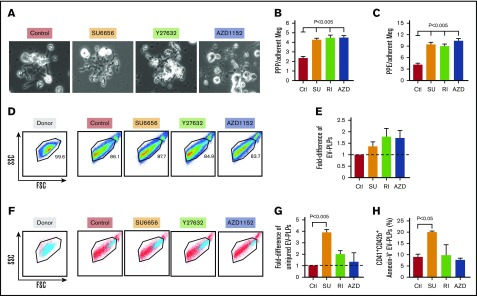
Size and granularity of in vitro–grown megakaryocytes in the presence of inhibitors. (A) Representative data of FSC on day 12 of in vitro–grown, CD42bHigh megakaryocytes. Cells have a wide size distribution, but with most of them being small megakaryocytes. Horizontal lines in the graphs indicate size range of the megakaryocytes classified as “large” megakaryocytes with FSC >70% of FSC of the control sample. (B) Mean percentage ± 1 SEM of the day 12, in vitro–grown, CD42bHigh inhibitor-treated and control megakaryocytes. P values were determined using 1-way ANOVA in comparison with the control. (C) Representative data of SSC of day 12, in vitro–grown, CD42bHigh megakaryocytes. Cells have wide granularity distribution, with most of them being low-granular megakaryocytes. Horizontal lines in the graphs indicate granularity range of the megakaryocytes classified as high-granular megakaryocytes with SSC >70% of the SSC in the control sample. (D) Mean percentage ± 1 SEM of the day 12, in vitro–grown, CD42bHigh inhibitor-treated and control megakaryocytes that are high-granular (HG). In both panels B and D, N = ≥6 independent experiments. P values were determined using 1-way ANOVA in comparison with the control.
Figure 4.
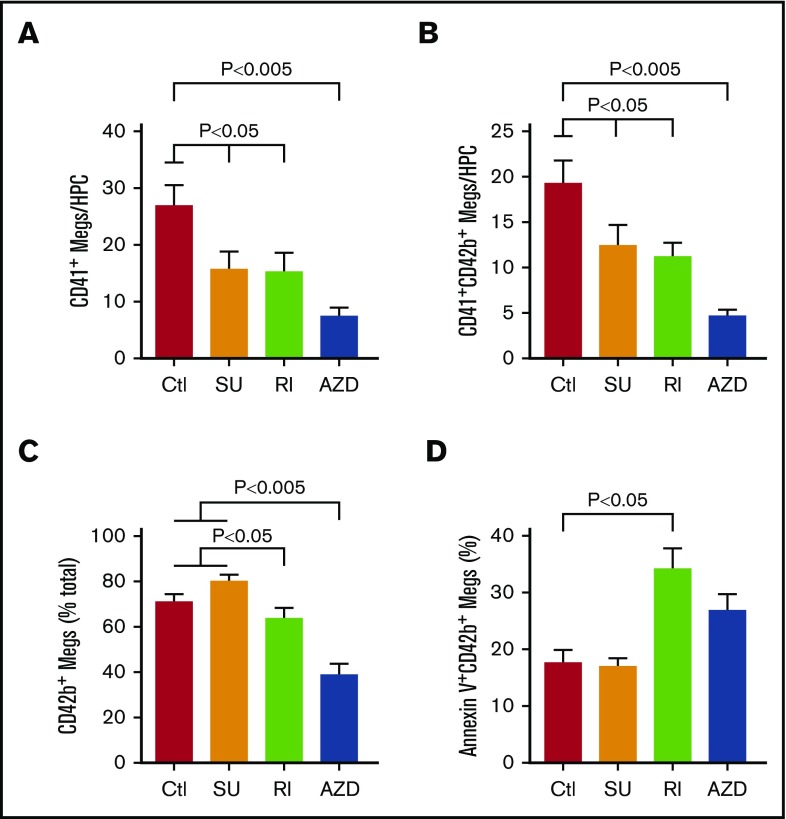
Inhibitors effect on yield and injury of in vitro–grown megakaryocytes. (A) Yield of viable day 12 CD41+ megakaryocytes per HPC plated with or without the addition of SU6656, Y27632, and AZD1152. Mean yield ± 1 SEM is shown for N = 6 independent experiments. P values were determined using 1-way ANOVA in comparison with the control. (B) Yield of viable day 12 CD41+CD42bHigh megakaryocytes per HPC plated with or without the addition of SU6656, Y27632, and AZD1152 as in panel A. Mean yield ± 1 SEM is shown for N = 6 independent experiments. P values were determined using 1-way ANOVA in comparison with the control. (C) Percentage of viable CD41+ megakaryocytes that were also CD42bHigh on day 12 of culture. (D) Percentage of Annexin V+ CD41+CD42bHigh day 12 megakaryocytes was done as in panel B, but for Annexin binding to the megakaryocytes at day 12. For panels C and D, mean percentage-positive cells ± 1 SEM is shown for N ≥ 7 independent experiments. P values were determined using 1-way ANOVA in comparison with the control.
