Abstract
Many women with breast cancer experience symptoms of pain, fatigue, and depression, collectively known as psychoneurologic (PN) symptoms, during and after chemotherapy treatment. Evidence that inflammatory dysfunction related to cancer and its treatments contributes to the development and persistence of PN symptoms through several interrelated pathways is accumulating. However, a major limiting factor in more precisely identifying the biological mechanisms underlying these symptoms is the lack of biological measures that represent a holistic spectrum of biological responses. Metabolomics allows for examination of multiple, co-occurring metabolic pathways and provides a systems-level perspective on biological mechanisms that may contribute to PN symptoms.
Methods:
In this pilot study, we performed serum metabolome analysis using liquid chromatography high-resolution mass spectrometry of global and targeted metabolomics from the tryptophan pathway from archived samples from 19 women with early-stage breast cancer. We used paired t tests to compare metabolite concentrations and Pearson’s correlation coefficients to examine concomitant changes in metabolite concentrations and PN symptoms before and after chemotherapy.
Results:
Levels of pain, fatigue, and depression increased after chemotherapy. Compared with pre-chemotherapy, global metabolites post-chemotherapy were characterized by higher concentrations of acetyl-l-alanine and indoxyl sulfate and lower levels of 5-oxo-l-proline. Targeted analysis indicated significantly higher kynurenine levels and kynurenine/tryptophan ratios post-chemotherapy. Symptoms of pain and fatigue had strong associations with multiple global and several targeted metabolites.
Conclusion:
Results demonstrated that metabolomics may be useful for elucidating biological mechanisms associated with the development and severity of PN symptoms, specifically pain and fatigue, in women with early-stage breast cancer.
Keywords: breast cancer, metabolomics, psychoneurologic symptoms, chemotherapy
Individuals with cancer have a number of distressing symptoms during treatment and, for some, into survivorship. In particular, the administration of systemic chemotherapy is associated with multiple, co-occurring symptoms (Dodd, Miaskowski, & Paul, 2001). Investigators have identified pain, fatigue, depression, insomnia, and cognitive disturbance as components of a notable cluster of symptoms (Miaskowski et al., 2017) that tend to occur together during active treatment and remain stable over time. Collectively, these symptoms are described as psychoneurological (PN) symptoms (Lyon et al., 2014; A. R. Starkweather et al., 2013). PN symptoms are prominent in women with breast cancer and are associated with adverse outcomes including significant declines in physical functioning (Dodd et al., 2001; Langford et al., 2016), quality of life (Bower et al., 2000; Langford et al., 2016; Jones et al., 2015), work life, and employment outcomes (Blinder, Eberle, Patil, Gany, & Bradley, 2017; Kamal et al., 2017; Lindbohm et al., 2014). As women frequently report PN symptoms throughout treatment and, in some cases, for years into survivorship, a significant number of research studies have focused on identifying the mechanisms that influence PN symptom severity and duration.
Accumulating evidence suggests that several interrelated biological mechanisms related to inflammatory activation by cancer and its treatments may contribute to the development and persistence of PN symptoms in women with breast cancer (A. Starkweather et al., 2017). Immune activation related to cancer and its treatments results in increased production of pro-inflammatory cytokines, contributing to the development and maintenance of sickness behavior, which includes PN symptoms. Cytokines cross the blood–brain barrier via active transport mechanisms in the choroid plexus and circumventricular organs and have direct effects on neurotransmitters, neural processing (Dantzer, 2017), and neuronal/glial cell modulation, suggesting an association with mood disorders and sickness behaviors (Liu, Adibfar, Herrman, Gallagher, & Lanctôt, 2017). Inflammatory activation by tumor cells and cancer treatments may also affect mood and behavior indirectly by activating the tryptophan (TRP)-degrading enzyme indoleamine 2,3-dioxygenase (IDO; Savitz et al., 2015). TRP, an essential amino acid, is a building block of protein as well as a substrate for two important biosynthetic pathways: the generation of neurotransmitter 5-hydroxytryptamine (serotonin) by tryptophan 5-hydroxylase and the formation of kynurenine derivatives and nicotinamide adenine dinucleotides (NADs; Figure 1). Elevated levels of IDO activity increase the degradation of TRP, a precursor to the synthesis of biogenic amines including serotonin, as mentioned above, and melatonin (Kim, Miller, Stefanek, & Miller, 2015). The degradation of TRP leads to the production of the coenzyme NAD and, through the kynurenine pathway, of neuroactive intermediates, including 3-hydroxykynurenine and quinolinic acid (QA), neurotoxic metabolites that have been associated with mood and behavioral symptoms across conditions including major depressive disorder, cognitive dysfunction (Savitz et al., 2015), multiple sclerosis (Aeinehband et al., 2016), brain aging (Török, Majláth, Fülöp, Toldi, & Vécsei, 2016), and sleep disturbances (Cho et al., 2017). The metabolic steps of the kynurenine pathway (Figure 1) that lead to an increase in QA concurrently lead to a decrease in kynurenic acid, the neuroprotective branch of this pathway, thus contributing further to a pathogenic inflammatory process (Cho et al., 2017). Because of the frequent co-occurrence of chronic pain, fatigue, depression, and sleep disturbance (PN symptoms) and the reported interrelationships of these symptoms, whether individual or clustered, with inflammation (Laird et al., 2013; Miaskowski et al., 2017), authors have suggested that these conditions may share a common biological mechanism represented by inflammation-induced IDO activation and the downstream metabolic steps of the kynurenine pathway and resultant decreases in the bioavailability of TRP for the synthesis of serotonin (Cho et al., 2017; Dantzer, 2017; Laumet et al., 2017).
Figure 1.
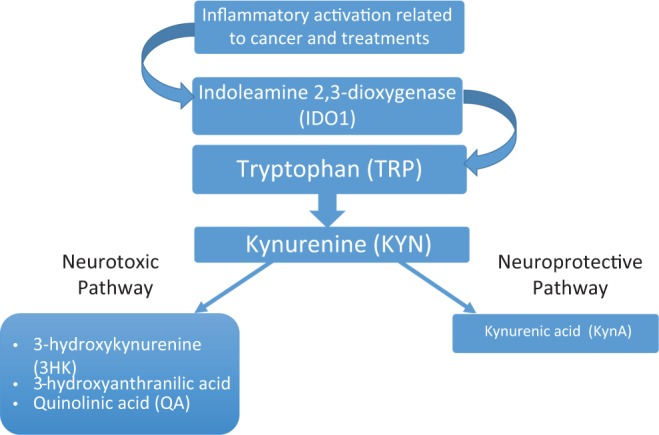
Effect of inflammatory activation on tryptophan/kynurenine pathways. Adapted, with permission, from Cho et al. (2017).
