Abstract
Since the generation of the first mouse model of Fragile X Syndrome (FXS) a broad range of neurophysiological phenotypes have been reported. However, it remains unclear which phenotypes are casually related to the cognitive deficits associated with FXS. Indeed, because many of these phenotypes are known to be modulated by experience, a confounding factor in the interpretation of many studies is whether some phenotypes are an indirect consequence of abnormal development and experience. To help diminish this confound we first conducted an in vitro developmental study of spontaneous neural dynamics in cortical organotypic cultures. A significant developmental increase in network activity and Up states was observed in both WT and Fmr1−/y circuits, along with a specific developmental delay in the emergence of Up states in knockout circuits. To determine whether Up state regulation is generally impaired in FXS circuits, we examined Up state plasticity using chronic optogenetic stimulation. WT and Fmr1−/y stimulated circuits exhibited a significant decrease in overall spontaneous activity including Up state frequency; however, no significant effect of genotype was observed. These results demonstrate that developmental delays characteristic of FXS are recapitulated during in vitro development, and that Up state abnormalities are likely a direct consequence of the disease, and not an indirect consequence of abnormal experience. But the fact that Fmr1−/y circuits exhibited normal homeostatic modulation of Up states, suggests that these plasticity mechanisms are largely intact, and that some of the previously reported plasticity deficits could reflect abnormal experience or the engagement of compensatory mechanisms.
Keywords: spontaneous activity, organotypic slices, homeostatic plasticity, optogenetic stimulation
Introduction
Fragile X syndrome (FXS) is caused by the loss of the fragile X mental retardation protein (FMRP) (Verkerk et al., 1991; O’Donnell and Warren, 2002; Santoro et al., 2012). In mice, deletion of the gene Fmr1, which codes for FMRP, generates a diverse array of neuronal phenotypes, including: abnormalities in dendritic spine morphology and stabilization (Irwin et al., 2000; Nimchinsky et al., 2001; Cruz-Martin et al., 2010; Portera-Cailliau, 2012), mGluR-LTD (Huber et al., 2002; Bear et al., 2004), synaptic plasticity (Larson et al., 2005; Desai et al., 2006; Meredith et al., 2007; Kim et al., 2013), homeostatic plasticity (Soden and Chen, 2010), short-term synaptic plasticity (Deng et al., 2011; Deng et al., 2013) axonal development (Antar et al.; Bureau et al., 2008), GABAergic inhibition (Curia et al., 2009; Vislay et al., 2013; He et al., 2014), inter-neuronal connectivity (Hanson and Madison, 2007; Gibson et al., 2008), channelopathies (Brown et al., 2010; Gross et al., 2011; Brager et al., 2012; Lee and Jan, 2012; Deng et al., 2013; Routh et al., 2013; Zhang et al., 2014), and imbalanced excitation/inhibition (Gibson et al., 2008; Harlow et al., 2010; Paluszkiewicz et al., 2011a; Goncalves et al., 2013). The sheer diversity of reported neural phenotypes has led some to suggest that FXS may be best understood under the light of abnormal network function (Belmonte and Bourgeron, 2006). Moreover, because cognition and behavior are not the products of isolated neurons, network level approaches provide an important framework for understanding and treating FXS. Indeed, FXS patients exhibit increased incidents of epilepsy (Musumeci et al., 1999; Hagerman and Stafstrom, 2009), and hypersensitivity to sensory stimuli (Miller et al., 1999; Frankland et al., 2004; Van der Molen et al., 2012). In addition, several studies in Fmr1 KO mice have reported enhanced spontaneous and evoked activity in neural networks from Fmr1 animals (Gibson et al., 2008; Hays et al., 2011; Goncalves et al., 2013).
A further challenge in interpreting the diversity of neural phenotypes observed in the mouse model of FXS is that most of the phenotypes are also associated with experience-dependent plasticity (Buonomano and Merzenich, 1998; Holtmaat and Svoboda, 2009). For example, spine density and stability are altered as a function of environmental enrichment and learning (Greenough et al., 1985; Trachtenberg et al., 2002; Holtmaat and Svoboda, 2009), and the magnitude and direction of synaptic plasticity can be modulated by experience (Kirkwood et al., 1995; Rioult-Pedotti et al., 2000). Indeed, the same plasticity protocol that leads to LTP in control animals, can generate LTD in animals deprived of normal sensory experience (Wang et al., 2012). Thus, one must consider the possibility that some of the neural phenotypes observed in Fmr1 knockout (KO) animals are a consequence of abnormal sensory experience—for example, impaired sensory perception, learning, and social interactions.
In vitro developmental studies provide one approach towards addressing these confounds since observed phenotypes are more likely to arise from the genotype and not from abnormal experience or development. Thus, we conducted in vitro developmental studies of spontaneous neural activity and Up states in cortical organotypic cultures from male knockout (Fmr1−/y) and WT littermates. We also characterized network-level plasticity of spontaneous activity in order to study how chronic external stimuli alter activity in Fragile X circuits. We used an optogenetic approach: cortical slices expressing ChR2 were optically stimulated over the course of days to induce a homeostatic down-regulation of network activity. The focus on internal network dynamics and Up states provides an effective approach to study network-level abnormalities, because emergent properties such as Up states ultimately reflect the net interaction of many of the different reported neural phenotypes (Chauvette et al., 2010; Crunelli and Hughes, 2010).
MATERIALS AND METHODS
Experimental animals
All experiments were conducted according the US National Institutes of Health guidelines for animal research, and approved by the Chancellor’s Animal Research Committee at the University of California, Los Angeles. Wild-type males (Fmr1+/y [#4828]) and Fmr1 knockout females (Fmr1−/− [#4624]) mice on the FVB background (FVB.129P2) were obtained from the Jackson Laboratory. A colony was established by breeding heterozygous (Fmr1−/+) females and WT (Fmr1+/y) males. Postnatal day 5–6 (PD5–6) WT (Fmr1+/y) and knockout (Fmr1−/y) male-littermates were used in all experiments. Mice were housed in the DLAM vivarium under a 12-h light/dark cycle up to 1 hr before brain tissue collection.
Organotypic slice preparation
Organotypic slices were prepared using the interface method (Stoppini et al., 1991; Buonomano, 2003) from (PD5–6) littermate Fmr1−/y and WT male mice. Mice were anesthetized with isoflurane and decapitated. The brain was removed and placed in chilled cutting media. Coronal slices (400 Km thickness) containing primary somatosensory and primary auditory cortex were cut using a vibratome and placed on Millipore (Billerica, MA) filters (MillicellCM) with 1 ml of culture media. Culture media was changed 1 and 24 h after cutting and every 2–3 d thereafter. Cutting media consisted of Eagle’s minimum essential medium (EMEM; catalog number 15-010; MediaTech, Herndon, VA) plus 3 mM MgCl2, 10 mM glucose, 25 mM HEPES, and 10 mM Tris base. Culture media consisted of EMEM plus 4 mM glutamine, 0.6 mM CaCl2, 1.85 mM MgSO4, 30 mM glucose, 30 mM HEPES, 0.5 mM ascorbic acid, 20% horse serum, 10 U/I penicillin, and 10 Kg/L streptomycin. Slices were incubated in 5% CO2 at 35°C for 7–30 d. Immediately after mice were anesthetized a tail sample from each mouse was collected for PCR analysis of tail DNA (genotyping by Transnetyx).