Analysis of infused hdPlts and platelets released from infused megakaryocytes
Baseline human platelet activation and responsiveness to agonist were assessed by surface PAC-1 and CD62P (P-selectin) levels in the absence and presence of thrombin as described.32 NSG mice were infused via tail vein with 2 × 108 hdPlts isolated as previously described16 or ∼107 megakaryocytes. After 4 hours, ∼1 mL of whole blood was collected from the inferior vena cava, and washed platelets were resuspended in platelet final resuspension buffer (134 mmol/L NaCl, 3 mmol/L KCl, 0.3 mmol/L NaH2PO4, 2 mmol/L MgCl2, 5 mmol/L N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid [HEPES], 5 mmol/L D−(+) glucose, 0.75% NaHCO3, and 0.2% bovine serum albumin) as described.33 Platelet activation with 10 U/mL thrombin (Sigma) was done at 37°C for 30 minutes, after which fluorescence-activated cell sorter analysis was done using anti-hCD41 and anti-hCD42b to detect human platelets, anti-hCD62P antibody for CD62P surface exposure, and fluorescein isothiocyanate–labeled PAC-1 antibody for fibrinogen receptor activation.33
To examine released human platelet incorporation into thrombi in mice, hdPlts or day 12 megakaryocytes were incubated with 2 μM calcein AM (Invitrogen) for 15 minutes at 37°C. hdPlts were washed with 10 mL of wash buffer with PGE1 to remove excess calcein AM, centrifuged at 2500 rpm for 10 minutes, and resuspended in 200 μL of platelet final resuspension buffer. Megakaryocytes were washed with 40 mL of Iscove modified Dulbecco medium to remove excess calcein AM, centrifuged at 200g for 5 minutes, and resuspended in 200 μL of Dulbecco phosphate-buffered saline (2.66 mM KCL, 1.47 mM KH2PO4, 138 mM NaCl, and 8.06 mM Na2HPO4; Life Technologies). Washed platelets and cells were then infused into the tail vein of NSG mice. Alexa 647–conjugated rat anti-mouse CD41 Fab fragments (BD Pharmingen) were injected IV to visualize the clot. Laser injuries were induced in the cremaster arterioles 4 hours after megakaryocyte infusion, and the number of human platelets incorporated per thrombus was determined by confocal microscopy, and standardized for the percentage of human platelets in the mice blood.16
Statistical analysis
Means ± standard error of the mean (SEM) are shown. Differences were analyzed by 1-way analysis of variance (ANOVA) using GraphPad Prism 5 and were significant if the P value was <.05 comparing drug-treated megakaryocytes to control.
Institutional approval of studies
Approval was obtained from the institutional internal review board for the volunteer blood samples and from the animal care and use committee for murine studies. The study was conducted in accordance with the Declaration of Helsinki.
Supplemental materials
See supplemental Materials and methods for evaluation of demarcation membrane system (DMS) and factor V (FV) uptake, discussion of the ex vivo analysis of EV-PLPs, and manual measurement of megakaryocyte number prior to infusion.
Results
Inhibitors influence on in vitro–grown megakaryocytes
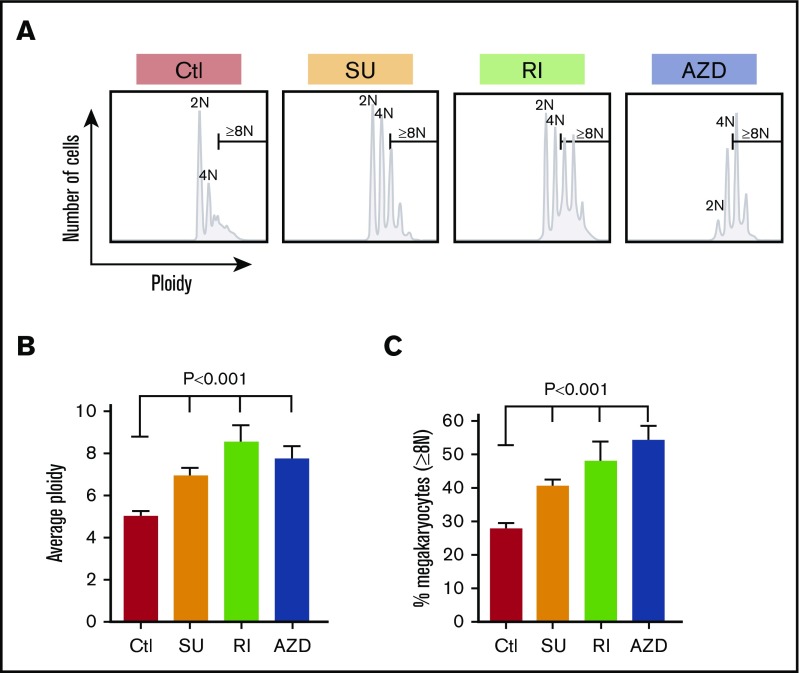
Enhancing yield and functionality of platelets derived from in vitro–grown megakaryocytes is important for developing a non–donor-based source of clinical platelets. Each small inhibitor, SU6656, Y27632, and AZD1152, effects distinct intracellular pathways, yet all enhance the degree of polyploidism of the resultant megakaryocytes.22-27 To examine whether these inhibitors would improve in vivo yield and/or function of platelets derived from such treated in vitro–grown megakaryocytes, we generated megakaryocytes from CD34+ HPCs (Figure 1) and studied their biology. We started by checking their influence on ploidy and confirmed that all 3 significantly increased ploidy of day 12 megakaryocytes (Figure 2A-B), and percentage of megakaryocytes ≥8N (Figure 2C). Such increased nuclear maturation should be accompanied by increased cell size measurable by forward scatter (FSC) on flow cytometry and increased cytoplasmic maturation as indicated by granularity and side scatter (SSC).34,35 Using flow cytometry, we defined a population of large megakaryocytes with FSC ≥70th percentile for untreated megakaryocytes grown (Figure 3A-B). We confirmed that all 3 drugs significantly increased the percentage of large megakaryocytes. We also examined megakaryocytes for granular content based on SSC and defined the ≥70th percentile of untreated cells (Figure 3C). All 3 inhibitors increased granularity of treated megakaryocytes (Figure 3D). Additionally, increased granularity and increased cytoplasmic maturation of drug-treated megakaryocytes were also evident by an increase DMS content (supplemental Figure 1A-C). The development of the DMS has been linked to the ability to shed platelets previously.36 Another indicator of the ability to shed platelets has been the ability to take up FV by in vitro–grown megakaryocytes.37,38 All in vitro–grown megakaryocytes were confirmed to be able to take up FV, with Y27632 and AZD1152 significantly increasing the amount of FV uptake compared with untreated megakaryocytes (supplemental Figure 1D-F), suggesting a beneficial effect on megakaryocyte maturation by these 2 inhibitors.
