Abstract
Androgen deprivation is the primary therapy for recurrent prostate cancer, and agents targeting the androgen receptor (AR) pathway continue to be developed. Because androgen-deprivation therapy (ADT) has immmunostimulatory effects as well as direct antitumor effects, AR-targeted therapies have been combined with other anti-cancer therapies, including immunotherapies. Here, we sought to study whether an antigen-specific mechanism of resistance to ADT (overexpression of the AR) may result in enhanced AR-specific T-cell immune recognition, and whether this might be strategically combined with an antitumor vaccine targeting the AR. Androgen deprivation increased AR expression in human and murine prostate tumor cells in vitro and in vivo. The increased expression persisted over time. Increased AR expression was associated with recognition and cytolytic activity by AR-specific T cells. Furthermore, ADT combined with vaccination, specifically a DNA vaccine encoding the ligand-binding domain of the AR, led to improved antitumor responses as measured by tumor volumes and delays in the emergence of castrate-resistant prostate tumors in two murine prostate cancer models (Myc-CaP and prostate-specific PTEN-deficient mice). Together, these data suggest that ADT combined with AR-directed immunotherapy targets a major mechanism of resistance, overexpression of the AR. This combination may be more effective than ADT combined with other immunotherapeutic approaches.
Keywords: Androgen deprivation, androgen receptor, DNA vaccine, mechanism of resistance
Introduction
Advances in the field of tumor immunology have provided clinical benefit to patients with a variety of solid malignancies. However, although pre-clinical studies have shown that antitumor vaccines hold promise for augmenting antitumor immune responses, translating these immunization strategies into clinical responses has been challenging. Despite trials demonstrating that immune responses can be generated with immunization, the clinical responses are difficult to measure and randomized studies are few (1). Even for prostate cancer, despite the approval of sipuleucel-T for immunotherapy, the lack of interim biomarkers of clinical efficacy has limited its use in clinical practice. The lack of effective treatment has spurred interest in combining immunization with other therapeutic modalities, including both immunotherapeutic approaches and standard antitumor therapies, which have both direct antitumor activity as well as immunostimulatory effects (2).
One approach to treating prostate cancer has been to combine immunization with androgen-deprivation therapy. Androgen deprivation, the gold-standard treatment for patients with recurrent prostate cancer for more than sixty years, leads to tumor cell death and clinical responses in greater than 80% of patients with recurrent disease. In addition to its on-target antitumor effects, androgen deprivation also has immunostimulatory effects. These include the induction of thymic regrowth and increased release of naïve T cells, an increase in immune cell infiltration into the prostate (both myeloid and lymphocyte populations), decreased numbers of regulatory T cells, and increased antibody responses to prostate antigens (3-8). Preclinical studies have shown that androgen deprivation can enhance the efficacy of various immunotherapeutic approaches, including checkpoint blockade (9), irradiated tumor cell vaccines (10), T-cell adoptive transfer (11), and antigen-specific vaccines (12,13). These combinatorial approaches have also been evaluated in early stage clinical trials, where the addition of androgen deprivation may improve responses to checkpoint blockade (14), sipuleucel-T (15), and PROSTVAC (16). Thus, harnessing the antitumor and immunostimulatory effects of androgen-deprivation therapy can enhance the efficacy of immunotherapy in prostate cancer.
Although the development of vaccine approaches for prostate cancer have focused on antigens expressed only in the prostate, approaches to other solid malignancies have often targeted antigens whose function is critical to the growth and progression of tumor cells. One such antigen in prostate cancer is the androgen receptor (AR), required for prostate cancer oncogenicity. The AR is immunologically recognized by cytolytic CD8+ T cells in patients with prostate cancer (17). A DNA vaccine encoding the ligand-binding domain (LBD) of the AR augmented antigen-specific CD8+ T cells, leading to decreased tumor development, delayed tumor growth, and increased overall survival in preclinical models in vivo (18).
Although androgen-deprivation therapy elicits antitumor responses in most patients with recurrent disease, these patients will invariably experience disease relapse and the development of castrate-resistant disease. Although ADT is aimed at eliminating the activity of the AR, prostate tumors develop a variety of mechanisms to overcome this therapeutic approach, including AR overexpression (which occurs in more than half of prostate cancer patients (19)). However, although AR overexpression can drive prostate cancer cell proliferation in the absence of androgens, it may conceivably render prostate tumor cells more susceptible to AR-directed immune responses. This could be due to both increased expression of the AR target antigen and also enhanced T-cell cytotoxicity and other immunostimulatory effects that occur following ADT (20). Here, we sought to determine if androgen-deprivation therapy makes prostate tumor cells more susceptible to AR-specific T-cell recognition, as determined by enhanced T cell-mediated tumor cell recognition as well as delayed time to castration resistance following AR-directed immunization.
