Abstract
Aims
To describe the real‐world use and effectiveness of IDegLira, a fixed‐ratio combination of the basal insulin degludec, and the glucagon‐like peptide‐1 receptor agonist (GLP‐1RA) liraglutide.
Materials and Methods
This European, multicentre, retrospective chart review comprised adults (n = 611) with type 2 diabetes, who started IDegLira ≥6 months before data collection. Clinical characteristics were assessed at baseline (defined as the most recent recording during the 6 months before the first IDegLira prescription) and 3, 6, 9 and 12 months (± 45 days for each time point) after commencing IDegLira, where data were available.
Results
Baseline regimens included non‐injectable medications (19%), basal insulin (19%), GLP‐1RA (10%), free combination therapy (insulin/GLP‐1RA, 24%) and multiple daily‐dose insulin injections (MDI, 28%), all ± oral antidiabetic drugs. After 6 months, significant glycated haemoglobin (HbA1c) reductions were observed in patients overall and in all subgroups (−10 mmol/mol [−0.9%] overall; P < .0001), and a significant reduction in mean body weight (−0.7 kg; P < .05) was observed in patients overall and in patients receiving MDI (−2.4 kg; P < .0001). The mean IDegLira dose was 22, 30 and 32 dose steps at initiation, and at 6 and 12 months follow‐up, respectively. In total, only 67 patients reached the maximum 50 dose steps, with most coming from the free combination therapy (n = 31) or MDI (n = 15) baseline regimen groups. Hypoglycaemia rates were reduced by 82% (rate ratio 0.18; P < .0001) in the 6‐month period after vs before IDegLira initiation. Overall, a total of 12 patients experienced 15 events in the 6 months after IDegLira initiation.
Conclusion
In real‐world practice, after 6 months and at a moderate dose, IDegLira resulted in substantial reductions in HbA1c and body weight, with a reduced risk of hypoglycaemia.
Keywords: GLP‐1 analogue, glycaemic control, insulin therapy, observational study, type 2 diabetes
1. INTRODUCTION
IDegLira is an injectable, fixed‐ratio combination of the basal insulin degludec, and the glucagon‐like peptide‐1 receptor agonist (GLP‐1RA) liraglutide, for the management of type 2 diabetes (T2D).1 The benefits of the free combination of basal insulin and GLP‐1RA therapy are well established,2, 3, 4, 5, 6, 7, 8 and are recommended in the latest guidelines for the management of T2D.9, 10 The more recent availability of fixed‐ratio insulin/GLP‐1RA combinations allows the combination of basal insulin and GLP‐1RA without the burden of multiple daily injections.11 Specifically, IDegLira is administered once daily, independent of meals, as dose steps. One dose step contains 1 unit (U) of insulin degludec and 0.036 mg of liraglutide. The maximum daily dose of IDegLira is 50 dose steps (50 U insulin degludec and 1.8 mg liraglutide).12
The efficacy and safety of IDegLira has been evaluated in patients with T2D and suboptimal glycaemic control on oral antidiabetic drugs (OADs), GLP‐1RA therapy and basal insulin therapy in the phase III DUAL clinical trial programme.1, 13 DUAL I–VII demonstrated that IDegLira combined the clinical advantages of both mono‐components to reduce both fasting and prandial blood glucose levels and, furthermore, mitigated the major side effects associated with insulin (hypoglycaemia, weight gain) and GLP‐1RA therapy (nausea).14, 15, 16, 17, 18, 19, 20
Based on findings from these trials, IDegLira has been approved for use in Europe (since September 2014) and the United States (since November 2016).12, 13, 21 In the United States, IDegLira is approved as an adjunct to diet and exercise to improve glycaemic control in adults with T2D inadequately controlled on basal insulin (<50 U daily) or liraglutide (≤1.8 mg).21 In Europe, IDegLira is approved to achieve glycaemic control in combination with OADs when these alone, or when combined with a GLP‐1RA or basal insulin, do not provide adequate glycaemic control.12 Additional DUAL trials are planned or ongoing, including those that will compare the safety and efficacy of IDegLira with other intensification strategies.22, 23
In contrast to the accumulating data from clinical trials, real‐world evidence on IDegLira is limited. Findings from an observational study in patients with T2D and suboptimal glycaemic control on >2 OADs and >1 injection/day at a single Swiss centre (n = 61) showed that IDegLira resulted in glycated haemoglobin (HbA1c) reductions, weight loss and a reduced total insulin dose after 6 months' treatment compared with baseline.24 Similar results were also observed in a small (n = 12) UK study25; however, real‐world data from a larger patient population that is more varied, in terms of baseline therapy, region and number of centres involved, is needed and may help to inform the prescribing decisions of clinicians. The aim of the present European Xultophy Treatment Retrospective Audit (EXTRA) study was to evaluate the clinical use and effectiveness of IDegLira in a real‐world population of adults with T2D, under conditions that reflect routine clinical care.