Electrophysiology
Whole-cell recordings were made from layer (L)-II/III regular-spiking, supragranular pyramidal neurons using infrared differential interference contrast visualization. Recording were performed at 7–30 DIV in ACSF composed of (in mM): 125 NaCl, 5.1 KCl, 2.6 MgSO4, 26.1 NaHCO3, 1 NaH2PO4, 25 glucose, and 2.6 CaCl2. This ACSF was formulated to match the standard culture media (Goel and Buonomano, 2013). The internal solution for whole-cell recordings contained (in mM) 100 K-gluconate, 20 KCl, 4 ATP-Mg, 10 phospho-creatine, 0.03 GTP-Na, and 10 HEPES and was adjusted to pH 7.3 and 300 mOsm. Temperature was maintained at 32°C, and the ACSF perfusion rate was set to 3–4 ml/min.
Only cells that satisfied the following criteria were accepted for analysis: membrane potential less than −60 mV, input resistance between 100 and 300 MR, and series resistance of less than 25 MR. Cells were discarded if membrane potential changed by more than 10 mV during the course of recording. Intrinsic excitability was measured as the number of action potentials evoked during a 250 ms current step at intensities of: 0.05, 0.1 0.15, 0.2 nA.
Induction of homeostatic plasticity
At 4–6 days-in-vitro (DIV) organotypic slices were transfected with AAV5-CaMKIIa-ChR2(H134R)-EYFP (University of North Carolina Vector Core). For each slice 1 KL of the viral solution was gently delivered via a glass pipette into 4–5 different locations in L-II/III. Slices were virally transfected only for the homeostatic plasticity experiments (data presented in Fig. 4–5). To reduce variability, these experiments relied on “sister” slices: derived from the same batch of animals (littermates), maintained with the same culture medium and serum, placed in the same incubator and virally transfected on the same session. In each experiment, 2 slices (from the same animal) of each genotype (WT or Fmr1−/y) were placed in the “stimulating incubator” on the day of experiment (total of 4 slices from two mice - sister slices). One slice per genotype received chronic stimulation via a blue LED (Stimulated slice, Super Bright LEDs) while the other was kept in the same incubator but did not receive stimulation. Optical stimulation consisted of a 50 ms flash of blue light (457 nm) delivered every 30 sec for either 2 or 4 days.
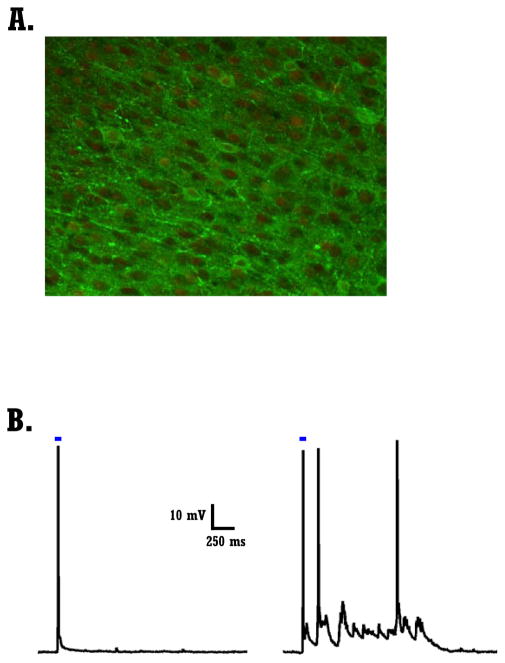
Figure 4. Optogenetic transfection and chronic stimulation of organotypice cultures.
A) Confocal image of a transfected slice containing cells expressing ChR2-EYFP. Slice was counterstained with anti-GFP and anti-NeuN. Overlay of green channel (GFP, ChR+ cells) and red channel (anti-NeuN) to label all neurons.
B) Range of light evoked responses in two ChR+ cells. Left cell shows a direct light evoked response, while the cell on the right shows both a direct and indirect light evoked responses.
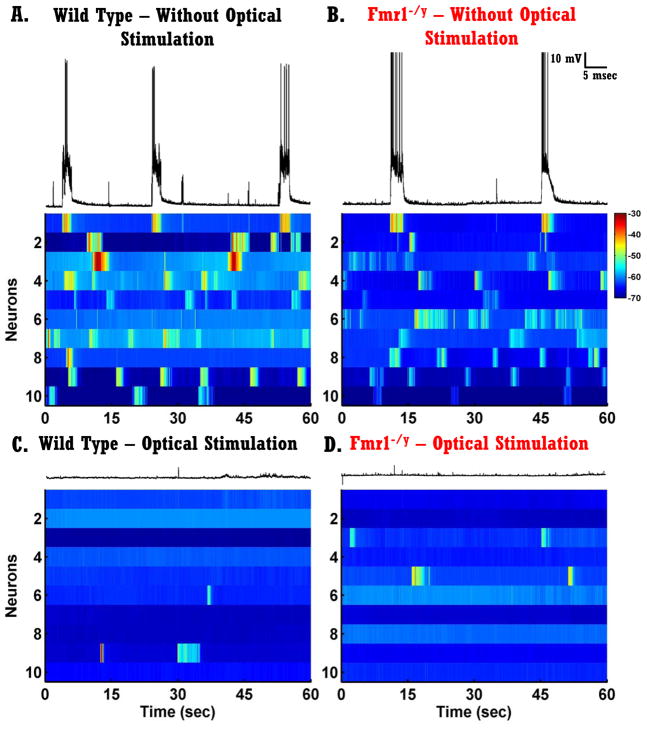
Figure 5. Plasticity of Spontaneous Network activity in vitro.
A–B) Each voltagegram represents one trace from ten randomly selected neurons recorded from slices that did not receive optical stimulation. Left panels, neurons from WT circuits; right panels, neurons from Fmr1−/y circuits. The trace above voltagegrams presents the same trace as the first row of the voltagegram.
C–D) Same as A–B but from slices that received optical stimulation for 2 or 4 days.
Quantification of spontaneous activity
For each neuron a minimum of 6 min of spontaneous activity was recorded. Recordings were sampled at 10 kHz for the developmental study (6–12 min of recording - data presented in Fig. 2–3) and at 5 kHz for the homeostatic study (10 min of recording – data presented in Fig. 4–5). Data was saved for off-line analysis using custom-written software in Matlab (MathWorks, Natick, MA).
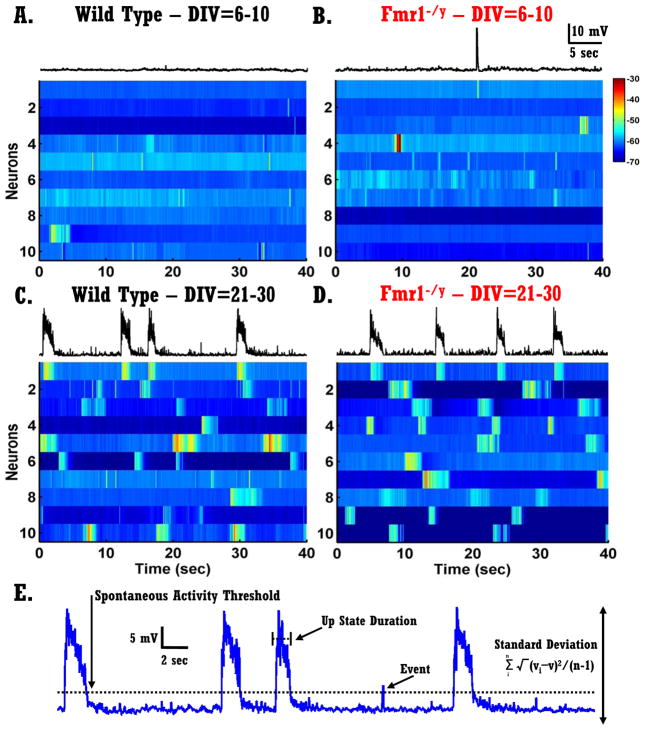
Figure 2. Development of spontaneous network activity in vitro.
