Lay Summary
Here, we show that one species of leaf-footed bug can self-amputate an injured limb to reduce the cost of injury. Other benefits of self-amputation include escaping predation and escaping entrapment. By identifying different benefits of this behavior, we stand to gain a more comprehensive understanding of how such an extreme trait evolves.
Keywords: autotomy, Coreidae, Hemiptera, injury, natural selection, regeneration
Abstract
Autotomy, self-induced limb loss, is an extreme trait observed throughout the animal kingdom; lizards drop their tails, crickets release their legs, and crabs drop their claws. These repeated evolutionary origins suggest that autotomy is adaptive. Yet, we do not have a firm understanding of the selective pressures that promote and maintain this extreme trait. Although multiple adaptive hypotheses exist, research has generally focused on autotomy’s adaptive value as a form of predator escape. However, autotomy could also be selected to reduce the cost of an injured limb, which we investigate here. Previously, this alternative hypothesis has been challenging to directly test because when an injury occurs on an autotomizable limb, that limb is almost always dropped (i.e., autotomy is behaviorally fixed within populations). Recently, however, we have identified a species, Narnia femorata (Insecta: Hemiptera: Coreidae), where some individuals autotomize limbs in response to injury, but some do not. This natural variation allowed us to investigate both the survival costs of retaining an injured limb and the benefits of autotomizing it. In this study, we find a positive association between autotomizing injured limbs and survival, thereby quantifying a new and likely widespread benefit of autotomy—reducing the cost of injury.
INTRODUCTION
Sacrificing a limb by self-amputation (i.e., autotomy) has evolved throughout the animal kingdom despite the enormous costs associated with this behavior (Table 1). Minimally, the cost of self-amputation includes the potential loss of blood or comparable bodily fluid (Lawrence 1992; Foelix 1996; Wilkie 2001; Fleming et al. 2007) and the potential for infection (Fleming et al. 2007; Slos et al. 2009; Bely and Nyberg 2010). Additionally, autotomy comes with costs that coincide with the function of the lost limb (Maginnis 2006; Fleming et al. 2007; Tsurui et al. 2014; Domínguez et al. 2016). These costs can be especially substantial should they decrease an individual’s future reproductive success (Smith 1992; Maginnis 2006; Fleming et al. 2007). Yet, given autotomy’s evolutionary persistence (McVean 1982; Zani 1996; Fleming et al. 2007), the benefits must outweigh the substantial costs (Arnold 1984). Thus, to better understand how this extreme trait evolves, we must identify the adaptive benefits of self-induced limb loss.
Table 1.
A taxonomic overview of autotomy, including anecdotal evidence of autotomy in response to injury
| Phyla | Group | Autotomizable appendage | Autotomy to escape | Autotomy in response to injury | Citations |
|---|---|---|---|---|---|
| Coelenterata | Jellyfish | Tentacles | Yes | — | Bickell-Page & Mackie 1991 |
| Mollusca | Nudibranchs | Cerata | Yes | — | Marín & Ros 2004 |
| Bivalves | Tentacles | Yes | — | Donovan et al 2004 | |
| Squid | Tentacles | Yes | — | Bush 2012 | |
| Annelida | Earthworms | Tail | Yes | — | Fiore et al 2004 |
| Arthropoda | Spiders | Legs, pedipalps | Yes | Yes | Savory 1928, Punzo 1997 |
| Scorpions | Tail | Yes | — | Mattoni et al 2015 | |
| Crabs | Claws, legs | Yes | Yes | McVean 1973, McVean 1982 | |
| Centipedes | Legs | Yes | Yes | Lewis 1981 | |
| Crickets | Legs | Yes | — | Bateman and Fleming 2006a | |
| True bugs | Legs | Yes | Yes | Luscher 1948, Emberts et al 2016 | |
| Echinodermata | Sea stars | Arms | Yes | Yes | Glynn 1982 |
| Brittlestars | Arms | Yes | — | Wilkie 2001 | |
| Chordata | Salamanders | Tail | Yes | Yes | Wake and Dresner 1967 |
| Lizards | Tail | Yes | Yes | Elwood et al 2012; Congdon et al. 1974 | |
| Mice | Tail skin | Yes | — | Shargal et al 1999 |
One benefit of autotomy is its ability to help an individual escape predation. In this context, individuals use autotomy to break free from a predator’s grasp and, in some cases, to distract the predator. Predator distraction occurs when the predator spends time handling and/or consuming an autotomized limb as oppose to trying to catch the surviving individual. Post-autotomy tail movement (observed in some lizards and salamanders) exemplifies this benefit, as autotomized tails that wiggle have been shown to increase predator handling and consumption time, thereby allowing the individual more time to escape (Dial and Fitzpatrick 1983). Although the means of predator escape can vary, the ultimate benefit has been demonstrated in numerous taxa, including lizards (Congdon et al. 1974; Downes and Shine 2001), starfish (Bingham et al. 2000), decapods (Lawton 1989; Wasson et al. 2002), spiders (Punzo 1997; Brueseke et al. 2001), and crickets (Bateman and Fleming 2006a). However, it is important to recognize that autotomy has additional benefits beyond that of escaping predation.
Other benefits of autotomy include escaping nonpredatory entrapment (Foelix 1996) and reducing the cost of envenomation (Eisner and Camazine 1983; Ortego and Bowers 1996). Nonpredatory entrapment is frequently observed in arthropods who undergo a complex molting process (Robinson et al. 1991; Juanes and Smith 1995; Foelix 1996; Johnson and Jakob 1999; Maginnis 2008). During this process, limbs, especially elaborated and elongated ones, may get stuck and autotomy provides a viable option for escaping (Maginnis 2008). Moreover, there are also benefits of autotomy that are not related to survival. For instance, self-amputated limbs can be used as copulatory plugs to increase a male’s reproductive success (Knoflach and van Harten 2001; Knoflach 2002; Fromhage and Schneider 2006; Snow et al. 2006; Nessler et al. 2007; Uhl et al. 2009). Therefore, when considering the evolution of autotomy in a broader context, it is critically important to separate autotomy from the assumption that its sole adaptive function is to escape predation.
