Abstract
Objective
To assess the feasibility and efficacy of implementing a treat‐to‐target approach versus usual care in a US‐based cohort of rheumatoid arthritis patients.
Methods
In this behavioral intervention trial, rheumatology practices were cluster‐randomized to provide treat‐to‐target care or usual care. Eligible patients with moderate/high disease activity (Clinical Disease Activity Index [CDAI] score >10) were followed for 12 months. Both treat‐to‐target and usual care patients were seen every 3 months. Treat‐to‐target providers were to have monthly visits with treatment acceleration at a minimum of every 3 months in patients with CDAI score >10; additional visits and treatment acceleration were at the discretion of usual care providers and patients. Coprimary end points were feasibility, assessed by rate of treatment acceleration conditional on CDAI score >10, and achievement of low disease activity (LDA; CDAI score ≤10) by an intent‐to‐treat analysis.
Results
A total of 14 practice sites per study arm were included (246 patients receiving treat‐to‐target and 286 receiving usual care). The groups had similar baseline demographic and clinical characteristics. Rates of treatment acceleration (treat‐to‐target 47% versus usual care 50%; odds ratio [OR] 0.92 [95% confidence interval (95% CI) 0.64, 1.34]) and achievement of LDA (treat‐to‐target 57% versus usual care 55%; OR 1.05 [95% CI 0.60, 1.84]) were similar between groups. Treat‐to‐target providers reported patient reluctance and medication lag time as common barriers to treatment acceleration.
Conclusion
This study is the first to examine the feasibility and efficacy of a treat‐to‐target approach in typical US rheumatology practice. Treat‐to‐target care was not associated with increased likelihood of treatment acceleration or achievement of LDA, and barriers to treatment acceleration were identified.
Introduction
The ultimate goal of treatment of rheumatoid arthritis (RA) is achievement of clinical remission, or at least low disease activity (LDA) when remission is not possible due to comorbidities that prohibit treatment intensification or in cases of established RA 1. There is a growing consensus that the use of a treat‐to‐target approach optimizes clinical outcomes in patients with RA. Treat‐to‐target care requires frequent assessment of patient disease activity using validated measures and acceleration of treatment until remission or LDA is achieved 1. Such frequent monitoring can be used to measure and document treatments and assess whether a treatment is effective or should be altered 2.
Significance & Innovations.
This was the first study to examine both feasibility and efficacy of implementing a treat‐to‐target approach to the care of patients with rheumatoid arthritis in typical clinical practice in the US.
The frequency of treatment acceleration was similar between the treat‐to‐target and usual care approaches, with acceleration occurring at approximately 47% of visits at which a patient had a Clinical Disease Activity Index (CDAI) score >10 in either arm; the most frequent reasons for not accelerating therapy in treat‐to‐target patients with CDAI score >10 were patient preference and physicians suspecting a lag in response to medication.
Treat‐to‐target and usual care were equally effective in achievement of low disease activity (LDA) over time and at the 12‐month end point, with approximately 60% of patients in either arm achieving LDA at 12 months.
These results provide important insights into improving implementation of a treat‐to‐target approach in everyday practice, particularly the need for patient engagement.
Multiple studies have suggested an advantage of treat‐to‐target management over usual care. The Tight Control of Rheumatoid Arthritis (TICORA) trial demonstrated that mandated treat‐to‐target treatment led to greater reduction in disease activity and higher remission rates than usual care 3. The TICORA findings have been supported by several other studies, including data from the Dutch Rheumatoid Arthritis Monitoring Registry and the Computer Assisted Management in Early Rheumatoid Arthritis trial, which demonstrated greater improvement in the 28‐joint Disease Activity Score (DAS28) in patients with early RA treated with a treat‐to‐target strategy 4, 5. Importantly, these trials were conducted in Europe, and it is possible that differences in health care systems, physician practice structure, and patient characteristics may affect applicability of these results to US rheumatology. Additionally, these trials were predominantly conducted in patients with early RA and, in some cases, prior to the use of biologic agents.
The feasibility of a treat‐to‐target approach for the routine care of patients in typical clinical practice in the US has not been evaluated. Specifically, adapting a treat‐to‐target approach to RA care for US rheumatologists and their patients in busy clinical practices needs to be assessed. Potential barriers to widespread implementation of a treat‐to‐target strategy include the increased frequency of visits mandated by treat‐to‐target recommendations, which may be challenging given limited provider availability (e.g., limited followup appointments) and busy patient and clinic schedules 6. Routine acceleration of therapy may be challenging due to patient out‐of‐pocket expenses for travel and copays at the time of additional appointments, denials or delays in medications by insurers, clinical inertia on the part of physicians, and patient reluctance to increase or change medication frequently.
Considering these potential barriers, evaluating the challenges and feasibility of treat‐to‐target implementation in a US patient population is important. We designed a cluster‐randomized behavioral intervention trial targeting rheumatologists to facilitate the implementation of the treat‐to‐target approach for all patients, irrespective of disease duration, in a variety of US rheumatology practices. The coprimary objectives were to assess the feasibility of implementing a treat‐to‐target approach in US rheumatology practices and to compare the efficacy of treat‐to‐target care versus usual care in achievement of LDA; these were selected as coprimary objectives because efficacy is likely related to the feasibility of implementing the treat‐to‐target approach.