2. MATERIALS AND METHODS
2.1. Study population and methods
This European, multicentre, retrospective, non‐interventional chart review study collected data from 61 sites in five countries, between April and October 2016. A contract research organization (ICON) was responsible for investigational site selection and training, independently of the study sponsor. Potential investigators were randomly drawn (sequentially) from databases of IDegLira prescribers and contacted about participation. Confirmed investigators invited eligible patients (consecutively) to participate in the study, starting with the patient who had attended the clinic most recently and working backwards. Competitive recruitment was used until the sample size was reached. Informed written consent was obtained from all participants, in accordance with the requirements of the Declaration of Helsinki,26 before any data were extracted from medical records. Data were collected from patient medical records at outpatient clinics in Germany (n = 450 patients, 35 sites), Switzerland (n = 84 patients, 9 sites), the United Kingdom (n = 44 patients, 8 sites), Austria (n = 19 patients, 4 sites) and Sweden (n = 14 patients, 5 sites). At treatment initiation, IDegLira therapy was reimbursed with restrictions in the United Kingdom (varying restrictions apply), Sweden (for patients with T2D uncontrolled on basal insulin) and Switzerland (for patients with T2D uncontrolled on metformin or basal insulin and with body mass index >28 kg/m2), whereas in Austria, individual reimbursement was required and in Germany, full reimbursement was available. Eligible participants were adults with T2D who had initiated IDegLira at least 6 months before enrolment in the study (Figure S1). All patients who received at least one prescription of IDegLira were considered for study participation, including those who had discontinued IDegLira at the time of patient selection. The minimum available data were: HbA1c readings at (1) the time of initial IDegLira prescription (or if unavailable, the most recent HbA1c value within 6 months before the first IDegLira prescription), and (2) 6 months ±45 days after the first IDegLira prescription. Patients were excluded if they had previously participated in this study or any clinical trial within 6 months before or 12 months after the first IDegLira prescription. Baseline values were defined as the most recent values during the 6‐month period before the first prescription of IDegLira. Outcome data were collected retrospectively with a window of ±45 days around the 3‐, 6‐, 9‐ and 12‐month time points.
2.2. Outcome measures
The primary objective was to assess IDegLira's effectiveness in controlling glycaemia, 6 months after initiation, in patients with T2D in routine clinical practice. Secondary objectives included assessment of other diabetes‐related effectiveness measures in patients initiating IDegLira treatment, including change in HbA1c at 3, 9 and 12 months and change in reference blood glucose measurement (e.g. fasting blood glucose), body weight and blood pressure at 6 and 12 months, where data were available in medical records. The proportion of patients achieving HbA1c thresholds (≤48, <53, <59 and <64 mmol/mol [≤6.5%, <7%, <7.5% and <8%]) and the incidence of hypoglycaemia were also assessed.
Other secondary objectives were to describe how IDegLira is used in the real‐world setting, including patient characteristics and reasons for initiation and discontinuation of IDegLira. Dose of IDegLira, total daily insulin and total daily GLP‐1RA dose were also assessed at baseline and follow‐up, together with use of OAD(s). To compare total GLP‐1RA dose at baseline and after initiation of IDegLira, GLP‐1RA dose was converted from mcg or mg to defined daily dose (DDD), according to the World Health Organization's ATC/DDD Index 2017,27 with adjustment as required for products that are not dosed once daily.
Hypoglycaemic events were those recorded by the investigator, based on the information in the medical records. Severe hypoglycaemia was defined as an episode requiring the assistance of another person to actively administer carbohydrate, glucagon or other corrective actions. Nocturnal hypoglycaemia was defined as any event in which the words “nocturnal” or “night” (or their equivalent in the local language) were used in the patient records.
2.3. Statistical analyses
Baseline characteristics and demographics were reported as mean (SD) or percentage, as appropriate, and were based on the full analysis set (FAS), as were reasons for initiation and discontinuation of IDegLira. All effectiveness and safety data were based on the effectiveness analysis set (EAS; all patients already included in the FAS having continued IDegLira for at least 6 months after initiation), unless otherwise stated. Descriptive statistics of continuous variables were compared using a two‐tailed paired t test. Logistic regression was used to model the achievement of specific HbA1c targets before and after exposure to IDegLira. Hypoglycaemia rates for the first 6 months after initiation of IDegLira treatment were compared with the 6‐month period before IDegLira initiation, and analysed using a Poisson regression model.
3. RESULTS
3.1. Study population demographics and clinical characteristics
A total of 611 patients were included in the study (FAS), of whom 566 (93%) continued IDegLira for at least 6 months (EAS). Baseline demographics and clinical characteristics are presented in Table 1. Baseline regimens included non‐injectable therapies (OADs only or treatment‐naïve; n = 118 [19%]), GLP‐1RA therapy (n = 60 [10%]), basal insulin therapy (n = 115 [19%]), free combination of insulin (basal/basal–bolus/premix) and GLP‐1RA (n = 145 [24%]), and multiple daily‐dose insulin injections (MDI; basal–bolus, premix or other insulin; n = 173 [28%]), all with or without OADs (Figure S2). Comorbidities present at baseline are shown in Table S1. The majority of patients (87%) presented with at least one comorbidity at baseline, the most common being hypertension (76%), dyslipidaemia (48%) and sensory‐motor neuropathy (38%).
Table 1.
