Abstract
Muscle thickness (MT) measured by ultrasound has been used to estimate cross‐sectional area (measured by CT and MRI) at a single time point. We tested whether MT could be used as a valid marker of MRI determined muscle anatomical cross‐sectional area (ACSA) and volume changes following resistance training (RT). Nine healthy, young, male volunteers (24 ± 2 y.o., BMI 24.1 ± 2.8 kg/m2) had vastus lateralis (VL) muscle volume (VOL) and ACSA mid (at 50% of femur length, FL) assessed by MRI, and VL MT measured by ultrasound at 50% FL. Measurements were taken at baseline and after 12 weeks of isokinetic RT. Differences between baseline and post‐training were assessed by Student's paired t test. The relationships between MRI and ultrasound measurements were tested by Pearson's correlation. After RT, MT increased by 7.5 ± 6.1% (P < .001), ACSA mid by 5.2 ± 5% (P < .001), and VOL by 5.0 ± 6.9% (P < .05) (values: means ± SD). Positive correlations were found, at baseline and 12 weeks, between MT and ACSA mid (r = .82, P < .001 and r = .73, P < .001, respectively), and between MT and VOL (r = .76, P < .001 and r = .73, P < .001, respectively). The % change in MT with training was correlated with % change in ACSA mid (r = .69, P < .01), but not % change in VOL (r = .33, P > .05). These data support evidence that MT is a reliable index of muscle ACSA mid and VOL at a single time point. MT changes following RT are associated with parallel changes in muscle ACSA mid but not with the changes in VOL, highlighting the impact of RT on regional hypertrophy.
Keywords: anatomical cross‐sectional area, magnetic resonance imaging, ultrasound, volume
1. INTRODUCTION
Skeletal muscle is the largest adipose tissue‐free mass in humans, constituting a substantial portion of the whole‐body mass, and it is crucial for locomotion and metabolic health. Over the last four decades, the quantification of skeletal muscle mass has been revolutionized by the introduction of imaging techniques such as computer tomography (CT), magnetic resonance imaging (MRI) and dual‐energy X‐ray absorptiometry (DXA), which facilitate the accurate quantification of whole‐body and regional muscle masses.1 These techniques have been used in a variety of settings, yet can be expensive, often inaccessible and, in the case of CT and DXA, involve ionizing radiation.
MRI is regarded as the gold standard for clinical and research imaging of skeletal muscle, allowing investigators to accurately assess muscle mass at an individual time point and its changes over time.2, 3 However, besides its accuracy, estimation of whole‐body muscle mass is not as cheap and accessible as with other techniques. DXA, for example, can provide estimates of regional and total lean masses at a lower cost than MRI and involves minimal radiation exposure compared to CT.1 Nonetheless, repeated DXA scanning in longitudinal studies does raise ethical concerns because of the stochastic risk posed by repeated radiation exposure.
Over the last 20 years, the use of ultrasound has been advocated as a potentially reliable tool for the quantification of skeletal muscle mass in young and older healthy volunteers4, 5, 6 and in clinical populations, such as intensive care patients.7, 8, 9, 10 Previous studies report a positive relationship between muscle thickness (MT) and lean mass (measured by DXA),11, 12 MT and anatomical cross‐sectional area (ACSA, measured by MRI),13 and between MT and muscle volume (VOL, measured by MRI)14, 15, 16 at a single time point. However, as far as we are aware, no study has reported the utility of MT measurements for detecting changes in muscle size or volume induced by resistance exercise training (RT).
Hence, the aim of this study was to examine whether muscle thickness measurements from ultrasound could be used to accurately estimate changes in muscle size and volume (assessed with MRI) following a RT protocol. It was hypothesized that vastus lateralis (VL) MT assessed at a single time point using ultrasound would be positively correlated with quantification of VL ACSA and VOL using MRI at the same time point. The second hypothesis was that the RT‐induced change in VL MT would be positively correlated to changes in VL ACSA and VOL.
2. METHODS
2.1. Participant characteristics and study design
Nine recreationally active, young, healthy males (age = 24 ± 2 years, BMI = 24.1 ± 2.8 kg/m2) volunteered for this study. Each participant underwent 12 weeks of unilateral RT performing maximal knee extensions on a Cybex® isokinetic dynamometer. One leg was trained concentrically (5 sets × 30 repetitions at 90 deg/s), whereas the contralateral limb was trained both concentrically (2 sets × 30 repetitions at 90 deg/s) and eccentrically (3 sets × 30 repetitions at 90 deg/s). This protocol was used to vary the training stimulus between legs and thereby potentially induce different increases in muscle mass between legs. Both legs performed knee extensions throughout the whole range of motion. Training frequency was 3 times per week. The choice of leg exercise combination was randomized. VL ACSA and VOL were measured at baseline and at 12 weeks using MRI (GE, 3T 750 Discovery, Chalfont Saint Giles UK). MT was also measured by ultrasonography at the same time points. Thus, a total of 18 (ie, 9 volunteers, both legs tested and trained differently) values of VL MT, ACSA and VOL were obtained per time point. The study was approved by the University of Nottingham Medical School Ethics Committee, in accordance with the Declaration of Helsinki, and informed consent was obtained from all participants.
