Abbreviations
- cccDNA
covalently closed circular DNA
- HCC
hepatocellular carcinoma
- HBV
hepatitis B virus
There is a growing interest in the discovery and development of new therapeutics that will cure chronic hepatitis B virus (HBV) infection, due to the recent establishment of new cell culture–based and small animal–based models. These new systems create unprecedented opportunities to study the entire viral life cycle and to search for vulnerabilities that can be exploited for curative purposes. Here, we propose a scientific pathway that we believe will lead to the development of curative therapies for chronic HBV infection and its associated diseases.
HBV is a hepatotropic, noncytopathic, partially double‐stranded DNA virus that replicates by reverse transcription of a greater‐than‐genome‐length RNA and causes acute and chronic necro‐inflammatory liver disease (hepatitis), cirrhosis of the liver, liver failure, and hepatocellular carcinoma (HCC).1, 2 Approximately 2 billion people alive today have been infected by HBV 250 million of whom are currently chronically infected.3, 4 Antiviral drugs that suppress viral replication and retard disease progression are available; however, treatment is generally not curative and is lifelong, expensive, and limited by the extent to which it fails to reduce the risk of death due to liver disease. A highly effective protective vaccine is available, leading the World Health Organization and the National Academies of Science, Engineering, and Medicine to recently declare that elimination of HBV is possible if a curative therapy can be developed to supplement the protective effect of the vaccine.3, 5, 6 This document introduces the challenge and presents a roadmap for the discovery of a cure.
Development of a cure for any viral infection requires a sufficiently deep understanding of the virus life cycle and its interaction with the host to identify vulnerabilities that can be exploited to eradicate the cccDNA from infected cells. The key word here is “eradicate.” In general, the targetable steps in the HBV life cycle include entry, uncoating, delivery of the viral DNA genome to the nucleus, establishment and maintenance of the covalently closed circular DNA (cccDNA) transcriptional template in the nucleus of the cell, followed by transcription, translation, replication, viral and subviral particle assembly, transport and release, and the recycling of cytoplasmic capsid particles to the nucleus to amplify the intracellular pool of cccDNA. Eradication also requires engagement of the host immune response to kill infected cells, to prevent viral spread from any residual infected cells and to counteract any evasive strategies deployed by the virus to defeat the host response.
Despite its discovery 50 years ago, most steps in the HBV life cycle and the nature of its interaction with its host are only partially understood because the experimental systems required for such experiments have not been available.7 Furthermore, despite the ability of currently available direct‐acting antiviral drugs to suppress HBV DNA replication, they are rarely curative because they do not prevent the establishment or maintenance of the long‐lived HBV cccDNA transcriptional template—the stable nuclear form of the viral genome, which must be eliminated or permanently silenced to achieve a durable HBV cure.8
Luckily, experimental systems that permit detailed analysis of cccDNA biogenesis, homeostasis, and decay; and all other steps in the viral life cycle were recently developed.9 Thus, we are now on the threshold of a period of exploration that, if focused on eliminating the cccDNA transcriptional template of the virus, could lead to a cure of chronic HBV infection, once and for all.
We encourage the scientific community to focus on research leading to discovery of a cure for chronic HBV infection based on these principles, as summarized here and highlighted in Fig. 1:
The surest way to cure HBV is to eliminate or permanently silence its cccDNA.
The most important impediment to this achievement is our limited understanding of the fundamental molecular mechanisms that control cccDNA biogenesis, homeostasis, and decay.
Understanding these mysteries is now within reach, thanks to recent technological advances that enable definition of these mechanisms.
Vulnerabilities in the cccDNA “life cycle” that are discovered in the course of these studies can be exploited to develop small molecule and other molecular strategies to eradicate or permanently silence the cccDNA.
Because these studies will explore the unknown, the outcome, like all great adventures, cannot be predicted. Thus, we suggest that in addition to approaches that directly target cccDNA, independent approaches that target other vulnerabilities in the viral life cycle and either indirectly repress HBV cccDNA or safely establish a curative antiviral immune response be pursued in parallel.