Population demographics and baseline characteristics
| Overall | Non‐injectable therapy | GLP‐1RA ± OADs | Basal insulin ± OADs | Insulin + GLP‐1RA ± OADs | MDI ± OADs | |
|---|---|---|---|---|---|---|
| FAS, N | 611 | 118 | 60 | 115 | 145 | 173 |
| Ethnicity, % | ||||||
| Asian | 0.8 | 1.7 | 0 | 1.7 | 0.7 | 0 |
| Black/African American | 0.2 | 0 | 0 | 0.9 | 0 | 0 |
| White | 97.4 | 94.9 | 96.7 | 95.7 | 98.6 | 99.4 |
| Missing | 1.6 | 3.4 | 3.3 | 1.7 | 0.7 | 0.6 |
| Male, % | 60.4 | 66.9 | 55.0 | 53.0 | 64.1 | 59.5 |
| Age, years [N] | 61.9 (10.5) [611] | 60.8 (10.0) [118] | 61.6 (9.3) [60] | 61.4 (10.7) [115] | 61.0 (10.1) [145] | 63.8 (11.2) [173] |
| Weight, kg [N] | 102.8 (21.2) [534] | 106.2 (21.0) [98] | 103.9 (20.1) [52] | 95.4 (20.0) [101] | 106.2 (22.8) [127] | 102.3 (19.9) [156] |
| BMI, kg/m2 [N] | 35.1 (6.5) [534] | 35.6 (6.5) [98] | 36.2 (7.8) [52] | 33.0 (5.9) [101] | 35.9 (6.8) [127] | 35.0 (5.9) [156] |
| HbA1c, mmol/mol [N] | 69 (16.4) [611] | 74 (19.7) [118] | 72 (15.3) [60] | 67 (16.4) [115] | 67 (14.2) [145] | 68 (16.4) [173] |
| Duration of diabetes, years [N] | 13.2 (7.5) [611] | 9.7 (6.2) [118] | 11.0 (5.1) [60] | 12.4 (7.1) [115] | 14.1 (8.0) [145] | 16.3 (7.7) [173] |
| BP, mm Hg [N] | [483] | [82] | [46] | [93] | [110] | [152] |
| Systolic | 141.6 (20.6) | 145.2 (21.1) | 143.5 (21.2) | 142.8 (21.2) | 141.5 (20.1) | 138.3 (19.9) |
| Diastolic | 83.0 (11.9) | 84.4 (11.9) | 86.7 (12.3) | 85.1 (11.4) | 82.4 (11.7) | 80.3 (11.7) |
| Lipids, mg/dL [N] | [180‐208] | [52‐58] | [20‐22] | [33‐41] | [39‐46] | [36‐41] |
| LDL cholesterol | 109.3 (41.1) | 124.1 (41.0) | 108.2 (43.0) | 109.2 (40.7) | 106.4 (38.6) | 92.9 (38.2) |
| HDL cholesterol | 45.7 (14.2) | 42.3 (8.8) | 45.5 (12.8) | 47.5 (13.9) | 46.8 (14.3) | 47.8 (20.2) |
| Total cholesterol | 183.7 (48.1) | 190.4 (53.9) | 182.0 (50.2) | 190.6 (48.0) | 186.3 (44.7) | 165.2 (38.6) |
| Triglycerides | 211.9 (103.8) | 222.8 (101.8) | 223.0 (97.0) | 204.4 (104.4) | 214.4 (116.3) | 194.2 (98.2) |
| Treated with OADs, N (%) | 520 (85.1) | 104 (88.1) | 57 (95.0) | 109 (94.8) | 127 (87.6) | 123 (71.1) |
| Dose of previous insulin treatment, U | ||||||
| Basal, U [N] | 32.7 (18.7) [369] | ‐ | ‐ | 31.4 (21.1) [114] | 33.4 (17.7) [132] | 33.1 (17.4) [123] |
| Prandial, U [N] | 46.5 (31.5) [163] | ‐ | ‐ | ‐ | 46.4 (33.0) [31] | 46.6 (31.3) [132] |
| Premix, U [N] | 42.4 (24.3) [40] | ‐ | ‐ | ‐ | 46.8 (33.2) [10] | 40.9 (21.1) [30] |
| Total daily insulin, U [N] | 50.1 (38.0) [426] | ‐ | ‐ | 31.4 (21.1) [114] | 44.2 (33.9) [143] | 67.7 (42.5) [169] |
| Dose of previous GLP‐1RA treatment | ||||||
| Exenatide (mcg) [N] | 16.7 (4.9) [12] | ‐ | 15.0 (7.1) [2] | ‐ | 17.0 (4.8) [10] | ‐ |
| Exenatide once weekly, mg [N] | 1.9 (0.3) [10] | ‐ | 2.0 (0.0) [4] | ‐ | 1.8 (0.4) [6] | ‐ |
| Lixisenatide, mcg [N] | 20.0 (0.0) [2] | ‐ | 20.0 (−) [1] | ‐ | 20.0 (−) [1] | ‐ |
| Dulaglutide, mg [N] | 1.5 (0.0) [30] | ‐ | 1.5 (0.0) [14] | ‐ | 1.5 (0.0) [16] | ‐ |
| Liraglutide, mg [N] | 1.6 (0.8) [145] | 1.4 (0.4) [37] | ‐ | 1.6 (0.9) [108] | ‐ | |
BMI, body mass index; BP, blood pressure; FAS, full analysis set; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; MDI, multiple daily‐dose insulin injections; OADs, oral antidiabetic drugs; SD, standard deviation.
Data are mean (SD), except where otherwise indicated, and based on FAS, where available. The number of participants with data available [N] is shown in square brackets.
3.2. IDegLira use in real‐world setting
The primary reason for initiating IDegLira (Figure 1) was insufficient efficacy of the previous treatment regimen (71.8%), followed by problems with weight gain (26.5%).
Figure 1.
