Abstract
We performed a meta‐analysis of randomized controlled trials (RCTs) to compare the long‐term glycaemic durability of dipeptidyl‐peptidase 4 (DPP‐4) inhibitors vs that of sulphonylureas (SUs) in patients with type 2 diabetes mellitus (T2DM), in terms of the changes in glycated haemoglobin (HbA1c) levels from an intermediate time point (26 or 52 weeks) to 104 weeks of treatment. The Medline (PubMed), Embase (Ovid), and CENTER (Cochrane Library) databases were searched for relevant RCTs. Eight RCTs were included. Compared with SUs, DPP‐4 inhibitors were associated with significantly smaller increases in the HbA1c level from 24 to 28 weeks to 104 weeks (mean difference [MD]: −0.16%, 95% confidence interval [CI]: −0.21 to −0.11; P < .001) and from 52 weeks to 104 weeks (MD −0.06%, 95% CI −0.10 to −0.02; P = .001). No significant heterogeneities were detected among the included comparisons (I2 = 0%). These results suggest that long‐term treatment with DPP‐4 inhibitors confers better durability of glycaemic response than treatment with SUs in patients with T2DM, which may indicate that DPP‐4 inhibitors better preserve islet β‐cell function compared with SUs.
Keywords: dipeptidyl peptidase‐4 inhibitors, glycaemic durability, glycated haemoglobin, meta‐analysis, sulphonylureas
1. INTRODUCTION
Sulphonylureas (SUs) are conventionally chosen as the treatment for type 2 diabetes mellitus (T2DM) after metformin failure1; however, the glucose‐lowering effect of SUs tends to diminish as a function of time. Dipeptidyl peptidase‐4 (DPP‐4) inhibitors, a novel class of OADs that function to inhibit the DPP‐4 enzyme‐induced degradation of glucagon‐like peptide‐1, have become a widely accepted option for T2DM treatment in the past decade.2 Regarding the long‐term efficacy of DPP‐4 inhibitors for glycaemic control, although a recent meta‐analysis combining the results of long‐term clinical trials suggested that the effect of DPP‐4 inhibitors on glycated haemoglobin (HbA1c) levels in patients with T2DM may decline during the second year of treatment,3 conclusive results have not been obtained. More importantly, the results of pilot studies evaluating the comparative glycaemic durability of DPP‐4 inhibitors and SUs were not consistent4, 5, 6, 7, 8, 9, 10, 11; therefore, the aim of the present study was to perform a meta‐analysis of long‐term randomized controlled trials (RCTs) to compare the glycaemic durability of DPP‐4 inhibitors and SUs in patients with T2DM, as reflected by the change in HbA1c levels from an intermediate time point (26 or 52 weeks) to 104 weeks of treatment.
2. METHODS
This meta‐analysis was performed in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses)12 and the Cochrane Handbook guidelines.13
2.1. Search strategy
The Medline (PubMed), Embase (Ovid), and CENTER (Cochrane Library) databases were systematically searched using the terms “DPP4,” “DPP‐4,” “dipeptidyl peptidase‐4 inhibitors,” “sitagliptin,” “vildagliptin,” “linagliptin,” “saxagliptin,” “alogliptin,” “dutogliptin,” “aemigliptin,” “anagliptin,” “teneligliptin,” “trelagliptin,” or “omarigliptin” coupled with “glimepiride,” “glipizide,” “gliclazide,” “glibenclamide,” “glyburide,” “gliguidone,” “sulphonylureas,” “sulfonylureas,” and “random,” “randomly,” or “randomized.” The final search was performed on January 25, 2017. The search was limited to clinical trials in humans. We also manually analysed the references of original and review articles to identify additional studies.
2.2. Criteria for study selection
Studies were included if they: (1) were full‐length articles in English or Chinese; (2) were RCTs with a parallel design; (3) recruited people with confirmed T2DM; (4) assigned patients to either an oral DPP‐4 inhibitor treatment group or an oral SU group; (5) had a treatment duration of at least 2 years (104 weeks); and (6) provided information necessary for extraction or estimation of data for changes in HbA1c levels from an intermediate time point (26 or 52 weeks) to 104 weeks.