Evidence that the inflammatory dysfunction related to cancer and its treatments contributes to the development and persistence of PN symptoms through several interrelated pathways has been growing. However, a major limiting factor in more precisely identifying the biological mechanisms of PN is the lack of biological measures that represent a holistic spectrum of biological responses. To date, researchers have studied the systems and pathways underlying PN symptoms primarily by focusing on an individual biological pathway, often in isolation from other relevant and concordant biological responses. Metabolomics provides a powerful array of tools to study hundreds of key molecules simultaneously and quantitatively (Beger et al., 2016; Quinones & Kaddurah-Daouk, 2009). In addition, many biological substances that are difficult to measure using traditional laboratory procedures, such as monoamines, can be measured more precisely and simultaneously using metabolomic methods. Thus, metabolomics is a promising method for differentiating biological pathways of complex phenotypes such as PN symptoms in individuals with cancer. In fact, the metabolome may offer the most revealing real-time view of biological heterogeneity and variation in response to treatment of all of the omics, and it does so at a systems level (Beger et al., 2016).
In the present pilot study, we performed serum metabolome analysis using liquid chromatography high-resolution mass spectrometry to characterize global metabolites and targeted metabolites in the TRP/kynurenine pathway of breast cancer patients prior to and after they received standard chemotherapy and to identify metabolites associated with changes in levels of symptoms after chemotherapy. We selected the TRP/kynurenine pathway for targeted analysis due to existing reports indicating a role for this pathway in the development and persistence of PN symptoms. Because reports have also suggested that other pathways play a role in PN symptoms, we also performed global metabolomic profiling to examine changes related to chemotherapy that might be associated with PN symptoms.
Method
This pilot study was part of a larger, randomized, double-blind clinical trial that tested a noninvasive complementary modality for symptom management (Cranial Microcurrent Electrical Stimulation (CES study)) in women with early-stage breast cancer. In the parent study, investigators noted no difference in levels of symptoms between the intervention and sham-control groups (Lyon et al., 2015). For the parent study, researchers enrolled women with early-stage breast cancer attending oncology clinics affiliated with a major academic cancer center in the mid-Atlantic region between 2009 and 2013. All participants provided voluntary written informed consent before entering the CES study. The institutional review board at the original study site approved the CES protocol, and the University of Florida board approved the adjunctive metabolomics analysis as nonhuman research. The primary analyses of the current study relied on symptom measures and biological samples from participants recruited as part of the CES trial (clinicaltrials.gov number NCT00902330).
The 19 participants in the present study were a subset of participants in the parent study. In that study, researchers collected blood samples and symptom questionnaires prior to the initial chemotherapy infusion (T1) and 1–2 weeks following the final chemotherapy infusion (T2). Frozen samples from the parent study were shipped using standard procedures to the Southeast Center for Integrated Metabolomics (SECIM) in Gainesville, FL, where they were stored and analyzed.
Metabolite Profiling
Sample preparation at SECIM involved the addition of extraction internal standards and protein precipitation by organic solvent without other steps. After brief centrifugation, the solvent was evaporated from the supernatant and the residue was resuspended in water containing injection standards for analysis of polar compounds. Untargeted metabolomics profiling was performed on a Thermo Q-Exactive Orbitrap mass spectrometer with Dionex ultra high performance liquid chromatography (UHPLC) and autosampler. All samples were analyzed in positive and negative heated electrospray ionization with a mass resolution of 35,000 at m/z 200. Chromatographic separation was achieved on an ACE 18-pfp 100 × 2.1 mm, 2 μm column with mobile phase A as 0.1% formic acid in water and mobile phase B as acetonitrile, with a flow rate of 350 μl/min and a column temperature of 25°C. Total run time per sample was 21 minutes. In mass spectroscopy (MS) analysis, positive and negative ion polarities were collected separately, as some species such as organic acids are detected as anions, while the amino and organic acids containing nitrogen atoms are detected as cations. Therefore, the laboratory used both negative and positive ionization modes to analyze these two kinds of compounds, respectively. Alignment and feature selection of metabolites was performed using MZmine 2 (Pluskal, Castillo, Villar-Briones, & Orešič, 2010), an open-source software developed for use in metabolomic studies. Raw data from the instrument were first converted to an open-source format (mzXML) using MS Convert from Proteowizard and then loaded into MZmine. Metabolite identification was performed by reference to an internal metabolite library of 700 compounds characterized by retention time and mass-to-charge (m/z).
Targeted quantitation was performed for selected metabolites (TRP, serotonin, and kynurenine) within the TRP/kynurenine pathway. Samples were prepared by aliquoting 100 μl to a microcentrifuge tube and adding 20 μl of internal standard solution (caffeine-D3, leucine-D10, creatine-D3, tryptophan-D3, and kynurenine-D4). All of the SECIM standard operating procedures are available from the metabolomics workbench as part of the National Institutes of Health sharing plan between metabolomics centers.
PN Symptom Measures
Symptom measurements included well-established, valid, and reliable instruments used frequently in individuals with cancer. In the present study, we focused on the symptoms of pain, fatigue, and depression. The Brief Pain Inventory, a 9-item questionnaire, was used to assess pain severity and interference with daily activities over the past 24 hours using a self-report Likert-type scale anchored at 0 and 10. Cronbach’s α ranges from .70 to .91 (Cleeland & Ryan, 1994). The Brief Fatigue Inventory, a 10-item questionnaire, was used to assess fatigue severity and interference with daily activity over the past 24 hours using a self-report Likert-type scale anchored at 0 and 10. Cronbach’s α ranges from .82 to .97 (Mendoza et al., 1999). The depression subscale (7 items) of the 14-item Hospital Anxiety and Depression Scale (Zigmond & Snaith, 1983) was used to assess the presence and severity of depressive symptoms over the past week using a self-report rating scale of 0–3. Cronbach’s α ranges from .82 to .90. Because it was developed for use in medically ill patients, it does not rely upon somatic symptoms of depression and anxiety such as pain and weight loss. A complete description of symptom measures used in the parent study appeared in prior publications (Lyon et al., 2015).