Patients and methods
Study population
The design and rationale for the study have been reported previously 7. Briefly, rheumatology practices that were affiliated with the CORRONA RA registry (comprising >160 private and academic practice sites across 40 US states) and practices that were not affiliated were approached regarding participation 8, 9. Eligible sites that agreed to participate were stratified by the number of patients they anticipated enrolling and cluster‐randomized 1:1 to administer treat‐to‐target care or usual care to enrolled patients, with the patient as the unit of analysis. All patients enrolled at a site received the same study arm assignment of care. This cluster‐randomization approach was used to address the concern of unintended crossover, because for rheumatologists to give usual care if they were also caring for patients randomized to the treat‐to‐target arm would be challenging. This approach was also used to address potential overlapping influence that might introduce bias if the same staff members at a study site were assigned to different treatment arms. Based on the power analyses described previously, our goal was to enroll a total of 530 patients, with 265 (17–18 patients at each of 15 sites) randomized per study arm 7. Sample size was determined based on the primary outcome of achievement of LDA, using historical CORRONA data that revealed an LDA achievement rate of 40% using the study inclusion criteria. The comparison of treat‐to‐target versus usual care had 80% power to detect an odds ratio (OR) of ≥1.50, which represents anticipated LDA rates of 60% in the treat‐to‐target arm versus 40% in the usual care arm.
Patients were invited to participate by their practicing rheumatologist. Eligible patients were outpatients ages ≥18 years who met the 2010 revised American College of Rheumatology criteria for the diagnosis of RA 10, had moderate to severe disease activity based on Clinical Disease Activity Index (CDAI) score >10, and were deemed medically appropriate for treatment acceleration by their rheumatologist. Patients were required to agree to the schedule of study visits (including the possibility of more frequent visits in the treat‐to‐target arm), be willing to have their therapy escalated as appropriate, and provide informed consent. Patients were included regardless of RA disease duration or prior medication use to ensure a typical practice sample of participants. Patients considered ineligible for treatment acceleration were excluded from the study 7. Practices were recruited from June 2011 through June 2013, with the first patient visit on July 29, 2011, and the last on July 29, 2014. The study was conducted per the current (2013) version of the Declaration of Helsinki. Institutional review board (IRB) approvals were obtained from local IRBs of participating academic sites and a central IRB (New England IRB) for private practice sites, and were required before study participation and randomization.
Study design
Both treat‐to‐target and usual care patients had mandated research visits every 3 months, at which time they completed CORRONA questionnaires (Figure 1). Clinical data, including CDAI score and medication use, were collected at each visit. Frequencies of visits, all changes in medication, and any adverse events (AEs) were tracked throughout the course of the study.
Figure 1.
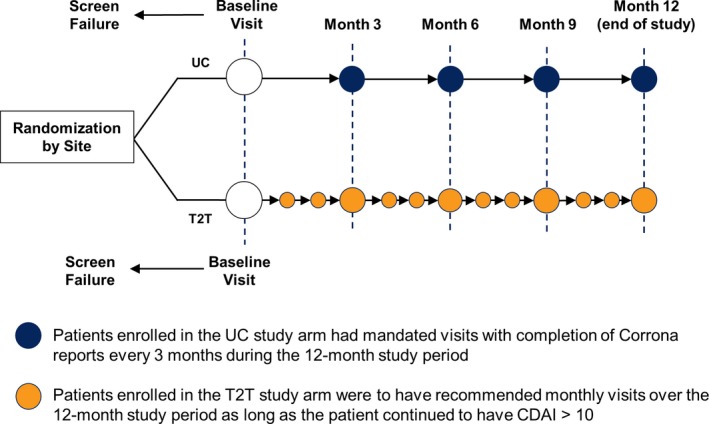
Study design. UC = usual care; T2T = treat‐to‐target; CDAI = Clinical Disease Activity Index score.
Intervention
All treat‐to‐target providers and sites received a training session on the treat‐to‐target treatment paradigm, including measuring disease activity using the CDAI score at every visit, and were asked to schedule monthly visits until LDA (CDAI score ≤10) was achieved according to the 2010 international recommendations 11. Prompts for treatment acceleration in the setting of elevated disease activity were provided through study questionnaires in the treat‐to‐target arm. Training was supplemented with informational e‐mails, newsletter reminders, site feedback, and telephone discussions to reinforce the treat‐to‐target philosophy. Providers in the treat‐to‐target arm were to escalate therapy no less frequently than every 3 months if LDA had not been achieved, unless good clinical practice dictated otherwise in the event of a contraindication or patient unwillingness to change therapy. Reasons for not accelerating treatment were recorded by the investigators. Treatment acceleration was defined as a dosage increase, the addition of or switch to a conventional synthetic disease‐modifying antirheumatic drug (csDMARD) or biologic agent, or a change from oral to subcutaneous methotrexate. Initiation or increased dosing of glucocorticoids was not considered treatment acceleration. Due to the behavioral intervention design of the trial, providers in the treat‐to‐target arm were not audited for adherence to the treat‐to‐target approach during the study and received no feedback on their performance. In the usual care group, the frequency of any additional visits and treatment acceleration was left to the discretion of investigators and patients. Usual care providers were made aware prior to receiving their treatment arm assignment that a treat‐to‐target intervention would be compared with usual care.