A–B) Each voltagegram represents ten randomly selected neurons recorded at an early developmental age (DIV 6–10) - each row represents a 40 sec recording from one neuron. Left panels: neurons from WT circuits; right panels: neurons from Fmr1−/y circuits. The trace above voltagegrams presents the same trace as the first row of the voltagegram.
C–D) Same as in A–B but from mature circuits (DIV 21–30).
E) Different quantitative measures of spontaneous network activity. A 40-second voltage trace of spontaneous activity of a pyramidal neuron.
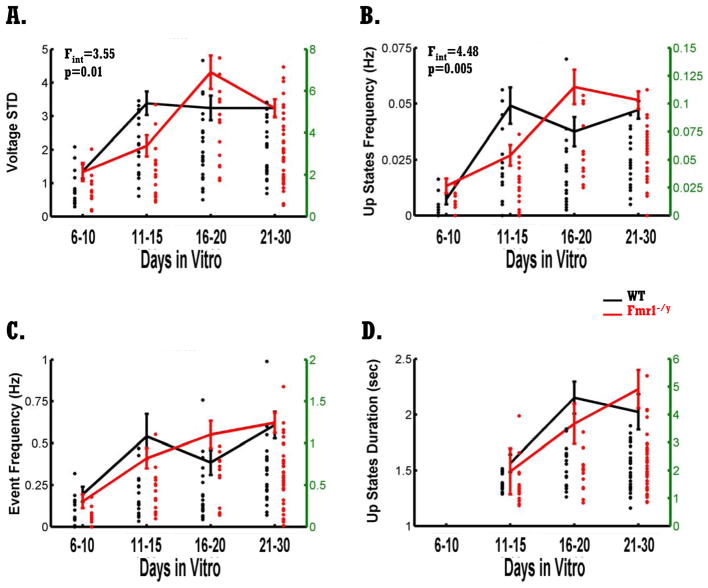
Figure 3. Developmental delay of Up states in Fmr1-/y circuits.
A) Littermate WT (black) and Fmr1−/y (red) circuits exhibit a developmental increase in the standard deviation of the voltage (F3,151=13.89, p<10−7). There was a developmental delay in the Fmr1−/y circuits as revealed by a significant interaction between genotype and age (F3,151=3.55, p=0.016). Dots represent single neurons and are plotted against the green y axes (right side; some dots are overlapping). Total number of cells recorded from WT circuits was 80: 15 (DIV 6–10), 17 (DIV 11–15), 22 (DIV 16–20), 26 (DIV 21–30). Total number of cells recorded from Fmr1−/y circuits was 79: 10 (DIV 6–10), 17 (DIV 11–15), 14 (DIV 16–20), 38 (DIV 21–30).
B) Up state frequency increased significantly with development for WT and Fmr1−/y circuits (F3,151=16.18, p<10−8). Neurons from Fmr1−/y circuits exhibited a significant delay in the emergence of Up states as indicated by the significant interaction between genotype and age (F3,151=4.48, p=0.004). Up state frequency was significantly reduced in Fmr1−/y circuits compared to WT circuits at DIV=11–15 (F1,32=5.59, p=0.02). Number of cells is as in A.
C) Overall spontaneous activity as quantified by event frequency was not different between genotypes, but increased significantly with development (F3,151=8.73, p<10−4). Number of cells is as in A.
D) Mean Up states duration increased significantly with development for both WT and Fmr1−/y circuits (F3,151=6.05, p=0.003). No difference between the two genotypes was observed. Number of cells is as in A without the cells at DIV=6–10.
Spontaneous network events and Up states were quantified based on previously defined criteria (Johnson and Buonomano, 2007; Goel and Buonomano, 2013). The criterion for classifying a spontaneous network event was a voltage deflection of 5 mV above resting membrane potential (‘threshold crossing’). This threshold excluded mini and unitary EPSPs from the analyses and captured primarily network events arising from the activity of multiple presynaptic neurons (see Fig. 2E). To prevent counting a single event that crossed threshold multiple times within a short window as multiple events, a minimum inter-event interval of 100 ms was used—thus, if the voltage fell and crossed threshold again within less than 100 ms, it was still classified as being the same event. Up states were defined as an event that remained above threshold for no less than 500 ms. During a network activation such as an Up state, the membrane potential would often make multiple brief passes above and below this threshold before returning to resting potential. For this reason and in order to prevent counting a single Up state that crossed threshold multiple times within a short window as multiple Up states, a minimum inter-event interval of 100 ms was used.
To provide an assumption independent measure of overall spontaneous activity we calculated the standard deviation of the voltage (vSTD): simply the standard deviation of the recorded membrane potential of a cell.
Statistics
Data are represented by the mean + SEM. Statistical significance was determined using two-way Analyses of Variance (ANOVA) for Fig. 3 & 5 and two-way ANOVA with repeated-measures t-tests for intrinsic excitability data. To contrast differences between specific groups, post hoc tests were preformed using F-tests for simple effects (Kintz, 1970). For reporting the statistical analyses, the value n refers to the number of cells.