Statistical Analysis
Statistical analysis was performed using statistical software R, Version 3.3.1 (https://www.r-project.org/). Descriptive statistics (mean, standard deviation, frequency, and percentage) were generated for demographic variables as well as PN symptoms and targeted metabolites before and after chemotherapy. We compared PN symptom levels before and after chemotherapy using paired Wilcoxon signed rank tests. Log transformations were applied to both targeted and global metabolomics data before further analysis. Paired t tests were used to compare metabolite concentrations before and after chemotherapy. Pearson correlations were used to analyze the changes in metabolite concentrations and PN symptoms from “before” to “after” chemotherapy. To account for multiple comparisons, we utilized the approach of false discovery rate (FDR), where we obtained for each test both the observed significance (p value) and the estimated FDR (q value). The q value associated with a test represents the expected proportion of false positives if the test, along with all tests with equal or lower p values, are declared significant. In the present analysis, we adopted the most conservative assumption of a prior null probability of 1 when computing the q values (Storey, 2002). Given the small sample and preliminary nature of the study, we considered an FDR of 20% to be significant (Benjamini & Hochberg, 1995). There were no missing data for patient demographic and PN variables. The amounts of missing data in targeted and global metabolites were 19% and 5%, respectively. Using an R software package called mice, we performed multivariate imputation by chained equation to generate 50 completed data sets (van Buuren & Groothuis-Oudshoorn, 2011). Statistical inference was performed on these completed data sets and then aggregated according to Rubin’s rules (Barnard & Rubin, 1999).
Results
Table 1 lists participant demographics. Participants were White and African American, ranged in age from 49 to 67 years, and had been diagnosed with one of multiple categories of early-stage, nonmetastatic breast cancer. In Table 2, we list the mean (SD) levels of the PN symptoms before and after chemotherapy. Generally, levels of pain, fatigue, and depression were mild to moderate, with fatigue and depression both increasing from T1 to T2.
Table 1.
Participants’ Demographic Characteristics.
| Variable | n (%) |
|---|---|
| Race | |
| White | 13 (68.4) |
| Black | 6 (31.6) |
| Ethnicity | |
| Hispanic | 1 (5.3) |
| Non-Hispanic | 18 (94.7) |
| Breast cancer stage | |
| I | 8 (42.1) |
| IIA | 6 (31.6) |
| IIB | 3 (15.8) |
| IIIA | 2 (10.5) |
| Mean (SD) | |
| Age | 58.7 (5.2) |
| BMI | 30.5 (6.8) |
Note. N = 19. BMI = body mass index.
Table 2.
Participant Psychoneurologic Symptoms Before and After Chemotherapy.
| Symptom | Before Chemo (N = 19) | After Chemo (N = 19) | p | q |
|---|---|---|---|---|
| Paina | 1.1 ± 1.9 | 1.3 ± 2.4 | .851 | .851 |
| Fatigueb | 1.5 ± 2.9 | 2.2 ± 2.7 | .074 | .113 |
| Depressionc | 2.9 ± 1.9 | 4.3 ± 3.1 | .075 | .113 |
aBrief Pain Inventory. bBrief Fatigue Inventory. cHospital Anxiety and Depression Scale, depression subscale.
Targeted Metabolites Pre- and Post-chemotherapy
Using a gas chromatography-time-of-flight mass spectrometry platform, we quantified levels of kynurenine, serotonin, and TRP and calculated the kynurenine/tryptophan ratio (K/T ratio; Table 3). Levels of kynurenine increased from T1 to T2, while levels of serotonin and TRP decreased across the same period. The K/T ratio also increased. Only the changes in kynurenine and the K/T ratio were statistically significant.
Table 3.
Levels of Targeted Metabolites Before and After Chemotherapy (Mean ± SD).
| Metabolite | Before Chemo (N = 19) | After Chemo (N = 19) | p | q a |
|---|---|---|---|---|
| Kynurenine (ng/dl) | 209 ± 62 | 272 ± 70 | .026b | .052b |
| Serotonin (ng/dl) | 164 ± 121 | 95 ± 66 | .104 | .104 |
| Tryptophan (ng/dl) | 8,549 ± 2,127 | 6,849 ± 2,135 | .066 | .088 |
| K/T ratio | 2.6 ± 0.9 | 4.5 ± 2.0 | .009b | .036b |
Note. K/T ratio = ratio of kynurenine to tryptophan.
aAn estimate of the false discovery rate, which is given by the q value, was calculated to take into account the multiple comparisons that normally occur in metabolomic-based studies. A low q value (q < .10) is an indication of high confidence in a result. bStatistically significant change from before to after chemotherapy.
Correlations Between Changes in PN Symptoms and Targeted Metabolites From Pre- to Post-chemotherapy
Table 4 provides the correlations between the changes from pre- to post-chemotherapy of targeted metabolite concentrations and K/T ratio and the changes in PN symptoms of pain, fatigue, and depression. While we found moderate-to-strong correlations between changes in pain and TRP concentration and the K/T ratio and between changes in depression and serotonin concentration, only the negative correlation between changes in TRP and pain was statistically significant (p = .009, q = .108).
Table 4.
Correlations of Changes in Psychoneurologic (PN) Symptom and Targeted Metabolite Concentrations From Pre- to Post-chemotherapy.
| Targeted Metabolite | Pain |
Fatigue |
Depression |
||||||
|---|---|---|---|---|---|---|---|---|---|
| r | p | q a | r | p | q a | r | p | q a | |
| Kynurenine | −.251 | .309 | .662 | .065 | .927 | .994 | −.176 | .585 | .800 |
| Serotonin | .139 | .600 | .800 | .377 | .215 | .645 | .429 | .165 | .645 |
| Tryptophan | −.676 | .009b | .108b | −.182 | .474 | .800 | −.133 | .716 | .859 |
| K/T ratio | .572 | .072 | .432 | .265 | .331 | .662 | .136 | .994 | .994 |
Note. Pearson’s correlations for the changes in metabolite concentrations and the changes in PN symptoms before and after chemotherapy.
aAn estimate of the false discovery rate, which is given by the q value, was calculated to take into account the multiple comparisons that normally occur in metabolomic-based studies. A low q value (q < .10) is an indication of high confidence in a result. bStatistically significant correlation.
Changes in Global Metabolite Concentrations From Pre- to Post-chemotherapy
Using a gas chromatography-time-of-flight mass spectrometry platform, we quantified 200 structurally known metabolites among 13 participants pre- and post-chemotherapy. Samples from six participants were contaminated with iodine and thus did not meet quality control standards. Overall, we annotated approximately 200 metabolites representing amino acid metabolism (48%), lipid metabolism (6%), organic acid metabolism (23%), purines (12%), and sugars (11%). Several metabolites had q values <20% and p values <.01. Levels of the α amino acid N-acetyl-l-alanine (p = .000, q = .134) and the indole n-indoxyl sulfate (IS; p = .001, q = .145) increased significantly post-chemotherapy, while levels of the carboxylic acid 5-oxo-l-proline decreased significantly (p = .001, q = .145).