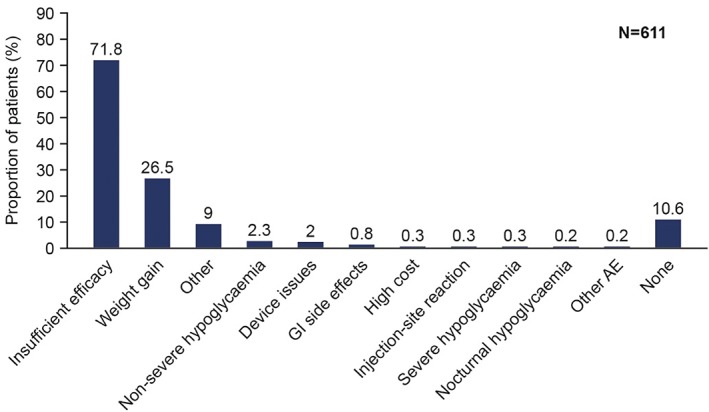
Reasons for initiation of insulin degludec/liraglutide combination (IDegLira). Reasons were not mutually exclusive. Data based on full analysis set. AE, adverse event; GI, gastrointestinal
The starting dose of IDegLira varied from 10 to 80 dose steps, with the majority (83.7%) of patients being initiated on between 10 and 30 dose steps IDegLira, and 10 patients (1.8%) receiving >50 dose steps (Table 2). Overall, the mean (SD) dose of IDegLira was 30.2 (12.9) dose steps at 6 months (n = 566), and 32.0 (13.6) dose steps at 12 months (n = 323). In all baseline therapy subgroups, titration of IDegLira was moderate after 6 months; change in IDegLira dose from baseline to 6 months was 8.5 dose steps (non‐injectables), 10.6 dose steps (GLP‐1RA ± OADs), 8.5 dose steps (basal insulin ± OADs), 6.0 dose steps (free combination of insulin + GLP‐1RA ± OADs) and 8.5 dose steps (MDI; Figure 2A). The maximum dose of 50 dose steps IDegLira was reached or exceeded by 38 patients at initiation and 62 patients at 6 months' follow‐up. In total, 67 patients received ≥50 dose steps IDegLira at either initiation or within 6 months (11.8%). Of these patients, most came from the free combination of insulin and GLP‐1RA (n = 31) or MDI subgroups (n = 15) and the majority (n = 47) did not increase their dose of either IDegLira or any other injectable therapy once they had reached 50 dose steps IDegLira.
Table 2.
Insulin degludec/liraglutide (IDegLira) combination starting dose
| Starting dose | Number of patients at baseline | Proportion of patients at baseline (%) |
|---|---|---|
| EAS, N | 566 | 100 |
| 10 dose steps | 86 | 15.2 |
| >10 to ≤20 dose steps | 271 | 47.9 |
| >20 to ≤30 dose steps | 117 | 20.7 |
| >30 to ≤40 dose steps | 47 | 8.3 |
| >40 to ≤50 dose steps | 35 | 6.2 |
| >50 dose steps | 10 | 1.8 |
EAS, effectiveness analysis set.
Data based on EAS.
Figure 2.
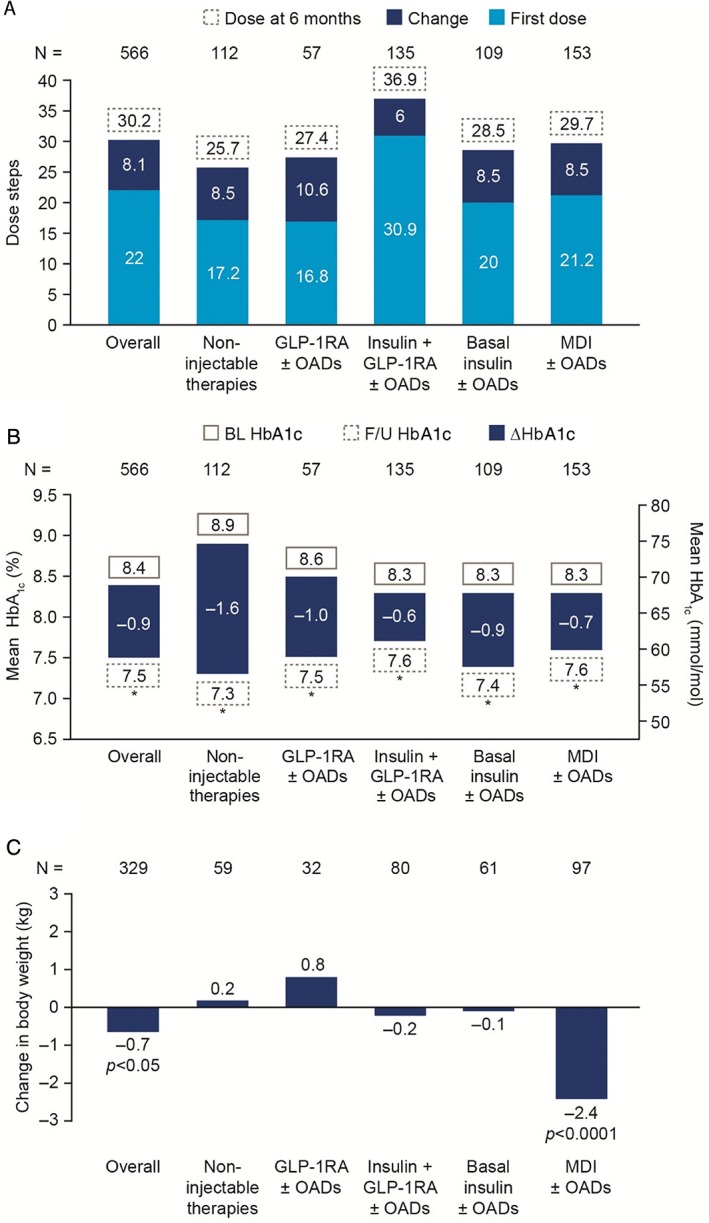
Change from baseline to 6 months in A, insulin degludec/liraglutide combination (IDegLira) dose; B, glycated haemoglobin (HbA1c) and C, body weight by baseline therapy subgroup. *P < .0001. Data based on effectiveness analysis set. Significance assessed using a two‐tailed paired t test. BL, baseline; F/U, follow‐up; GLP‐1RA, glucagon‐like peptide‐1 receptor agonist; MDI, multiple daily‐dose insulin injections; N, number of patients with data at both time points; OADs, oral antidiabetic drugs
3.3. Effectiveness outcomes
After 6 months of treatment with IDegLira, HbA1c was significantly reduced by 10 mmol/mol (95% confidence interval [CI] −11, −9), or 0.9% (95% CI −1.0, −0.8), in the overall population (P < .0001), corresponding to a mean (SD) HbA1c of 59 (12) mmol/mol (7.5 [1.1]%) at follow‐up. Significant reductions in HbA1c were also observed at all other time points assessed, with a reduction of 7 mmol/mol (0.6%) to 61 mmol/mol (7.7%) after 12 months of treatment (P < .0001, n = 238). Significantly greater proportions of patients reached HbA1c thresholds (≤48, <53, <59 and <64 mmol/mol [≤6.5%, <7%, <7.5% and <8%]) at 6 months (17.3%, 33.6%, 54.6% and 71.6%, respectively) vs baseline (6.2%, 12.5%, 27.6% and 43.3%, respectively; P < .0001 for all). After 6 months, significant reductions in HbA1c were observed across all baseline therapy subgroups (Figure 2B).