RESULTS
Up states are highly correlated across neurons
Spontaneous neural activity occurs at both the synaptic (e.g., mEPSPs) and network level. Network-level spontaneous activity reflects the orchestrated regulation of numerous synaptic and cellular properties and it provides an important indicator of normal network function (Thompson, 1997; Johnson and Buonomano, 2007; Destexhe, 2011; Runfeldt et al., 2014)—e.g., connectivity, synaptic strength, the balance of excitation and inhibition, and intrinsic excitability. Spontaneous network activity can take on at least two qualitatively different forms: brief events that reflect bouts of simultaneous activity in a subset of neurons, and Up states
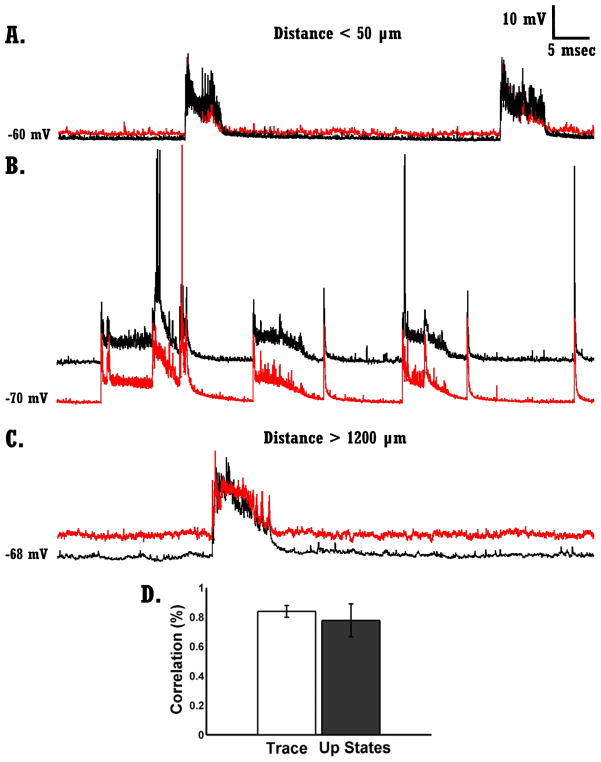
Up states are network-wide events, as demonstrated by the fact that during an Up state the vast majority of neurons within a network participate in the Up state. Furthermore, there is a high degree of correlation between voltage waveforms recorded in different neurons during Up states (MacLean et al., 2005; Johnson and Buonomano, 2007; Paluszkiewicz et al., 2011b; Poskanzer and Yuste, 2011; Runfeldt et al., 2014). Thus the spontaneous voltage activity recorded from a single neuron provides an excellent readout of network dynamics. Fig.1A–C shows 3 examples of dual current-clamp recordings performed in three different organotypic slices from WT animals. Each pair shows a 40 second recording of spontaneous voltage activity. The voltage activity depicted in any two simultaneously recorded neurons is highly correlated, and when an Up state occurs in one neuron it occurs in the second neuron as well—we never observed an Up state in one neuron but not in the other. The high correlation, holds true even when neurons were more than 1000 Km apart (Fig.1C). Fig.1D shows that the mean correlation between cells, as measured over the entire trace or a window around an Up state is above 0.75. These results are in accordance with previous data (MacLean et al., 2005; Poskanzer and Yuste, 2011) and confirm that intracellular recordings from a single pair of cells provide an accurate measure of network level Up state activity.
Figure 1. Network activity – Up states are highly correlated across neurons.
A) Voltage traces from two simultaneously recorded neurons in WT circuits (< 50 μm apart). Up states in both neurons occurred synchronously.
B) Same as A.
C) Same as A–B but from two neurons that were more than 1200 μm apart.
D) Mean correlation of entire traces (white bar) and mean correlation of Up states (dark bar). Data was collected from 7 pairs of neurons from 7 different slices of WT circuits.
Fmr1−/y circuits exhibit a developmental delay of the emergence of spontaneous activity and Up states
To examine potential network-level abnormalities in FMRP KO circuits we characterized the in vitro development of spontaneous activity in cortical organotypic slices from Fmr1−/y and WT male-littermate mice. Whole-cell recordings were performed from L-II/III pyramidal neurons from slices cultured for 7–30 days. The voltagegrams shown in Fig. 2A–B indicate that neurons of both WT and Fmr1−/y circuits exhibited very little spontaneous activity at early ages (6–10 days-in-vitro, DIV) as indicated by the low incidents of deflections from resting membrane potentials. Interestingly, mature circuits (21–30 DIV) exhibited significantly more and richer forms of spontaneous activity (Fig. 2C–D). Each voltagegram shows data from ten randomly selected neurons and each row represent a 40 sec recording from one neuron.
To quantify the development of spontaneous activity we used four measures illustrated in Fig. 2E: standard-deviation of voltage (vSTD), Up state frequency, event frequency, and Up state duration. In general, vSTD provides an assumption-independent measure of spontaneous activity, while the other measures are dependent on the choice of voltage and duration thresholds (see Methods). A two-way ANOVA analysis revealed a significant increase in vSTD with development (F3,151=13.89, p<10−7; Fig. 3A) as well as a significant interaction between the age and genotype factors (F3,151=3.55, p=0.016; Fig. 3A). Post hoc analyses of this interaction revealed that in WT circuits vSTD increased significantly between DIV of 6–10 and DIV of 11–15 (F1,30=22.34, p<10−5, F-test for simple effects) while no significant increase was observed in Fmr1−/y circuits during this same period (p=0.10). However, while vSTD did not increase in the WT circuits between DIV 11–15 and 16–20 (p=0.78), it did in the Fmr1−/y circuits (F1,29=14.86, p=0.001, F-test for simple effects). Additionally, at DIV 11–15 vSTD of WT circuits was significantly higher than in Fmr1−/y circuits (F1,32=13.68, p=0.011, F-test for simple effects), while at DIV 16–20 vSTD of WT was marginally lower than in Fmr1−/y circuits (F1,35=3.65, p=0.06, F-test for simple effects). These results show that during in vitro development there is a significant increase in network activity in both WT and Fmr1−/y circuits. However, the emergence of spontaneous activity in Fmr1−/y circuits is developmentally delayed. The vSTD measure, however, does not discern whether this delay reflects a general abnormality in the development of all types of spontaneous network activity, or of specific forms of spontaneous activity, such as spontaneous Up states.
Quantification of spontaneous Up states (see Methods) revealed a strong interaction effect between the age and genotype factors (F3,151=4.48, p=0.004; Fig. 3B) as well as the expected increase with development (F3,151=16.18, p<10−8; Fig. 3B). Post hoc analyses revealed that between DIV of 6–10 and DIV of 11–15, Up state frequency of WT circuits increased dramatically (F1,30=21.59, p<10−5, F-test for simple effects) whereas in the Fmr1−/y circuits Up state frequency only increased when circuits reached DIV of 16–20 (F1,29=12.43, p=0.001, F-test for simple effects).
There was no significant genotype or interaction effect on spontaneous activity as measured by event frequency, although there was again a significant developmental increase in event frequency (F3,151=8.73, p<10−4; Fig. 3C). Note that this measure was defined as a voltage deflection higher than 5 mV that was not limited to a certain amount of time (as opposed to Up States—which tend to cross and stay above threshold for longer periods), thus this measure contains both deflections that are Up states and short bouts of network activity. The robust developmental delay of Up state frequency, and the absence of any effect on general forms of spontaneous activity, suggest that the developmental delay in vSTD is mainly driven by changes in Up states. Additionally, these results indicate that the abnormalities found in Fmr1−/y circuits do not reflect a nonspecific alteration of all forms of spontaneous network activity, but rather that these effects are specific to the development of Up states.
Previous results, both in vitro (acute slices) and in vivo have found that the duration of spontaneously occurring Up states are altered in Fmr1 KO mice (Gibson et al., 2008; Hays et al., 2011). We thus analyzed Up state duration as a function of age and genotype—because circuits at 6–10 DIV exhibited few Up states we excluded this time point from our analysis (Fig. 3D). Analysis of Up state duration did not reveal a significant genotype or genotype and age interaction effect, although there was again a significant increase in Up state duration with development (F3,151=6.05, p=0.003).
Together, these results show that both in WT and Fmr1−/y circuits spontaneous activity increases with development, further confirming previous result showing a progressive transition, from mostly single events occurring during the first week in vitro to the presence of more frequent and longer-duration Up states at older ages in vitro (Johnson and Buonomano, 2007). However, Fmr1−/y circuits exhibit a significant developmental delay in the emergence of spontaneous network activity that was specific to Up states occurrence.