Another hypothesized benefit of autotomy, one that has gone untested, is that autotomy can limit damage associated with wounded body parts. In other words, if injury occurs on an autotomizable limb it is hypothesized that individuals can self-amputate (autotomize) the injured limb to reduce the cost of the injury. This hypothesis is largely inspired by physiological and behavioral observations. Physiologically, self-induced injuries (i.e., injuries induced through autotomy) are thought to quickly heal (Wake and Dresner 1967; Foelix 1996; Wilkie 2001). Therefore, if an externally induced injury was severe, self-amputating the injured limb may reduce the loss of blood and the chance of infection, ultimately increasing survival. Still, despite the anecdotal but taxonomically widespread observance of this behavior (Table 1), the benefit of autotomizing in response to injury has yet to be investigated.
For this behavior to be beneficial, the cost of the injury has to exceed the cost of autotomy. Thus, our first aim is to investigate whether injury has a higher survival and/or developmental cost than autotomy. Then, we experimentally investigate whether injured individuals can reduce this cost differential by autotomizing their damaged limb (i.e., reduce the cost of injury via autotomy).
METHODS
Study organism
To investigate whether autotomy can indeed reduce the cost of injury, we used the leaf-footed cactus bug, Narnia femorata (Insecta: Hemiptera: Coreidae; Figure 1). Previously, N. femorata has been shown to use autotomy to escape from entrapment (Emberts et al. 2016). Furthermore, the behavior normally occurs within 60 s suggesting that individuals could also use this trait to escape from predation (Emberts et al. 2016). However, the role autotomy plays in reducing the cost of injury remains unclear.
Figure 1.
A juvenile Narnia femorata.
In Hemipterans, potential responses to limb injury include autotomizing the injured limb or (retaining and) regenerating it, but not both (Luscher 1948; Shaw and Bryant 1974). In the case of autotomy, the limb is dropped at the trochanter-femur joint (Luscher 1948; Emberts et al. 2016), a location from which regeneration has not been shown to occur (Luscher 1948; Shaw and Bryant 1974). The alternative, regeneration, is only available to individuals who retain (i.e., do not autotomize) their damaged limb and have molts remaining to regrow the lost structure (i.e., juveniles). Furthermore, the regenerative capabilities are quite limited as juveniles have only been shown to partially regenerate their tibia and tarsi (Luscher 1948; Shaw and Bryant 1974). Consequently, injury location may factor into an individual’s decision to autotomize or retain an injured limb. Additionally, for N. femorata, the loss of a male’s hind leg may have costly implications for reproductive success, as males use their hind legs in intrasexual competition (Procter et al. 2012). Thus, our study takes sex, injury location, and the ability to regenerate into consideration.
Study design
Insect rearing
For our experiments, we used first-generation lab-reared individuals. The populations were founded in November of 2015 with 29 mating pairs collected from Live Oak, Florida (30.26°N, −83.18°W). Individuals were reared in deli cups containing Opuntia mesacantha subsp. lata cladodes (cactus pads) and fruit collected from the same location throughout the experiment. Before experimentation, individuals were reared with siblings in a greenhouse (set temperature: 21–32°C, and photoperiod: 14:10 h L:D) until their second instar.
Experiment 1
To investigate how injury and/or autotomy affects survival and development (e.g., time to reach adulthood, regeneration, and terminal body size) we randomly assigned second instar juveniles with all of their legs to one of six treatments (final sample sizes ranged from 19 to 25 per treatment): 1) control (no injury/no autotomy), 2) experimentally induced autotomy at the trochanter-femur joint, 3) incision (i.e., cut completely through the leg) at the trochanter-femur joint (henceforth referred to as Injury A to reflect that this experimentally induced injury occurred at the same location as self-induced autotomy), 4) incision at the femur-tibia joint (henceforth referred to as Injury 1), 5) incision through the middle of the tibia (henceforth referred to as Injury 2), and 6) incision at the tibia-tarsus joint (henceforth referred to as Injury 3; Figure 2). We only used individuals with all of their legs because limb loss has been shown to affect the propensity to autotomize additional limbs (Bateman and Fleming 2005). Autotomy was induced by gripping the insect’s right hind femur with reverse-action forceps while the insect was in contact with a piece of wood (38 × 44 × 305 mm; Emberts et al. 2016). For a comparative baseline, individuals in the control (no injury/no autotomy) treatment underwent a sham autotomy protocol (i.e., their legs were held for a shorter amount of time (1 s) with reverse-action forceps), but were not induced to autotomize. For the remaining treatments (e.g., injury A, 1, 2, and 3), injury was induced with iridectomy scissors at the specified location following previous regeneration protocols in other species (Luscher 1948; Shaw and Bryant 1974). After an individual’s respective procedure, it was moved into its own deli cup and placed into an incubator (temperature: 32°C, photoperiod: 14:10 h L:D). As an ethical note, animals were treated as humanely as possible by inducing injury in a disinfected environment and by providing them with species-specific optimal living conditions. Individuals were monitored daily (maximum 32 days) for developmental rate (i.e., molt timing), limb loss, and death. On becoming an adult, individuals were sexed, and their body and legs were photographed using a Canon EOS 50D digital camera attached to a Leica M165 C dissecting microscope. Pronotal width (a body size metric) and hind leg length were measured to the nearest micrometer using ImageJ v1.46.
Figure 2.
The right hind leg of a juvenile N. femorata, depicting the location of each injury site.
Experiment 2
To experimentally test if autotomizing an injured leg increases survival, we randomly assigned juveniles (second instars) into 1 of 3 treatment groups in which the right hind leg was 1) experimentally induced to autototomize (n = 38), 2) injured then experimentally induced to autotomize (n = 39), or 3) injured without experimental autotomy (n = 40). This experiment involved 2 stages separated by 1 hour. First, injury was induced in treatments 2 and 3 at the femur-tibia joint with disinfected iridectomy scissors. Individuals in treatment 1 were handled in the same manner, but injury was not induced. If unplanned autotomy occurred due to handling, the individual was removed from the experiment and replaced. In the second stage (1 hour later), individuals in treatments 1 and 2 were induced to autotomize their right hind leg with reverse-action forceps, whereas individuals in treatment 3 underwent a sham autotomy protocol, as detailed above for Experiment 1. After treatment manipulations, each individual was placed into a separate deli cup with O. mesacantha subsp. lata fruit and water and moved into an incubator (temperature: 32°C, photoperiod: 14:10 h L:D). Individuals were checked at 12 h intervals over 48 h and survival and limb loss were recorded.