Figure 2.
Ploidy of in vitro–grown megakaryocytes. (A) Example of ploidy distribution of day 12 CD42bHigh megakaryocytes after the indicated treatments. (B) Same as panel A, but showing mean ploidy ± 1 SEM. (C) Same as in panel B but mean percentage ± 1 SEM of megakaryocytes that were ≥8N. In both panels B and C, N = ≥5 independent experiments. P values were determined using 1-way ANOVA in comparison with the control. AZD, exposed to the aurora kinase inhibitor AZD1152; Ctl, control exposed to DMSO only; RI, exposed to the ROCK inhibitor Y27632; SU, exposed to the Src inhibitor SU6656.
Influence on yield of uninjured megakaryocytes
We examined the influence of the inhibitors on number of mature megakaryocytes (Figure 4). A decrease in the yield of megakaryocytes was seen with each, but was greatest for AZD treatment with a more than threefold decrease (Figure 4A). An important marker of injury to megakaryocytes in vitro is the metalloproteinase cleavage of the glycocalyx portion of glycoprotein Ibα resulting in loss of surface CD42b.39,40 Exposure of differentiating CD34+ progenitors to all 3 inhibitors diminished the yield of CD42bHigh megakaryocytes with again the greatest decrease being after AZD1152 treatment (Figure 4B). The decrease in CD42bHigh megakaryocyte yield for both Y27632 and AZD1152 was not only due to suppression of total megakaryocyte yield, but also to a decrease in percentage of megakaryocytes that were CD42bHigh (Figure 4C).
Another indicator of injured in vitro–grown megakaryocytes is the appearance of apoptotic markers, including binding of Annexin V.41 Y27632 and AZF1152 treatment led to significantly more Annexin V+ megakaryocytes by day 12 than no treatment or SU6656 treatment (Figure 4D).
Influence of inhibitors on proplatelet formation and EV-PLPs
In vitro proplatelet release and the release of EV-PLPs have been used to judge the thrombopoiesis potential of cultured megakaryocytes.5 SU6656, Y27632, and AZD1152 have been shown to increase in vitro proplatelet formation.22,24,26,42 We confirmed these findings by counting the number of shafts extended per megakaryocyte (Figure 5A-B) and branching extensions (Figure 5C). Released EV-PLPs by in vitro–grown megakaryocytes have also been reported to reflect platelet-releasing potential,5-7 but we had shown previously14 that <10% of released EV-PLPs have the size distribution, surface markers, and functionality of hdPlts. Similar to that study, total released EV-PLPs from day 12 megakaryocytes, with or without treatment with any of the drugs, demonstrated a difference in size from hdPlts (Figure 5D). All 3 drugs insignificantly increased the total number of EV-PLPs that were hdPlt in size (Figure 5E). Only big EV-PLPs at the upper end of the hdPlt size range were CD41+CD42bhigh (Figure 5F). CD41+CD42+AnnexinV− EV-PLPs are likely functional, and the yield of such PLPs was significantly increased more than fourfold after SU6656 treatment, but not Y27632 and AZD1152 (Figure 5G). After SU6656 treatment, the percentage of all EV-PLPs that were CD41+CD42+AnnexinV− and thus could be functional platelets after transfusion approached 20%, twice as high as without drug treatment (Figure 5H).
Figure 5.