Outcome measures
The predefined coprimary outcomes were the feasibility of implementing a treat‐to‐target approach to care in US rheumatology practice, and the efficacy of treat‐to‐target care versus usual care in the achievement of LDA. Feasibility of the treat‐to‐target approach was assessed by comparing the rates of treatment acceleration conditional on moderate or high disease activity in the 2 groups. Efficacy was evaluated by the achievement of LDA as defined by CDAI score ≤10 at 12 months by an intent‐to‐treat analysis.
Secondary objectives were to compare between the 2 study arms: the change in CDAI score from baseline to 12 months, the proportion of patients in LDA at each mandated research visit (3, 6, 9, and 12 months), the mean CDAI score over time in the total population and in patients not in LDA overall and those with no treatment accelerations in the prior 3 months, trends in treatment acceleration over time, and the rates of infection, cardiovascular, and malignancy AEs over the study period. In the treat‐to‐target arm, the reasons for lack of acceleration for all visits with a CDAI score >10 were evaluated. Subanalyses limiting the sample to those visits without a treatment acceleration in the prior 3 months were also performed.
Statistical analysis
The characteristics of patients enrolled into the treat‐to‐target and usual care arms were compared using standardized differences, which are less influenced by sample size. Feasibility of the treat‐to‐target approach was assessed by comparing the rate of treatment acceleration conditional on CDAI score >10 between the treat‐to‐target and usual care arms. The rate of achievement of LDA (CDAI score ≤10) at 12 months was analyzed using an intent‐to‐treat approach. In a subanalysis, the rate of achievement of LDA at 12 months was examined using only those patients who completed the study. The rates of acceleration and achievement of LDA were adjusted for clustering of patients by physician and physicians by practice sites, as well as baseline covariates with |standardized differences| >0.1, using linear random‐effects mixed models. A standardized difference <0.1 has been taken to indicate a negligible difference in the mean or prevalence of a covariate between treatment groups 12, 13. Multiple imputation using chained equations was performed to replace missing values for covariates associated with the primary efficacy outcome, achievement of LDA 14.
Change in CDAI score at 12 months was compared between groups using linear mixed models and an intent‐to‐treat approach adjusting for clustering and covariates as outlined for the primary outcome. The proportion of patients with LDA in both the treat‐to‐target and usual care groups at each research visit (3, 6, 9, and 12 months) was determined in the intent‐to‐treat population. The mean CDAI score for all visits in the total study population and among patients who did not achieve LDA overall and those with no treatment accelerations in the previous 3 months was examined. The occurrence of infection, cardiovascular, and malignancy AEs was compared between groups using a time to first event in the category approach and the log‐rank test to evaluate differences. The probability of acceleration based on CDAI score >10 at each visit was calculated by identifying all visits for each patient at which CDAI score >10 occurred (all eligible accelerations) and determining how many of these visits were associated with a treatment acceleration by the end of the visit or by the next visit. In a subanalysis, we excluded visits where there had been an acceleration in the prior 3 months (eligible new accelerations), as these patients were likely not candidates for acceleration due to the recent medication change. As supplementary information, we provided both unadjusted and adjusted (for clustering and baseline values) changes in World Health Organization/International League of Associations of Rheumatology (WHO/ILAR) core set outcomes (tender and swollen joint counts, pain, physician and patient global assessments, Health Assessment Questionnaire score, and erythrocyte sedimentation rate) 15.
Results
Patient demographics and baseline characteristics
A total of 106 sites were approached. Thirty‐one sites agreed to participate and were able to comply with the study protocol, 16 of which were randomized to the treat‐to‐target arm and 15 to the usual care arm. Two practices in the treat‐to‐target group and 1 in the usual care group did not enroll any patients; 14 study sites per group were included in the final analysis. The total number of patients eligible for inclusion in the analysis was 246 (median [range] 12 [5–41] patients per site) in the treat‐to‐target arm and 286 (median [range] 13.5 [2–40] patients per site) in the usual care arm. Of the eligible enrolled patients, 197 patients (80%) in the treat‐to‐target arm and 239 patients (84%) in the usual care arm completed the study (Figure 2).
Figure 2.
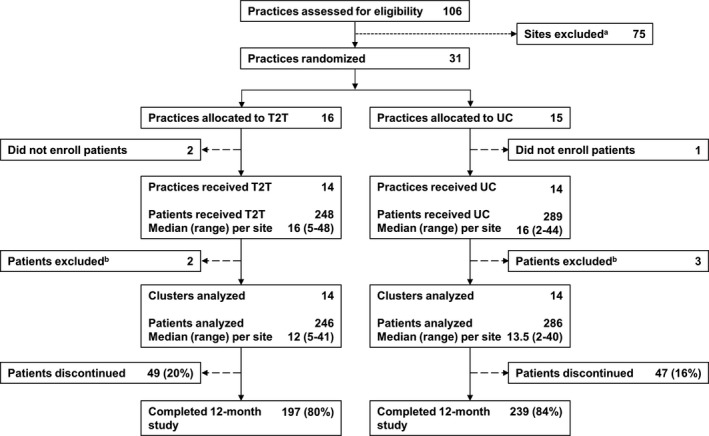
Study flow diagram. T2T = treat‐to‐target; UC = usual care; a = reasons for site exclusion were refusal to participate, inability to initiate in time to recruit, or lack of response to outreach; b = reasons for patient exclusion were low disease activity (T2T, n = 2; UC, n = 2) and missing Clinical Disease Activity Index score (UC, n = 1).