2.2. VOL and ACSA assessments
Axial plane scans of each thigh were obtained using an MRI scanner (GE, 3T 750 Discovery). A T1‐weighted Spin Echo protocol was used (repetition time 600 ms, echo time 15.2 ms, field of view 512 × 512 mm, slice thickness 10 mm, no gap between slices). Participants were asked to lie supine on the MRI bed for 20 minutes to allow body fluid shift stabilization. Thereafter, 38 axial plane scans along the entire length of the VL were collected. From these scans, the contours of the VL muscle of each MRI scan were digitized offline using the Osirix DICOM image analysis software (Pixmeo, Geneva, Switzerland) (Figure 1). When it was difficult to distinguish the contours of VL and vastus intermedius muscles (ie, usually close to the very proximal insertions), the remaining ACSA (n = 2‐3 circa) were estimated by fitting the other obtained values into a spline curve.17, 18 Subsequently, VL VOL was calculated as previously described19 using the following equation:
where ΣACSA is the sum of contiguous ACSA, and slice thickness refers to the thickness of each individual MRI axial image with no gap between contiguous slices.
Figure 1.
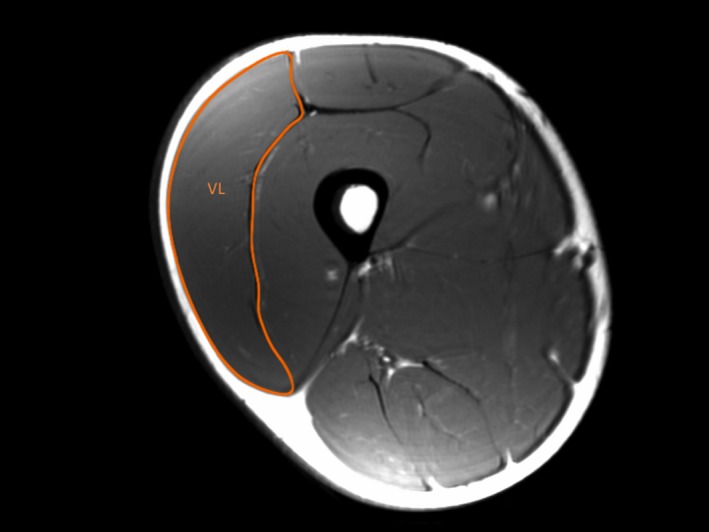
Magnetic resonance image scan of the right thigh at 50% of femur length from a representative subject. The contours that comprise the vastus lateralis (VL) anatomical cross‐sectional area are shown
VL muscle ACSA was measured at 50% of femur length (ACSAmid), defined as the distance from the greater trochanter to the lateral border of the femoral condyle (which was previously measured from a single coronal image). To ensure that ultrasound measurements were performed at the same anatomical location, this reference point was marked on the skin using an indelible pen.
2.3. MT assessment
VL MT was assessed by the same investigator from images obtained in vivo at rest using B‐mode ultrasonography (Mylab 25; Esaote Biomedica, Genova, Italy), with a 50 mm, 7.5 MHz, linear‐array probe. MT has previously been assessed by placing the ultrasound probe transversally in relation to the limb and evaluated as the perpendicular distance between the skeletal muscle interfaces.20 Longitudinal ultrasound scans (ie, with the probe aligned with the fascicle plane) have also been used to detect changes in muscle size and growth as well as skeletal muscle architecture.21, 22, 23
In this study, resting ultrasound images were taken at 50% of femur length, applying the same reference point used for the MRI scanning. The participant was resting supine on an examination bed with the knee in full extension (ie, anatomical zero).24 The transducer was placed longitudinally to the thigh along the mid‐sagittal axis of the VL, and carefully aligned to the fascicle plane to clearly visualize fascicles on the ultrasound screen. The experienced operator was careful in applying as little pressure as possible when placing the probe on the skin. Three images were acquired and stored for offline analysis. VL MT was measured as the distance between superficial and deep aponeuroses, in the proximal, central, and distal portions of the acquired image22, 23 (Figure 2), using the image analysis software ImageJ 1.42q (National Institutes of Health, USA). The mean of the three measures was calculated for statistical analysis.
Figure 2.
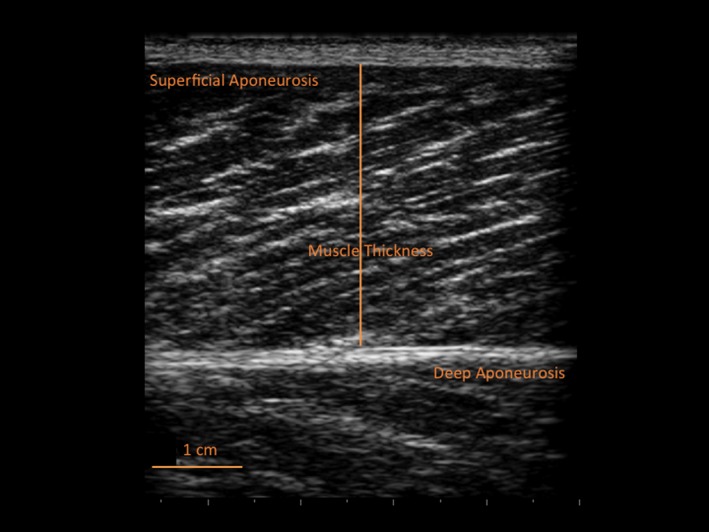
Ultrasound image of the vastus lateralis muscle (at 50% of femur length) from a representative subject with the muscle thickness measurement highlighted (solid line between aponeuroses)
The reliability of this ultrasound technique has been previously validated by cadaver anatomical inspection.25 Moreover, previous studies assessed the reliability of in vivo measurements of fascicle length26 and pennation angle.27 In this study, the interday reliability of MT was also assessed. All subjects were tested on two different days before the start of the training period. Volunteers were tested at the same hour of the day, and a permanent marker was used to trace the ultrasound probe contours in order to ensure that MT was assessed at the same VL site on both days. All images were collected and digitally analyzed by the same operator.