Influence of inhibitors on in vitro proplatelet formation and released EV-PLPs. (A) Representative fields of megakaryocytes extending proplatelet shafts and branching extensions on fibronectin-coated plates. Original magnification ×200. (B) Proplatelet protrusions (PPP) and (C) proplatelet extensions (PPE) were quantitated on day 12 megakaryocytes that had or had not been exposed to the various inhibitors. Mean ± 1 SEM are shown with N = ≥20 cells counted per condition. P values were determined using 1-way ANOVA in comparison with the control. (D) Representative FSC vs SSC for hdPlts (Donor, left), and PLPs harvested from the media from day 12 plates of control and treated megakaryocytes (right 4 graphs). (E) Shows a difference in number of EV-PLPs (from the normal donor Plt FSC/SSC window) obtained from treated megakaryocytes in reference to control ones. (F) Representative FSC vs SSC for hdPlt (Donor, left), and EV-PLPs harvested from the media from day 12 plates of control and treated megakaryocytes (right 4 graphs). The blue indicates CD42b+ hdPlts and EV-PLPs among all others released particles. (G) Fold-difference in number of CD41+CD42b+AnnexinV− (uninjured) EV-PLPs obtained from treated megakaryocytes in reference to control ones. Mean ± 1 SEM are shown with N = 3 independent experiments. P values were determined using 1-way ANOVA in comparison with the control. (H) Percentage of healthy EV-PLPs (as in panel G) compared with the entire EV-PLP population. Mean ± 1 SEM are shown with N = 3 independent experiments. P values were determined using 1-way ANOVA in comparison with the control.
Influence of inhibitors on released platelets from infused megakaryocytes
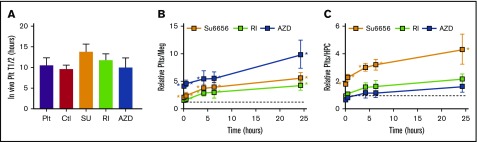
Previous work from our group demonstrated that hdPlts infused into NSG mice could be detected in their blood over the next 1 to 2 days.14 These platelets had a Gaussian-size distribution by FSC analysis consistent with the known 10-fold larger volume of human to mouse platelets.14 In contrast, infused human megakaryocytes initially have 2 CD41+ components: (1) a transient component that had a wide, non-Gaussian-size distribution and (2) a longer-lasting component that emerged after a delay of a few minutes to hours and displayed the same Gaussian-size distribution and half-life as infused hdPlts. This longer-lasting pool represents platelets newly released in the lungs14 likely by a mechanism similar to endogenous megakaryocyte-released platelets.15 Using the FSC parameter together with specific anti-hCD41 and anti-hCD42b antibodies, we evaluated the effect of drug treatment on such released platelets and found that these platelets lasted as long as those released from control megakaryocytes and hdPlts (Figure 6A). We found that all 3 inhibitors significantly increased the number of platelets released per infused CD41+CD42bHigh megakaryocytes compared with control (Figure 6B; Table 1). When expressed per initial HPCs, SU6656 had the greatest increase in released platelets seen with a more than fourfold increase in platelet yield per initial HPC differentiated (Figure 6C; Table 1). The absolute number of platelets released from a nontreated “large” (see Figure 3A) megakaryocyte was 7.0 ± 1.1. This yield is lower that what we published before,14 but would be unchanged if we had used the prior definition (data not shown). This new definition of large megakaryocyte captures most of the large megakaryocyte population. For the drug-treated megakaryocytes, yields were approximately doubled (Table 1). Moreover, the percentage of thiazole orange–positive platelets, an indication of young platelets, was as high in the drug-treated as in the control megakaryocyte infusion studies (supplemental Figure 2A), and the level of Annexin V binding as an indicator of apoptosis was as low (supplemental Figure 2B).
Figure 6.
In vivo–released platelets from infused megakaryocytes. (A) Determined released or infused platelet half-lives. Mean ± 1 SEM are shown. N = 9 for hdPlt infusions. N ≥ 6 for megakaryocyte infusions. (B-C) Fold-difference in the number of human platelets released after infusing megakaryocytes expressed relative to control megakaryocyte infusions corrected for (B) the number of CD41+CD42bHigh megakaryocytes infused or (C) the number of HPCs initially plated. Mean ± 1 SEM are shown; N = ≥6 per arm. Horizontal lines are the control megakaryocytes value at 1. *P ≤ .05 compared with control by 1-way ANOVA analysis. Plt, hdPlt infusion.
Table 1.
Number of healthy platelets obtained from infused megakaryocytes
| No. of healthy platelets per | |||
|---|---|---|---|
| CD42b+Meg | Big CD42+Meg | HPC | |
| Control (5 min) | 2.9 ± 1.5 | 29 ± 15 | 54.8 ± 28.35 |
| SU (4 h) | 10.3 ± 5.4 | 77.25 ± 40.5 | 129.78 ± 68.9 |
| RI (4 h) | 8.5 ± 3.12 | 42.5 ± 15.5 | 89.3 ± 44.3 |
| AZD (4 h) | 14.8 ± 5.18 | 71 ± 25.8 | 56.2 ± 19.7 |
Number of CD41+CD42+ platelets obtained per CD42b+ megakaryocyte (left column), per large (as defined in Figure 3A) megakaryocyte (center column), and per initial HPC at 4 hours after infusion. Values are mean ± 1 SEM.