Correlations Between Changes in PN Symptoms and Global Metabolites From Pre- to Post-chemotherapy
Pain
Table 5 shows the correlations between changes in levels of the PN symptom of pain and global metabolite concentrations from T1 to T2. Increased pain was associated with increased levels of betaine and decreased levels of the α amino acids acetyl-arginine, guanidinoacetate, and citrulline; the γ amino acid 4-guanidinobutanoate; the essential amino acids TRP and n-TRP; and the branched-chain amino acid l-valine.
Table 5.
Correlations Between Changes in the Level of Pain and the Concentrations of Global Metabolites From Pre- to Post-chemotherapy.
| Metabolite | Correlation With Change in the Level of Pain |
Biofunction | ||
|---|---|---|---|---|
| p | q | r | ||
| α Amino acid | ||||
| Acetyl-arginine | .000 | .008 | −.917 | Protein synthesis, amino acid biosynthesis |
| Citrulline | .005 | .176 | −.752 | Component of alanine, aspartate, arginine, and proline metabolism |
| Guanidinoacetate | .001 | .041 | −.829 | Component of metabolic pathway for glycine, serine, threonine, arginine, and proline metabolism |
| Branched-chain amino acid | ||||
| l-valine | .001 | .041 | −.832 | Stress, energy metabolism |
| Essential amino acid | ||||
| Tryptophan | .000 | .041 | −.847 | Component of aminoacyl-tRNA biosynthesis |
| N-tryptophan | .001 | .056 | −.805 | See tryptophan. Negative ion. |
| γ Amino acid | ||||
| 4-Guanidinobutanoate | .000 | .010 | −.898 | Protein synthesis, amino acid biosynthesis |
| Small N-trimethylated amino acid | ||||
| Betaine | .001 | .041 | .834 | Liver function, cellular replication, and detoxification reactions |
Note. Only metabolites with q values <20% are listed.
Fatigue
Decreases in the levels of multiple bile-related compounds (bile acids and bile salts) were significantly associated with increases in the level of fatigue post-chemotherapy, including taurochenodeoxycholic acid (T-CDCA), n-T-CDCA, T-CDCA-SF1, T-CDCA-SF2, taurocholic acid (TCA), n-TCA, glycocholic acid (GCA), n-GCA, GCA-SF1, glycocholate, glycodeoxycholic acid (GDCA), n-GDCA, n-chenodeoxycholyglycine, n-taurodeoxycholic acid, ursodeoxycholic acid, chenodeoxycholyglycine, and n-tauroursodeoxycholic acid (Table 6).
Table 6.
Correlations Between Changes in the Level of Fatigue and Concentrations of Bile Acid Metabolites From Pre- to Post chemotherapy.
| Metabolite | Correlation With Change in Level of Fatigue |
||
|---|---|---|---|
| P | q | r | |
| Chenodeoxycholyglycine | .019 | .162 | −.65 |
| Glycocholate | .015 | .146 | −.74 |
| Glycocholic acid (GCA) | .005 | .103 | −.74 |
| Glycocholic acid (GCA)-SF1 | .005 | .103 | −.74 |
| Glycodeoxycholic acid (GDCA) | .015 | .146 | −.67 |
| N-chenodeoxycholyglycine | .010 | .127 | −.70 |
| N-glycocholic acid (GCA) | .003 | .103 | −.77 |
| N-glycodeoxycholic acid (GDCA) | .015 | .146 | −.67 |
| N-taurochenodeoxycholic acid (T-CDCA) | .004 | .103 | −.75 |
| N-taurocholic acid (TCA) | .004 | .103 | −.77 |
| N-taurodeoxycholic acid | .012 | .146 | −.68 |
| N-tauroursodeoxycholic acid | .023 | .182 | −.63 |
| Taurochenodeoxycholic acid (T-CDCA) | .000 | .083 | −.86 |
| Taurochenodeoxycholic acid (T-CDCA)-SF1 | .009 | .127 | −.71 |
| Taurochenodeoxycholic acid (T-CDCA)-SF2 | .008 | .127 | −.72 |
| Taurocholic acid (TCA) | .002 | .103 | −.82 |
| Ursodeoxycholic acid | .016 | .146 | −.67 |
Note. Only metabolites with q values <20% are listed.
Other classes of metabolites significantly associated with higher levels of fatigue post-chemotherapy included diazines, monosaccharides, and fatty acids (Table 7). Increased levels of the diazines uracil, n-uracil, and 5,6-dihydrouracil were associated with increased levels of fatigue. Decreased levels of monosaccharides were associated with higher levels of fatigue post-chemotherapy, specifically n-glucose/fructose, n-glucose/fructose [M + Cl]−, and aldo/keto-hexose. Fatty acids that were associated with fatigue post-chemotherapy included arachidonic acid, which was negatively associated with fatigue, and n-malate, citramalate, and n-citramalate, which were positively associated with fatigue. In addition to these classes, higher levels of the peptide n-cys-gly were associated with higher levels of fatigue. The steroid tetracosahexaenoic acid and quinolones n-3,4-dihydroxybenzenesulfonic acid and 6-quinoline carboxylic acid were negatively associated with fatigue. Two carbohydrates and carbohydrate conjugates were also negatively associated with fatigue: n-d-glucoronic acid/d-glucoronolactone/d-(+)-galacturonic acid and n-disaccharide (6C/6C). A carbonyl compound, 5-hydroxymethyl-2-furaldehyde, and the amine histamine were negatively associated with fatigue, as were two carboxylic acids and derivatives, threonine/homoserine, and l-arginine. Higher levels of 5-oxo-l-proline were associated with higher levels of fatigue.
Table 7.