Patient demographics and baseline disease characteristics were mostly similar between intervention groups (Table 1), except for a larger proportion of self‐reported Hispanic patients in the treat‐to‐target arm (22%) compared with the usual care arm (8%). In the treat‐to‐target arm, mean ± SD age was 57 ± 12.8 years, 80% of patients were women, and mean ± SD RA disease duration was 7.3 ± 9.5 years. In the usual care arm, mean ± SD age was 58 ± 13.1 years, 79% of patients were women, and mean ± SD RA disease duration was 8.4 ± 9.4 years.
Table 1.
Baseline demographics of the treat‐to‐target and usual care study populationsa
| Treat‐to‐target (n = 246) | Usual care (n = 286) | Standardized differences | |||
|---|---|---|---|---|---|
| No. | Value | No. | Value | ||
| Age, years | 240 | 57.0 ± 12.8 | 271 | 58.0 ± 13.1 | 0.08 |
| Female, no. (%) | 246 | 196 (79) | 286 | 224 (79) | 0.01 |
| Hispanic, no. (%) | 210 | 47 (22) | 219 | 18 (8) | 0.40 |
| Race, no. (%) | |||||
| White | 206 | 181 (88) | 250 | 224 (90) | 0.06 |
| African American | 206 | 15 (7) | 250 | 14 (6) | 0.07 |
| Asian | 206 | 3 (2) | 250 | 3 (1) | 0.02 |
| Other/mixed | 206 | 7 (3) | 250 | 9 (4) | 0.01 |
| College educated, no. (%) | 236 | 133 (56) | 277 | 160 (58) | 0.03 |
| Part‐ or full‐time employment, no. (%) | 243 | 119 (49) | 280 | 141 (50) | 0.03 |
| Insurance, no. (%) | |||||
| Private | 224 | 168 (75) | 264 | 202 (77) | 0.04 |
| Medicare | 224 | 60 (27) | 264 | 86 (33) | 0.13 |
| Medicaid | 224 | 18 (8) | 264 | 12 (5) | 0.14 |
| No insurance | 224 | 10 (5) | 264 | 9 (3) | 0.05 |
| RA characteristics | |||||
| Disease duration, years | 245 | 7.3 ± 9.5 | 282 | 8.4 ± 9.4 | 0.12 |
| RF seropositivity, no. (%) | 184 | 124 (67) | 207 | 153 (74) | 0.14 |
| HAQ DI (0–3) | 234 | 1.1 ± 0.7 | 266 | 1.0 ± 0.7 | 0.07 |
| Disease activity | |||||
| CDAI (0–76) | 246 | 26.7 ± 13.4 | 286 | 25.5 ± 11.8 | 0.09 |
| CDAI disease activity category, no. (%) | |||||
| Moderate (CDAI >10 to ≤22) | 246 | 121 (49) | 286 | 139 (49) | 0.01 |
| High (CDAI >22) | 246 | 125 (51) | 286 | 147 (51) | 0.01 |
| TJC (0–28) | 246 | 8.0 ± 7.0 | 286 | 7.8 ± 5.5 | 0.03 |
| SJC (0–28) | 246 | 8.1 ± 5.6 | 286 | 7.3 ± 5.1 | 0.14 |
| ESR, mm/hour | 211 | 28.5 ± 24.9 | 240 | 29.8 ± 23.8 | 0.05 |
| CRP, mg/dl | 72 | 32.6 ± 83.7 | 166 | 24.6 ± 87.8 | 0.09 |
| Medication, no. (%) | |||||
| Biologic agent naive | 246 | 140 (57) | 286 | 158 (55) | 0.03 |
| Current glucocorticoid | 246 | 92 (37) | 286 | 103 (36) | 0.03 |
| Current biologic agent/small moleculeb | 246 | 54 (22) | 286 | 79 (28) | 0.13 |
| Current csDMARDsc | 246 | 85 (35) | 286 | 120 (42) | 0.15 |
| Current MTX | 246 | 165 (67) | 286 | 186 (65) | 0.04 |
Values are the mean ± SD unless indicated otherwise. RA = rheumatoid arthritis; RF = rheumatoid factor; HAQ DI = Health Assessment Questionnaire Disability Index score; CDAI = Clinical Disease Activity Index score; TJC = tender joint count; SJC = swollen joint count; ESR = erythrocyte sedimentation rate; CRP = C‐reactive protein; csDMARDs = conventional synthetic disease‐modifying antirheumatic drugs; MTX = methotrexate.
All patients undergoing treatment with a biologic agent or small molecule, with or without concurrent use of csDMARDs, at enrollment.
Patients treated with csDMARDs without a concurrent biologic agent or small molecule at enrollment.