2.3. Data extraction and quality assessment
Two authors independently performed the literature search, data extraction and quality assessment. Discrepancies were resolved by discussion with the corresponding author. The Cochrane Risk of Bias Tool with seven domains13 was applied for study quality evaluation.
2.4. Statistical analysis
REVMAN (Version 5.1; Cochrane Collaboration, Oxford, UK) and STATA software (version 12.0; Stata Corporation, College Station, Texas) were used for statistical analysis. The primary outcome of this meta‐analysis was the change in HbA1c level from an intermediate time point (26 or 52 weeks) to 104 weeks of treatment with DPP‐4 inhibitors and SUs. If data for HbA1c levels at a certain intermediate time point were not reported, data for the nearest time‐point were used. The combined effect was presented as a mean difference (MD) with the 95% confidence interval (CI). Heterogeneity was evaluated with Cochrane's Q test,13 and significant heterogeneity was considered if P < .10. The I2 statistic was determined to describe the percentage of total variation across studies. A random effects model was used because it is a more conservative method which considers heterogeneity among the included studies.13 Data from studies with more than one interventional arm with DPP‐4 inhibitors were considered as multiple comparisons and were included in the meta‐analysis after the sample sizes of the control groups had been equally split, as indicated by the Cochrane Handbook guideline.13 Publication bias was assessed by visual inspection of the asymmetry of the funnel plot and Egger's regression asymmetry test.13 P values were two‐tailed, and statistical significance was set at P < .05.
3. RESULTS
3.1. Study selection
A flow chart outlining the study selection process is presented in Figure S1. A final total of eight RCTs were included in our study.4, 5, 6, 7, 8, 9, 10, 11
3.2. Study characteristics and quality evaluation
Of the eight included RCTs, one included two interventional arms with different doses of alogliptin (12.5 and 25 mg once daily), and these were included as two comparisons. The clinical and baseline patient characteristics for the included studies are presented in Table 1. All of the RCTs included patients already taking metformin, except for one study that included medication‐naïve patients,4 and there was considerable variation in HbA1c levels at baseline (7.5%‐11.0%). Different DPP‐4 inhibitors were used, including vildagliptin, sitagliptin, alogliptin, linagliptin and saxagliptin. The drop‐out rate was substantial for the included RCTs, and the strategies of considering observed cases and last observation carried forward were applied to handle this issue. The details of the quality evaluation are shown in Table S1. All of the included studies were double‐blind RCTs.
Table 1.
Baseline patient and clinical characteristics of the included studies