Abnormal intrinsic excitability in Fmr1−/y circuits
In vivo and ex vivo studies have previously reported that cortical networks from Fmr1 KO mice are hyperexcitable (Gibson et al., 2008; Curia et al., 2009; Olmos-Serrano et al., 2010; Testa-Silva et al., 2012; Goncalves et al., 2013). Such hyperexcitability, could be attributed to a number of different synaptic, cellular, and network mechanisms, including, intrinsic neuronal excitability (Gibson et al., 2008; Zhang et al., 2014). Therefore, we also measured intrinsic excitability as the number of action potentials elicited by 250 ms current steps at intensities between 0.05 and 0.2 nA. As expected, a three-way ANOVA (age, genotype, current intensity - repeated measures) revealed a significant decrease in excitability with development for both genotypes (F3,151=6.94; p<10−4, data not shown) (Johnson and Buonomano, 2007), as well as a significant difference between Fmr1−/y and WT circuits as revealed by the main genotype effect (F1,151=10.67; p=0.001) and the significant interaction between genotype and age (F3,151=9.53; p<10−6). Post hoc analyses revealed that Fmr1−/y neurons were significantly more excitable compared to WT neurons at DIV 6–10 (F1,23=9.28, p=0.006) and at 16–20 (F1,34=20.4, p<10−4). In contrast at DIV 11–15 Fmr1−/y circuits exhibited reduced excitability compared to WT circuits (F1,32=58.24, p=0.017). Although intrinsic excitability could contribute to the differences seen in Up state frequency it cannot account for the developmental delay in network activity for a number of reasons, including, the absence of any change in excitability in WT cells during the period of the largest increase in Up state frequency (DIV 6–10 – DIV 11–15), raising the possibility that the increase in excitability in Fmr1−/y could be a compensatory mechanism triggered by the delay in Up state development (see Discussion).
Homeostatic down-regulation of spontaneous activity by optogenetic stimulation
The developmental delay of Up state emergence in Fmr1−/y circuits, together with the absence of a delay in the development of general features of spontaneous activity, suggests potential abnormalities in one or more of the mechanisms governing Up state dynamics. To determine whether this is a general and long-lasting abnormality we next asked whether homeostatically induced plasticity of Up states is also altered in Fmr1−/y circuits.
Previous studies using chronic electrical stimulation (Goel and Buonomano, 2013) demonstrated a homeostatic down-regulation of spontaneous Up state frequency. Here we examined the effects of long-term optical stimulation, on spontaneous activity and Up states in WT and Fmr1−/y circuits. We used an optogenetic approach to stimulate slices, and emulate the increase in externally driven activity that occurs during development. Slices were transfected with AAV5-CaMKIIa-ChR2(H134R)-EYFP on DIV 4–6 (Fig. 4A) and optically stimulated for two or four days at DIV>22 (see Methods). Two types of light-evoked responses were elicited in L-II/III pyramidal neurons: a short-latency depolarization (Fig. 4B - Left cell), or a slow latency prolonged depolarization—produced by the recurrent activation of light responsive neurons (Fig. 4B – Right cell). Cells that exhibited a short latency (<15 ms) depolarization of at least 5 mV, were designated as ChR+ cells.
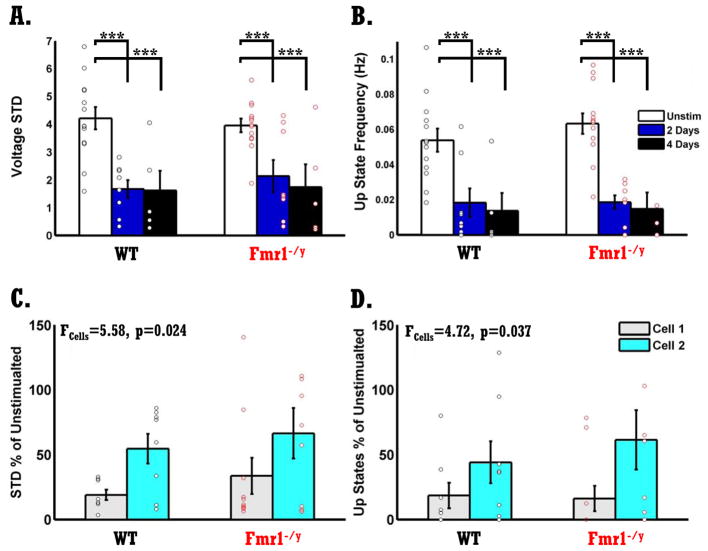
The voltagegrams shown in Fig. 5A–D reveal that chronic optical stimulation of both WT and Fmr1−/y circuits resulted in a significant reduction of network spontaneous activity. Each voltagegram represents ten randomly selected neurons from four conditions: Unstimulated WT slices (Fig. 5A), Unstimulated Fmr1−/y slices (Fig. 5B), Stimulated WT slices (2 or 4 days) (Fig. 5C) and Stimulated Fmr1−/y slices (Fig. 5D). A two-way ANOVA of sister slices (see Methods) revealed a significant reduction in vSTD following chronic optical stimulation for both WT and Fmr1−/y circuits (F2,46=18.76, p<10−5, Fig. 6A), and no significant difference between the two genotypes. Quantifications of Up state frequency also revealed a significant stimulation effect indicating that Up state frequency is significantly reduced following chronic optical stimulation (F2,46=25.42, p<10−7), and no genotype effect. Additionally, no significant difference was found between 2 or 4 days of stimulation meaning that 2 days of optical stimulation was enough to induce a significant reduction in spontaneous activity. Chronic optical stimulation also induced a significant decrease in intrinsic excitability, and the degree of this plasticity was normal in the Fmr1−/y circuits (data not shown).
Figure 6. Normal homeostatic plasticity of Up states in Fmr1−/y circuits.
A) Chronic optical stimulation resulted in a significant reduction in vSTD in both WT and Fmr1− /y circuits (F2,46=18.76, p<10−5 ), with no significant difference between two or four days of stimulation. There was no difference in homeostatic plasticity of vSTD between WT and Fmr1−/y circuits. White bars represent no optical stimulation, blue bars, two days of optical stimulation, and black bars, four days of optical stimulation. Black dots represent neurons from WT circuits, and red dots neurons from Fmr1−/y circuits. Number of neurons in WT and Fmr1−/y groups are the same due to sister-slice design: Unstimulated slices, n=13; two days of stimulation, n=8; four days-stimulation, n=5. Note that number of neurons is higher than number of dots due to overlapping data.
B) Same as in A but for Up state frequency. Stimulation induced a decrease in Up state frequency (F2,46=25.42, p<10−7), and no difference between genotypes. Same number of neurons as in A.
C) Same data from A but analyzed according to the order the cells were recorded, and collapsed across 2 and 4 days of stimulation. Data is normalized to the sister unstimulated slice. A significant difference in vSTD was found between cell order, neurons from the first recorded cell (Cell 1) exhibited reduced values compared to the second cell (Cell 2) (F1,35=5.58, p=0.024). Activity induced plasticity was the same in WT and Fmr1−/y circuits. Gray bars, Cell 1.; Cyan, Cell 2. Number of cells in each group was similar, n=8.
D) Same as C but for Up state frequency. There was a significant effect of stimulation on Up state frequency (F1,35=4.72, p=0.037). Number of cells in each group was similar, n=10.
These results demonstrate that chronic optical stimulation results in a significant reduction in all measures of spontaneous network activity, and that this form of homeostatic plasticity was normal in Fmr1−/y circuits. To the best of our knowledge, these are the first experiments to show that chronic optical stimulation can induce homeostatic down-regulation of spontaneous network activity and Up states—although other labs have used optogenetic approaches to induce synaptic homeostatic plasticity (Goold and Nicoll, 2010).