Primary outcomes
The frequency of visits was significantly greater in the treat‐to‐target arm than the usual care arm, with a mean number of visits per person‐year of followup of 7.68 and 5.58, respectively (P < 0.001). However, there was no difference in the overall probability of treatment accelerations for patients with CDAI score >10 between the treat‐to‐target and usual care arms (Figure 3A), with approximately 47% of visits in either arm associated with therapy escalation.
Figure 3.
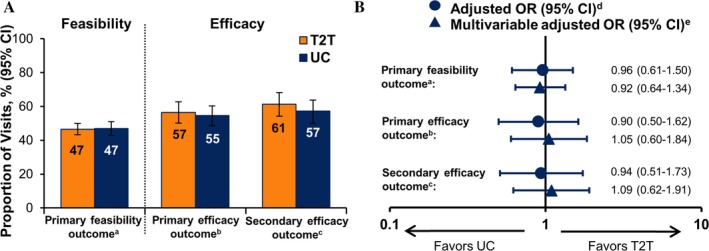
Outcomes in the treat‐to‐target (T2T) versus usual care (UC) groups at 12 months. A, Unadjusted response rates at 12 months. B, Odds ratio (OR) comparing response rates with T2T versus UC at 12 months, adjusted for clustering and patient baseline characteristics. 95% CI = 95% confidence interval; a = the primary feasibility outcome was the probability of treatment acceleration conditional on Clinical Disease Activity Index score >10; b = the primary efficacy outcome was overall achievement of low disease activity (LDA); c = the secondary efficacy outcome was achievement of LDA among patients who completed the study (analysis of those who completed); d = T2T versus UC adjusted for clustering by physician for the efficacy outcomes and for clustering by patient, physician, and practice site for the feasibility outcomes; e = adjusted for age, Hispanic ethnicity, Medicare insurance, rheumatoid factor seropositivity, disease duration, number of prior biologic agents, number of prior conventional synthetic disease‐modifying antirheumatic drugs, current biologic agent/small molecule use, and clustering.
Similarly, there was no significant difference between the treat‐to‐target and usual care groups in the achievement of LDA (P = 0.665) (Figure 3A). At last followup, 139 patients (57%) in the treat‐to‐target group and 156 (55%) in the usual care group had achieved LDA (CDAI score ≤10). This similarity in efficacy was consistent when rates of LDA were restricted to only those patients who completed the study (P = 0.434). Models adjusting for clustering and baseline characteristics revealed no significant difference between the treat‐to‐target and usual care arms for either the feasibility or efficacy outcomes (Figure 3B).
Secondary outcomes
Secondary analyses comparing change in CDAI score in the treat‐to‐target versus usual care arms, adjusted for clustering and baseline characteristics, showed no significant difference at 12 months (0.61 [95% confidence interval −2.02, 3.24]). There were no differences between the 2 groups in the proportion of patients with LDA at each visit (see Supplementary Figure 1, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.23294/abstract) or in the mean CDAI score over time in the total population or in patients eligible for acceleration (CDAI score >10) (see Supplementary Figure 2, available on the Arthritis Care & Research web site). There were no differences in the change in WHO/ILAR core set outcomes (see Supplementary Table 1, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.23294/abstract). The rates of serious infections (4.7 per 100 patient‐years versus 4.3 per 100 patient‐years), cardiovascular events (2.3 per 100 patient‐years versus 3.1 per 100 patient‐years), and cancers (0.5 per 100 patient‐years versus 0.8 per 100 patient‐years) were similar in the treat‐to‐target versus usual care groups (see Supplementary Table 2, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.23294/abstract).
Treatment acceleration over time
Over time, there was a decrease in the rate of accelerations per visit in both groups. In the treat‐to‐target arm, the proportion of all eligible acceleration visits at which treatment acceleration occurred was 70% at enrollment, 43% at 3 months, 39% at 6 months, and 29% at 9+ months; in the usual care arm, it was 60% at enrollment, 41% at 3 months, 39% at 6 months, and 28% at 9+ months (Figure 4A). A similar decrease in accelerations over time was observed when visits with treatment acceleration in the prior 3 months were excluded to account for patients who were less‐likely candidates for acceleration due to a recent medication change. The proportion of eligible new acceleration visits at which treatment acceleration occurred in the treat‐to‐target arm was 73% at enrollment, 46% at 3 months, 42% at 6 months, and 31% at 9+ months; in the usual care arm it was 61% at enrollment, 42% at 3 months, 42% at 6 months, and 25% at 9+ months (Figure 4B). There was no significant difference between the groups in the proportion of visits with treatment acceleration over time following enrollment (Figure 4).
Figure 4.
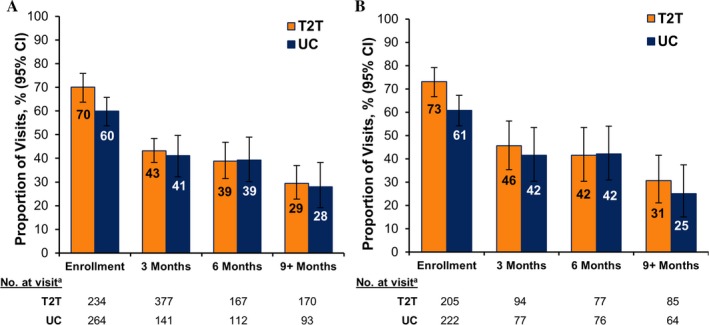
Patterns of treatment acceleration over time. A, Proportion of all eligible acceleration visits at which treatment was accelerated. B, Proportion of eligible new acceleration visits at which treatment was accelerated. T2T = treat‐to‐target; UC = usual care; 95% CI = 95% confidence interval; a = total number of patient visits within the indicated time period with Clinical Disease Activity Index score >10 and no accelerations within the previous 3 months.