Such projects could include genetic approaches to cccDNA mutagenesis, epigenetic modification, or other strategies that can suppress cccDNA transcription (e.g., HBV‐targeted antisense and small interfering RNA, HBV X protein inhibition, etc.) or to prevent its recycling (e.g., capsid inhibitors).
Figure 1.
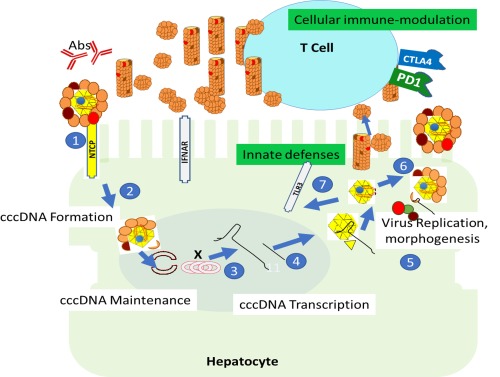
HBV life cycle, emphasizing opportunities to suppress viral cccDNA and restore immune control. Host and viral functions that could be exploited for therapeutic purposes are illustrated, beginning with binding of the virus to the sodium–taurocholate cotransporting polypeptide receptor on hepatocytes (1), followed by translocation of the nucleocapsid to the nucleus and formation of cccDNA (2) and synthesis steps (3,4), leading to either egress (6) of newly formed virions or recycling of cccDNA‐containing nucleocapsids to the nucleus (7). Opportunities for cccDNA suppression and immune control are categorized as either acting upon the viral gene products (white boxed text) or acting upon host innate and adaptive immune systems (green boxed text), noting that in many cases these different pathways overlap. Humoral responses are also indicated (Abs). Orange, red, and brown circles indicate small, medium, and large hepatitis B surface proteins, respectively; yellow triangle (core protein), blue circle (pol), “X” (x protein), red semicircles, cccDNA and black line, HBV 3.2 kb and subgenomic HBV RNA; 22‐nM in diameter spherical and rod‐shaped subviral envelope particles and infectious 42‐nM virions are also illustrated. The examples of virus life cycle steps and immune modulators are representative and not comprehensive. Toll‐like receptor 3 is shown because it is present in hepatocytes, but other toll‐like receptors may also be exploited therapeutically. Abbreviations: Abs, antibodies; CTLA4, cytotoxic T lymphocyte antigen 4; IFNAR, type 1 interferon receptor; NTCP, sodium–taurocholate cotransporting polypeptide; PD1, programmed death 1; TLR3, Toll‐like receptor 3.
Of course, a vigorous basic and translational research effort to better define the nature of the immune response to HBV in chronically infected patients is also essential. Ideally, the antibody response would be examined at the single B‐cell level to reveal the extent to which neutralizing antibodies are produced by chronically infected patients and to determine whether patients who are “cured” of HBV by direct‐acting antivirals will require active immunization to prevent intrahepatic viral spread from any infected cells that remain after treatment and to protect them from future exposure.
Similarly, the functional and phenotypic characteristics of the HBV‐specific T‐cell response must be studied before, during, and after curative treatment to determine the extent to which it contributes to the durability or the failure of a given therapeutic strategy.
In addition, a wide variety of immune‐based strategies that can induce T cell–mediated elimination of HBV‐infected cells should be explored, including diverse approaches including therapeutic immunization, targeted delivery of antiviral effector molecules to infected cells, T‐cell checkpoint blockade of T cell inhibitory checkpoint pathways, and targeted delivery of HBV‐specific effector T cells to the HBV‐infected liver by T cell receptor‐based or chimeric antigen receptor (CAR)‐T cell technology, etc, should be explored.