Correlations Between Changes in the Level of Fatigue and Concentrations of Global Metabolites From Pre- to Post-chemotherapy.
| Metabolite | Correlation With Level of Fatigue | Biofunction | ||
|---|---|---|---|---|
| p | q | r | ||
| Amine | ||||
| Histamine | .018 | .162 | −.66 | Aids in local immunity and gut physiology |
| Carbohydrate | ||||
| N-d-glucoronic acid/d-glucoronolactone/ d-(+)-galacturonic acid | .012 | .146 | −.68 | Main energy source |
| N-disaccharide (6C/6C) | .023 | .182 | −.63 | Main energy source |
| Carbonyl | ||||
| 5-Hydroxymethyl-2-furaldehyde | .015 | .146 | −.67 | Functional group |
| Carboxylic acid | ||||
| Threonine/homoserine | .020 | .162 | −.69 | Functional group |
| l-arginine | .020 | .162 | −.65 | Functional group |
| 5-Oxo-l-proline | .024 | .186 | .63 | Functional group |
| Diazine | ||||
| Uracil | .002 | .103 | .79 | Hydrogenation of alkenes and alkynes |
| N-uracil | .002 | .103 | .78 | Hydrogenation of alkenes and alkynes |
| 5,6-Dihydrouracil | .003 | .103 | .77 | Hydrogenation of alkenes and alkynes |
| Fatty acid | ||||
| Arachidonic acid | .005 | .103 | −0.76 | Aids in biological structure and processes |
| N-malate | .008 | .127 | .71 | Aids in biological structure and processes |
| Citramalate | .009 | .127 | .71 | Aids in biological structure and processes |
| N-citramalate | .013 | .146 | .68 | Aids in biological structure and processes |
| Monosaccharide | ||||
| N-glucose/fructose | .004 | .103 | −.75 | Energy |
| N-glucose/fructose [M + Cl]− | .005 | .103 | −.74 | Energy |
| Aldo/keto-hexose | .009 | .127 | −.70 | Energy |
| Peptide | ||||
| N-cys-gly | .002 | .103 | .79 | Forms proteins in amino acid chains |
| Quinolone | ||||
| N-3,4-dihydroxybenzenesulfonic acid | .009 | .127 | −.70 | Inhibits DNA replication |
| 6-Quinoline carboxylic acid | .015 | .146 | −.68 | Inhibits DNA replication |
| Steroid | ||||
| Tetracosahexaenoic acid | .006 | .113 | −.73 | Regulates metabolism |
Note. Only metabolites with q values <20% are listed.
Depression
The only metabolite with a significant correlation with depression was nicotinamide (NAA; r = .834, p = .0007, q = .200).
Discussion
To the best of our knowledge, this study is the first to examine global and targeted metabolites from the TRP pathway in women with early-stage breast cancer prior to and after completing chemotherapy as well as the relationships of these metabolites with symptoms of pain, depression, and fatigue. We designed this investigation as a pilot study to examine archived plasma samples for metabolomic measures and to detect differences in metabolites prior to and post-chemotherapy. There were several noteworthy findings. First, there were significant changes in the concentrations of TRP-related metabolites from before to after chemotherapy including a statistically significant increase in kynurenine and an increase in the K/T ratio. The increase in the ratio indicates increased TRP breakdown, which has a neurotoxic effect. These results are similar to those researchers found in a sample of patients with non-small cell lung cancer who had decreases in melatonin, TRP, and 6-hydroxymelatonin sulfate (6-OH-MLT) concentrations over time during chemotherapy (Hu, Shen, Yin, Xu, & Hu, 2009). In the psychiatric literature, there is support for an imbalance between neuroprotective and neurodegenerative metabolites in the kynurenine pathway due to immune activation leading to sickness behaviors including depressive symptoms, anxiety, fatigue, and sleep disturbances (Myint et al., 2007).
Levels of multiple metabolites changed significantly from pre- to post-chemotherapy. Levels of the α amino acid n-N-acetyl-l-alanine and the IS increased post-chemotherapy while the carboxylic acid n-5-oxo-l-proline decreased. Acetylalanine has been associated with adverse health outcomes. In one study, researchers found that high levels of N-acetylalanine had modest associations with all-cause mortality in African Americans (Yu, Heiss, Alexander, Grams, & Boerwinkle, 2016). The biological functions and health effects of this amino acid, however, require further investigation. Researchers have also reported neurotoxic effects of IS after chemotherapy administration in animal models, raising the possibility that specific chemotherapeutic agents may leave a metabolomic fingerprint (Iwata et al., 2007). In a study evaluating cisplatin administration, increased levels of IS were associated with nephro- and central nervous system toxicity including dysregulation of the circadian rhythm (Russcher et al., 2015). In another study, higher serum IS level was independently associated with lower executive function in patients with chronic kidney disease (F = 12.76, p < .0001; Yeh et al., 2016). Among various uremic toxins, researchers posit that IS and p-cresyl sulfate are most likely to negatively affect this cerebro–renal interaction (Yeh et al., 2016).
Increased levels of pain and fatigue after chemotherapy were associated with multiple metabolites in the present study. Increases in pain levels from pre- to post-chemotherapy were associated with increased concentrations of betaine. Betaine is an n-trimethylated amino acid and important cofactor in methylation, including the synthesis of dopamine and serotonin, neurotransmitters involved in pain processing. In addition, decreased levels of α, γ, essential, and branched amino acids were associated with increased pain from pre- to post-chemotherapy. Deficiencies in many of these amino acids, including N-acetyl-arginine, guanidinoacetate, citrulline, and n-citrulline have been associated with joint and musculoskeletal pain, and amino acid supplements are often used to treat the associated pain (Ursini & Pipicelli, 2009). Decreased levels of the essential amino acids TRP and n-tryptophan have been associated with increases in the affective dimension of pain (Labus et al., 2011; Shell et al., 2016).
Increased levels of fatigue post-chemotherapy were associated with decreased levels of multiple bile-related metabolites in the present study. Primary bile acids are secreted by hepatocytes and transformed by the intestinal microbiota into secondary bile acids, such as GCA. Bile acids facilitate excretion, absorption, and transportation of fats and sterols in the liver and intestines and play a role in cholesterol homeostasis and microbiome signaling (Kelly et al., 2015). Researchers reported similar findings of decreased bile acid synthesis in patients with chronic fatigue syndrome, which they attributed to a state of hypometabolism known as dauer (Naviaux et al., 2016). Dauer is an evolutionarily conserved response that authors have described in invertebrates that is triggered by exposure to adverse environmental conditions and confers survival advantage.