Although the induction of homeostatic plasticity was normal, it is possible that in Fmr1−/y circuits, a difference was present in the reversibility of this plasticity once stimulation has ceased. Therefore, we analyzed vSTD and Up state frequency according to the order cells were recorded. The first cell of a slice (Cell 1) was recorded up to 20 minutes after the end of chronic stimulation, while the second cell (Cell 2) was recorded over the next 20–40 minutes (Fig. 6C–D). The decrease in spontaneous Up state frequency was significantly larger in Cell 1 compared to Cell 2 (vSTD: F1,35=5.58, p=0.024; Up state frequency: F1,35=4.72, p=0.037), but there was no difference between WT and Fmr1−/y circuits. These results clearly indicate that the mechanisms that control homeostatic plasticity of network activity and Up state modulation are intact in Fmr1−/y circuits, even though these circuits exhibited a developmental delay in the emergence of Up states.
DISCUSSION
The diversity of neural phenotypes that have been reported in animal models of Fragile X highlight the challenge in determining which phenotypes are a primary consequence of the disease, and those that arise as a secondary consequence of abnormal development and experience. To help overcome this challenge we used organotypic slices, which undergo a developmental process that recapitulates (Bolz, 1994; Echevarria and Albus, 2000; De Simoni et al., 2003; Johnson and Buonomano, 2007) many aspects of in vivo development (Hubel and Wiesel, 1963; Colonnese et al., 2010).
Our results revealed an in vitro developmental delay of Up state emergence in Fmr1−/y circuits. Critically, this delay did not reflect a generalized delay of all forms of spontaneous network activity. The alternations in Up state dynamics are consistent with previous reports of Up state abnormalities in acute slices and in vivo (Gibson et al., 2008; Hays et al., 2011; Goncalves et al., 2013). We did not observe any abnormalities in Up state duration or firing frequency during Up states, as in some previous studies. Such differences are likely in part a consequence of the use of in vivo/acute preparations versus organotypic slices. But the previous studies, together with the current study, clearly indicate that across preparations Up states represent a significant neural phenotype in FXS. Furthermore, the nature of the current in vitro developmental study, suggests that the alterations in Up state development are not a secondary consequence of experience or developmental abnormalities but a direct result of FMRP loss. Thus, our data confirm previous results and show significant network abnormalities in FXS KO circuits that are more likely to arise from the absence of the FMRP and not indirect result of abnormal development or experience.
Consistent with previous studies, we also observed abnormalities in intrinsic excitability (Gibson et al., 2008; Zhang et al., 2014). While intrinsic excitability in WT neurons decreased throughout development, excitability in Fmr1−/y neurons increased from DIV 11–15 to DIV 16–20, before decreasing again. These results are the first to show that FXS circuits are not always more excitable than WT circuits and that neurons’ intrinsic excitability depends greatly on the time of test. Previous work in rat organotypic slices (Johnson and Buonomano, 2007) suggests that the developmental increase in Up states is not produced by changes in intrinsic excitability. Indeed, in the current study there was no change in excitability in the WT circuits during the period of the largest increase in Up state frequency (DIV 6–10 – DIV 11–15), nor was there a correlation between excitability and Up state frequency (data not shown). Additionally, we did not find any genotypic difference in firing frequency during Up states. Together, these observations raise the possibility that the abnormal developmental increase in intrinsic excitability in Fmr1−/y networks (DIV 16–20) reflected a compensatory mechanism in response to the delayed increase in Up states at DIV 11–15.
Homeostatic plasticity of spontaneous network activity in response to global pharmacological manipulations and electrical stimulation has been established before (Ramakers et al., 1990; Johnson and Buonomano, 2007; Goel and Buonomano, 2013). However, it was not known whether spontaneous activity of Fmr1−/y circuits is sensitive to chronic stimulation. We examined this issue using an optogenetic approach in which slices expressing ChR were stimulated while in the incubator. Both WT and Fmr1−/y circuits exhibited reduced network activity in response to chronic stimulation and no difference was found between the two circuits. In addition, both WT and Fmr1−/y circuits exhibited a significant but similar reduction in intrinsic excitability following stimulation. Together, these results indicate that mature Fmr1−/y circuits exhibit normal homeostatic plasticity of Up states.
A number of studies have reported developmental delays of neural phenotypes in FXS (Nimchinsky et al., 2001; Bureau et al., 2008; Cruz-Martin et al., 2010; Harlow et al., 2010; He and Portera-Cailliau, 2013; Padmashri et al., 2013). Here, we report for the first time, a delay of a neural phenotype during in vitro development. Thus providing strong evidence that Up state abnormalities are a direct consequence of the absence of FMRP, rather than an indirect product of altered development or experience. Importantly, despite abnormal development of Up states, a fairly complex network level form of plasticity—homeostatic plasticity of Up state frequency—was normal in mature in vitro circuits. This was unexpected because many of the neural phenotypes associated with FXS, including abnormal LTP/LTD, STDP, homeostatic plasticity, and the balance of excitation and inhibition, would be expected to alter the ability of networks to homeostatically adjust Up state dynamics (Haider et al., 2006; Mann et al., 2009; Chen et al., 2013). Thus the observation that chronic stimulation appears to produce normal homeostatic plasticity of Up state frequency, raises the interesting possibility that mature cortical circuits may have largely normal forms of plasticity in place. These results also suggest that some reported neural phenotypes could indeed reflect compensatory mechanisms, and that in vitro studies could provide an approach to address such confounds.