The inhibitors did not interfere with functionality of released platelets
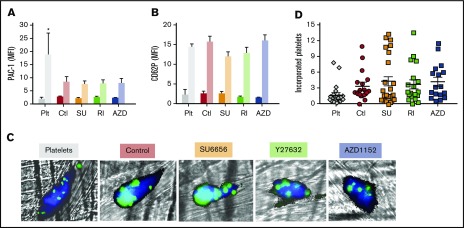
To assess the functional responsiveness of released platelets from infused, drug-exposed megakaryocytes, we studied their responsiveness to agonists in whole murine blood and their selective incorporation into in situ thrombi. Activation of platelets causes a conformational change in the surface αIIbβ3 receptor detectable using PAC-1 antibody binding33 and an activation-dependent degranulation that can be detected by CD62P (P-selectin) surface expression.33 Human and murine platelets isolated from whole murine blood from mice that were infused with hdPlts or released from megakaryocytes were similarly quiescent prior to activation with thrombin showing very low baseline activation level by mean fluorescence intensity (MFI) of PAC-1 binding and surface CD62P expression (Figure 7A and B, respectively). Following high-dose thrombin stimulation (10 U/mL),43 platelet activation was seen as PAC-1 binding increased approximately sixfold and threefold for hdPlts and in vivo–released platelets, respectively. Also, CD62P surface expression MFI increased approximately fivefold both for hdPlts and in vivo–released platelets. There was also no difference in PAC-1 binding and CD62P exposure in response to a range of thrombin dosages supporting equal agonist responsiveness independent of whether the megakaryocytes had been treated with any of the 3 small drugs (supplemental Figure 3A and B, respectively).
Figure 7.
Functionality of in vivo–released platelets. (A-B) Studies with whole murine blood isolated 4 hours after human megakaryocytes or hdPlts were infused. Mean ± 1 SEM of (A) PAC-1 binding as measured by MFI and (B) CD62P surface expression MFI pre- and post-high dose (10 U/mL) thrombin activation. N = 3 independent studies for hdPlt infusion, and N = ≥6 independent studies for infused megakaryocytes arms. *P < .05 compared with hdPlts by 1-way ANOVA analysis. (C) Representative confocal images of laser-induced cremaster arteriole thrombi formed in mice infused with either hdPlts or the various treated in vitro–grown megakaryocytes. Mouse platelets are labeled with CD41–Alexa 647 (dark blue). Human platelets derived from infused donor-derived platelets or megakaryocytes are calcein-AM labeled (green or cyan blue when overlapped with mouse platelets). Original magnification ×20. (D) Quantification of the incorporation into the mice clot standardized per the percentage of human platelets in the mice circulation and thrombus size after infused hdPlts or megakaryocytes. Individual study results are shown with N ≥ 16 independent studies per arm. Mean value is shown as a single horizontal line in each arm.
We then asked whether these released platelets are as well incorporated into thrombi as hdPlts, which we previously showed were actively incorporated into laser-induced cremaster arteriole thrombi.14 Platelets released from either control or treated with each of the inhibitor megakaryocytes were incorporated into thrombi at least as well as hdPlts (Figure 7C-D).
Discussion
Despite the discovery of thrombopoietin2-4 and advances in culture protocols,8-11 in vitro–cultured megakaryocytes are still smaller, have lower ploidy, and release fewer EV-PLPs than primary bone marrow–derived megakaryocytes.18,21 SU6656, Y27632, and AZD1152 are distinct inhibitors. Each enhances in vitro ploidy, size and granularity, and proplatelet yield.22-27,42 These findings have raised the possibility that 1 or more may have clinical application in enhancing the generation of platelets from in vitro–grown megakaryocytes to displace hdPlts.
However, there has been a growing body of insights into how to interpret the quality of in vitro–grown megakaryocytes and their released EV-PLPs. One such insight is that in vitro–grown megakaryocytes undergo terminal apoptosis and consequently loss of CD42b surface expression.34 Unlike primary megakaryocytes that exit the marrow, in vitro–cultured megakaryocytes likely transition beyond peak capacity, undergoing apoptosis and having their surface glycoprotein Ibα extracellular glycocalicin domain cleaved.41 These “over-ripe” and damaged megakaryocytes likely shed much of the EV-PLPs.18 Our studies confirm that all 3 inhibitors enhance ploidy, size, granularity, DMS content, FV uptake (Y27632 and AZD1152 only), and proplatelet yield. However, we also found that all 3 inhibitors decreased the yield of megakaryocytes per initial HPC, perhaps by accelerating maturation of the differentiating cells. More importantly, Y27632 and AZD1152 enhanced apoptosis of the developing megakaryocytes and glycocalicin cleavage, whereas this was not seen for SU6656. Our group has published that surface expression of ADAM17, the metalloproteinase responsible for CD42b shedding, is increased during terminal apoptosis,34 requiring phosphatidylserine exposure to cleave.44 Thus, the increased apoptosis seen for Y27632- and AZD1152-treated megakaryocytes (Figure 4D) explains the lower percentage of CD42b+ on the surface of those megakaryocytes (Figure 4C). In contrast, SU6656-treated megakaryocytes had the same level of apoptosis and percentage of CD42b+ megakaryocytes as untreated, control samples. The consequence of increased terminal megakaryocyte injury following Y27632 and AZD1152 is that there was a greater release of EV-PLPs in culture that are CD41+CD42b− that would be rapidly cleared if infused. Only after SU6656 treatment was there an increase in uninjured CD41+CD42b+ EV-PLPs; however, in spite of this improvement, only ∼20% of these EV-PLPs would be useful for platelet infusion.