2.4. Statistical analysis
All statistical analyses were conducted using the SPSS 23.0 software (SPSS® Inc., Chicago, IL, USA). Data are reported as mean ± SD. Normality of distribution was checked by the Shapiro‐Wilk's test. Differences between pre‐ and post‐training were statistically analyzed for muscle VOL, ACSAmid, and MT values using paired Student's t test. Differences between legs at both time points were statistically analyzed using a two‐way repeated‐measures ANOVA. Correlations were tested using the Pearson's product moment correlation coefficient (r). The level of significance was set at P < .05.
The magnitude of the changes between baseline and 12 weeks was determined using effect size (ES) statistics with 90% confidence intervals (CI), or partial eta‐squared (η2) statistics when appropriate. ES was classified as trivial for ES values <0.20, small between 0.20 and 0.60, moderate between 0.61 and 1.20, large between 1.21 and 2.0, and very large when >2.0.28
The interday reliability of MT measurements was tested with the intraclass correlation coefficient (ICC, two‐way random, absolute agreement),29 with 95% CI, and the calculation of the relative standard error of measurement (SEM%). The minimum detectable change at 95% confidence as a percentage (MDC95%) was also determined.30 ICC values were considered as very high if >0.90, high if between 0.70 and 0.89, and moderate if between 0.50 and 0.69.31
3. RESULTS
3.1. MT reliability
Interday measurements of MT yielded an ICC of 0.99 (95% CI = 0.96‐0.99), with a SEM% of 1.65 and a MDC95% of 4.6%.
3.2. Morphological adaptations
No differences were found between legs at both baseline and after training (P = .83). Following the RT protocol, all parameters were significantly different from baseline (Table 1). ACSAmid increased by 5.2 ± 5%, (P < .001, ES = 1.05 ± 0.11, moderate), VOL by 5.0 ± 6.9%, (P < .05, ES = 0.69 ± 0.14, moderate), and MT by 7.5 ± 6.1%, (P < .001, ES = 1.28 ± 0.13, large). The observed mean changes in MT were greater than the requested MDC95% (4.6%).
Table 1.
Vastus lateralis anatomical cross‐sectional area measured at midpoint of femur length (ACSAmid) and total volume (VOL) measured by magnetic resonance imaging, and muscle thickness (MT) measured by ultrasound at the same site, before and after 12 wk of resistance exercise training
| Baseline | 12 wk | P‐value | Effect size | |
|---|---|---|---|---|
| ACSAmid (cm2) | 32.5 (5.4) | 34.6 (4.6) | <.001 | 1.05 |
| VOL (cm3) | 668 (121) | 695 (100) | <.05 | 0.69 |
| MT (cm) | 2.54 (0.4) | 2.73 (0.34) | <.001 | 1.28 |
When plotting ACSAmid against MT (Figure 3A), significant positive correlations were found at baseline (r = .82 P < .001) and 12 weeks (r = .73 P < .001). Likewise, significant positive correlations were found between VOL and MT at both time points (baseline: r = .76, P < .001, very large; 12 weeks: r = .73, P < .001, very large; Figure 3B).
Figure 3.
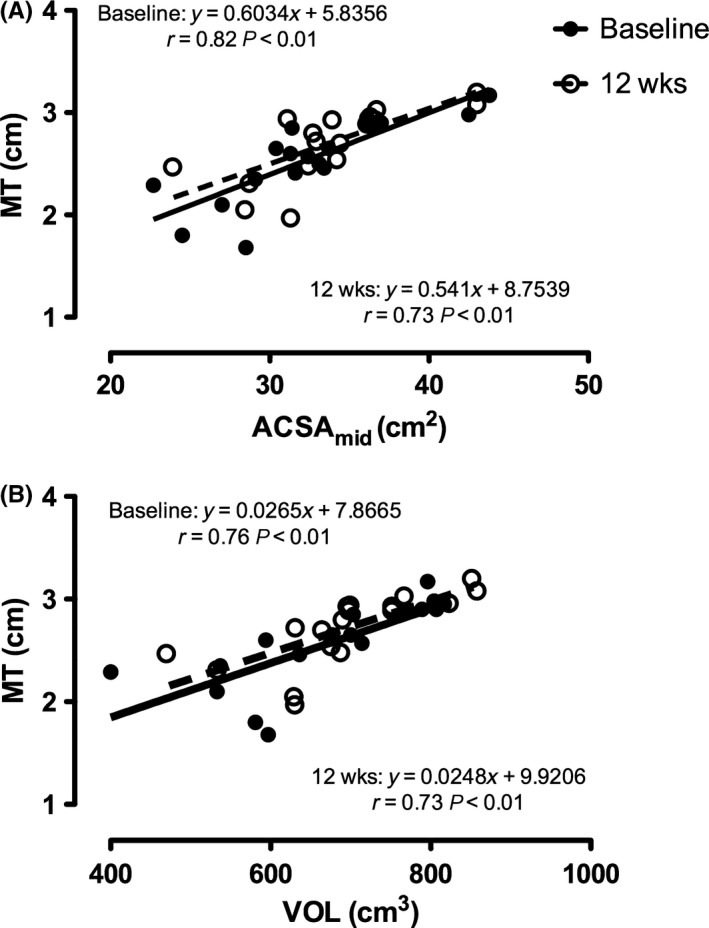
(A) Correlations between vastus lateralis cross‐sectional area measured at midpoint of femur length (ACSA mid) by magnetic resonance imaging and muscle thickness (MT) measured by ultrasound at the same site, before (filled circles, black line) and after 12 wk (empty circles, dashed line) of resistance training (RT). (B) Correlations between vastus lateralis whole volume (VOL) measured by magnetic resonance imaging and MT before and after 12 wk of RT. Participants N = 9. Data represent both legs for each participant (18 points)
A significant positive correlation was found between the percentage increase in VL ACSAmid and percentage increase in MT (r = .69, P = .001 large; Figure 4A). However, no significant relationship was found between the percentage increases in VOL and MT (r = .33, P = .207, Figure 4B).