Overall, and across all baseline therapy groups, baseline HbA1c was a significant factor for change in HbA1c after 6 months; the extent of HbA1c reduction being dependent on baseline HbA1c. When patients were placed into subgroups according to baseline HbA1c category (<53 mmol/mol, ≥53 to <59 mmol/mol, ≥59 to <70 mmol/mol, ≥70 to <80 mmol/mol, and ≥80 mmol/mol [<7.0%, ≥7.0% to <7.5%, ≥7.5% to <8.5%, ≥8.5% to <9.5%, and ≥9.5%]), HbA1c reductions were significantly greater at 6 months in patients from all baseline HbA1c categories >59 mmol/mol (≥59 to <70 mmol/mol [−7 mmol/mol], ≥70 to <80 mmol/mol [−10 mmol/mol], ≥80 mmol/mol [−27 mmol/mol]), or >7.5% (≥7.5% to <8.5% [−0.6%], ≥8.5% to <9.5% [−0.9%], ≥9.5% [−2.5%]), compared with patients with HbA1c <53 mmol/mol (<7%) at baseline (all between‐group comparisons P < .0001). A similar trend was observed in all baseline therapy groups with the exception of MDI. There was no association between change in HbA1c and IDegLira dose at baseline (P = .76) and at 6‐month follow‐up (P = .24); reductions in HbA1c were similar, irrespective of baseline IDegLira dose. When grouping patients according to follow‐up dose at 6 months (<10 U, ≥10 to <20 U, ≥20 to <30 U, ≥30 to <40 U, ≥40 to <50 U, and ≥50 U), reductions in HbA1c were observed across all subgroups (−22, −10, −9, −11, −12, and −9 mmol/mol [−2.0%, −0.9%, −0.8%, −1.0%, −1.1%, and −0.8%], respectively).
In the overall population, body weight was significantly reduced by 0.7 (4.8) kg at 6 months vs baseline (P = .0127, n = 329), but there were insufficient data available for time points thereafter. Subgroup analysis showed, however, that weight loss was only statistically significant in the MDI group (−2.4 kg; P < .0001; Figure 2C).
Changes in reference blood glucose (or fasting plasma glucose) and proportions of patients achieving composite endpoints of HbA1c targets with no hypoglycaemia and/or weight gain are not reported here because of the substantial amount of missing data in patients' medical records for these endpoints.
IDegLira initiation was associated with a reduction in the need for glucose‐lowering therapies (Table S2). Specifically, of the 481 patients across all subgroups using ≥1 OAD at baseline, 154 (32%) discontinued ≥1 OAD after IDegLira initiation. In addition, in patient subgroups receiving GLP‐1RA as part of their regimen, IDegLira initiation was associated with a reduction in GLP‐1RA dose from 1.3 (0.6) DDD at baseline to 1.0 (0.4) DDD after 6 months (P < .0001; Figure 3). Across all patients receiving insulin at baseline, total daily insulin dose was reduced by 9.2 U (95% CI −12.1, −6.3; P < .0001), but reductions varied depending on the previous regimen. Total daily insulin dose was significantly reduced vs baseline (P < .0001; Figure 3) in patients previously receiving MDI; this was largely because 60.5% (78 of 129) of the patients who were receiving a prandial insulin at baseline discontinued prandial insulin within 6 months of IDegLira initiation.
Figure 3.
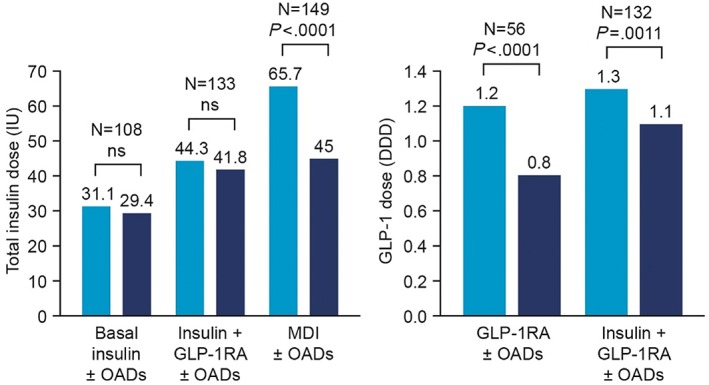
Changes in total insulin and glucagon‐like peptide‐1 receptor agonist (GLP‐1RA) dose from baseline to 6 months. Baseline is defined as last recorded insulin dose before insulin degludec/liraglutide combination (IDegLira) initiation. Data based on effectiveness analysis set. Significance assessed using a two‐tailed paired t test. DDD, defined daily dose; IU, international unit; MDI, multiple daily‐dose insulin injections; ns, non‐significant; OADs, oral antidiabetic drugs
Significant reductions were observed in systolic blood pressure at 6 months, while diastolic blood pressure remained similar to baseline (Table S3).