Materials and Methods
Mice and cell lines
Human prostate cancer cells were obtained from ATCC, and cultured in RPMI-1640 medium with 200U/mL penicillin/streptomycin, 1mM sodium pyruvate, and 0.1mM β-mercaptoethanol. Cell identity and mycoplasma testing was confirmed by DDC Medical (Fairfield, OH). Myc-CaP/AS or Myc-CaP/CR cells (androgen-sensitive and castrate-resistant variants of the Myc-CaP parental line originally generated by Charles Sawyers) and culture conditions have been previously described (21). Cell lines were maintained in either 10% complete fetal calf serum (FCS) or charcoal-stripped serum (CSS) for androgen-replete or androgen-deprived conditions.
Tumor studies using Myc-CaP tumor cells were conducted in wild-type male FVB mice (Jackson Laboratory, Bar Harbor, ME). PTEN knock-out mice were generated by crossing Ptenloxp/loxp animals with Probasin-Cre (PB-Cre4+) animals, as has been described (22). Mice were screened by PCR for the floxed or wild-type PTEN alleles (forward primer: CAA GCA CTC TGC GAA CTG AG; reverse primer: AAG TTT TTG AAG GCA AGA TGC) and PB-Cre transgene (forward primer: CTG AAG AAT GGG ACA GGC ATT G; reverse primer: CAT CAC TCG TTG CAT CGA CC). Mice were maintained under aseptic conditions and all experiments were conducted under an IACUC-approved protocol.
Tumor studies
FVB mice were inoculated subcutaneously with 106 Myc-CaP/AS tumor cells, and followed daily for the presence of palpable tumors. Once tumors were palpable, mice were treated subcutaneously with either degarelix (25mg/kg) or a vehicle sham treatment every four weeks. For immunization studies, degarelix-treated animals were randomized to weekly immunization with 100μg pTVG4 or pTVG-AR beginning one day after receiving degarelix. Where indicated, some groups also received 200 μg of anti-CD8 (BioXCell, clone 2.43) or IgG administered i.p. twice weekly. Tumor growth was measured at least three times weekly, and tumor volumes calculated as previously published (17). At the time of euthanasia, tumors and spleens were collected. For studies using PTEN-deficient mice, animals began receiving degarelix (25mg/kg) at 20 weeks (+/- two weeks) of age, followed by immunization every 2 weeks with 100μg pTVG4 or pTVG-AR beginning one day after ADT. Animals were treated until 40 weeks (+/- two weeks) of age before tissue collection.
Androgen receptor enzyme-linked immunosorbent assay (ELISA)
Cultured prostate cancer cells were collected, cell lysates prepared, and analyzed for protein expression using the PathScan androgen receptor ELISA per manufacturer's instructions (Cell Signaling Technology, Danvers, MA). Briefly, microwell strips (pre-coated with anti-AR antibody) were incubated with 2mg/mL protein lysates in triplicate, and incubated overnight. AR was detected using a detection antibody followed by HRP-linked secondary antibody and TMB substrate development. A standard curve using purified AR LBD protein (Invitrogen, Carlsbad, CA) was generated, and used to obtain relative AR concentration per mg cell lysate.
Flow cytometry
For androgen receptor intracellular staining, cells were stained with a Live/Dead GhostDye 780 Live/Dead Stain (Tonbo Biosciences, San Diego, CA) and CD45 (clone 30-F11, Tonbo Biosciences) for dissociated tumor samples, and intracellularly stained with antibodies directed against the AR ligand-binding domain (clone EP670Y, Abcam, Cambridge, United Kingdom) and amino terminal domain (clone D6F11, Cell Signaling Technologies), or isotype controls. For HLA-A2 and PD-L1 expression, cells were stained with HLA-ABC (clone W6/32, eBioscience, San Diego, CA) and PD-L1 (clone MIH-5, eBioscience) antibodies.