The most frequent reason for not accelerating treatment was physician concern about medication response time lag (53%), when the provider thought more time was needed to allow the medication to have maximal effect (Table 2). Patient preference to not accelerate treatment was reported as the reason for nonacceleration in approximately one‐third of cases. Over time, medication response lag time was less likely to account for nonacceleration, while patient preference was more likely to be reported as the reason for nonacceleration. Patient preference was the most frequent reason for not accelerating treatment (52%), followed by medication response time lag (30%), when only eligible new acceleration visits were considered.
Table 2.
Possible reasons for nonacceleration in patients with Clinical Disease Activity Index (CDAI) score >10 in the treat‐to‐target study arma
| Reasonb | All visits, month no.c | Visits without treatment acceleration in prior 3 months, month no.c | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1–3 (n = 150) | 4–6 (n = 98) | 7–9 (n = 70) | 10–12+ (n = 69)d | Overall | 1–3 (n = 48) | 4–6 (n = 42) | 7–9 (n = 30) | 10–12+ (n = 45)d | Overall | |
| Medication response time lag | 68.0 | 56.1 | 48.6 | 21.7 | 53.2 | 39.6 | 38.1 | 26.7 | 13.3 | 29.7 |
| Patient preference | 24.7 | 28.6 | 38.6 | 52.2 | 33.1 | 50.0 | 38.1 | 53.3 | 53.3 | 52.1 |
| Comorbid conditions | 6.7 | 8.2 | 5.7 | 5.8 | 6.7 | 8.3 | 11.9 | 6.7 | 4.4 | 7.9 |
| Disagree with CDAI | 2.7 | 10.2 | 5.7 | 11.6 | 6.7 | 2.1 | 11.9 | 10.0 | 11.1 | 8.5 |
| Surgery | 2.7 | 1.0 | 5.7 | 7.3 | 3.6 | 4.2 | 2.4 | 13.3 | 6.7 | 6.1 |
| Nonrheumatoid arthritis pain | 0 | 1.0 | 0 | 4.4 | 1.0 | 0 | 0 | 0 | 2.2 | 0.6 |
| Pregnancy | 0 | 1.0 | 1.4 | 2.9 | 1.0 | 0 | 0 | 0 | 2.2 | 0.6 |
| Tuberculosis | 0 | 0.0 | 0 | 1.5 | 0.3 | 0 | 0 | 0 | 2.2 | 0.6 |
Values are percentages. Possible reasons for nonacceleration were a new or worsening comorbid condition, anticipated medication response time lag (e.g., time for the medication to have the maximal effect was inadequate), physician disagreement with CDAI score (considered the patient as not having moderate/high disease activity), nonrheumatoid arthritis pain was influencing the disease activity measure, recent or pending surgical procedure, pregnancy, breastfeeding, or planning to become pregnant, history or new diagnosis of human immunodeficiency virus, hepatitis B virus, or hepatitis C virus, history of positive tuberculin test or equivalent or had not received treatment for latent tuberculosis, and patient preference.
More than 1 reason could be reported.
Number of visits with a CDAI score >10.
The 10–12+ month group includes visits that occurred after 12 months.
Discussion
To our knowledge, this is the first study that examines both the feasibility and efficacy of implementing a treat‐to‐target approach to care of patients with RA in typical US clinical practices using a cluster‐randomized approach. Specifically, we found 47% of visits with a CDAI score >10 were associated with treatment acceleration. Analysis using an intent‐to‐treat approach showed that almost 60% of patients in either arm achieved LDA at 12 months. There was no difference between the treatment arms with respect to feasibility, defined by treatment acceleration in the setting of active disease, or efficacy. Primary reasons for nonacceleration were patient preference and a perceived need for additional time for a medication to fully take effect.
Both the usual care and treat‐to‐target groups had higher rates of LDA at trial completion than anticipated based on historical data from CORRONA and from other treat‐to‐target trials 2. Further evaluation of the number of visits among patients in the usual care group suggests that many of these patients had treat‐to‐target–like care. This fact may not be surprising because to participate in the trial, sites had to be willing to provide treat‐to‐target care prior to the cluster‐randomization process. Of note, the participating usual care providers were aware that outcomes were being compared between the treat‐to‐target and usual care groups. Given the growing awareness of the merits of treat‐to‐target care, the physicians at usual care sites may have had greater awareness of the treat‐to‐target philosophy as part of routine care compared with physicians participating in earlier treat‐to‐target trials. Thus, these physicians may be early adopters of a treat‐to‐target–like approach to care or may have incorporated this approach during the study. Analyses are underway to explore these hypotheses by comparing the treatment patterns of the usual care and treat‐to‐target arms over time in the years before and during the treat‐to‐target trial.