3.4. Hypoglycaemia
The overall number of hypoglycaemic events in this study (including events that were non‐severe, nocturnal and were not defined according to blood glucose threshold) was low. Nine patients experienced a total of 66 events of hypoglycaemia (0.28 events per patient‐year) in the 6 months before IDegLira initiation vs 12 patients experiencing a total of 15 events (0.06 events per patient‐year) in the 6 months after IDegLira initiation. The reduction was significant (rate ratio 0.18; P < .0001). Of the subset of patients who had follow‐up data available for months 6 to 12 after IDegLira initiation, 4 patients experienced a total of 6 events of hypoglycaemia (0.04 events per patient‐year) in this period. There was one severe hypoglycaemic event during treatment with IDegLira, which occurred between 6 and 12 months after IDegLira initiation.
3.5. Discontinuation
At 6 months, 7.4% of patients (n = 45) had discontinued IDegLira. The main reason for discontinuation (31 of 45, 68.9%) was classified under “other” and was related to ceased distribution of IDegLira in Germany from October 2016, following a change in reimbursement status. Other reasons were insufficient efficacy, and cost and availability. Overall reasons for discontinuation during study are summarized in Figure S3.
4. DISCUSSION
The present study is the first large, multicentre, multi‐country observational study on real‐world initiation of IDegLira for the treatment of T2D in Europe. As such, it builds on the positive findings from the DUAL clinical trial programme but also describes real‐world use of IDegLira. In the EXTRA study, patients were not selected for study inclusion based on previous therapy. Hence, this study included patients with T2D receiving non‐injectable therapy, basal insulin, GLP‐1RA, free combination of insulin + GLP‐1RA, and MDI therapy. This suggests that IDegLira was not always introduced as early in the treatment pathway as has been investigated in the DUAL clinical trial programme and as is recommended in the prescribing information.12, 21 The reasons for this are unknown, but might reflect a willingness by physicians to first try this relatively recently approved agent in patients for whom other treatment options have been exhausted and who did not previously have access to IDegLira, before broadening their experience in less severe patient cases. Importantly, however, the findings presented here do show that, while safety and effectiveness measures vary with pre‐study regimens, real‐world IDegLira use resulted in improved glycaemic control across all baseline therapy groups, with significant reductions in HbA1c after 6 months. Furthermore, real‐world IDegLira use was associated with a low risk of hypoglycaemia, no weight gain and reductions in at least one class of concomitant diabetic medication across all baseline therapy groups. Also of note is that the above‐mentioned results were achieved at a moderate dose of IDegLira.
Both the EU summary of product characteristics and the US prescribing information recommend initiation of IDegLira at 16 dose steps/U (16 U insulin degludec + 0.6 mg liraglutide) in patients receiving basal insulin or GLP‐1RA therapy, with twice‐weekly titration to a maximum of 50 dose steps.12, 21 In Europe, insulin‐naïve patients are to be initiated at 10 dose steps.12 The present findings describe real‐world clinical initiation of IDegLira at a mean starting dose of 22 dose steps in the overall population. The starting dose of IDegLira varies across baseline therapy groups; unsurprisingly, it is higher (30.9 dose steps) in patients who are already receiving a combination of insulin + GLP‐1RA at baseline and would therefore not be expected to experience the transient gastrointestinal side effects associated with GLP‐1RA initiation. Similarly, real‐world titration of IDegLira differed from that of clinical trials, with modest increases to 30.2 dose steps (overall) and 36.9 dose steps (free combination) by follow‐up.
Importantly, as already mentioned, real‐world titration of IDegLira resulted in significant HbA1c reductions after 6 months across all baseline therapy groups. In the overall population, mean HbA1c was reduced to 58 mmol/mol (7.5%) after 6 months of IDegLira treatment and mean IDegLira dose was 30.2 dose steps. Similar results were observed across all baseline therapy groups; HbA1c generally plateaued at 58 mmol/mol (7.5%) with limited titration thereafter. The mean dose reported here is lower than those reported in clinical trials, while HbA1c is higher, which suggests that glycaemic control could be further improved with more aggressive titration. For example, this study demonstrated that real‐world use of IDegLira in patients receiving non‐injectable therapies (including various OADs and treatment‐naïve patients) achieved HbA1c of 56 mmol/mol (7.3%) at a mean dose of 26 dose steps after 6 months. In 26‐ (DUAL I) and 32‐week (DUAL VI) clinical trials involving patients uncontrolled on metformin ± pioglitazone, end‐of‐trial HbA1c ranged from 42 mmol/mol to 46 mmol/mol (6.0% to 6.4%) and was achieved at a mean dose of 38 to 41 U.17, 28 Similar differences between real‐world data presented here and clinical trial data were observed for patients on basal insulin15, 18 and GLP‐1RA.19 It is not clear why more stringent glycaemic targets were not pursued more aggressively, but reasons might relate to country‐specific guidelines, the expense/availability of further uptitration, patient‐led titration being guided by fear of hypoglycaemia, and other patient characteristics (e.g. old age, concomitant therapy associated with hypoglycaemia). Nevertheless, this retrospective chart review study shows that initiating IDegLira in a real‐life setting significantly improves glycaemic control, together with other safety and effectiveness variables, at a moderate dose.
With regard to other effectiveness measures, the benefit of IDegLira in terms of weight change was more substantial in some patient subgroups than others, similar to results from the DUAL clinical trial programme.1, 13 In clinical trials, IDegLira is weight‐neutral in patients uncontrolled on OADs,16, 17, 20 whereas it is associated with weight gain in patients uncontrolled on GLP‐1RAs,19 and weight loss in patients uncontrolled on basal insulin therapy.15, 18 Similar trends are observed in this real‐world study, with the greatest benefit being observed in patients receiving MDI at baseline, in whom a significant decrease in mean body weight was observed. The significant reduction in total insulin dose in this subgroup is likely to have contributed to the weight loss observed. Conversely, the non‐significant mean increase in body weight in insulin‐naïve subgroups is probably a result of the addition of insulin when IDegLira is initiated and is consistent with trial data.