References
- Antar LN, Li C, Zhang H, Carroll RC, Bassell GJ. Local functions for FMRP in axon growth cone motility and activity-dependent regulation of filopodia and spine synapses. Molecular and Cellular Neuroscience. 2006;32:37–48. doi: 10.1016/j.mcn.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Bourgeron T. Fragile X syndrome and autism at the intersection of genetic and neural networks. Nat Neurosci. 2006;9:1221–1225. doi: 10.1038/nn1765. [DOI] [PubMed] [Google Scholar]
- Bolz J. Cortical circuitry in a dish. Curr Opinion Neurobio. 1994;4:545–549. doi: 10.1016/0959-4388(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Brager DH, Akhavan AR, Johnston D. Impaired dendritic expression and plasticity of h-channels in the fmr1(-/y) mouse model of fragile X syndrome. Cell Rep. 2012;1:225–233. doi: 10.1016/j.celrep.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MR, Kronengold J, Gazula VR, Chen Y, Strumbos JG, Sigworth FJ, Navaratnam D, Kaczmarek LK. Fragile X mental retardation protein controls gating of the sodium-activated potassium channel Slack. Nat Neurosci. 2010;13:819–821. doi: 10.1038/nn.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomano DV. Timing of neural responses in cortical organotypic slices. Proc Natl Acad Sci U S A. 2003;100:4897–4902. doi: 10.1073/pnas.0736909100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annual Rev Neuroscience. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Bureau I, Shepherd GMG, Svoboda K. Circuit and Plasticity Defects in the Developing Somatosensory Cortex of Fmr1 Knock-Out Mice. J Neurosci. 2008;28:5178–5188. doi: 10.1523/JNEUROSCI.1076-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvette S, Volgushev M, Timofeev I. Origin of active states in local neocortical networks during slow sleep oscillation. Cereb Cortex. 2010;20:2660–2674. doi: 10.1093/cercor/bhq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Rochefort NL, Sakmann B, Konnerth A. Reactivation of the same synapses during spontaneous up states and sensory stimuli. Cell Rep. 2013;4:31–39. doi: 10.1016/j.celrep.2013.05.042. [DOI] [PubMed] [Google Scholar]
- Colonnese MT, Kaminska A, Minlebaev M, Milh M, Bloem B, Lescure S, Moriette G, Chiron C, Ben-Ari Y, Khazipov R. A conserved switch in sensory processing prepares developing neocortex for vision. Neuron. 2010;67:480–498. doi: 10.1016/j.neuron.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V, Hughes SW. The slow (<1 Hz) rhythm of non-REM sleep: a dialogue between three cardinal oscillators. Nat Neurosci. 2010;13:9–17. doi: 10.1038/nn.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Martin A, Crespo M, Portera-Cailliau C. Delayed stabilization of dendritic spines in fragile X mice. J Neurosci. 2010;30:7793–7803. doi: 10.1523/JNEUROSCI.0577-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curia G, Papouin T, Seguela P, Avoli M. Downregulation of tonic GABAergic inhibition in a mouse model of fragile X syndrome. Cereb Cortex. 2009;19:1515–1520. doi: 10.1093/cercor/bhn159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simoni A, Griesinger CB, Edwards FA. Development of rat CA1 neurones in acute versus organotypic slices: role of experience in synaptic morphology and activity. J Physiol. 2003;550:135–147. doi: 10.1113/jphysiol.2003.039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Sojka D, Klyachko VA. Abnormal presynaptic short-term plasticity and information processing in a mouse model of fragile X syndrome. J Neurosci. 2011;31:10971–10982. doi: 10.1523/JNEUROSCI.2021-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng PY, Rotman Z, Blundon JA, Cho Y, Cui J, Cavalli V, Zakharenko SS, Klyachko VA. FMRP regulates neurotransmitter release and synaptic information transmission by modulating action potential duration via BK channels. Neuron. 2013;77:696–711. doi: 10.1016/j.neuron.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai NS, Casimiro TM, Gruber SM, Vanderklish PW. Early Postnatal Plasticity in Neocortex of Fmr1 Knockout Mice. Journal of Neurophysiology. 2006;96:1734–1745. doi: 10.1152/jn.00221.2006. [DOI] [PubMed] [Google Scholar]
- Destexhe A. Intracellular and computational evidence for a dominant role of internal network activity in cortical computations. Curr Opin Neurobiol. 2011 doi: 10.1016/j.conb.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Echevarria D, Albus K. Activity-dependent development of spontaneous bioelectric activity in organotypic cultures of rat occipital cortex. Brain Res Dev Brain Res. 2000;123:151–164. doi: 10.1016/s0165-3806(00)00089-4. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Wang Y, Rosner B, Shimizu T, Balleine BW, Dykens EM, Ornitz EM, Silva AJ. Sensorimotor gating abnormalities in young males with fragile X syndrome and Fmr1-knockout mice. Mol Psychiatry. 2004;9:417–425. doi: 10.1038/sj.mp.4001432. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Bartley AF, Hays SA, Huber KM. Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. J Neurophysiol. 2008;100:2615–2626. doi: 10.1152/jn.90752.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Buonomano DV. Chronic electrical stimulation homeostatically decreases spontaneous activity, but paradoxically increases evoked network activity. J Neurophysiol. 2013;109:1824–1836. doi: 10.1152/jn.00612.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves JT, Anstey JE, Golshani P, Portera-Cailliau C. Circuit level defects in the developing neocortex of Fragile X mice. Nat Neurosci. 2013;16:903–909. doi: 10.1038/nn.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goold CP, Nicoll RA. Single-Cell Optogenetic Excitation Drives Homeostatic Synaptic Depression. Neuron. 2010;68:512–528. doi: 10.1016/j.neuron.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, Hwang HM, Gorman C. Evidence for active synapse formation or altered postsynaptic metabolism in visual cortex of rats reared in complex environments. Proc Natl Acad Sci U S A. 1985;82:4549–4552. doi: 10.1073/pnas.82.13.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Yao X, Pong DL, Jeromin A, Bassell GJ. Fragile X mental retardation protein regulates protein expression and mRNA translation of the potassium channel Kv4.2. J Neurosci. 2011;31:5693–5698. doi: 10.1523/JNEUROSCI.6661-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman PJ, Stafstrom CE. Origins of epilepsy in fragile X syndrome. Epilepsy Curr. 2009;9:108–112. doi: 10.1111/j.1535-7511.2009.01309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider B, Duque A, Hasenstaub AR, McCormick DA. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J Neurosci. 2006;26:4535–4545. doi: 10.1523/JNEUROSCI.5297-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JE, Madison DV. Presynaptic FMR1 genotype influences the degree of synaptic connectivity in a mosaic mouse model of fragile X syndrome. J Neurosci. 2007;27:4014–4018. doi: 10.1523/JNEUROSCI.4717-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow EG, Till SM, Russell TA, Wijetunge LS, Kind P, Contractor A. Critical period plasticity is disrupted in the barrel cortex of FMR1 knockout mice. Neuron. 2010;65:385–398. doi: 10.1016/j.neuron.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays SA, Huber KM, Gibson JR. Altered neocortical rhythmic activity states in Fmr1 KO mice are due to enhanced mGluR5 signaling and involve changes in excitatory circuitry. J Neurosci. 2011;31:14223–14234. doi: 10.1523/JNEUROSCI.3157-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He CX, Portera-Cailliau C. The trouble with spines in fragile X syndrome: density, maturity and plasticity. Neuroscience. 2013;251:120–128. doi: 10.1016/j.neuroscience.2012.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Nomura T, Xu J, Contractor A. The Developmental Switch in GABA Polarity Is Delayed in Fragile X Mice. The Journal of Neuroscience. 2014;34:446–450. doi: 10.1523/JNEUROSCI.4447-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive Fields of Cells in Striate Cortex of Very Young, Visually Inexperienced Kittens. J Neurophysiol. 1963;26:994–1002. doi: 10.1152/jn.1963.26.6.994. [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin SA, Galvez R, Greenough WT. Dendritic spine structural anomalies in fragile-X mental retardation syndrome. Cereb Cortex. 2000;10:1038–1044. doi: 10.1093/cercor/10.10.1038. [DOI] [PubMed] [Google Scholar]