Data and statistical analyses
Experiment 1
To investigate the effect injury has on survivorship (live/die), we conducted planned contrasts in the context of a binary, generalized linear mixed model with family as a random factor (GLMM; logit-link function assuming a binomial distribution). Since we hypothesized that injury would have a negative effect on survivorship regardless of injury site, we contrasted all of our injury treatments (injury A, 1, 2, and 3) against the control treatment (treatment 1). Using this same approach (i.e., binary GLMM using contrasts), we also investigated the effect autotomy has on survivorship by contrasting the autotomy treatment versus the control treatment. Finally, we contrasted autotomy and injuries, to evaluate whether their effects differed. Comparable analyses were done, using a GLMM with contrasts (identity-link function assuming a Gaussian distribution), to investigate whether injury and/or autotomy affected the number of days to reach adulthood or terminal body size.
To investigate whether autotomizing an injured limb resulted in higher survivorship, we compared the survival of those that autotomized their injured limbs versus those that retained them using a binary GLMM (logit-link function with assumed binomial distribution) for all 3 injury treatments combined (injury 1, 2, and 3). We excluded injury A from this and subsequent analyses because we could not determine whether individuals in this treatment autotomized their injured limb; as injury was induced at the same location where autotomy occurs. Similarly, to see whether autotomizing an injured limb affects the time to reach adulthood and/or terminal body size, we compared these metrics for individuals that retained their injured limbs versus those that autotomized their injured limb using a GLMM (identity-link function assuming a Gaussian distribution). We also investigated, using a binary GLMM (logit-link function with assumed binomial distribution), whether injury site or sex could explain any variation in the propensity to autotomize.
By using landmark locations on the legs (e.g., joints), and by measuring leg length from these landmarks, we were also able to quantify regenerative ability. If there was no growth beyond an injury site, then we classified the injury as nonregenerative.
Experiment 2
Our goal with the second experiment was to compare the probability of survival for those retaining injured limbs, autotomizing injured limbs, and autotomizing uninjured limbs. However, injured individuals in the “retain injured limb treatment” cannot be prevented from self-autotomizing their damaged limbs. Thus, we proceeded with a series of analyses designed to test differences across and within our treatment groups. For these tests, we used binary GLMMs (logit-link function with assumed binomial distribution) and, where relevant, used contrasts in our models to compare groups of interest for which we had developed a priori hypotheses of their relationships.
Across both experiments, sample sizes varied because we were unable to retrieve some individuals (n = 15) at the end of the experiment. Although there are multiple reasons that might explain how these individuals were lost, we believe that most of these individuals became buried in the soil on death, making it extremely challenging to locate them. Still, we took the most conservative approach and excluded these individuals from our analyses. However, even making the reasonable assumption that all 15 of these individuals died, excluding or including these individuals had no effect on our conclusions.
RESULTS
Experiment 1
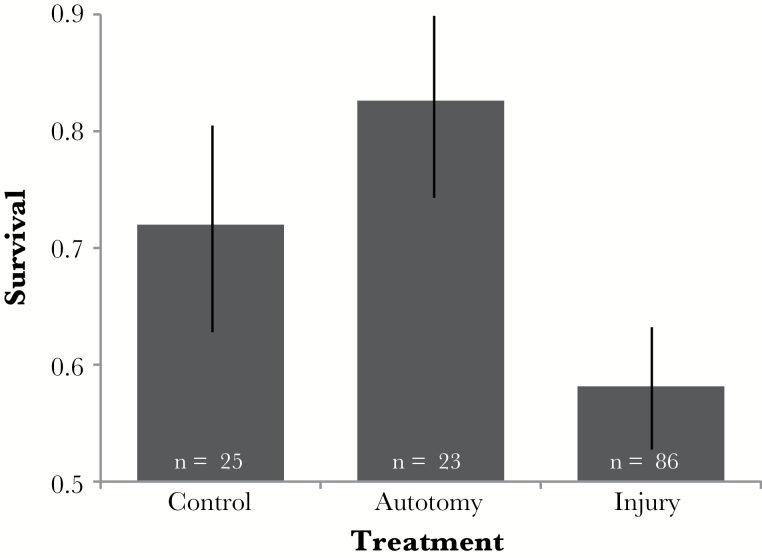
We first compared survival for injured insects (injury locations A, 1, 2, and 3), insects experiencing experimentally induced autotomy, and individuals in our control group. We found that injured insects had approximately 25% lower survival on average than those that were experimentally induced to autotomize (GLMM with a priori contrasts: χ2 = 5.43, df = 1, P = 0.020, Figure 3). Insects that were not injured and not experimentally induced to autotomize (control group) did not differ in survival relative to the injured insects (binary GLMM with a priori contrasts: χ2 = 1.64, df = 1, P = 0.200, Figure 3) or those experiencing induced autotomy (binary GLMM with a priori contrasts: χ2 = 0.48, df = 1, P = 0.476, Figure 3). When we compared terminal body size and the number of days it took to reach adulthood across our contrasted treatments we did not find any significant differences (Table 2).
Figure 3.
Experiment 1—contrast of treatments to investigate the effects of autotomy and injury on the proportion of individuals (±SE) surviving to adulthood.
Table 2.
Experiment 1—developmental differences between autotomy, injury, and our control (no autotomy/no injury)
| χ 2 | df | P | |
|---|---|---|---|
| Days until adulthood | |||
| Autotomy vs. control | 0.285 | 1 | 0.593 |
| Injury vs. control | 1.265 | 1 | 0.261 |
| Autotomy vs. injury | 3.37 | 1 | 0.067 |
| Terminal body size (PW) | |||
| Autotomy vs. control | 1.2 | 1 | 0.274 |
| Injury vs. control | 0.992 | 1 | 0.319 |
| Autotomy vs. injury | 0.223 | 1 | 0.637 |
PW, pronotal width.