One limitation inherent to all non‐interventional studies is that the introduction of a new drug might be associated with improved adherence and/or closer follow‐up simply by virtue of its novelty. However, HbA1c reductions were maintained over 12 months, indicating that a non‐specific initial improvement in adherence was not responsible for the observed results. This study had several other limitations, some of which are typical for observational studies; for example, a lack of a head‐to‐head comparator or control group, the impact of missing data in medical records and the variability between data abstractors. Minimal available data for this study were such that HbA1c was the only endpoint available for all patients. Hypoglycaemic events were limited to those recorded by the investigator, based on the information in the medical records. Other endpoints pertinent to IDegLira use, such as patient‐reported outcomes, were not assessed so it is not possible to comment on the benefit of fewer injections from a patient's perspective. A lack of diversity in this study population warrants further studies in patients from other countries, ethnicities and backgrounds. The majority of patients (n = 450) were from Germany, where IDegLira distribution was discontinued during the study period. Consequently, the most common reason for discontinuation of IDegLira was “other” and not related to safety issues. Further analysis is therefore required to establish the effect of discontinuation of IDegLira distribution in Germany on the results.
In conclusion, the findings from this study show that in a real‐world European population, IDegLira is used as an intensification strategy for patients receiving a broad variety of treatment regimens. IDegLira initiation results in a reduction of HbA1c, low rates of hypoglycaemia and no weight gain in patients with T2D in a real‐world setting. Based on findings in patients uncontrolled on OADs either alone or in combination with basal insulin or GLP‐1RA from the IDegLira clinical trial programme, greater improvements might be expected when introducing IDegLira at an earlier point in the treatment pathway, but the results described here suggest that patients can derive benefit from IDegLira at a later stage than currently appreciated. The results of this study are therefore pertinent to physicians who are considering the most appropriate therapy for patients who are failing to achieve glycaemic control targets or struggling with side effects such as weight gain and hypoglycaemia.
Supporting information
Figure S1 Study design
Figure S2. Baseline regimens
Figure S3. Reasons for discontinuing IDegLira during whole study
Table S1. Baseline characteristics: prevalence of comorbidities.
Table S2. Changes in concomitant antidiabetic therapies in the 6 months following IDegLira initiation
Table S3. Change in blood pressure at 6 months
ACKNOWLEDGMENTS
The authors would like to thank the investigators, study staff and patients for their participation. The authors also thank Trine W. Boesgaard, Esther Zimmermann and Andrei‐Mircea Catarig (Novo Nordisk) for their review and input to the manuscript, Loes Vanden Eynde (Novo Nordisk) for European project co‐ordination and ICON (Ireland, UK) for their project management and statistical analyses services. Medical writing and submission support were provided by Victoria Atess and Daria Renshaw of Watermeadow Medical, an Ashfield company, part of UDG Healthcare plc. This support was funded by Novo Nordisk.
Parts of this study were presented as a poster presentation at the American Diabetes Association, 77th Annual Scientific Sessions, 9–13 June 2017, San Diego, California.
Conflict of interest
H.P. has received lecture fees from MSD, AstraZeneca, Lilly, Sanofi, Novo Nordisk and Boehringer Ingelheim, conference travel from Novo Nordisk, and received honoraria for advisory board or steering committee participation from Sanofi and Novo Nordisk. M.B. received honoraria for presentations and advisory board participations from Amgen, AstraZeneca, Boehringer Ingelheim, Bayer, Lilly, Novartis, Novo Nordisk and Sanofi. R.P. has received honoraria for advisory board participation from Amgen, AstraZeneca, Boehringer Ingelheim, Lilly, MSD, Novartis, Novo Nordisk and Sanofi. T‐M.P. was an employee of Novo Nordisk Region Europe A/S and owned stocks/shares in the company at the time of this study. B.L.T. is an employee and shareholder of Novo Nordisk A/S. B.S. has received honoraria for advisory board and speakers' bureau participation from Novo Nordisk, Sanofi, AstraZeneca, Lilly, Bayer and Boehringer Ingelheim, and for speakers' bureau participation from Sanofi, Lilly, MSD, Bayer and Boehringer Ingelheim.
Author contributions
H.P., M.B., R.P. and B.S. contributed to the analysis and manuscript writing for this study.
B.L.T. and T‐M.P. contributed to the design, conduct/data collection, analysis and manuscript writing for this study.
Price H, Blüher M, Prager R, et al. Use and effectiveness of a fixed‐ratio combination of insulin degludec/liraglutide (IDegLira) in a real‐world population with type 2 diabetes: Results from a European, multicentre, retrospective chart review study. Diabetes Obes Metab. 2018;20:954–962. https://doi.org/10.1111/dom.13182
Tra‐Mi Phan MD, MBA was an employee of Novo Nordisk at the time of this study but has since left the company.
Funding information This study was funded by Novo Nordisk.