These studies should be iterative, where results from the clinical work guide the laboratory work and vice versa. In this way, the immunobiology, number of infected cells, and other clinical parameters of chronic hepatitis B as a function of medical intervention can be followed.
We also note that HCC can be a consequence of chronic hepatitis B. Therefore, to comprehensively address the problems associated with chronic viral hepatitis B, an improved understanding of the molecular basis of HCC to guide early detection and treatment is vital. Clinical collaborative networks should also be reinforced and expanded to allow for evaluation of new early detection strategies of HCC and therapeutics of HCC and HBV.
It is also important to note that any intervention that directly or indirectly activates the cytotoxic T‐cell response to HBV could kill all the infected hepatocytes. This would be good if only a few hepatocytes in a given patient are infected and the functional hepatic reserve in that patient is robust. On the other hand, it could be fatal, inducing an acute on chronic liver disease event, if many hepatocytes are infected and hepatic reserve is tenuous. Thus, if the cytotoxic T‐cell response is activated by any therapeutic intervention, it must be in a “Goldilocks zone,” where it kills just enough hepatocytes at just the right rate to clear the infection without either triggering acute hepatic insufficiency or worsening the underlying chronic liver disease. It is imperative, therefore, to do these studies if we hope to predict how infected patients will respond immunologically to curative therapy before treatment begins, keeping in mind that the physician's first responsibility is primum non nocere, “first, do no harm.”
A recent review article from Revill et al. specified broad goals for HBV research and has since led to the establishment of an international coalition of scientists, clinicians, and stakeholders committed to the elimination of HBV (International Coalition to Eliminate HBV; http://ice-hbv.org/).10 Our intention is to support and build upon their effort by adding detail to create a roadmap for policy makers from government and other funding institutions and for planning long‐term research.
A cure for hepatitis B is also likely to greatly reduce morbidity and mortality associated with hepatitis delta virus infection, end‐stage liver disease, and HCC, although it is appreciated that these clinical problems deserve a specific research agenda of their own.
It is clearly important to explore multiple viral gene products and life cycle steps for intervention opportunities. To date, of all of the candidate approaches considered, elimination of HBV cccDNA is most likely to produce a durable cure of chronic HBV infection, after a finite course of antiviral therapy. The extent to which this can be achieved with drugs, biologicals, genetic manipulations, immunomodulation, etc. is the major question to be answered. While transcriptional silencing of cccDNA may be easier to achieve than physical cccDNA elimination, it would probably require lifelong treatment to produce lifelong effects unless it triggers some unpredictable durable downstream effect like immune‐mediated destruction or noncytolytic elimination of cccDNA from the infected cells. Thus, a vigorous, comprehensive, adequately funded research effort involving multiple, complementary approaches must be taken, with the results being shared in the public domain as quickly as possible.
Luckily, experimental systems that permit detailed analysis of the cccDNA and other steps in the viral life cycle are now available to the scientific community for these challenges. In our opinion, a concerted discovery effort that is both encouraged and enabled by governmental and nongovernmental funding agencies can make a huge difference in the lives of hundreds of millions of people worldwide. Let us not let this chance to do so much good slip away.
Acknowledgment
The Hepatitis B Foundation (HBF), a non profit organization, initiated the conversation that led to tbis article by asking the authors about their research priorities. The authors thank HBF staff members Theresa Weizman, Ph.D. and Judith Marchand for invaluable help in manuscript preparation.