Fatigue was also associated with changes in diazines, monosaccharides, and fatty acids. Diazines, notably uracil, are essential for the synthesis of enzymes required for cellular function. The pyrimidine metabolism pathway leads to uracil, while 5,6-dihydrouracil is a metabolite from the intermediate breakdown of uracil. The monosaccharide metabolite aldo-keto/hexose (HMDB) was also associated with fatigue in the present study. The main source of HMDB is lactose found in the milk of mammals but also in some fruits and vegetables. Enzymatic action initiates its utilization for energy. In addition, lower levels of glucose/fructose and glucose/fructose [M + Cl−] were associated with increased fatigue, suggesting a possible link between fatigue and bioenergetic inefficiency. Amino acids in the fatty acids class that were associated with fatigue in the present study include the hydroxyl fatty acids citramalate and malate and a polyunsaturated fatty acid arachidonic acid. The hydroxyl fatty acids are similar to each other in structure except there is an additional CH3 on citramalate acid. Malate is the ionized form of malic acid, which is an intermediate of the tricarboxylic acid (TCA) cycle. When combined with nicotinamide adenine dinucleotide (NAD+) during the cycle, malate generates NADH, a form of NAD that has two additional electrons, through oxidation, which is the equivalent to 2.5 adenosine triphosphates, the main energy source in humans. The polyunsaturated fatty acid arachidonic acid is found in human adipose tissue, liver, and brain. This acid mediates inflammation and is the substrate for the synthesis of a range of biologically active compounds involved in cytokine production and immune function. The metabolites associated with increased fatigue in this pilot study thus are involved in human energy metabolism by involvement in bile production and synthesis, carbohydrate metabolism, and/or as part of the TCA cycle. Given these preliminary findings, which support the relationships among metabolites in women with breast cancer who experience fatigue, additional studies are needed to verify the alterations in bioenergetics metabolites associated with chemotherapy.
The only metabolite associated with depression in the present study was NAA, the amide of nicotinic acid (niacin). NAA is a key component of both the production of energy and signal transduction—processes that undergo crucial changes in cancer cells (Chiarugi, Dolle, Felici, & Ziegler, 2012). However, NAA may boost the effects of chemotherapy by acting as a sensitizing agent and increasing blood flow to tumors. NAA also has anxiolytic properties and has been shown to reduce cellular apoptosis.
Limitations
In this pilot study, we did not include potential effects of other covariates or confounding variables in our analysis. The parent study used a “pragmatic trial” design to ensure that study participants were similar to those who would receive the intervention if it became usual care (Ford & Norrie, 2016). Research in individuals undergoing breast cancer treatment using “real-life” participants involves differences in treatment regimens driven by patient and provider choices. These differences can include type of infusion, infusion interval, and use of premedications for chemotherapy such as Zofran. Other covariates that may have influenced study findings include individual variations in lifestyle behaviors that may have changed with the emotional impact of a cancer diagnosis, presence or absence of a support system, and lifestyle choices such as dietary adjustments, increased or decreased levels of physical activity, or changes in risky behavior such as increased or decreased smoking or alcohol intake (Suhre et al., 2010). We also could not control for the potential for a variety of pharmacologic agents to have influenced the metabolomics landscape in this patient population, including analgesics, cardiovascular protective medications, antidiabetic medications, mood-stabilizing agents (e.g., anxiolytics, antidepressants, and anti-epileptics), or antirheumatic drugs (e.g., immunosuppressants; Dougados et al., 2014). In addition, several of our samples were contaminated with iodine. We speculate that betadine (povidine/iodine swabs) may have been used in the sample collection as part of the protocol to cleanse porta-catheters prior to chemotherapy infusion. Although we have not found reference to iodine contamination in other reports, results from this pilot study illuminate the need for strict attention to presample collection protocol (Gika & Theodoridis, 2011; Guest & Rahmoune, 2017).
Despite these limitations, information gained through this examination of metabolites in relation to pain, fatigue, and depression in women with breast cancer can serve as a foundation for a study that includes healthy controls, comparative disease processes, and control for comorbidities and other potential covariates in women with breast cancer, so that the biological processes associated with cancer chemotherapy that contribute to the severity of PN can be fully elucidated. These findings, in turn, could be used for the development of targeted therapies to reduce the suffering associated with breast cancer and its treatments.
Conclusion
Preliminary data from this pilot study support the further exploration of global and targeted metabolomic profiling for hypothesis generation regarding the biological mechanisms associated with PN symptoms in women with breast cancer. We found multiple significant changes among global and targeted metabolites from pre- to post-chemotherapy and significant correlations between metabolites and changes in the symptoms of pain and fatigue. However, given the limited sample size and preliminary design of the study, the findings need to be confirmed with larger samples of women with breast cancer, preferably in a study with a longitudinal design that follows subjects through active treatment and into survivorship. Careful attention to sample collection protocols and documentation of potential covariates, such as physical activity, medications, and diet, will help to elucidate relevant metabolic pathways of the symptom trajectory.
Compliance With Ethical Requirements
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the parent study.
Footnotes
Author Contributions: D. Lyon contributed to conception, design, acquisition, analysis, and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. A. Starkweather contributed to conception, analysis, and interpretation; critically revised the manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Y. Yao contributed to design, analysis, and interpretation; drafted the manuscript and critically revised the manuscript; and gave final approval, and agreed to be accountable for all aspects of work ensuring integrity and accuracy. T. Garrett contributed to conception, design, and analysis; drafted the manuscript; gave final approval, and agreed to be accountable for all aspects of work ensuring integrity and accuracy. D. Lynch Kelly contributed to conception, design, and interpretation; drafted the manuscript; and critically revised the manuscript; gave final approval, and agreed to be accountable for all aspects of work ensuring integrity and accuracy. V. Menzies contributed to acquisition, drafted the manuscript and critically revised the manuscript, gave final approval, and agreed to be accountable for all aspects of work ensuring integrity and accuracy. P. Dereziński contributed to acquisition and analysis, critically revised the manuscript, gave final approval, and agreed to be accountable for all aspects of work ensuring integrity and accuracy. S. Datta contributed to design and analysis, drafted the manuscript and critically revised the manuscript, gave final approval, and agreed to be accountable for all aspects of work ensuring integrity and accuracy. S. Kumar contributed to interpretation, drafted the manuscript and critically revised the manuscript, gave final approval, and agreed to be accountable for all aspects of work ensuring integrity and accuracy. C. Jackson-Cook contributed to conception, acquisition, and analysis; drafted the manuscript and critically revised the manuscript; gave final approval, and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: R01CA127446 Cranial Stimulation for Chemo Symptoms in Breast CA, NIH Grant (# U24 DK097209), and UF PRICE-UF Health Cancer Center Seed Grant Program.