| Study | Design | N | Mean age, years | Sex, % men | BMI, kg/m2 | Baseline HbA1c, % | T2DM duration, years | Add‐on therapy | Extension study | DPP‐4 inhibitor dose | SU dose | Intermediate time point, weeks | Final time point, weeks | Drop out, %, and handling strategy |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Foley 2009 | R, DB | 811 | 54.8 | 55.8 | 30.7 | 8.7 | 2.2 | N | N | Vildagliptin, 50 mg twice daily | Gliclazide, 80‐320 mg once daily | 24, 52 | 104 | 25.7%, LOCF |
| Ahren 2010 | R, DB | 258 | 57.5 | 53.6 | 31.8 | 7.3 | 5.7 | Y, Metformin | Y | Vildagliptin, 50 mg twice daily | Glimepiride, 2‐6 mg once daily | 24, 52 | 104 | NR |
| Matthews 2010 | R, DB | 1357 | 57.5 | 53.5 | 31.6 | 7.2 | 5.7 | Y, Metformin | N | Vildagliptin, 50 mg twice daily | Glimepiride, 2‐6 mg once daily | 24, 53 | 104 | 37.6%, LOCF |
| Seck 2010 | R, DB | 504 | 57.3 | 60.1 | 31.1 | 7.3 | 5.8 | Y, Metformin | N | Sitagliptin, 100 mg once daily | Glipizide, 5‐20 mg once daily | 24, 54 | 104 | 56%, OC |
| Gallwitz 2012 | R, DB | 504 | 59.8 | 60.5 | 30.3 | 7.7 | NR | Y, Metformin | N | Linagliptin, 5 mg once daily | Glimepiride, 1‐4 mg once daily | 28, 52 | 104 | 23%, LOCF |
| Goke 2013 | R, DB | 312 | 57.5 | 52.4 | 31.4 | 7.7 | 5.5 | Y, Metformin | Y | Saxagliptin, 5 mg once daily | Glipizide, 5‐20 mg once daily | 24, 52 | 104 | 73%, LOCF |
| Ahren 2014 | R, DB | 602 | 55.1 | 50.1 | NR | 8.2 | 6.2 | Y, Metformin | N | Sitagliptin, 100 mg once daily | Glimepiride, 2‐4 mg once daily | 24, 52 | 104 | 33%, LOCF |
| Del 2014, 12.5 mg | R, DB | 1317 | 55.4 | 48.9 | 31.2 | 7.6 | 5.6 | Y, Metformin | N | Alogliptin, 12.5 mg once daily | Glipizide, 5‐20 mg once daily | 26, 52 | 104 | 22%, LOCF |
| Del 2014, 25 mg | R, DB | 1322 | 55.4 | 50.8 | 31.2 | 7.6 | 5.5 | Y, Metformin | N | Alogliptin, 25 mg once daily | Glipizide, 5‐20 mg once daily | 26, 52 | 104 | 22%, LOCF |
Abbreviations: BMI, body mass index; DB, double‐blind; LOCF, last observation carried forward; N, no; NR, not reported; OC, observed cases; R, randomized; Y, yes.
3.3. Comparative glycaemic durability of DPP‐4 inhibitors and SUs
Treatment with DPP‐4 inhibitors was associated with significantly smaller changes in HbA1c levels from 24 to 28 weeks to 104 weeks (MD −0.16%, 95% CI −0.21 to −0.11; P < .001; Figure 1A) and 52 weeks to 104 weeks (MD −0.06%, 95% CI −0.10 to −0.02; P = .001; Figure 1B) compared with SUs, with no considerable heterogeneity (I2 = 0%). A sensitivity analysis based on the omission of the study including medication‐naïve patients showed similar results (24‐28 weeks: MD −0.15%, 95% CI −0.20 to −0.10, P < .001; 52 weeks: MD −0.06%, 95% CI −0.10 to −0. 02, P = .003).
Figure 1.
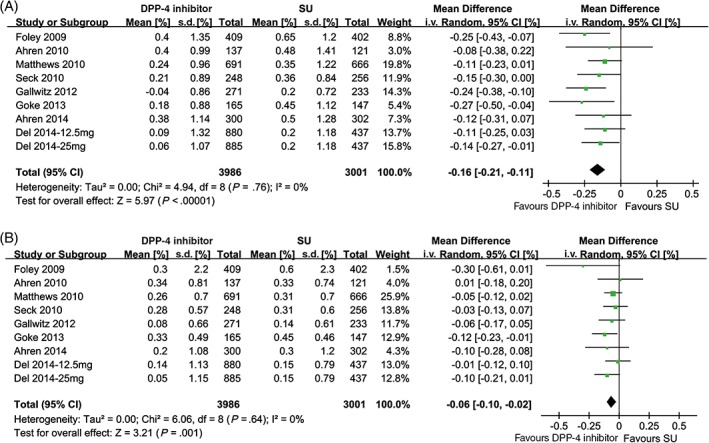
Forest plots for the comparative glycaemic durability of DPP‐4 inhibitors and SUs. A, Changes in HbA1c levels from 24 to 28 to 104 weeks of treatment; B, changes in HbA1c levels from 52 to 104 weeks of treatment. i.v., intravenous; s.d., standard deviation
3.4. Publication bias
The funnel plots for the comparative effectiveness of DPP‐4 inhibitors and SUs for long‐term glycaemic control (Figure S2) were symmetrical on visual inspection, suggesting no significant publication bias. Egger's significance test also did not indicate the existence of publication bias (P = .542 and P = .388, respectively).