Potential conflict of interest: Dr. Block owns stock, holds intellectual property rights, and received grants from Arbutus. He is employed by and owns stock in Contravir. He is director and has equity in Glycotest. Dr. Chisari consults for Gilead. Dr. H. Guo owns stock and received grants from Arbutus. He consults for Assembly. He received grants from Alios. Dr. J. Guo received grants from Arbutus. Dr. Lok received grants from Bristol‐Myers Squibb and Gilead. Dr. Kowdley consults, advises, and is on the speakers' bureau for Gilead. Dr. Glenn consults, is employed by, and owns stock in Riboscience. He is employed by and owns stock in Eiger. Dr. Feld consults and received grants from AbbVie, Gilead, Janssen, and Merck. He consults for Contravir. He received grants from Abbott. Dr. Hu consults and received grants from Gilead and Roche. He consults for Sanofi and Arbutus. Dr. El‐Serag received grants from Wako, Gilead, and Merck. Dr. Mason consults for Gilead. Dr. Revill received grants from Gilead. Dr. Chang advises Arbutus and Alnylam. Dr. Locarnini consults and received grants from Gilead and Arrowhead. He consults for Roche and Janssen. He received grants from Spring Bank. Dr. Zoulim consults and received grants from Gilead, Janssen, and Roche. He consults for Assembly, Arbutus, Contravir, and Transgene. Dr. Mehta owns stock and holds intellectual property rights in Glycotest. He consults for Insta‐Diagnostics. He owns stock in N‐Zyme. Dr. Brosgart consults for Dynavax. She is employed by and owns stock in Galmed and Contravir. She was employed by and owns stock in Allergan (formerly Tobira). Dr. Gish consults, advises, is on the speakers' bureau, and received grants from Gilead. He consults, advises, and is on the speakers' bureau for AbbVie and Merck. He consults, advises, and owns stock in Arrowhead. He consults, is on the speakers' bureau, and owns stock in Alexion. He consults and advises AstraZeneca, Contravir, Eiger, Enyo, Genentech, Humabs, Intellia, Intercept, Ionis, Janssen, MedImmune, Nanogen, and Theranos. He consults and is on the speakers' bureau for Bayer. He consults and owns stock in CoCrystal. He consults for Abbott, AccessBiologics, Novira, Springbank, Transgene, and VitalTherapies. He advises Quest, Biocollections, and Prometheus. He is on the speakers' bureau for Salix/Valeant. He owns stock in Kinex and RiboSciences.
REFERENCES
- 1. Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol 1995;13:29‐60. [DOI] [PubMed] [Google Scholar]
- 2. McMahon BJ. Natural history of chronic hepatitis B. Clin Liver Dis 2010;14:381‐396. [DOI] [PubMed] [Google Scholar]
- 3.National Academies of Sciences, Engineering, and Medicine. 2016. Eliminating the public health problem of hepatitis B and C in the United States: Phase one report. Washington, DC: The National Academies Press. doi: 10.17226/23407. [PubMed]
- 4. Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age‐specific HBsAg seroprevalence and endemicity. Vaccine 2012;30:2212‐2219. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization . Combating hepatitis B and C to reach elimination by 2030: An advocacy brief. Geneva, Switzerland: World Health Organization; 2016. http://apps.who.int/iris/bitstream/10665/206453/1/WHO_HIV_2016.04_eng.pdf?ua=1 [Google Scholar]
- 6. Ryerson ABE, Eheman CR, Altekruse SF, Ward JW, Jemal A, Sherman RL, et al. Annual Report to the Nation on the Status of Cancer, 1975‐2012, Featuring the Increasing Incidence of Liver Cancer. Cancer 2016;122(9):1312–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Block TM, Alter HJ, London WT, Bray M. A historical perspective on the discovery and elucidation of the hepatitis B virus. Antiviral Res 2016;131:109‐123. [DOI] [PubMed] [Google Scholar]
- 8. Seeger C, Mason WS. Molecular biology of hepatitis B virus infection. Virology 2015;479‐480:672‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liang TJ, Block TM, McMahon BJ, Ghany MG, Urban S, Guo JT, et al. Present and future therapies of hepatitis B: from discovery to cure. Hepatology 2015;62:1893‐1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Revill P, Testoni B, Locarnini S, Zoulim F. Global strategies are required to cure and eliminate HBV infection. Nat Rev Gastroenterol Hepatol 2016;13:239‐248. [DOI] [PubMed] [Google Scholar]