- Johnson HA, Buonomano DV. Development and Plasticity of Spontaneous Activity and Up States in Cortical Organotypic Slices. J Neurosci. 2007;27:5915–5925. doi: 10.1523/JNEUROSCI.0447-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Gibboni R, Kirkhart C, Bao S. Impaired Critical Period Plasticity in Primary Auditory Cortex of Fragile X Model Mice. The Journal of Neuroscience. 2013;33:15686–15692. doi: 10.1523/JNEUROSCI.3246-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintz JLBaB. Computational Handbook of Statistics - 1970 [Google Scholar]
- Kirkwood A, Lee H-K, Bear MF. Co-regulation of long-term potentiation and experience-dependent synaptic plasticity in visual cortex by age and experience. Nature. 1995;375:328–331. doi: 10.1038/375328a0. [DOI] [PubMed] [Google Scholar]
- Larson J, Jessen RE, Kim D, Fine AK, du Hoffmann J. Age-dependent and selective impairment of long-term potentiation in the anterior piriform cortex of mice lacking the fragile X mental retardation protein. J Neurosci. 2005;25:9460–9469. doi: 10.1523/JNEUROSCI.2638-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY, Jan LY. Fragile X syndrome: mechanistic insights and therapeutic avenues regarding the role of potassium channels. Curr Opin Neurobiol. 2012;22:887–894. doi: 10.1016/j.conb.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean JN, Watson BO, Aaron GB, Yuste R. Internal dynamics determine the cortical response to thalamic stimulation. Neuron. 2005;48:811–823. doi: 10.1016/j.neuron.2005.09.035. [DOI] [PubMed] [Google Scholar]
- Mann EO, Kohl MM, Paulsen O. Distinct roles of GABA(A) and GABA(B) receptors in balancing and terminating persistent cortical activity. J Neurosci. 2009;29:7513–7518. doi: 10.1523/JNEUROSCI.6162-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith RM, Holmgren CD, Weidum M, Burnashev N, Mansvelder HD. Increased Threshold for Spike-Timing-Dependent Plasticity Is Caused by Unreliable Calcium Signaling in Mice Lacking Fragile X Gene Fmr1. Neuron. 2007;54:627–638. doi: 10.1016/j.neuron.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Miller LJ, McIntosh DN, McGrath J, Shyu V, Lampe M, Taylor AK, Tassone F, Neitzel K, Stackhouse T, Hagerman RJ. Electrodermal responses to sensory stimuli in individuals with fragile X syndrome: a preliminary report. Am J Med Genet. 1999;83:268–279. [PubMed] [Google Scholar]
- Musumeci SA, Hagerman RJ, Ferri R, Bosco P, Dalla Bernardina B, Tassinari CA, De Sarro GB, Elia M. Epilepsy and EEG findings in males with fragile X syndrome. Epilepsia. 1999;40:1092–1099. doi: 10.1111/j.1528-1157.1999.tb00824.x. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Oberlander AM, Svoboda K. Abnormal Development of Dendritic Spines inFMR1 Knock-Out Mice. The Journal of Neuroscience. 2001;21:5139–5146. doi: 10.1523/JNEUROSCI.21-14-05139.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell WT, Warren ST. A decade of molecular studies of fragile X syndrome. Annu Rev Neurosci. 2002;25:315–338. doi: 10.1146/annurev.neuro.25.112701.142909. [DOI] [PubMed] [Google Scholar]
- Olmos-Serrano JL, Paluszkiewicz SM, Martin BS, Kaufmann WE, Corbin JG, Huntsman MM. Defective GABAergic neurotransmission and pharmacological rescue of neuronal hyperexcitability in the amygdala in a mouse model of fragile X syndrome. J Neurosci. 2010;30:9929–9938. doi: 10.1523/JNEUROSCI.1714-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmashri R, Reiner BC, Suresh A, Spartz E, Dunaevsky A. Altered structural and functional synaptic plasticity with motor skill learning in a mouse model of fragile X syndrome. J Neurosci. 2013;33:19715–19723. doi: 10.1523/JNEUROSCI.2514-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluszkiewicz SM, Martin BS, Huntsman MM. Fragile X syndrome: the GABAergic system and circuit dysfunction. Dev Neurosci. 2011a;33:349–364. doi: 10.1159/000329420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluszkiewicz SM, Olmos-Serrano JL, Corbin JG, Huntsman MM. Impaired inhibitory control of cortical synchronization in fragile X syndrome. J Neurophysiol. 2011b;106:2264–2272. doi: 10.1152/jn.00421.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portera-Cailliau C. Which comes first in fragile x syndrome, dendritic spine dysgenesis or defects in circuit plasticity? Neuroscientist. 2012;18:28–44. doi: 10.1177/1073858410395322. [DOI] [PubMed] [Google Scholar]
- Poskanzer KE, Yuste R. Astrocytic regulation of cortical UP states. Proc Natl Acad Sci U S A. 2011;108:18453–18458. doi: 10.1073/pnas.1112378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakers GJ, Corner MA, Habets AM. Development in the absence of spontaneous bioelectric activity results in increased stereotyped burst firing in cultures of dissociated cerebral cortex. Exp Brain Res. 1990;79:157–166. doi: 10.1007/BF00228885. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- Routh BN, Johnston D, Brager DH. Loss of functional A-type potassium channels in the dendrites of CA1 pyramidal neurons from a mouse model of fragile X syndrome. J Neurosci. 2013;33:19442–19450. doi: 10.1523/JNEUROSCI.3256-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runfeldt MJ, Sadovsky AJ, MacLean JN. Acetylcholine functionally reorganizes neocortical microcircuits. J Neurophysiol. 2014;112:1205–1216. doi: 10.1152/jn.00071.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro MR, Bray SM, Warren ST. Molecular mechanisms of fragile X syndrome: a twenty-year perspective. Annu Rev Pathol. 2012;7:219–245. doi: 10.1146/annurev-pathol-011811-132457. [DOI] [PubMed] [Google Scholar]
- Soden ME, Chen L. Fragile X Protein FMRP Is Required for Homeostatic Plasticity and Regulation of Synaptic Strength by Retinoic Acid. J Neurosci. 2010;30:16910–16921. doi: 10.1523/JNEUROSCI.3660-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Testa-Silva G, Loebel A, Giugliano M, de Kock CP, Mansvelder HD, Meredith RM. Hyperconnectivity and slow synapses during early development of medial prefrontal cortex in a mouse model for mental retardation and autism. Cereb Cortex. 2012;22:1333–1342. doi: 10.1093/cercor/bhr224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson I. Cortical development: a role for spontaneous activity? Curr Biol. 1997;7:R324–326. doi: 10.1016/s0960-9822(06)00150-3. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- Van der Molen MJ, Van der Molen MW, Ridderinkhof KR, Hamel BC, Curfs LM, Ramakers GJ. Auditory and visual cortical activity during selective attention in fragile X syndrome: a cascade of processing deficiencies. Clin Neurophysiol. 2012;123:720–729. doi: 10.1016/j.clinph.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF, Zhang FP, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Vislay RL, Martin BS, Olmos-Serrano JL, Kratovac S, Nelson DL, Corbin JG, Huntsman MM. Homeostatic Responses Fail to Correct Defective Amygdala Inhibitory Circuit Maturation in Fragile X Syndrome. The Journal of Neuroscience. 2013;33:7548–7558. doi: 10.1523/JNEUROSCI.2764-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Fontanini A, Maffei A. Experience-Dependent Switch in Sign and Mechanisms for Plasticity in Layer 4 of Primary Visual Cortex. The Journal of Neuroscience. 2012;32:10562–10573. doi: 10.1523/JNEUROSCI.0622-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bonnan A, Bony G, Ferezou I, Pietropaolo S, Ginger M, Sans N, Rossier J, Oostra B, LeMasson G, Frick A. Dendritic channelopathies contribute to neocortical and sensory hyperexcitability in Fmr1-/y mice. Nat Neurosci. 2014;17:1701–1709. doi: 10.1038/nn.3864. [DOI] [PubMed] [Google Scholar]