4. DISCUSSION
The present meta‐analysis showed that treatment of patients with T2DM with DPP‐4 inhibitors was associated with a significantly smaller change in the HbA1c level from an intermediate time point to the end of 104 weeks of follow‐up as compared with treatment with SUs, suggesting that DPP‐4 inhibitors offer better glycaemic durability than SUs. Considering the other advantages of DPP‐4 inhibitors over SUs, such as the lower incidence of adverse events, including hypoglycaemia, and the lesser impact on body weight, the results of the present study indicate that DPP‐4 inhibitors might be a preferable treatment choice for patients with T2DM as an add‐on medication with metformin.
Diminished β‐cell function has been recognized as an important step in the pathogenesis of T2DM.14 Insulin resistance, as an initial event during the pathogenesis of T2DM, leads to accelerated insulin secretion by islet β cells, which eventually results in the overwork of the β cells and subsequent deterioration of β‐cell function.15 Because the primary pharmacological effects of SUs are the stimulation of insulin secretion by β cells, the significant glucose‐lowering effect that can be observed during the early phase of the treatment eventually fades or even disappears with long‐term treatment. This so‐called monotherapy failure with SUs has been linked to continuous loss of functional islet β cells and impaired β‐cell function during the progression of T2DM.16 Our finding that DPP‐4 inhibitors have better glycaemic durability than SUs is consistent with the results of previous studies demonstrating that DPP‐4 inhibitors may have beneficial effects on the preservation of β‐cell numbers and β‐cell functions. An early study in rats with streptozotocin‐induced diabetes found that the DPP‐4 inhibitor P32/98 had a dose‐dependent preventive effect on streptozotocin‐induced β‐cell apoptosis, suggesting that the therapeutic effects of DPP‐4 inhibitors may involve the prevention of functional β‐cell loss.17 By contrast, treatment with glipizide, was not associated with restoration of β‐cell mass.18 Subsequent experimental studies also indicated that some DPP‐4 inhibitors had protective effects on β‐cell mass and function.19 These results suggest that the preservation of β‐cell mass and function is a common effect of DPP‐4 inhibitors. Further studies are needed to confirm our hypothesis that DPP‐4 inhibitors confer better protective effects on β‐cell mass and function in humans, particularly as compared with SUs.
Our results have limitations that should be considered when interpreting the results. First, we tested our hypothesis that DPP‐4 inhibitors offer better glycaemic durability than SUs using the change in the HbA1c level from an intermediate time point to the final time point as the outcome. To minimize the influence of the choice of the intermediate time point on the meta‐analysis results, we chose two intermediate time points (26 and 52 weeks) for evaluation. Since a generalized index for glycaemic durability is lacking for current medical research, our approach may be a practical method for evaluating glycaemic durability of OADs in diabetes research. As mentioned in a previous meta‐analysis with the outcome of glycaemic durability3 and the curves of treatment effects of our included RCTs,4, 5, 6, 7, 8, 9, 10, 11 the maximum reduction in HbA1c by DPP‐4 inhibitors appeared at ~24 to 28 weeks after the initiation of the treatment; therefore, it is logical to evaluate the glycaemic durability after the stabilization of the DPP‐4 inhibitors' therapeutic effects. In addition, our conclusions were confirmed by the consistent results obtained for changes in HbA1c levels from two intermediate time points to the final visit. Secondly, the difference in treatment effect size was not particularly large, and the clinical relevance of the results deserves further confirmation; however, because HbA1c is a relatively stable variable that is considered a reflection of glucose levels within 3 months and has been linked to the risk of diabetes complications, the results of our study may indicate the benefits of long‐term administration of DPP‐4 inhibitors over SUs on clinical outcome. Thirdly, the total follow‐up duration was limited to 104 weeks. Obviously, changes in glycaemic control after treatment with DPP‐4 inhibitors vs SUs beyond the 2‐year duration deserve further investigation. Fourthly, our study included two extension studies based on original studies with a 52‐week follow‐up, which may introduce selection bias because patients with unsatisfactory glucose control during follow‐up for the original studies may not have participated in the extension studies; however, a meta‐analysis limited to studies originally designed with a 104‐week follow‐up produced similar results. Fifthly, the participant drop‐out rates varied among the RCTs, which may have influenced the quality of the study. Finally, although no significant statistical heterogeneities were observed among the included RCTs, these studies varied with regard to the study and patient characteristics.