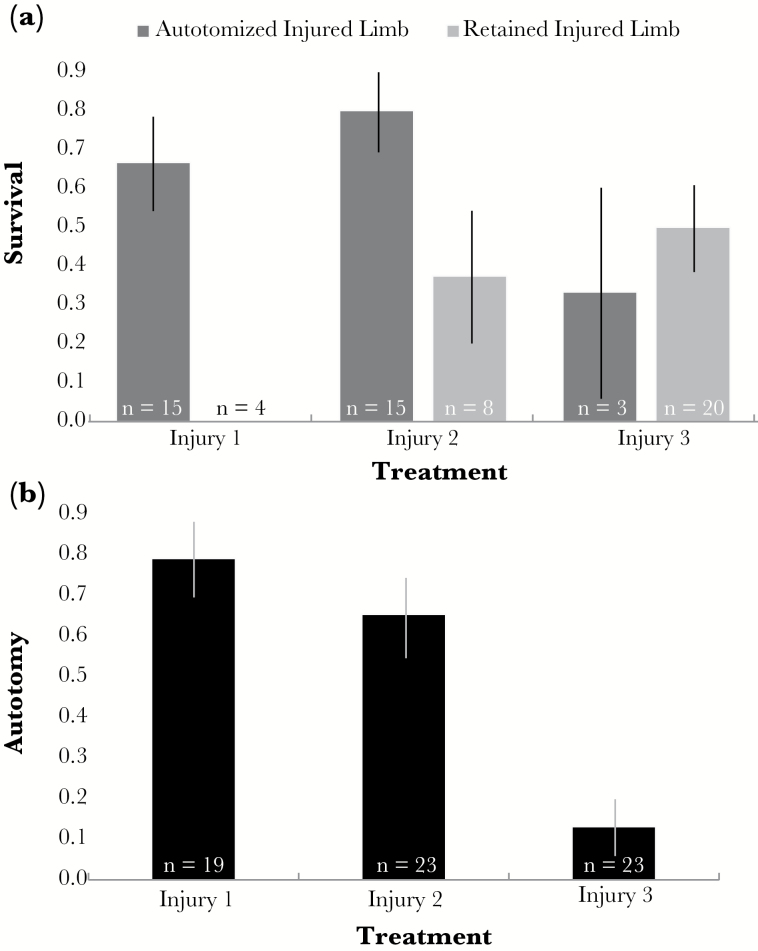
For all of the injury treatments where autotomy was possible (injury 1, 2, and 3), a large fraction (50.7%) of individuals responded to their injury by autotomizing. In general, individuals who autotomized their injured limb had higher survival than those that retained their injured limb (binary GLMM: χ2 = 5.67, df = 1, P = 0.017), but the location of the injury also tended to affect this benefit (binary GLMM: χ2 = 5.53, df = 2, P = 0.063). In particular, autotomy of limbs injured at the femur-tibia joint (injury 1) and tibia (injury 2) led to higher survival, whereas autotomy after an injury on the tibia-tarsus joint (injury 3) did not (Figure 4).
Figure 4.
Experiment 1—effect of injury location on autotomy and survival. (a) Depicts the proportion of individuals (±SE) that survived based on their behavioral decision to autotomize or retain their injured limb for each injury location. (b) Illustrates the variation in the proportion of individuals (±SE) that autotomized at each injury location. Individuals in the injury 3 treatment had a significantly lower propensity to autotomize then those in the injury 1 and injury 2 treatments. Furthermore, autotomizing limbs injured at the injury 3 location did not increase survival.
Injury location also had an effect on the propensity to autotomize (GLMM: χ2 = 23.03, df = 2, P < 0.001; Figure 4). Specifically, individuals injured at the tibia-tarsus joint (injury 3) were significantly less likely to autotomize than individuals injured at the femur-tibia joint (injury 1; GLMM: χ2 = 14.79, df = 1, P < 0.001) and individuals injured through their tibia (injury 2; GLMM: χ2 = 20.04, df = 1, P < 0.001; Figure 4). Sex did not explain any of the variation in the expression of autotomy (GLMM: χ2 = 0.07, df = 1, P = 0.793).
Of those individuals that retained their injured limb, less than half (40.6%) survived to adulthood. Compared to those that autotomized their injured limbs, those that retained them required fewer days to reach adulthood (Table 3) and showed some form of regeneration. Individuals who retained their injured limb in the injury 3 treatment regenerated their first tarsal segment, whereas individuals in the injury 2 treatment regenerated both their tibia and their first tarsal segment. The regenerated tarsi in both treatments were 55% shorter than our control (Supplementary Table S1). Thus, we conclude that N. femorata has partial regenerative capabilities. None of the individuals that retained their injured limb in the injury 1 treatment survived to adulthood (0 out of 4); therefore, we were unable to quantify the potential for regeneration from this injury location.
Table 3.
Experiment 1—developmental differences between self-autotomizing and retaining an injured limb
| χ 2 | df | P | |
|---|---|---|---|
| Days until adulthood | |||
| Autotomy | 9.085 | 1 | 0.003 |
| Injury location | 3.445 | 2 | 0.179 |
| Autotomy × injury location | 16.404 | 1 | <0.001 |
| Terminal body size (PW) | |||
| Autotomy | 3.554 | 1 | 0.059 |
| Injury location | 1.350 | 2 | 0.509 |
| Autotomy × injury location | 4.171 | 1 | 0.041 |
We investigated how injury location, the decision to autotomize, and their interaction affected the number of days it took a juvenile to reach adulthood and terminal body size. Means and standard errors are reported in Supplementary Table 2. PW, pronotal width.