The recent in situ microscopy studies of the murine pulmonary bed demonstrated that a significant percentage of daily platelet production occurs in the lungs from marrow-derived megakaryocytes.15 Given the poor yield of functional platelets in EV-PLPs with or without the studied drugs, we examined the ability of these megakaryocytes to release platelets after being infused. We previously demonstrated that both murine and human megakaryocytes infused IV rapidly become entrapped in the lungs, and release, over the following few hours, a shower of platelets that have a similar Gaussian-size distribution, circulating half-life, and functionality as infused hdPlts. We now show that the 3 drug-treated megakaryocyte lineages also release such platelets, and the resulting platelets are as good as hdPlts in terms of circulating half-life and almost as good as hdPlts in terms of responsiveness to agonist and functionality as determined by incorporation into laser-induced thrombi in cremaster arterioles. The yield of these platelets per infused megakaryocyte was increased after treatment with SU6656, Y27632, or AZD1152 compared with infused megakaryocytes not exposed to these drugs. This increase would be consistent with the larger cytoplasmic size of these megakaryocytes. However, because the yield of such healthy, terminal megakaryocytes per initial HPC was reduced for Y27632 and AZD1152, these 2 drugs did not significantly increase yield of circulating platelets per HPC, compared with control studies. On the other hand, SU6656-treated megakaryocytes had a better yield of healthy, mature megakaryocytes per initial HPC, resulting in a yield of released platelets per HPC that is approximately fourfold higher than from untreated megakaryocytes.
These studies clearly depended on a standardized course of treatment of the initial HPCs with the tested drugs as shown in Figure 1, which is based on prior studies.22-27,42 It is unclear why treatment with the Src inhibitor SU6656 had less late deleterious effects on megakaryocytes than the ROCK inhibitor Y27632 or the aurora B kinase inhibitor AZD1152, and clearly the underlying mechanism(s) needs further study. One simple explanation is that Y27632 and AZD1152 speed up differentiation, and the megakaryocytes may need to be harvested earlier to avoid both injury and consequent decreased platelet yield per HPC. Another explanation for the increased platelet yield may be that these drugs enhance maturation of the megakaryocytes (see Lannutti et al,22 Gandhi et al,23 Avanzi et al,24 Lordier et al,25 Avanzi et al,26 Lordier et al,27 Figures 2 and 3, and supplemental Figure 1). Polyploidism depends on synchronous contributions from microtubules, the mitotic spindle, actin/myosin action, and cleavage furrow formation. Each of the drugs studied here is known to effect 1 or more of these events. Aurora B kinase inhibitors, like AZD1152, lead to microtubule destruction via stathmin and mitotic centromere-associated kinesins.45,46 Rho-associated kinase inhibitors, like Y27632, may effect megakaryocyte cytokinesis by slowing cleavage furrow formation.45 Rho not only acts on Rock, but can intersect the Src-signaling pathway through its interactions with LIM kinase and cofilin,46 both of which decrease actin polymerization. Besides effecting actin formation, SU6656 has been shown to effect a motor protein involved in cytokinesis, Dynamin 3,47 as well as Stat5-based pathways.48 Thus, these effectors alter multiple pathways, which in aggregate may affect ploidy and size of the final mature megakaryocyte as a final common pathway. The labeling of the studied inhibitors as affecting megakaryopoiesis by targeting a specific pathway was done based on usage in the literature. Clearly, a more extensive analysis of the specific events by each inhibitor is now needed to better understand how they alter megakaryopoiesis. Such an analysis will also help better explain studies that suggest that the combination of these drugs may enhance overall activity by altering different pathways involved in megakaryocyte ploidy.24
In summary, our data show that a subset of drugs that enhance in vitro human megakaryocyte ploidy resulted in an increased yield of healthy, mature megakaryocytes and more in vitro–released, healthy EV-PLPs. Infusion of these megakaryocytes also released an increased number of circulating platelets per initial HPC that have as good half-life and functionality as infused hdPlts. The timing and dosing of the use of such drugs may require optimization. Studies to define the pathways being enhanced need better definition, but these studies support that at least 1 such drug, SU6656, may be useful for pretreating in vitro–grown megakaryocytes for clinical use.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants R01 HL130698 (M.P.) and U01 HL099656 (M.P.).
Authorship
Contribution: D.J. carried out the majority of the described studies, designed and performed experiments, interpreted data, and prepared the manuscript and figures; K.K.V. and R.B.L. helped with initial in vitro and in vivo cell studies; V.H. performed the cremaster laser-injury studies and helped with manuscript review; R.M.C. provided labeled FV; and M.P. provided overall scientific guidance and manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mortimer Poncz, Children’s Hospital of Philadelphia, 3615 Civic Center Blvd, Abramson Research Center, Room 317, Philadelphia, PA 19104; e-mail: poncz@email.chop.edu.