Figure 4.
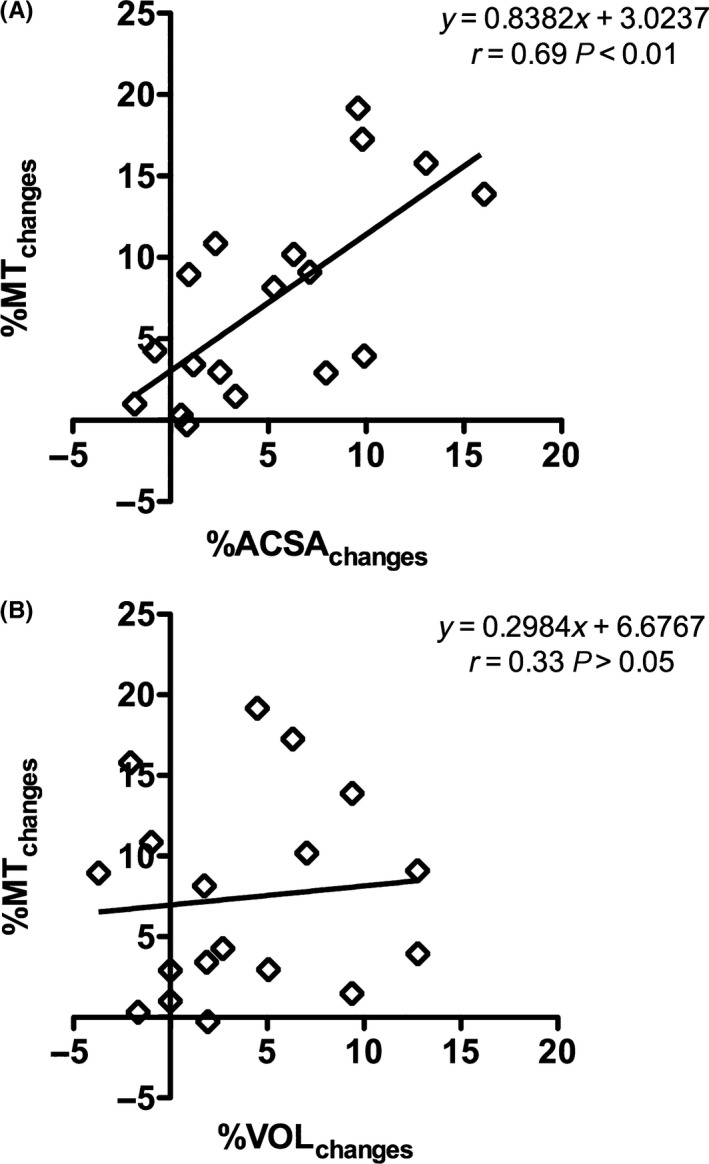
(A) Correlations between the percentage changes in vastus lateralis cross‐sectional area measured at midpoint of femur length (%ACSA changes) by magnetic resonance imaging and muscle thickness (%MT changes) measured by ultrasound at the same site induced by 12 wk of resistance training (RT). (B) Correlations between the percentage changes in vastus lateralis whole volume (%VOL changes) measured by magnetic resonance imaging and %MT changes induced by 12 wk of RT. Participants N = 9. Data represent both legs for each participant (18 points)
4. DISCUSSION
The present study demonstrated that VL MT measured mid‐thigh level using ultrasound before and after a knee extension training protocol was significantly correlated to ACSAmid and VOL assessed by MRI at the same time points. However, when changes in muscle size were expressed as a percentage increase over the training period, only the increase in ACSAmid significantly correlated with the increase in MT. These data support evidence that MT can be regarded as a readily available measure of muscle size that is related to skeletal muscle ACSAmid and VOL when assessed at a single time point. Moreover, the results demonstrate that MT changes following RT are associated with parallel changes in muscle ACSAmid. However, the lack of association between relative changes in MT and relative changes in VOL highlights the impact of RT on regional hypertrophy.
Several studies have reported the measurement of MT in different scenarios to assess muscle hypertrophy,22, 23, 32, 33, 34, 35, 36 atrophy, and/or sarcopenia.34, 37, 38, 39, 40 Other studies have compared ultrasound MT measurement to muscle mass/volume assessed by either DXA or MRI.6, 11, 13, 14, 16, 41 However, to the best of the authors' knowledge, the present study is the first to investigate RT‐induced changes in MT and ACSA or VOL, respectively, assessed by ultrasound and MRI.