Experiment 2
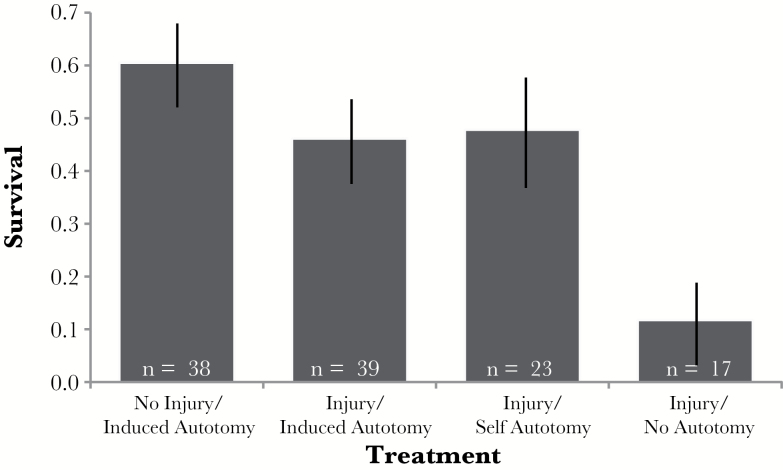
Experiment 2 involved 3 treatment groups: 1) experimentally induced autotomy of a noninjured limb, 2) experimentally induced autotomy of an injured limb, and 3) injured without experimental autotomy. When we compared treatment 2 to treatment 3, we did not find that experimental autotomy of injured limbs significantly increased survival (contrasted GLMM: χ2 = 1.58, df = 1, P = 0.209). However, over half (57.50%) of the individuals in treatment 3 self-autotomized their injured limb. This difference in behavior allowed us to additionally consider the behavioral decision to self-autotomize an injured limb. Those that self-autotomized their injured limb had higher survivorship than those that maintained their injured limb (GLMM: χ2 = 6.10, df = 1, P = 0.014; Figure 5). Furthermore, those that self-autotomized their injured limbs and those that were experimentally induced to autotomize their injured limbs had similar survivorship (contrasted GLMM: χ2 < 0.001, df = 1, P = 0.985; Figure 5), as we hypothesized. Thus, we compared all the individuals that autotomized their injured limb, whether it was self-induced or experimentally induced, to those individuals who maintained their injured limb; we found that those who autotomized their injured limb had significantly higher survival than those who did not (contrasted GLMM: χ2 = 4.02, df = 1, P = 0.045; Figure 5).
Figure 5.
Experiment 2—proportion of individuals (±SE) that survived in each treatment based on their autotomy behavior. In treatments where autotomy was experimentally induced, individuals did not have a behavioral choice. However, when only injury was induced, an individual could have self-autotomized or retained (no autotomy) the injured limb.
DISCUSSION
Here, we have shown that autotomy can reduce the cost of injury. Autotomy after injury has been observed across taxa (Table 1), but the benefits of the behavior have only been assumed, not tested (Savory 1928; Lewis 1981; Glynn 1982; Bulliére and Bulliére 1985; Johnson and Jakob 1999; Bingham et al. 2000; Ramsay et al. 2001). Thus, this study is the first to provide evidence of a novel benefit of autotomy—reducing the (survival) cost of injury. Other widespread benefits of autotomy include escaping predation (Congdon et al. 1974; Carlberg 1986; Lawton 1989; Punzo 1997; Bingham et al. 2000; Brueseke et al. 2001; Downes and Shine 2001; Sword 2001; Wasson et al. 2002; Bateman and Fleming 2006b) and escaping nonpredatory entrapment (Robinson et al. 1991; Juanes and Smith 1995; Foelix 1996; Johnson and Jakob 1999; Maginnis 2008). Our results, and others, highlight that there are multiple benefits of autotomy, which may select for and maintain the trait. As this evidence grows it becomes crucial that we abandon the assumption that autotomy’s sole, or even primary, adaptive benefit is escaping predation. By doing so, we stand to gain a more comprehensive understanding of how such an extreme trait evolves.
Another major implication of this study is that autotomy is less costly than injury, but only with respect to survival as we did not find injury to have an effect on the time to reach adulthood nor terminal body size. Regardless, these results highlight that autotomy and injury should not be considered synonymous. Specifically, autotomy is self-induced, or self-controlled (Fleming et al. 2007), injury. Recognizing this distinction is vital to understanding how autotomy can reduce the costs of injury. To elaborate, injuries, whether self-induced or externally induced, can result in blood loss and infection, both of which may ultimately result in death. Thus, there should be selection to minimize these effects, such as selection on an immune system (Medzhitov and Janeway 1997; Cooper and Alder 2006; Cerenius and Söderhäll 2011). However, what differentiates self-controlled injury from externally induced injury is that self-controlled injury can consistently occur at a very precise location. This consistency allows selection, over time, to potentially act on morphology to reduce the risk of infection and the loss of blood. Consequently, self-induced injury at a predetermined breakage plane may be less severe then externally induced injury. Previous studies have noted that self-induced injuries (i.e., due to autotomy) quickly seal and result in negligible amounts of blood loss (Wake and Dresner 1967; Foelix 1996; Wilkie 2001). However, the differences in blood loss and immune response between autotomy and externally induced injury have yet to be measured. Although we have not quantified these effects, the differences in survival observed in our study likely result from such differences.
The benefit of autotomizing an injured limb appears to vary by injury location. For example, we did not find a survival benefit of autotomy if the injury occurred at the tibia-tarsus joint, our most distal injury. This lack of a benefit might explain why insects experiencing injuries at that site rarely autotomized their limb. By retaining their injured limb, individuals could regenerate part of their tarsus and have a resulting hind leg that was only 19% shorter than our comparative baseline (Supplementary Table S1). This pattern highlights the likely trade-off individuals face between autotomizing an injured limb and retaining it. That is to say, when autotomizing a limb does not increase survival (i.e., the severity of the injury is minimal), few individuals should autotomize their limbs. However, when autotomy does increase survival (i.e., the injury is severe) individuals should readily drop their limb, even though it comes with the cost of permanently losing their leg.