References
- 1.Estcourt LJ. Why has demand for platelet components increased? A review. Transfus Med. 2014;24(5):260-268. [DOI] [PubMed] [Google Scholar]
- 2.Kaushansky K, Broudy VC, Lin N, et al. Thrombopoietin, the Mp1 ligand, is essential for full megakaryocyte development. Proc Natl Acad Sci USA. 1995;92(8):3234-3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lok S, Kaushansky K, Holly RD, et al. Cloning and expression of murine thrombopoietin cDNA and stimulation of platelet production in vivo. Nature. 1994;369(6481):565-568. [DOI] [PubMed] [Google Scholar]
- 4.Kuter DJ, Beeler DL, Rosenberg RD. The purification of megapoietin: a physiological regulator of megakaryocyte growth and platelet production. Proc Natl Acad Sci USA. 1994;91(23):11104-11108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi ES, Nichol JL, Hokom MM, Hornkohl AC, Hunt P. Platelets generated in vitro from proplatelet-displaying human megakaryocytes are functional. Blood. 1995;85(2):402-413. [PubMed] [Google Scholar]
- 6.Reems J-A, Pineault N, Sun S. In vitro megakaryocyte production and platelet biogenesis: state of the art. Transfus Med Rev. 2010;24(1):33-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avanzi MP, Mitchell WB. Ex vivo production of platelets from stem cells. Br J Haematol. 2014;165(2):237-247. [DOI] [PubMed] [Google Scholar]
- 8.Matsunaga T, Tanaka I, Kobune M, et al. Ex vivo large-scale generation of human platelets from cord blood CD34+ cells. Stem Cells. 2006;24(12):2877-2887. [DOI] [PubMed] [Google Scholar]
- 9.Guerriero R, Testa U, Gabbianelli M, et al. Unilineage megakaryocytic proliferation and differentiation of purified hematopoietic progenitors in serum-free liquid culture. Blood. 1995;86(10):3725-3736. [PubMed] [Google Scholar]
- 10.Gaur M, Kamata T, Wang S, Moran B, Shattil SJ, Leavitt AD. Megakaryocytes derived from human embryonic stem cells: a genetically tractable system to study megakaryocytopoiesis and integrin function. J Thromb Haemost. 2006;4(2):436-442. [DOI] [PubMed] [Google Scholar]
- 11.Lu SJ, Li F, Yin H, et al. Platelets generated from human embryonic stem cells are functional in vitro and in the microcirculation of living mice. Cell Res. 2011;21(3):530-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambert MP, Sullivan SK, Fuentes R, French DL, Poncz M. Challenges and promises for the development of donor-independent platelet transfusions. Blood. 2013;121(17):3319-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sim X, Poncz M, Gadue P, French DL. Understanding platelet generation from megakaryocytes: implications for in vitro-derived platelets. Blood. 2016;127(10):1227-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Hayes V, Jarocha D, et al. Comparative analysis of human ex vivo-generated platelets vs megakaryocyte-generated platelets in mice: a cautionary tale. Blood. 2015;125(23):3627-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lefrançais E, Ortiz-Muñoz G, Caudrillier A, et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 2017;544(7648):105-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuentes R, Wang Y, Hirsch J, et al. Infusion of mature megakaryocytes into mice yields functional platelets. J Clin Invest. 2010;120(11):3917-3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebbe S, Boudreaux MK. Relationship of megakaryocyte ploidy with platelet number and size in cats, dogs, rabbits and mice. Comp Haematol Int. 1998;8(1):21-25. [Google Scholar]
- 18.Mattia G, Vulcano F, Milazzo L, et al. Different ploidy levels of megakaryocytes generated from peripheral or cord blood CD34+ cells are correlated with different levels of platelet release. Blood. 2002;99(3):888-897. [DOI] [PubMed] [Google Scholar]
- 19.Raslova H, Kauffmann A, Sekkaï D, et al. Interrelation between polyploidization and megakaryocyte differentiation: a gene profiling approach. Blood. 2007;109(8):3225-3234. [DOI] [PubMed] [Google Scholar]
- 20.Raslova H, Roy L, Vourc’h C, et al. Megakaryocyte polyploidization is associated with a functional gene amplification. Blood. 2003;101(2):541-544. [DOI] [PubMed] [Google Scholar]
- 21.Takayama N, Nishimura S, Nakamura S, et al. Transient activation of c-MYC expression is critical for efficient platelet generation from human induced pluripotent stem cells. J Exp Med. 2010;207(13):2817-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lannutti BJ, Blake N, Gandhi MJ, Reems JA, Drachman JG. Induction of polyploidization in leukemic cell lines and primary bone marrow by Src kinase inhibitor SU6656. Blood. 2005;105(10):3875-3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gandhi MJ, Drachman JG, Reems JA, Thorning D, Lannutti BJ. A novel strategy for generating platelet-like fragments from megakaryocytic cell lines and human progenitor cells. Blood Cells Mol Dis. 2005;35(1):70-73. [DOI] [PubMed] [Google Scholar]
- 24.Avanzi MP, Chen A, He W, Mitchell WB. Optimizing megakaryocyte polyploidization by targeting multiple pathways of cytokinesis. Transfusion. 2012;52(11):2406-2413. [DOI] [PubMed] [Google Scholar]