In light of the correlations between the changes in ACSAmid and MT, it can be concluded that a single longitudinal ultrasound snapshot is sensitive enough to indicate the presence of VL muscle hypertrophy after a 12‐week RT program. However, a significant correlation between the percentage increase in MT and ACSAmid is opposed to a non‐significant correlation between the changes in MT and VOL. This indicates that a single‐site ultrasound snapshot can detect changes in muscle size but also that these variations are not predictive of changes in muscle volume, which are affected by a heterogenous distribution of hypertrophy.19, 42, 43, 44 From a simple mathematical point of view, the three parameters (MT, ACSA, and VOL) would be expected to change proportionally only if the muscle had a perfectly regular geometrical shape (eg, ellipsoid); in fact, if assuming that the length and the width of a muscle are constant, the increases in muscle VOL should be reflected in a proportional increases in ACSA and MT.
However, the VL muscle does not seem to reflect these geometrical properties in response to knee extension training, as the present findings show different percentage changes among the three measurements. Therefore, as VL MT is canonically assessed at ~50% of the whole muscle length, the measured increase in muscle size at this site might not be representative of the changes occurring along the muscle belly, which reasonably reflect a regional distribution of hypertrophy.19, 42, 43, 44 Moreover, it should be noted that such relationships between MT, ACSA, and VOL could be specific to the type of training adopted in the present study (ie, knee extension). A previous investigation44 has reported regional hypertrophy in quadriceps muscles using a similar training protocol compared to this study, but it is a possibility that other typologies of RT that imply multijoints movements may elicit different regionally specific responses. However, similar regional hypertrophic responses have been reported for VL muscle when RT (concentric‐only vs eccentric‐only protocols) was performed using a leg press machine.19 Concentric‐only RT led to greater VL hypertrophy (in terms of relative increases in ACSA) in the middle of the muscle, and eccentric‐only RT presented more pronounced distal growth. In the present investigation, although both legs performed the same amount of repetitions, one leg was trained with an additional eccentric component. Thus, it is possible that regional adaptations similar to the ones previously reported19 may have occurred in the present study.
As suggested by the present data, ultrasound seems to represent a reliable and cheaper method alternative compared to MRI, for the estimation of changes in muscle mass with RT. The result of the present investigation seems to support the findings of two other training studies recently published by our group, in which we investigated the relationship between DXA‐derived thigh lean mass and MT.23, 45 Both studies showed good correlations between the increase in lean mass and MT just after 4 weeks of resistance training in young men23 and the increase/decrease in lean mass and MT after 31 days of high‐intensity interval training in an older population (males and females).45 However, even if considerably less expensive than MRI, and with the advantage of minimizing radiation exposure compared to CT, DXA presents some drawbacks. In fact, DXA seems to systematically underestimate the age‐related loss of lower limb lean mass compared to the loss in muscle mass assessed by MRI in older individuals.46 A lack of accuracy of DXA in assessing changes in lean mass with strength training compared to MRI‐derived muscle mass has also been demonstrated.47
When investigating muscle adaptations and metabolic aspects, DXA does not provide the possibility to measure separate muscles or muscle groups, whereas this can be easily obtained by ultrasonography. Moreover, many research facilities do not have direct access to a DXA suite and, in addition, it is still considerably more expensive than an ultrasound machine. Although DXA, compared to ultrasound, can provide more information on body composition than just the quantification of muscle mass, the aforementioned drawbacks of such a technique should be taken into account when assessing changes in muscle size.
Ultrasound‐derived MT has also been found to be a useful marker of muscle growth with RT. In fact, two previous studies demonstrated a positive correlation between MT and myofibrillar protein synthesis (expressed in terms of fractional synthetic rates) after just 348 and 4 weeks23 of RT. This reinforces the use of ultrasound as a reliable alternative to more expensive imaging techniques for the measurement of changes in MT as an index of long‐term changes in muscle mass.
Although ultrasound has often been questioned in terms of repeatability, our group and others have demonstrated that, with appropriate operator training, measures can be highly reproducible, as shown by the ICC values from the present study (0.99) and those of previous studies (ranging between 0.997 and 0.999).5 It should be acknowledged that, even if ultrasound is highly reproducible, when comparing MT measurements (obtained from a single plane) to muscle volume measurements, the former might not fully explain the changes in the latter. In fact, it is known from earlier studies (ie, cross‐sectional design, which compared ultrasound‐based measurements of muscle size to MRI‐derived muscle volumes) that MT is related to VOL but explains ~80% of VOL variance.14, 15 Nevertheless, while highlighting the heterogeneity of VL hypertrophic adaptations, the present study stresses that changes in MT are locally related to the ones in ACSA following RT. This is of interest especially for studies that investigate changes in muscle size together with molecular pathways that may regulate such responses, as the muscle site of where the biopsy is taken should then be the same where MT is assessed (~50% of VL length).