Although injury location influenced the tendency to autotomize injured limbs, sex did not. In N. femorata male hind legs have been shown to function as sexually selected weapons (Procter et al. 2012; Nolen et al. 2017). Thus, the permanent loss of a male hind leg potentially comes with a larger cost than the loss of a female hind leg. In previous studies, when the costs and benefits of autotomy differ between the sexes there is often a corresponding difference in the propensity to autotomize (Fox et al. 1998; Wasson and Lyon 2005). For our study, however, there are several possible explanations for why we did not observe such differences. First, it is possible that the loss of a hind leg comes with an equal cost to males and females, as males may compensate, behaviorally (e.g., Berzins and Caldwell 1983) and/or morphologically (e.g., Simmons and Emlen 2006), for the loss of their weapon, an intriguing future direction. Second, it is also possible that there was a sex difference in the propensity to autotomize (that occurred on the scale of seconds, minutes, or hours), but because of our experimental design we were unable to detect the difference. Still, even if such a difference existed, sex did not ultimately affect whether or not an individual autotomized their injured limb.
Additionally, in our study system, the consequences of autotomy and regeneration are not confounded as individuals may only autotomize or regenerate their injured limb, but not both. In other arthropods, autotomy (at a preformed breakage plane) often precedes regeneration. Therefore, in some instances, it can be challenging to differentiate consequences of regeneration from consequences of autotomy. One of these challenges is determining whether regeneration and/or autotomy alters developmental time. In arthropods, regeneration (preceded by autotomy) is often shown to increase the amount of time it takes to develop (Maginnis 2006). However, it is possible that this developmental delay is a consequence of autotomy, not regeneration. With our study species, we are presented with a unique opportunity to separately investigate the consequences of autotomy and (independently) the consequences of regeneration. In N. femorata, autotomy had no effect on the number of days it took to reach adulthood (Table 2, autotomy vs. control). However, regeneration did. When comparing those that regenerated their injured limbs (without being preceded by autotomy) to those that autotomized their injured limbs (without being followed by regeneration), we found that individuals who regenerated had shorter intermolt intervals (i.e., they developed from 3rd instars to adults more quickly). In Hemipterans, and other arthropods, regeneration coincides with molting. Consequently, by decreasing intermolt intervals an individual may be able to replace its missing limb more quickly (Maginnis 2006). These results could be interpreted to mean that regeneration accelerates development in N. femorata. However, it is also important to note that the data set from which we drew these conclusions inherently excluded individuals that did not survive to adulthood, and thereby disproportionally excluding individuals that retained their injured limb (Figure 4a). Thus, these results could also reflect that only quickly developing individuals can retain an injured limb (with subsequent regeneration) and survive until adulthood.
Our second experiment, although it did not fully demonstrate cause and effect, provides further support that autotomy of injured limbs increases survival. As with experiment 1, we found a positive association between self-autotomizing injured limbs and survival. This result could reflect (as we have postulated) that autotomizing injured limbs increases survival. However, because this result is correlative, it could also suggest that high-quality individuals (i.e., those predisposed to higher survival) are more likely to autotomize their injured limb. Thus, in our second experiment, we induced autotomy to directly investigate these alternatives. We found that experimentally inducing autotomy had the same effect as self-induced autotomy. This similarity suggests that the patterns of survivorship we observed are not due to variation in individual quality, but instead stem from autotomy of injured limbs; thereby strongly supporting the hypothesis that autotomy can indeed reduce the cost of injury.
In conclusion, the results of this study are the first to provide evidence that autotomy can reduce the cost of injury. Specifically, here, we observed a survival difference between individuals that autotomized their injured limb and those that retained it. Furthermore, in our second experiment, we observed this survival difference just 48 h post-injury, suggesting a relatively immediate benefit to autotomizing injured limbs. However, it is also possible that autotomizing injured limbs comes with long-term benefits too. For example, if a limb is severely damaged, an individual may be able to reduce the metabolic cost of carrying around a lame limb by autotomizing it. Moreover, if the species can regenerate, autotomizing a lame limb may promote the growth of a new, functional one. Such benefits of autotomizing injured limbs are not necessarily mutually exclusive alternatives. Instead, we hypothesize that these potential benefits may additionally contribute to the selection and maintenance of autotomy. Thus, to gain a better understand how this extreme trait evolves, we must continue to identify the adaptive benefits of self-inducing limb loss.
SUPPLEMENTARY MATERIAL
Supplementary data are available at Behavioral Ecology online.
Supplementary Material
Acknowledgments
We would like to thank P. Carlson, I. Meirom, and S. Abdullaj for all their help collecting and rearing coreids. We would also like to thank C. Howard, M. Forthman, and 2 anonymous reviewers for their comments on previous versions of the manuscript. Finally, we would like to thank the Suwannee High Future Farmers of America Chapter for allowing us to collect insects on their property. This work was supported by the National Science Foundation (grants IOS-0926855 and IOS-1553100 to C.W.M.). Publication of this article was funded in part by the University of Florida Open Access Publishing Fund.
Data accessibility: analyses reported in this article can be reproduced using the data provided by Emberts et al. (2017).