REFERENCES
- 1. Greig SL, Scott LJ. Insulin degludec/liraglutide: a review in type 2 diabetes. Drugs. 2015;75(13):1523‐1534. [DOI] [PubMed] [Google Scholar]
- 2. Buse JB, Bergenstal RM, Glass LC, et al. Use of twice‐daily exenatide in basal insulin‐treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2011;154(2):103‐112. [DOI] [PubMed] [Google Scholar]
- 3. Riddle MC, Aronson R, Home P, et al. Adding once‐daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24‐week, randomized, placebo‐controlled comparison (GetGoal‐L). Diabetes Care. 2013;36(9):2489‐2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Riddle MC, Forst T, Aronson R, et al. Adding once‐daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: a 24‐week, randomized, placebo‐controlled study (GetGoal‐Duo 1). Diabetes Care. 2013;36(9):2497‐2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ahmann A, Rodbard HW, Rosenstock J, et al. Efficacy and safety of liraglutide versus placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes: a randomized, placebo‐controlled trial. Diabetes Obes Metab. 2015;17(11):1056‐1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mathieu C, Rodbard HW, Cariou B, et al. A comparison of adding liraglutide versus a single daily dose of insulin aspart to insulin degludec in subjects with type 2 diabetes (BEGIN: VICTOZA ADD‐ON). Diabetes Obes Metab. 2014;16(7):636‐644. [DOI] [PubMed] [Google Scholar]
- 7. Rosenstock J, Fonseca VA, Gross JL, et al. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP‐1 receptor agonist, versus thrice‐daily prandial insulin lispro. Diabetes Care. 2014;37(8):2317‐2325. [DOI] [PubMed] [Google Scholar]
- 8. Diamant M, Nauck MA, Shaginian R, et al. Glucagon‐like peptide 1 receptor agonist or bolus insulin with optimized basal insulin in type 2 diabetes. Diabetes Care. 2014;37(10):2763‐2773. [DOI] [PubMed] [Google Scholar]
- 9. American Diabetes Association . Standards of Medical Care in Diabetes‐2017 abridged for primary care providers. Clin Diabetes. 2017;35(1):5‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm–2016 executive summary. Endocr Pract. 2016;22(1):84‐113. [DOI] [PubMed] [Google Scholar]
- 11. Hughes E. IDegLira: redefining insulin optimisation using a single injection in patients with type 2 diabetes. Prim Care Diabetes. 2016;10(3):202‐209. [DOI] [PubMed] [Google Scholar]
- 12. Novo Nordisk . Xultophy Summary of Product Characteristics. 2017. https://www.medicines.org.uk/emc/medicine/29493. Accessed July 31, 2017.
- 13. Vedtofte L, Knop FK, Vilsboll T. Efficacy and safety of fixed‐ratio combination of insulin degludec and liraglutide (IDegLira) for the treatment of type 2 diabetes. Expert Opin Drug Saf. 2017;16(3):387‐396. [DOI] [PubMed] [Google Scholar]
- 14. Billings LD, Gouet D, Oviedo A, et al. Efficacy and safety of insulin degludec/liraglutide (IDegLira) vs. basal‐bolus (BB) therapy in patients with type 2 diabetes (T2D): DUAL VII trial. Diabetes. 2017;66(suppl 1A):A36 (Abstract 136‐OR). [Google Scholar]
- 15. Buse JB, Vilsboll T, Thurman J, et al. Contribution of liraglutide in the fixed‐ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care. 2014;37(11):2926‐2933. [DOI] [PubMed] [Google Scholar]
- 16. Gough SC, Bode BW, Woo VC, et al. One‐year efficacy and safety of a fixed combination of insulin degludec and liraglutide in patients with type 2 diabetes: results of a 26‐week extension to a 26‐week main trial. Diabetes Obes Metab. 2015;17(10):965‐973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harris SB, Kocsis G, Prager R, et al. Safety and efficacy of IDegLira titrated once weekly versus twice weekly in patients with type 2 diabetes uncontrolled on oral antidiabetic drugs: DUAL VI randomized clinical trial. Diabetes Obes Metab. 2017;19(6):858‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lingvay I, Pérez Manghi F, Garcia‐Hernandez P, et al. Effect of insulin glargine up‐titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized clinical trial. JAMA. 2016;315(9):898‐907. [DOI] [PubMed] [Google Scholar]
- 19. Linjawi S, Bode BW, Chaykin LB, et al. The efficacy of IDegLira (insulin degludec/liraglutide combination) in adults with type 2 diabetes inadequately controlled with a GLP‐1 receptor agonist and oral therapy: DUAL III randomized clinical trial. Diabetes Ther. 2017;8(1):101‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rodbard HW, Bode BW, Harris SB, et al. Safety and efficacy of IDegLira added to sulphonylureas alone or sulphonylureas and metformin in insulin‐naïve people with type 2 diabetes: the DUAL IV trial. Diabet Med. 2017;34(2):189‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Novo Nordisk . Xultophy® Prescribing Information (PI). 2016. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208583s000lbl.pdf. Accessed July 31, 2017.
- 22.DUAL VIII trial. NLM identifier: NCT02501161. https://clinicaltrials.gov/ct2/show/NCT02501161. Accessed July 31, 2017.
- 23.DUAL IX trial. NLM identifier: NCT02773368. https://clinicaltrials.gov/ct2/show/NCT02773368. Accessed July 31, 2017.
- 24. Sofra D. Glycemic control in a real‐life setting in patients with type 2 diabetes treated with IDegLira at a Single Swiss Center. Diabetes Ther. 2017;8(2):377‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wyatt K, Hall L. No or Aye to Xultophy? Experience of fixed combination insulin Degludec and Liraglutide in Wishaw General Hospital. Diabet Med. 2017;34(suppl s1):189‐194. (Abstract P511).27589252 [Google Scholar]
- 26. World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191‐2194. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization ATC/DDD Index 2017. https://www.whocc.no/atc_ddd_index/?code=A10BJ. Accessed July 31, 2017.
- 28. Gough SC, Bode B, Woo V, et al. Efficacy and safety of a fixed‐ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open‐label, randomised, 26‐week, treat‐to‐target trial in insulin‐naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2(11):885‐893. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Study design
Figure S2. Baseline regimens
Figure S3. Reasons for discontinuing IDegLira during whole study
Table S1. Baseline characteristics: prevalence of comorbidities.
Table S2. Changes in concomitant antidiabetic therapies in the 6 months following IDegLira initiation
Table S3. Change in blood pressure at 6 months