Our results differ from those of other studies evaluating treat‐to‐target therapy due to the nature of our trial, which was designed as a behavioral intervention targeting US rheumatologists in typical clinical practice and their patients with moderate or high disease activity. In previous clinical trials, patients under treat‐to‐target management experienced significantly greater improvement in disease activity and higher rates of remission compared with patients under usual care 3, 5. However, these previous trials targeted patients with early disease with mandated treatment protocols.
Fransen et al 2 conducted a multicenter, cluster‐randomized trial to compare treat‐to‐target care with usual care in the achievement of LDA in 384 patients from 24 centers in The Netherlands. Similar to our study design, patients were not excluded based on disease duration or prior csDMARD use, and treatment acceleration strategy in both arms was left to physician and patient discretion. Patients were monitored over 24 weeks for achievement of LDA (DAS28 ≤3.2) and the frequency of changes in csDMARD treatment. Although significantly more patients in the treat‐to‐target arm achieved LDA at 24 weeks compared with the usual care arm, there was no significant difference in the mean change in DAS28 over the 24‐week study period between the study arms. Furthermore, while csDMARD changes occurred more frequently in the treat‐to‐target group than the usual care group, csDMARD treatment changes were made at only 20% of the visits at which DAS28 measured >3.2 in the treat‐to‐target arm.
Our study is the first to perform any US‐based treat‐to‐target trial, and the first to focus on feasibility of treat‐to‐target implementation in routine clinical practice in a contemporary US setting. Although prior European clinical trials demonstrated the benefits of a treat‐to‐target approach with mandated treatment acceleration, the implementation of this approach in the US is potentially challenging, given different health care payment systems, practice styles, and patient‐provider interactions. Additionally, we crafted a nonrigid, simple intervention that, if successful, could be widely disseminated. Of note, because this was a behavioral intervention trial, we did not intervene if providers were not following the predefined treat‐to‐target approach. While all treat‐to‐target sites received detailed instruction prior to study initiation and received study reminders, physicians were not audited for adherence during the study; patients had agreed conceptually to the principles of acceleration prior to enrollment.
One inherent limitation of the intervention design of our study was the inclusion of study sites based on their willingness to implement a treat‐to‐target approach, which may have selected for usual care sites predisposed to provide treat‐to‐target care and contributed to the similarity in acceleration outcomes between the study arms. Patients in both arms were seen more frequently and had higher rates of LDA than typically seen in clinical practice 2, 6. Lastly, we did not address patient‐ or systems‐related barriers to a treat‐to‐target approach, as our goal was to develop a simple intervention that could be disseminated widely to physicians.
We believe our observations provide unique insights that enhance the understanding of the challenges to providing treat‐to‐target care using clinical disease activity level as the cutoff metric. Both patient and physician preference and suspected time lags in medication effectiveness were frequently reported as reasons for nonacceleration. Patient reluctance to accelerate therapy suggests that practitioners should provide patient‐centric tools and the rationale for acceleration in patients who may feel the risk of unknown side effects of a new therapy outweighs the potential benefits of modest improvement in disease activity 16, 17. The psychological dynamic of avoiding a potential loss, which carries more emotional weight than a possible gain, has been well documented in decision theory 17. These insights into human behavior are likely relevant when rheumatologists try to convince a patient with low or moderate disease to accelerate to a new treatment and the patient resists due to fear of new side effects 18, 19. Approaches to work within the innate human propensity to avoid a loss (i.e., a new toxicity) are needed before widespread patient acceptance of treatment acceleration can be implemented in patients with moderate disease. These challenges may be more compelling in the US, where ubiquitous direct‐to‐consumer advertising is replete with a mandated litany of potential toxicities, which may influence patient decision‐making.
While treat‐to‐target management may be more effective than usual care under optimal conditions, circumstances in routine clinical practice are rarely optimal. Specifically, the results of this trial demonstrate the difficulty of convincing patients of the virtues of treat‐to‐target care. Based on the similar frequency of acceleration and treatment outcomes observed in the 2 treatment arms, one wonders whether benchmarking and motivating providers is sufficient to achieve a treat‐to‐target approach to care. Potentially greater transparency of physician practice performance may result in superior RA disease outcomes. We hope that these insights provide a fresh consideration of real‐world challenges associated with treat‐to‐target implementation in US patients with longstanding disease. These newly described insights provide a foundation for future investigations by identifying behavioral impediments to a treat‐to‐target approach in typical clinical practice in the US.
Author contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Harrold had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Harrold, Reed, John, Barr, Saunders, Haselkorn, Greenberg, Gibofsky, Harrington, Kremer.
Acquisition of data
Barr, Soe, Ruderman.
Analysis and interpretation of data
Harrold, Reed, John, Soe, Magner, Ruderman, Haselkorn, Greenberg, Gibofsky, Harrington, Kremer.
Supporting information
Acknowledgment
Support for third‐party writing assistance for this manuscript, furnished by Elizabeth Ohneck of Health Interactions, was provided by F. Hoffmann‐La Roche.