REFERENCES
- Arnold EN. 1984. Evolutionary aspects of tail shedding in lizards and their relatives. J Nat Hist. 18:127–169. [Google Scholar]
- Bateman PW, Fleming PA. 2005. Direct and indirect costs of limb autotomy in field crickets, Gryllus bimaculatus. Anim Behav. 69:151–159. [Google Scholar]
- Bateman PW, Fleming PA. 2006a. Increased susceptibility to predation for autotomized house crickets (Acheta domestica). Ethology. 112:670–677. [Google Scholar]
- Bateman PW, Fleming PA. 2006b. Sex and the single (-eared) female: leg function, limb autotomy and mating history trade-offs in field crickets (Gryllus bimaculatus). Biol Lett. 2:33–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bely AE, Nyberg KG. 2010. Evolution of animal regeneration: re-emergence of a field. Trends Ecol Evol. 25:161–170. [DOI] [PubMed] [Google Scholar]
- Berzins IK, Caldwell RL. 1983. The effect of injury on the agonistic behavior of the Stomatopod, Gonodactylus Bredini (manning). Mar Behav Physiol. 10:83–96. [Google Scholar]
- Bickell-Page LR, Mackie GO. 1991. Tentacle autotomy in the hydromedusa Aglantha digitale (Cnidaria): an ultrastructural and neurophysiological analysis. Philos Trans Biol Sci. 331:155–170. [Google Scholar]
- Bingham BL, Burr J, Head HW. 2000. Causes and consequences of arm damage in the sea star Leptasterias hexactis. Can J Zool. 78:596–605. [Google Scholar]
- Brueseke MA, Rypstra AL, Walker SE, Persons MH. 2001. Leg autotomy in the wolf spider Pardosa milvina: a common phenomenon with few apparent costs. Am Midl Nat. 146:153–160. [Google Scholar]
- Bulliére D, Bulliére F. 1985. Regeneration. In: Kerkut GA, Gilbert LI, editors. Comprehensive insect physiology, biochemistry and pharmacology 2. Oxford: Pergamon Press; p. 371–424. [Google Scholar]
- Bush SL. 2012. Economy of arm autotomy in the mesopelagic squid Octopoteuthis deletron. Mar Ecol Prog Ser. 458:133–140. [Google Scholar]
- Carlberg U. 1986. Thanatosis and autotomy as defence in Baculum sp. 1 (Insecta: Phasmida). Zool Anz. 217:39–53. [Google Scholar]
- Cerenius L, Söderhäll K. 2011. Coagulation in invertebrates. J Innate Immun. 3:3–8. [DOI] [PubMed] [Google Scholar]
- Congdon JD, Vitt LJ, King WW. 1974. Geckos: adaptive significance and energetics of tail autotomy. Science. 184:1379–1380. [DOI] [PubMed] [Google Scholar]
- Cooper MD, Alder MN. 2006. The evolution of adaptive immune systems. Cell. 124:815–822. [DOI] [PubMed] [Google Scholar]
- Dial BE, Fitzpatrick LC. 1983. Lizard tail autotomy: function and energetics of postautotomy tail movement in Scincella lateralis. Science. 219:391–393. [DOI] [PubMed] [Google Scholar]
- Domínguez M, Escalante I, Carrasco-Rueda F, Figuerola-Hernández CE, Ayup MM. 2016. Losing legs and walking hard: effects of autotomy and different substrates in the locomotion of harvestmen in the genus Prionostemma. BioOne. 44:76–82. [Google Scholar]
- Donovan DA, Elias JP, Baldwin J. 2004. Swimming behavior and morphometry of the file shell Limaria fragilis. Mar Freshw Behav Physiol. 37:7–16. [Google Scholar]
- Downes S, Shine R. 2001. Why does tail loss increase a lizard’s later vulnerability to snake predators? Ecology. 82:1293–1303. [Google Scholar]
- Eisner T, Camazine S. 1983. Spider leg autotomy induced by prey venom injection: an adaptive response to “pain”? Proc Natl Acad Sci USA. 80:3382–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwood C, Pelsinski J, Bateman PW. 2012. Anolis sagrei (Brown Anole). Voluntary autotomy. Herpetol Rev. 43:642. [Google Scholar]
- Emberts Z, Miller C, Kiehl D, St Mary C. 2017. Data from: cut your losses: self-amputation of injured limbs increases survival. Dryad Digital Repository. http://dx.doi.org/10.5061/dryad.65n35. [DOI] [PMC free article] [PubMed]
- Emberts Z, St Mary CM, Miller CW. 2016. Coreidae (Insecta: Hemiptera) limb loss and autotomy. Ann Entomol Soc Am. 109:678–683. [Google Scholar]
- Fiore C, Tull JL, Zehner S, Ducey PK. 2004. Tracking and predation on earthworms by the invasive terrestrial planarian Bipalium adventitium (Tricladida, Platyhelminthes). Behav Processes. 67:327–334. [DOI] [PubMed] [Google Scholar]
- Fleming PA, Muller D, Bateman PW. 2007. Leave it all behind: a taxonomic perspective of autotomy in invertebrates. Biol Rev Camb Philos Soc. 82:481–510. [DOI] [PubMed] [Google Scholar]
- Foelix RF. 1996. Biology of Spiders. New York: Oxford University Press. [Google Scholar]
- Fox SF, Conder JM, Smith AE. 1998. Sexual dimorphism in the ease of tail autotomy: Uta stansburiana with and without previous tail loss. Copeia. 1998:376–382. [Google Scholar]
- Fromhage L, Schneider JM. 2006. Emasculation to plug up females: the significance of pedipalp damage in Nephila fenestrata. Behav Ecol. 17:353–357. [Google Scholar]
- Glynn PW. 1982. Acanthaster population regulation by a shrimp and a worm. Proc Fourth Int Coral Reef Sym. 2:607–612. [Google Scholar]
- Johnson SA, Jakob EM. 1999. Leg autotomy in a spider has minimal costs in competitive ability and development. Anim Behav. 57:957–965. [DOI] [PubMed] [Google Scholar]
- Juanes F, Smith LD. 1995. The ecological consequences of limb damage and loss in decapod crustaceans: a review and prospectus. J Exp Mar Bio Ecol. 193:197–223. [Google Scholar]
- Knoflach B. 2002. Copulation and emasculation in Echinotheridion gibberosum (Kulczynski, 1899) (Araneae, Theridiidae). Eur Arachnol. 2000:139–144. [Google Scholar]
- Knoflach B, van Harten A. 2001. Tidarren argo sp. nov. (Araneae: Theridiidae) and its exceptional copulatory behaviour: emasculation, male palpal organ as a mating plug and sexual cannibalism. J Zool. 254:449–459. [Google Scholar]