In conclusion, long‐term treatment with DPP‐4 inhibitors conferred better glycaemic control compared with SUs in patients with T2DM, which may reflect that DPP‐4 inhibitors better preserve islet β‐cell function than do SUs.
Supporting information
Figure S1. Flow chart of study selection.
Figure S2. Funnel plot for the glycemic durability of DPP4 inhibitors and SUs. A, Changes in HbA1c levels from 24 to 28 to 104 weeks of treatment; B, changes in HbA1c levels from 52 to 104 weeks of treatment.
Table S1. Details of study quality as evaluated by Cochrane's risk of bias tool.
ACKNOWLEDGMENTS
Editorial assistance with the preparation of this review was provided by Medjaden Bioscience Limited. This assistance was funded by MSD China Holding Co., Ltd.
Conflict of interest
G. C., Y. Z. and R. Z. are employees of MSD China Holding Co., Ltd.
Author contributions
KC conceived and designed the study, collected the data, interpreted the results, wrote sections of the initial draft; DK conceived and designed the study, provided substantive suggestions for revision or critically reviewed subsequent iterations of the manuscript. MY conceived and designed the study, interpreted the results, provided substantive suggestions for revision or critically reviewed subsequent iterations of the manuscript. RZ and YZ conceived and designed the study, collected the data, performed analyses, interpreted the results, provided substantive suggestions for revision or critically reviewed subsequent iterations of the manuscript. GC interpreted the results, provided substantive suggestions for revision or critically reviewed subsequent iterations of the manuscript. YM conceived and designed the study, performed analyses, provided substantive suggestions for revision or critically reviewed subsequent iterations of the manuscript. All of the authors reviewed and approved the final version of the paper.
Chen K, Kang D, Yu M, et al. Direct head‐to‐head comparison of glycaemic durability of dipeptidyl peptidase‐4 inhibitors and sulphonylureas in patients with type 2 diabetes mellitus: A meta‐analysis of long‐term randomized controlled trials. Diabetes Obes Metab. 2018;20:1029–1033. https://doi.org/10.1111/dom.13147
Kang Chen and Deying Kang are co‐first authors.
Funding information MSD China Holding Co., Ltd.