References
- Aeinehband S., Brenner P., Ståhl S., Bhat M., Fidock M. D., Khademi M.…Piehl F. (2016). Cerebrospinal fluid kynurenines in multiple sclerosis; Relation to disease course and neurocognitive symptoms. Brain, Behavior, and Immunity, 51, 47–55. doi:10.1016/j.bbi.2015.07.016 [DOI] [PubMed] [Google Scholar]
- Barnard J., Rubin D. B. (1999). Miscellanea. Small-sample degrees of freedom with multiple imputation. Biometrika, 86, 948–955. doi:10.1093/biomet/86.4.948 [Google Scholar]
- Beger R. D., Dunn W., Schmidt M. A., Gross S. S., Kirwan J. A., Cascante M.…Kaddurah-Daouk R. (2016). Metabolomics enables precision medicine: “A white paper, community perspective.” Metabolomics, 12, 149 doi:10.1007/s11306-016-1094-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological), 57, 289–300. [Google Scholar]
- Blinder V., Eberle C., Patil S., Gany F. M., Bradley C. J. (2017). Women with breast cancer who work for accommodating employers more likely to retain jobs after treatment. Health Affairs, 36, 274–281. doi:10.1377/hlthaff.2016.1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower J. E., Ganz P. A., Desmond K. A., Rowland J. H., Meyerowitz B. E., Belin T. R. (2000). Fatigue in breast cancer survivors: Occurrence, correlates, and impact on quality of life. Journal of Clinical Oncology, 18, 743–743. [DOI] [PubMed] [Google Scholar]
- Chiarugi A., Dolle C., Felici R., Ziegler M. (2012). The NAD metabolome—A key determinant of cancer cell biology. Nature Reviews Cancer, 12, 741–752. [DOI] [PubMed] [Google Scholar]
- Cho H. J., Savitz J., Dantzer R., Teague T. K., Drevets W. C., Irwin M. R. (2017). Sleep disturbance and kynurenine metabolism in depression. Journal of Psychomatic Research, 99, 1–7. doi:10.1016/j.jpsychores.2017.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleeland C., Ryan K. (1994). Pain assessment: Global use of the Brief Pain Inventory. Annals of the Academy of Medicine, Singapore, 23, 129–138. [PubMed] [Google Scholar]
- Dantzer R. (2017). Role of the kynurenine metabolism pathway in inflammation-induced depression: Preclinical approaches In Dantzer R., Capuron L. (Eds.), Current topics in behavioral neuroscience 31: Inflammation-associated depression: Evidence, mechanisms and implications (pp. 117–138). Cham, Switzerland: Springer International Publishing AG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd M. J., Miaskowski C., Paul S. M. (2001). Symptom clusters and their effect on the functional status of patients with cancer. Oncology Nursing Forum, 28, 465–470. [PubMed] [Google Scholar]
- Dougados M., Soubrier M., Antunez A., Balint P., Balsa A., Buch M. H.…Emery P. (2014). Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: Results of an international, cross-sectional study (COMORA). Annals of the Rheumatic Diseases, 73, 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford I., Norrie J. (2016). Pragmatic trials. New England Journal of Medicine, 375, 454–463. [DOI] [PubMed] [Google Scholar]
- Gika H., Theodoridis G. (2011). Sample preparation prior to the LC–MS-based metabolomics/metabonomics of blood-derived samples. Bioanalysis, 3, 1647–1661. doi:10.4155/bio.11.122 [DOI] [PubMed] [Google Scholar]
- Guest P. C., Rahmoune H. (2017). Blood sampling and preparation procedures for proteomic biomarker studies of psychiatric disorders In Guest P. (Ed.), Proteomic methods in neuropsychiatric research (pp. 141–147). Cham, Switzerland: Springer. [DOI] [PubMed] [Google Scholar]
- Hu S., Shen G., Yin S., Xu W., Hu B. (2009). Melatonin and tryptophan circadian profiles in patients with advanced non-small cell lung cancer. Advances in Therapy, 26, 886–892. doi:10.1007/s12325-009-0068-8 [DOI] [PubMed] [Google Scholar]
- Iwata K., Watanabe H., Morisaki T., Matsuzaki T., Ohmura T., Hamada A., Saito H. (2007). Involvement of indoxyl sulfate in renal and central nervous system toxicities during cisplatin-induced acute renal failure. Pharmaceutical Research, 24, 662–671. [DOI] [PubMed] [Google Scholar]
- Jones S. M. W., LaCroix A. Z., Li W., Zaslavsky O., Wassertheil-Smoller S., Weitlauf J.…Danhauer S. C. (2015). Depression and quality of life before and after breast cancer diagnosis in older women from the Women’s Health Initiative. Journal of Cancer Survivorship, 9, 620–629. doi:10.1007/s11764-015-0438-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal K. M., Covvey J. R., Dashputre A., Ghosh S., Shah S., Bhosle M., Zacker C. (2017). A systematic review of the effect of cancer treatment on work productivity of patients and caregivers. Journal of Managed Care & Specialty Pharmacy, 23, 136–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. R., Kennedy P. J., Cryan J. F., Dinan T. G., Clarke G., Hyland N. P. (2015). Breaking down the barriers: The gut microbiome, intestinal permeability and stress-related psychiatric disorders. Frontiers in Cellular Neuroscience, 9, 392 doi:10.3389/fncel.2015.00392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Miller B. J., Stefanek M. E., Miller A. H. (2015). Inflammation-induced activation of the indoleamine 2,3-dioxygenase pathway: Relevance to cancer-related fatigue. Cancer, 121, 2129–2136. doi:10.1002/cncr.29302 [DOI] [PubMed] [Google Scholar]
- Labus J., Mayer E., Jarcho J., Kilpatrick L., Evers E., Backes W.…van Nieuwenhoven M. (2011). Acute tryptophan depletion alters the effective connectivity of emotional arousal circuitry during visceral stimuli in healthy women. Gut, 60, 1196–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird B. J., McMillan D. C., Fayers P., Fearon K., Kaasa S., Fallon M. T., Klepstad P. (2013). The systemic inflammatory response and its relationship to pain and other symptoms in advanced cancer. The Oncologist, 18, 1050–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford D. J., Paul S. M., Cooper B., Kober K. M., Mastick J., Melisko M.…Cartwright F. (2016). Comparison of subgroups of breast cancer patients on pain and co-occurring symptoms following chemotherapy. Supportive Care in Cancer, 24, 605–614. [DOI] [PubMed] [Google Scholar]
- Laumet G., Zhou W., Dantzer R., Edralin J. D., Huo X., Budac D. P.…Kavelaars A. (2017). Upregulation of neuronal kynurenine 3-monooxygenase mediates depression-like behavior in a mouse model of neuropathic pain. Brain, Behavior, and Immunity, 66, 94–102. doi:10.1016/j.bbi.2017.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindbohm M. L., Kuosma E., Taskila T., Hietanen P., Carlsen K., Gudbergsson S., Gunnarsdottir H. (2014). Early retirement and non-employment after breast cancer. Psycho-Oncology, 23, 634–641. [DOI] [PubMed] [Google Scholar]