Limitations of the present study were the low number and the age‐group specificity of the volunteers. However, both legs were specifically trained (unilaterally, performing different protocols) and assessed; hence, a total of 18 (ie, 9 volunteers, both legs tested and trained differently) values of VL MT, ACSA, and VOL were obtained per single time point. The advantage of training the same participants with different unilateral RT designs is that within‐subject variability in the training responses is minimized, which increases statistical power.23, 49, 50 This design is well established and has been adopted by several previous studies either in the knee extensors51, 52, 53, 54, 55 or in the elbow flexors.50, 56 Although we acknowledge that further studies are needed to specifically investigate the use of MT in different and larger populations (ie, sarcopenic and cachectic individuals, clinical settings), a good relationships between DXA‐assessed lean mass and MT measured by ultrasound in middle‐aged Japanese men and an elderly population (men and women) have been previously reported in cross‐sectional studies.6, 11 Thus, these findings seem to support the employment of ultrasound as tool for assessing skeletal muscle mass adaptations to RT by measuring MT even within an aging population.
5. PERSPECTIVES
The present study supports the use of ultrasound‐measured MT as a reliable tool for monitoring local long‐term hypertrophic responses (changes in VL skeletal muscle ACSAmid) induced by RT as an alternative to the more expensive MRI technique. However, the non‐significant correlation between the percentage changes of VL MT vs whole VOL highlights the importance of accounting for regional hypertrophy. Hence, MT should not be used to estimate changes in muscle volume. These considerations are of primary importance when assessing regional/local vs whole muscle hypertrophic adaptations, especially in relation to heterogeneous molecular/metabolic responses along the full muscle length, such as when ultrasound scan sites and muscle biopsy sites are not the same.
ACKNOWLEDGEMENTS
This work was supported by the Biotechnology and Biological Sciences Research Council, [grant number BB/I020713/1].
Franchi MV, Longo S, Mallinson J, et al. Muscle thickness correlates to muscle cross‐sectional area in the assessment of strength training‐induced hypertrophy. Scand J Med Sci Sports. 2018;28:846–853. https://doi.org/10.1111/sms.12961
Greenhaff and Narici are joint senior authors.
REFERENCES
- 1. Heymsfield SB, Adamek M, Gonzalez MC, Jia G, Thomas DM. Assessing skeletal muscle mass: historical overview and state of the art. J Cachexia Sarcopenia Muscle. 2014;5:9‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ross R, Pedwell H, Rissanen J. Effects of energy restriction and exercise on skeletal muscle and adipose tissue in women as measured by magnetic resonance imaging. Am J Clin Nutr. 1995;61:1179‐1185. [DOI] [PubMed] [Google Scholar]
- 3. Ross R, Rissanen J, Pedwell H, Clifford J, Shragge P. Influence of diet and exercise on skeletal muscle and visceral adipose tissue in men. J Appl Physiol. 1996;81:2445‐2455. [DOI] [PubMed] [Google Scholar]
- 4. Narici M. Human skeletal muscle architecture studied in vivo by non‐invasive imaging techniques: functional significance and applications. J Electromyogr Kinesiol. 1999;9:97‐103. [DOI] [PubMed] [Google Scholar]
- 5. Reeves ND, Maganaris CN, Narici MV. Ultrasonographic assessment of human skeletal muscle size. Eur J Appl Physiol. 2004;91:116‐118. [DOI] [PubMed] [Google Scholar]
- 6. Abe T, Loenneke JP, Thiebaud RS. Morphological and functional relationships with ultrasound measured muscle thickness of the lower extremity: a brief review. Ultrasound. 2015;23:166‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parry SM, El‐Ansary D, Cartwright MS, et al. Ultrasonography in the intensive care setting can be used to detect changes in the quality and quantity of muscle and is related to muscle strength and function. J Crit Care. 2015;30:1151.e9‐1151.e14. [DOI] [PubMed] [Google Scholar]
- 8. Bunnell A, Ney J, Gellhorn A, Hough CL. Quantitative neuromuscular ultrasound in intensive care unit‐acquired weakness: a systematic review. Muscle Nerve. 2015;52:701‐708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dias CP, Freire B, Goulart NBA, et al. Muscle architecture and torque production in stroke survivors: an observational study. Top Stroke Rehabil. 2017;24:206‐213. doi: 10.1080/10749357.2016.1210873. [DOI] [PubMed] [Google Scholar]
- 10. Cho KH, Lee HJ, Lee WH. Intra‐ and inter‐rater reliabilities of measurement of ultrasound imaging for muscle thickness and pennation angle of tibialis anterior muscle in stroke patients. Top Stroke Rehabil. 2017;24:368‐373. https://doi.org/10.1080/10749357.2017.1285745. [DOI] [PubMed] [Google Scholar]