REFERENCES
- 1. Turner LW, Nartey D, Stafford RS, Singh S, Alexander GC. Ambulatory treatment of type 2 diabetes in the U.S., 1997‐2012. Diabetes Care. 2014;37:985–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Drucker DJ, Nauck MA. The incretin system: glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. [DOI] [PubMed] [Google Scholar]
- 3. Esposito K, Chiodini P, Maiorino MI, Bellastella G, Capuano A, Giugliano D. Glycaemic durability with dipeptidyl peptidase‐4 inhibitors in type 2 diabetes: a systematic review and meta‐analysis of long‐term randomised controlled trials. BMJ Open. 2014;4:e005442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Foley JE, Sreenan S. Efficacy and safety comparison between the DPP‐4 inhibitor vildagliptin and the sulfonylurea gliclazide after two years of monotherapy in drug‐naive patients with type 2 diabetes. Horm Metab Res. 2009;41:905–909. [DOI] [PubMed] [Google Scholar]
- 5. Ahren B, Foley JE, Ferrannini E, et al. Changes in prandial glucagon levels after a 2‐year treatment with vildagliptin or glimepiride in patients with type 2 diabetes inadequately controlled with metformin monotherapy. Diabetes Care. 2010;33:730–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matthews DR, Dejager S, Ahren B, et al. Vildagliptin add‐on to metformin produces similar efficacy and reduced hypoglycaemic risk compared with glimepiride, with no weight gain: results from a 2‐year study. Diabetes Obes Metab. 2010;12:780–789. [DOI] [PubMed] [Google Scholar]
- 7. Seck T, Nauck M, Sheng D, et al. Safety and efficacy of treatment with sitagliptin or glipizide in patients with type 2 diabetes inadequately controlled on metformin: a 2‐year study. Int J Clin Pract. 2010;64:562–576. [DOI] [PubMed] [Google Scholar]
- 8. Gallwitz B, Rosenstock J, Rauch T, et al. 2‐year efficacy and safety of linagliptin compared with glimepiride in patients with type 2 diabetes inadequately controlled on metformin: a randomised, double‐blind, non‐inferiority trial. Lancet. 2012;380:475–483. [DOI] [PubMed] [Google Scholar]
- 9. Goke B, Gallwitz B, Eriksson JG, Hellqvist A, Gause‐Nilsson I. Saxagliptin vs. glipizide as add‐on therapy in patients with type 2 diabetes mellitus inadequately controlled on metformin alone: long‐term (52‐week) extension of a 52‐week randomised controlled trial. Int J Clin Pract. 2013;67:307–316. [DOI] [PubMed] [Google Scholar]
- 10. Ahren B, Johnson SL, Stewart M, et al. HARMONY 3: 104‐week randomized, double‐blind, placebo‐ and active‐controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metformin. Diabetes Care. 2014;37:2141–2148. [DOI] [PubMed] [Google Scholar]
- 11. Del Prato S, Camisasca R, Wilson C, Fleck P. Durability of the efficacy and safety of alogliptin compared with glipizide in type 2 diabetes mellitus: a 2‐year study. Diabetes Obes Metab. 2014;16:1239–1246. [DOI] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. www.cochranehandbook.org. Accessed January 25, 2017. [Google Scholar]
- 14. Kim DJ, Lee MS, Kim KW, Lee MK. Insulin secretory dysfunction and insulin resistance in the pathogenesis of korean type 2 diabetes mellitus. Metabolism. 2001;50:590–593. [DOI] [PubMed] [Google Scholar]
- 15. Matveyenko AV, Butler PC. Relationship between beta‐cell mass and diabetes onset. Diabetes Obes Metab. 2008;10(suppl 4):23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brereton MF, Rohm M, Ashcroft FM. beta‐Cell dysfunction in diabetes: a crisis of identity? Diabetes Obes Metab. 2016;18(suppl 1):102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pospisilik JA, Martin J, Doty T, et al. Dipeptidyl peptidase IV inhibitor treatment stimulates beta‐cell survival and islet neogenesis in streptozotocin‐induced diabetic rats. Diabetes. 2003;52:741–750. [DOI] [PubMed] [Google Scholar]
- 18. Mu J, Petrov A, Eiermann GJ, et al. Inhibition of DPP‐4 with sitagliptin improves glycemic control and restores islet cell mass and function in a rodent model of type 2 diabetes. Eur J Pharmacol. 2009;623:148–154. [DOI] [PubMed] [Google Scholar]
- 19. Moritoh Y, Takeuchi K, Hazama M. Combination treatment with alogliptin and voglibose increases active GLP‐1 circulation, prevents the development of diabetes and preserves pancreatic beta‐cells in prediabetic db/db mice. Diabetes Obes Metab. 2010;12:224–233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow chart of study selection.
Figure S2. Funnel plot for the glycemic durability of DPP4 inhibitors and SUs. A, Changes in HbA1c levels from 24 to 28 to 104 weeks of treatment; B, changes in HbA1c levels from 52 to 104 weeks of treatment.
Table S1. Details of study quality as evaluated by Cochrane's risk of bias tool.