Dr. Harrold holds shares in CORRONA, has received consulting fees from Roche (less than $10,000), and has received research support from Pfizer. Dr. Reed holds shares in CORRONA. Dr. Ruderman has received consulting fees from Amgen, AbbVie, Janssen, Eli Lilly, Novartis, Roche, and Seattle Genetics (less than $10,000 each) and from Pfizer (more than $10,000). Dr. Haselkorn has received consulting fees from Genentech (less than $10,000). Dr. Greenberg holds shares in CORRONA, and has received consulting fees from Eli Lilly, Genentech, Janssen, Novartis, and Pfizer (less than $10,000 each). Dr. Gibofsky holds shares in AbbVie, Amgen, Johnson & Johnson, and Pfizer, and has received consulting fees from AbbVie, Celgene, Eli Lilly, Medec, Novartis, Relbum, Pfizer, and UCB (less than $10,000 each). Dr. Kremer holds shares in CORRONA, and has received consulting fees from AbbVie, Amgen, Bristol‐Meyers Squibb, Genentech, GSK, Eli Lilly, Pfizer, Regeneron, and Sanofi (less than $10,000 each), and has received research support from AbbVie, Genentech, Eli Lilly, Novartis, and Pfizer.
References
- 1. Smolen JS, Breedveld FC, Burmester GR, Bykerk VP, Dougados M, Emery P, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis 2016;75:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fransen J, Moens HB, Speyer I, van Riel PL. Effectiveness of systematic monitoring of rheumatoid arthritis disease activity in daily practice: a multicentre, cluster randomised controlled trial. Ann Rheum Dis 2005;64:1294–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grigor C, Capell H, Stirling A, McMahon AD, Lock P, Vallance R, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single‐blind randomised controlled trial. Lancet 2004;364:263–9. [DOI] [PubMed] [Google Scholar]
- 4. Schipper LG, Vermeer M, Kuper HH, Hoekstra MO, Haagsma CJ, Den Broeder AA, et al. A tight control treatment strategy aiming for remission in early rheumatoid arthritis is more effective than usual care treatment in daily clinical practice: a study of two cohorts in the Dutch Rheumatoid Arthritis Monitoring registry. Ann Rheum Dis 2012;71:845–50. [DOI] [PubMed] [Google Scholar]
- 5. Verstappen SM, Jacobs JW, van der Veen MJ, Heurkens AH, Schenk Y, ter Borg EJ, et al. Intensive treatment with methotrexate in early rheumatoid arthritis: aiming for remission. Computer Assisted Management in Early Rheumatoid Arthritis (CAMERA, an open‐label strategy trial). Ann Rheum Dis 2007;66:1443–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arnold E, Arnold W, Conaway D, Crump G, LaCour E, Mossell J, et al. Rheumatoid arthritis practice performance project spots problems in RA management. Rheumatologist 2015. URL: http://www.the-rheumatologist.org/article/rheumatoid-arthritis-practice-performance-project-spots-problems-in-ra-management/. [Google Scholar]
- 7. Harrold LR, Reed GW, Harrington JT, Barr CJ, Saunders KC, Gibofsky A, et al. The rheumatoid arthritis treat‐to‐target trial: a cluster randomized trial within the Corrona rheumatology network. BMC Musculoskelet Disord 2014;15:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kremer JM. The CORRONA database. Clin Exp Rheumatol 2005;23:S172–7. [PubMed] [Google Scholar]
- 9. Curtis JR, Chen L, Bharat A, Delzell E, Greenberg JD, Harrold L, et al. Linkage of a de‐identified United States rheumatoid arthritis registry with administrative data to facilitate comparative effectiveness research. Arthritis Care Res (Hoboken) 2014;66:1790–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO III, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81. [DOI] [PubMed] [Google Scholar]
- 11. Smolen JS, Aletaha D, Bijlsma JW, Breedveld FC, Boumpas D, Burmester G, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis 2010;69:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Normand ST, Landrum MB, Guadagnoli E, Ayanian JZ, Ryan TJ, Cleary PD, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol 2001;54:387–98. [DOI] [PubMed] [Google Scholar]
- 14. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377–99. [DOI] [PubMed] [Google Scholar]
- 15. Boers M, Tugwell P, Felson DT, van Riel PL, Kirwan JR, Edmonds JP, et al. World Health Organization and International League of Associations for Rheumatology core endpoints for symptom modifying antirheumatic drugs in rheumatoid arthritis clinical trials. J Rheumatol 1994;41:86–9. [PubMed] [Google Scholar]
- 16. De Wit MP, Smolen JS, Gossec L, van der Heijde DM.Treating rheumatoid arthritis to target: the patient version of the international recommendations. Ann Rheum Dis 2011;70:891–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kahneman D. Thinking, fast and slow. New York: Farrar, Straus and Giroux; 2011. [Google Scholar]
- 18. Van Hulst LT, Hulscher ME, van Riel PL. Achieving tight control in rheumatoid arthritis. Rheumatology (Oxford) 2011;50:1729–31. [DOI] [PubMed] [Google Scholar]
- 19. Van Hulst LT, Kievit W, van Bommel R, van Riel PL, Fraenkel L. Rheumatoid arthritis patients and rheumatologists approach the decision to escalate care differently: results of a maximum difference scaling experiment. Arthritis Care Res (Hoboken) 2011;63:1407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