- 11. Takai Y, Ohta M, Akagi R, et al. Validity of ultrasound muscle thickness measurements for predicting leg skeletal muscle mass in healthy Japanese middle‐aged and older individuals. J Physiol Anthropol. 2013;32:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takai Y, Ohta M, Akagi R, et al. Applicability of ultrasound muscle thickness measurements for predicting fat‐free mass in elderly population. J Nutr Health Aging. 2014;18:579‐585. [DOI] [PubMed] [Google Scholar]
- 13. Abe T, Kawakami Y, Suzuki Y, Gunji A, Fukunaga T. Effects of 20 days bed rest on muscle morphology. J Gravit Physiol. 1997;4:S10‐S14. [PubMed] [Google Scholar]
- 14. Miyatani M, Kanehisa H, Kuno S, Nishijima T, Fukunaga T. Validity of ultrasonograph muscle thickness measurements for estimating muscle volume of knee extensors in humans. Eur J Appl Physiol. 2002;86:203‐208. [DOI] [PubMed] [Google Scholar]
- 15. Kanehisa H, Ito M, Kawakami Y, Fukunaga T, Miyatani M. The accuracy of volume estimates using ultrasound muscle thickness measurements in different muscle groups. Eur J Appl Physiol. 2004;91:264‐272. [DOI] [PubMed] [Google Scholar]
- 16. Sanada K, Kearns CF, Midorikawa T, Abe T. Prediction and validation of total and regional skeletal muscle mass by ultrasound in Japanese adults. Eur J Appl Physiol. 2006;96:24‐31. [DOI] [PubMed] [Google Scholar]
- 17. Seynnes OR, Maganaris CN, de Boer MD, di Prampero PE, Narici MV. Early structural adaptations to unloading in the human calf muscles. Acta Physiol. 2008;193:265‐274. [DOI] [PubMed] [Google Scholar]
- 18. Seynnes OR, Erskine RM, Maganaris CN, et al. Training‐induced changes in structural and mechanical properties of the patellar tendon are related to muscle hypertrophy but not to strength gains. J Appl Physiol. 2009;107:523‐530. http://jap.physiology.org/content/107/2/523#ref-49. [DOI] [PubMed] [Google Scholar]
- 19. Franchi MV, Atherton PJ, Reeves ND, et al. Architectural, functional, and molecular responses to concentric and eccentric loading in human skeletal muscle. Acta Physiol (Oxf). 2014;210:642‐654. https://doi.org/10.1111/apha.12225. [DOI] [PubMed] [Google Scholar]
- 20. Weiss LW. The Use of B‐mode ultrasound for measuring the thickness of skeletal muscle at two upper leg sites. J Orthop Sports Phys Ther. 1984;6:163‐167. [Google Scholar]
- 21. Kawakami Y, Abe T, Fukunaga T. Muscle‐fiber pennation angles are greater in hypertrophied than in normal muscles. J Appl Physiol. 1993;74:2740‐2744. [DOI] [PubMed] [Google Scholar]
- 22. Reeves ND, Maganaris CN, Longo S, Narici MV. Differential adaptations to eccentric versus conventional resistance training in older humans. Exp Physiol. 2009;94:825‐833. [DOI] [PubMed] [Google Scholar]
- 23. Franchi MV, Wilkinson DJ, Quinlan JI, et al. Early structural remodeling and deuterium oxide‐derived protein metabolic responses to eccentric and concentric loading in human skeletal muscle. Physiol Rep. 2015;3:e12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Akagi R, Iwanuma S, Fukuoka M, Kanehisa H, Fukunaga T, Kawakami Y. Methodological issues related to thickness‐based muscle size evaluation. J Physiol Anthropol. 2011;30:169‐174. [DOI] [PubMed] [Google Scholar]
- 25. Narici MV, Binzoni T, Hiltbrand E, Fasel J, Terrier F, Cerretelli P. In vivo human gastrocnemius architecture with changing joint angle at rest and during graded isometric contraction. J Physiol. 1996;496(Pt 1):287‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reeves ND, Narici MV, Maganaris CN. In vivo human muscle structure and function: adaptations to resistance training in old age. Exp Physiol. 2004;89:675‐689. [DOI] [PubMed] [Google Scholar]
- 27. Seynnes OR, de Boer M, Narici MV. Early skeletal muscle hypertrophy and architectural changes in response to high‐intensity resistance training. J Appl Physiol. 2007;102:368‐373. [DOI] [PubMed] [Google Scholar]
- 28. Batterham AM, Hopkins WG. Making meaningful inferences about magnitudes. Int J Sports Physiol Perform. 2006;1:50‐57. [PubMed] [Google Scholar]
- 29. Weir JP. Quantifying test‐retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res. 2005;19:231‐240. [DOI] [PubMed] [Google Scholar]
- 30. Donoghue D, Stokes E, Stokes EK. How much change is true change? The minimum detectable change of the Berg Balance Scale in elderly people. J Rehabil Med. 2009;41:343‐346. [DOI] [PubMed] [Google Scholar]
- 31. Munro BH. Statistical Methods for Health Care Research. Vol 5th edn Philadelphia, PA: Lippincott William and Wilkins; 2004. [Google Scholar]
- 32. Kawakami Y, Abe T, Kuno SY, Fukunaga T. Training‐induced changes in muscle architecture and specific tension. Eur J Appl Physiol Occup Physiol. 1995;72:37‐43. [DOI] [PubMed] [Google Scholar]
- 33. Starkey DB, Pollock ML, Ishida Y, et al. Effect of resistance training volume on strength and muscle thickness. Med Sci Sports Exerc. 1996;28:1311‐1320. [DOI] [PubMed] [Google Scholar]
- 34. Narici MV, Flueck M, Koesters A, et al. Skeletal muscle remodeling in response to alpine skiing training in older individuals. Scand J Med Sci Sports. 2011;21(Suppl 1):23‐28. [DOI] [PubMed] [Google Scholar]