- Liu C. S., Adibfar A., Herrman N., Gallagher D., Lactôt K. L. (2017). Evidence for inflammation-associated depression In Dantzer R., Capuron L. (Eds.), Current topics in behavioral neuroscience 31: Inflammation-associated depression: evidence, mechanisms and implications (pp. 3–30). Cham, Switzerland: Springer International Publishing AG. [DOI] [PubMed] [Google Scholar]
- Lyon D., Elmore L., Aboalela N., Merril-Schools J., McCain N., Starkweather A.…Jackson-Cook C. (2014). Potential epigenetic mechanism(s) associated with the persistence of psychoneurological symptoms in women receiving chemotherapy for breast cancer: A hypothesis. Biological Research for Nursing, 16, 160–174. doi:10.1177/1099800413483545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon D., Kelly D., Walter J., Bear H., Thacker L., Elswick R. K. (2015). Randomized sham controlled trial of cranial microcurrent stimulation for symptoms of depression, anxiety, pain, fatigue and sleep disturbances in women receiving chemotherapy for early-stage breast cancer. SpringerPlus, 4, 369 doi:10.1186/s40064-015-1151-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza T. R., Wang X. S., Cleeland C. S., Morrissey M., Johnson B., Wendt J., Huber S. (1999). The rapid assessment of fatigue severity in cancer patients: Use of the Brief Fatigue Inventory. Cancer, 85, 1186–1196. [DOI] [PubMed] [Google Scholar]
- Miaskowski C., Conley Y. P., Mastick J., Paul S. M., Cooper B. A., Levine J. D.…Kober K. M. (2017). Cytokine gene polymorphisms associated with symptom clusters in oncology patients undergoing radiation therapy. Journal of Pain and Symptom Management, 54, 305–316. e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myint A.-M., Kim Y. K., Verkerk R., Scharpé S., Steinbusch H., Leonard B. (2007). Kynurenine pathway in major depression: Evidence of impaired neuroprotection. Journal of Affective Disorders, 98, 143–151. doi:10.1016/j.jad.2006.07.013 [DOI] [PubMed] [Google Scholar]
- Naviaux R. K., Naviaux J. C., Li K., Bright A. T., Alaynick W. A., Wang L.…Gordon E. (2016). Metabolic features of chronic fatigue syndrome. Proceedings of the National Academy of Sciences, 113, E5472–E5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluskal T., Castillo S., Villar-Briones A., Orešič M. (2010). MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinformatics, 11, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones M. P., Kaddurah-Daouk R. (2009). Metabolomics tools for identifying biomarkers for neuropsychiatric diseases. Neurobiology of Disease, 35, 165–176. [DOI] [PubMed] [Google Scholar]
- Russcher M., Chaves I., Lech K., Koch B. C., Nagtegaal J. E., Dorsman K. F.…Gaillard C. A. (2015). An observational study on disturbed peripheral circadian rhythms in hemodialysis patients. Chronobiology International, 32, 848–857. doi:10.3109/07420528.2015.1048868 [DOI] [PubMed] [Google Scholar]
- Savitz J., Drevets W. C., Wurfel B. E., Ford B. N., Bellgowan P. S., Victor T. A.…Dantzer R. (2015). Reduction of kynurenic acid to quinolinic acid ratio in both the depressed and remitted phases of major depressive disorder. Brain, Behavior, and Immunity, 46, 55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shell W. E., Pavlik S., Roth B., Silver M., Breitstein M. L., May L., Silver D. (2016). Reduction in pain and inflammation associated with chronic low back pain with the use of the medical food theramine. American Journal of Therapeutics, 23, e1353–e1362. doi:10.1097/mjt.0000000000000068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkweather A., Kelly D. L., Thacker L., Wright M. L., Jackson-Cook C. K., Lyon D. E. (2017). Relationships among psychoneurological symptoms and levels of C-reactive protein over 2 years in women with early-stage breast cancer. Supportive Care in Cancer, 25, 167–176. doi:10.1007/s00520-016-3400-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkweather A. R., Lyon D. E., Elswick R., Jr, Montpetit A. J., Conley Y., McCain N. L. (2013). A conceptual model of psychoneurological symptom cluster variation in women with breast cancer: Bringing nursing research to personalized medicine. Current Pharmacogenomics and Personalized Medicine, 11, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey J. D. (2002). A direct approach to false discovery rates. Journal of the Royal Statistical Society: Series B (Statistical Methodology), 64, 479–498. doi:10.1111/1467-9868.00346 [Google Scholar]
- Suhre K., Meisinger C., Döring A., Altmaier E., Belcredi P., Gieger C.…Illig T. (2010). Metabolic footprint of diabetes: A multiplatform metabolomics study in an epidemiological setting. PLoS One, 5, e13953 doi:10.1371/journal.pone.0013953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Török N., Majláth Z., Fülöp F., Toldi J., Vécsei L. (2016). Brain aging and disorders of the central nervous system: Kynurenines and drug metabolism. Current Drug Metabolism, 17, 412–429. [DOI] [PubMed] [Google Scholar]
- Ursini F., Pipicelli G. (2009). Nutritional supplementation for osteoarthritis. Alternative and Complimentary Therapies, 15, 173–177. [Google Scholar]
- van Buuren S. V., Groothuis-Oudshoorn K. (2011). Mice: Multivariate imputation by chained equations in R. Journal of Statistical Software, 45 doi:10.18637/jss.v045.i03 [Google Scholar]
- Yeh Y.-C., Huang M.-F., Liang S.-S., Hwang S.-J., Tsai J.-C., Liu T.-L.…Chen C.-S. (2016). Indoxyl sulfate, not p-cresyl sulfate, is associated with cognitive impairment in early-stage chronic kidney disease. NeuroToxicology, 53, 148–152. doi:10.1016/j.neuro.2016.01.006 [DOI] [PubMed] [Google Scholar]
- Yu B., Heiss G., Alexander D., Grams M. E., Boerwinkle E. (2016). Associations between the serum metabolome and all-cause mortality among African Americans in the Atherosclerosis Risk in Communities (ARIC) study. American Journal of Epidemiology, 183, 650–656. doi:10.1093/aje/kwv213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond A. S., Snaith R. P. (1983). The Hospital Anxiety and Depression scale. Acta Psychiatrica Scandinavica, 67, 361–370. [DOI] [PubMed] [Google Scholar]