- 25.Lordier L, Jalil A, Aurade F, et al. Megakaryocyte endomitosis is a failure of late cytokinesis related to defects in the contractile ring and Rho/Rock signaling. Blood. 2008;112(8):3164-3174. [DOI] [PubMed] [Google Scholar]
- 26.Avanzi MP, Goldberg F, Davila J, Langhi D, Chiattone C, Mitchell WB. Rho kinase inhibition drives megakaryocyte polyploidization and proplatelet formation through MYC and NFE2 downregulation. Br J Haematol. 2014;164(6):867-876. [DOI] [PubMed] [Google Scholar]
- 27.Lordier L, Chang Y, Jalil A, et al. Aurora B is dispensable for megakaryocyte polyploidization, but contributes to the endomitotic process. Blood. 2010;116(13):2345-2355. [DOI] [PubMed] [Google Scholar]
- 28.Bain J, Plater L, Elliott M, et al. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408(3):297-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noh JY, Gandre-Babbe S, Wang Y, et al. Inducible Gata1 suppression expands megakaryocyte-erythroid progenitors from embryonic stem cells. J Clin Invest. 2015;125(6):2369-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bluteau D, Glembotsky AC, Raimbault A, et al. Dysmegakaryopoiesis of FPD/AML pedigrees with constitutional RUNX1 mutations is linked to myosin II deregulated expression. Blood. 2012;120(13):2708-2718. [DOI] [PubMed] [Google Scholar]
- 31.Kienast J, Schmitz G. Flow cytometric analysis of thiazole orange uptake by platelets: a diagnostic aid in the evaluation of thrombocytopenic disorders. Blood. 1990;75(1):116-121. [PubMed] [Google Scholar]
- 32.Sullivan SK, Mills JA, Koukouritaki SB, et al. High-level transgene expression in induced pluripotent stem cell-derived megakaryocytes: correction of Glanzmann thrombasthenia. Blood. 2014;123(5):753-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kowalska MA, Ratajczak MZ, Majka M, et al. Stromal cell–derived factor-1 and macrophage-derived chemokine: 2 chemokines that activate platelets. Blood. 2000;96(1):50-57. [PubMed] [Google Scholar]
- 34.Sim X, Jarocha D, Hayes V, et al. Identifying and enriching platelet-producing human stem cell-derived megakaryocytes using factor V uptake. Blood. 2017;130(2):192-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomer A, Harker LA, Burstein SA. Flow cytometric analysis of normal human megakaryocytes. Blood. 1988;71(5):1244-1252. [PubMed] [Google Scholar]
- 36.Schulze H, Korpal M, Hurov J, et al. Characterization of the megakaryocyte demarcation membrane system and its role in thrombopoiesis. Blood. 2006;107(10):3868-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Camire RM, Pollak ES, Kaushansky K, Tracy PB. Secretable human platelet-derived factor V originates from the plasma pool. Blood. 1998;92(9):3035-3041. [PubMed] [Google Scholar]
- 38.Gould WR, Simioni P, Silveira JR, Tormene D, Kalafatis M, Tracy PB. Megakaryocytes endocytose and subsequently modify human factor V in vivo to form the entire pool of a unique platelet-derived cofactor. J Thromb Haemost. 2005;3(3):450-456. [DOI] [PubMed] [Google Scholar]
- 39.Bergmeier W, Burger PC, Piffath CL, et al. Metalloproteinase inhibitors improve the recovery and hemostatic function of in vitro-aged or -injured mouse platelets. Blood. 2003;102(12):4229-4235. [DOI] [PubMed] [Google Scholar]
- 40.Bergmeier W, Piffath CL, Cheng G, et al. Tumor necrosis factor-α-converting enzyme (ADAM17) mediates GPIbalpha shedding from platelets in vitro and in vivo. Circ Res. 2004;95(7):677-683. [DOI] [PubMed] [Google Scholar]
- 41.Ryu KH, Chun S, Carbonierre S, et al. Apoptosis and megakaryocytic differentiation during ex vivo expansion of human cord blood CD34+ cells using thrombopoietin. Br J Haematol. 2001;113(2):470-478. [DOI] [PubMed] [Google Scholar]
- 42.Kaminska J, Klimczak-Jajor E, Skierski J, Bany-Laszewicz U. Effects of inhibitor of Src kinases, SU6656, on differentiation of megakaryocytic progenitors and activity of alpha1,6-fucosyltransferase. Acta Biochim Pol. 2008;55(3):499-506. [PubMed] [Google Scholar]
- 43.Zamora C, Cantó E, Nieto JC, et al. Binding of platelets to lymphocytes: a potential anti-inflammatory therapy in rheumatoid arthritis. J Immunol. 2017;198(8):3099-3108. [DOI] [PubMed] [Google Scholar]
- 44.Sommer A, Kordowski F, Büch J, et al. Phosphatidylserine exposure is required for ADAM17 sheddase function. Nat Commun. 2016;7:11523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geddis AE, Kaushansky K. Megakaryocytes express functional Aurora-B kinase in endomitosis. Blood. 2004;104(4):1017-1024. [DOI] [PubMed] [Google Scholar]
- 46.Normand G, King RW. Understanding cytokinesis failure. Adv Exp Med Biol. 2010;676:27-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang W, Gilligan DM, Sun S, Wu X, Reems JA. Distinct functional effects for dynamin 3 during megakaryocytopoiesis. Stem Cells Dev. 2011;20(12):2139-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drayer AL, Boer A-K, Los EL, Esselink MT, Vellenga E. Stem cell factor synergistically enhances thrombopoietin-induced STAT5 signaling in megakaryocyte progenitors through JAK2 and Src kinase. Stem Cells. 2005;23(2):240-251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.