- 35. Hernandez HJ, McIntosh V, Leland A, Harris‐Love MO. Progressive resistance exercise with eccentric loading for the management of knee osteoarthritis. Front Med. 2015;2:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bjørnsen T, Salvesen S, Berntsen S, et al. Vitamin C and E supplementation blunts increases in total lean body mass in elderly men after strength training. Scand J Med Sci Sports. 2016;26:755‐763. [DOI] [PubMed] [Google Scholar]
- 37. de Boer MD, Seynnes OR, di Prampero PE, et al. Effect of 5 weeks horizontal bed rest on human muscle thickness and architecture of weight bearing and non‐weight bearing muscles. Eur J Appl Physiol. 2008;104:401‐407. [DOI] [PubMed] [Google Scholar]
- 38. Pišot R, Narici MV, Šimunič B, et al. Whole muscle contractile parameters and thickness loss during 35‐day bed rest. Eur J Appl Physiol. 2008;104:409‐414. [DOI] [PubMed] [Google Scholar]
- 39. Li R, Narici MV, Erskine RM, et al. Costamere remodeling with muscle loading and unloading in healthy young men. J Anat. 2013;223:525‐536. https://doi.org/10.1111/joa.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Atkinson RA, Srinivas‐Shankar U, Roberts SA, et al. Effects of testosterone on skeletal muscle architecture in intermediate‐frail and frail elderly men. J Gerontol A Biol Sci Med Sci. 2010;65:1215‐1219. [DOI] [PubMed] [Google Scholar]
- 41. Sipila S, Suominen H. Ultrasound imaging of the quadriceps muscle in elderly athletes and untrained men. Muscle Nerve. 1991;14:527‐533. [DOI] [PubMed] [Google Scholar]
- 42. Narici MV, Roi GS, Landoni L, Minetti AE, Cerretelli P. Changes in force, cross‐sectional area and neural activation during strength training and detraining of the human quadriceps. Eur J Appl Physiol Occup Physiol. 1989;59:310‐319. [DOI] [PubMed] [Google Scholar]
- 43. Narici MV, Hoppeler H, Kayser B, et al. Human quadriceps cross‐sectional area, torque and neural activation during 6 months strength training. Acta Physiol Scand. 1996;157:175‐186. [DOI] [PubMed] [Google Scholar]
- 44. Seger JY, Arvidsson B, Thorstensson A. Specific effects of eccentric and concentric training on muscle strength and morphology in humans. Eur J Appl Physiol Occup Physiol. 1998;79:49‐57. [DOI] [PubMed] [Google Scholar]
- 45. Boereboom CL, Phillips BE, Williams JP, Lund JN. A 31‐day time to surgery compliant exercise training programme improves aerobic health in the elderly. Tech Coloproctol. 2016;20:375‐382. [DOI] [PubMed] [Google Scholar]
- 46. Maden‐Wilkinson TM, Degens H, Jones DA, McPhee JS. Comparison of MRI and DXA to measure muscle size and age‐related atrophy in thigh muscles. J Musculoskelet Neuronal Interact. 2013;13:320‐328. [PubMed] [Google Scholar]
- 47. Delmonico MJ, Kostek MC, Johns J, Hurley BF, Conway JM. Can dual energy X‐ray absorptiometry provide a valid assessment of changes in thigh muscle mass with strength training in older adults? Eur J Clin Nutr. 2008;62:1372‐1378. [DOI] [PubMed] [Google Scholar]
- 48. Brook MS, Wilkinson DJ, Mitchell WK, et al. Skeletal muscle hypertrophy adaptations predominate in the early stages of resistance exercise training, matching deuterium oxide‐derived measures of muscle protein synthesis and mechanistic target of rapamycin complex 1 signaling. FASEB J. 2015;29:4485‐4496. https://doi.org/10.1096/fj.15-273755. [DOI] [PubMed] [Google Scholar]
- 49. Hubal MJ, Gordish‐Dressman H, Thompson PD, et al. Variability in muscle size and strength gain after unilateral resistance training. Med Sci Sports Exerc. 2005;37:964‐972. [PubMed] [Google Scholar]
- 50. Moore DR, Young M, Phillips SM. Similar increases in muscle size and strength in young men after training with maximal shortening or lengthening contractions when matched for total work. Eur J Appl Physiol. 2012;112:1587‐1592. [DOI] [PubMed] [Google Scholar]
- 51. Moore DR, Phillips SM, Babraj JA, Smith K, Rennie MJ. Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions. Am J Physiol Endocrinol Metab. 2005;288:E1153‐E1159. [DOI] [PubMed] [Google Scholar]
- 52. Kim PL, Staron RS, Phillips SM. Fasted‐state skeletal muscle protein synthesis after resistance exercise is altered with training. J Physiol. 2005;568(Pt 1):283‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cuthbertson DJ, Babraj J, Smith K, et al. Anabolic signaling and protein synthesis in human skeletal muscle after dynamic shortening or lengthening exercise. Am J Physiol Endocrinol Metab. 2006;290:E731‐E738. [DOI] [PubMed] [Google Scholar]
- 54. Kostek MC, Chen Y‐W, Cuthbertson DJ, et al. Gene expression responses over 24 h to lengthening and shortening contractions in human muscle: major changes in CSRP3, MUSTN1, SIX1, and FBXO32. Physiol Genomics. 2007;31:42‐52. [DOI] [PubMed] [Google Scholar]
- 55. Kumar V, Atherton P, Smith K, Rennie MJ. Human muscle protein synthesis and breakdown during and after exercise. J Appl Physiol. 2009;106:2026‐2039. [DOI] [PubMed] [Google Scholar]
- 56. West DWD, Burd NA, Tang JE, et al. Elevations in ostensibly anabolic hormones with resistance exercise enhance neither training‐induced muscle hypertrophy nor strength of the elbow flexors. J Appl Physiol. 2010;108:60‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]