- Lawrence J. 1992. Arm loss and regeneration in Asteroidea (Echinodermata). In: Scalera-Liaci L, Canicatti C, editors. Echinoderm Research. Rotterdam, the Netherlands: A.A. Balkema; p. 39–52. [Google Scholar]
- Lawton P. 1989. Predatory interaction between the brachyuran crab Cancer Pagurus and Decapod Crustacean prey. Mar Ecol Ser. 52:169–179. [Google Scholar]
- Lewis JGE. 1981. The Biology of Centipedes. Cambridge: Cambridge University Press. [Google Scholar]
- Luscher M. 1948. The regeneration of legs in Rhodnius prolixus (Hempiptera). J Exp Biol. 25:334–343. [DOI] [PubMed] [Google Scholar]
- Maginnis TL. 2006. The costs of autotomy and regeneration in animals: a review and framework for future research. Behav Ecol. 17:857–872. [Google Scholar]
- Maginnis TL. 2008. Autotomy in a stick insect (Insecta: Phasmida): predation versus molting. Florida Entomol. 91:126–127. [Google Scholar]
- Marín A, Ros J. 2004. Chemical defenses in Sacoglossan Opisthobranchs: taxonomic trends and evolutive implications. Sci Mar. 68:227–241. [Google Scholar]
- Mattoni CI, García-Hernández S, Botero-Trujillo R, Ochoa JA, Ojanguren-Affilastro AA, Pinto-da-Rocha R, Prendini L. 2015. Scorpion sheds ‘tail’ to escape: consequences and implications of autotomy in scorpions (Buthidae: Ananteris). PLoS One. 10:e0116639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVean A. 1982. Autotomy. In: Sandeman DC, Atwood HL, editors. The Biology of Crustacea: Volume 4: Neural Integration and Behavior. New York: Academic Press; p. 107–132. [Google Scholar]
- McVean AR. 1973. Autotomy in Cavcinus maenas (Decapoda: Crustacea). J Zool Proc Zool Soc London. 169:349–364. [Google Scholar]
- Medzhitov R, Janeway CA., Jr 1997. Innate immunity: minireview the virtues of a nonclonal system of recognition. Cell. 91:295–298. [DOI] [PubMed] [Google Scholar]
- Nessler SH, Uhl G, Schneider JM. 2007. Genital damage in the orb-web spider Argiope bruennichi (Araneae: Araneidae) increases paternity success. Behav Ecol. 18:174–181. [Google Scholar]
- Nolen ZJ, Allen PE, Miller CW. 2017. Seasonal resource value and male size influence male aggressive interactions in the leaf footed cactus bug, Narnia femorata. Behav Processes. 138:1–6. [DOI] [PubMed] [Google Scholar]
- Ortego F, Bowers WS. 1996. Induction of autotomy in the American bird grasshopper Schistocerca americana (Drury) by the ecdysone agonist RH-5849 and investigation of its mode of action. Experientia. 52:42–50. [Google Scholar]
- Procter DS, Moore AJ, Miller CW. 2012. The form of sexual selection arising from male-male competition depends on the presence of females in the social environment. J Evol Biol. 25:803–812. [DOI] [PubMed] [Google Scholar]
- Punzo F. 1997. Leg autotomy and avoidance behavior in response to a predator in the wolf spider, Schizocoa avida (Araneae, Lycosidae). Am Arachnol Soc. 25:202–205. [Google Scholar]
- Ramsay K, Kaiser MJ, Richardson CA. 2001. Invest in arms: behavioural and energetic implications of multiple autotomy in starfish (Asterias rubens). Behav Ecol Sociobiol. 50:360–365. [Google Scholar]
- Robinson JV, Shaffer LR, Hagemier DD, Smatresk NJ. 1991. The ecological role of caudal lamellae loss in the larval damselfly, Ischnura posita (Hagen) (Odonata: Zygoptera). Oecologia. 87:1–7. [DOI] [PubMed] [Google Scholar]
- Savory TH. 1928. The Biology of Spiders. New York: Macmillan. [Google Scholar]
- Shargal E, Rath-Wolfson L, Kronfeld N, Dayan T. 1999. Ecological and histological aspects of tail loss in spiny mice (Rodentia: Muridae, Acomys) with a review of its occurrence in rodents. J Zool. 249:187–193. [Google Scholar]
- Shaw VK, Bryant PJ. 1974. Regeneration of appendages in the large milkweed bug, Oncopeltus fasciatus. J Insect Physiol. 20:1849–1857. [DOI] [PubMed] [Google Scholar]
- Simmons LW, Emlen DJ. 2006. Evolutionary trade-off between weapons and testes. Proc Natl Acad Sci USA. 103:16346–16351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slos S, De Block M, Stoks R. 2009. Autotomy reduces immune function and antioxidant defence. Biol Lett. 5:90–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LD. 1992. The impact of limb autotomy on mate competition in blue crabs Callinectes sapidus Rathbun. Oecologia. 89:494–501. [DOI] [PubMed] [Google Scholar]
- Snow LSE, Abdel-Mesih A, Andrade MCB. 2006. Broken copulatory organs are low-cost adaptations to sperm competition in redback spiders. Ethology. 112:379–389. [Google Scholar]
- Sword GA. 2001. Tasty on the outside, but toxic in the middle: grasshopper regurgitation and host plant-mediated toxicity to a vertebrate predator. Oecologia. 128:416–421. [DOI] [PubMed] [Google Scholar]
- Tsurui K, Narita S, Iwatani Y, Honma A. 2014. Change in take-off elevation angle after limb autotomy mitigates the reduction in jumping distance in rice grasshoppers Oxya yezoensis. Entomol Sci. 17:181–190. [Google Scholar]
- Uhl G, Nessler SH, Schneider JM. 2009. Securing paternity in spiders? A review on occurrence and effects of mating plugs and male genital mutilation. Genetica. 138:75–104. [DOI] [PubMed] [Google Scholar]
- Wake D, Dresner I. 1967. Functional morphology and evolution of tail autotomy in salamanders. J Morphol. 122:265–306. [DOI] [PubMed] [Google Scholar]
- Wasson K, Lyon BE. 2005. Flight or fight: flexible antipredatory strategies in porcelain crabs. Behav Ecol. 16:1037–1041. [Google Scholar]
- Wasson K, Lyon BE, Knope M. 2002. Hair-trigger autotomy in porcelain crabs is a highly effective escape strategy. Behav Ecol. 13:481–486. [Google Scholar]
- Wilkie IC. 2001. Autotomy as a prelude to regeneration in echinoderms. Microsc Res Tech. 55:369–396. [DOI] [PubMed] [Google Scholar]
- Zani P. 1996. Patterns of caudal-autotomy evolution in lizards. J Zool. 240:201–220. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.