Androgen receptor quantitative real-time PCR
Prostate tumor cells (cell lines or dissociated tumors) were collected, RNA was prepared (RNeasy RNA purification system; Qiagen, Hilden, Germany), used to synthesize cDNA (iScript cDNA synthesis kit; BioRad, Hercules, CA), and used as a template for qPCR reactions using SsoFast qPCR supermix (BioRad). Reactions were performed using a Bio-Rad MyiQ thermocycler, using an annealing temperature of 60°C and 40 cycles. Primer sets: full-length human androgen receptor (forward: ACATCAAGGAACTCGATCGTATCATTGC; reverse: TTGGGCACTTGCACAGAGAT), AR-V7 (forward: CCATCTTGTCGTCTTCGGAAATGTTATGAAGC; reverse: TTTGAATGAGGCAAGTCAGCCTTTCT), full length mouse AR (forward: GGACCATGTTTTACCCATCG; reverse: GGACCATGTTTTACCCATCG), mouse AR-V2 (forward: GGACCATGTTTTACCCATCG; reverse: TTGTTGTGGCAGCAGAGTTC), mouse AR-V4 (forward: GGACCATGTTTTACCCATCG; reverse: AAGTGGGGAACCACAGCAT), and β-actin (forward: TCATGAAGTGTGACGTTGACATCCGT; reverse: CTTAGAAGCATTTGCGGTGCACGATG) (23-25). Results were analyzed by the 2-ΔCt method relative to β-actin as a control gene, as published (25).
Gene Expression Analysis
22Rv1 cells cultured in medium containing 10% complete fetal calf serum (FCS) or charcoal-stripped serum (CSS) were evaluated in triplicate for differences in gene expression of a panel of 2568 genes by high-throughput mRNA quantitation (HTG EdgeSeq Oncology Biomarker Panel, HTG Molecular, Tucson, AZ). Individual target genes were compared between cell lines, and all genes significantly different were assessed for gene ontology using the Panther Classification System (version 12.0, available at www.pantherdb.org). Heatmap Builder software (version 1.1, Stanford University) was used to generate heatmaps displaying relative gene expression.
Immunology Assays
To study immune responses, human T-cell lines or splenocytes were collected as previously described (18), and used for intracellular cytokine staining assays and cytotoxicity assays. For intracellular cytokine staining, cells were stimulated for 18 hours with media alone, an ARLBD peptide pool (a pool of 15-mer peptides, overlapping by 11 residues, and covering the entire sequence of the AR LBD; LifeTein, Somerset, NJ), tumor cells, or a PMA/Ionomycin positive control. Cells were stained using a fixable live/dead marker (Tonbo Bioscience) and extracellular and intracellular antibodies. Human antibodies: CD3 (clone UCHT1, BD Biosciences), CD4 (clone RPA-T4, BD Biosciences), CD8 (clone RPA-T8, eBioscience), CD69 (clone FN50, BD Biosciences), CD107a (clone H4-A3, BD Biosciences), IL2 (clone MQ1-17H12, eBioscience), IFNγ (clone 4S.B3, BioLegend, San Diego, CA), TNFα (clone MAb11, BD Biosciences), GrB (clone GB11, BD Biosciences). Mouse antibodies: CD3 (clone 17A2, BD Biosciences), CD4 (clone GK1.5, BD Biosciences), CD8 (clone 53-6.7, BD Biosciences), CD45 (clone 30-F11, BD Biosciences), CD69 (clone H1.2F3, eBioscience), IFNγ(clone XMG1.2, BD Biosciences), TNFα (clone MP6-XT22, BD Biosciences). Cells were subsequently analyzed using an LSR II or Fortessa flow cytometer (BD Biosciences), and events were analyzed by gating CD3+CD4+ or CD3+CD8+ cells and analyzing this population for expression of CD69, CD107a, IFNγ, TNFα, IL2, and/or GrB. Cytotoxicity assays were performed as previously described (18). Briefly, splenocytes were restimulated for five days with an ARLBD peptide pool, and were cultured with tumor cell lines, after which LDH release was calculated using the Cytotox 96 Assay kit (Promega, Madison, WI), as previously published (17).
Immunohistochemistry
Paraffin-embedded MycCaP tumors were stained for CD3 expression by immunohistochemistry as described (18). Sections were stained with primary antibodies (CD3: clone SP7, Abcam), developed using the LSAB+ System-HRP (Agilent Technologies, Santa Clara, CA) and Metal Enhanced DAB Substrate Kit DAB metal concentration (Thermo Fisher Scientific, Waltham, MA), imaged using an Olympus BX51 fluorescent microscope (Olympus, Lombard, IL) in combination with SPOT RT analysis software (SPOT Imaging Solutions, Sterling Heights, MI), and quantified by the frequency of CD3+ cells per 10× field, counting at least five fields per tumor section per animal by a blinded investigator.
Positron Emission Tomography/Computed Tomography Imaging
Mice were intravenously administered between 5-8 MBq of 124I-CLR1404 and then micro positron emission tomography/computed tomography (PET/CT) scanned 96 hours post-injection. During scanning, mice were anesthetized with 2% isoflurane inhalation gas mixed with 1L/min of pure oxygen (26). Mice were scanned with the Siemens Inveon Hybrid microPET/CT (Siemens Medical Solutions, Knoxville, TN) in the prone position. Forty-million counts per mouse were collected for the PET scan to obtain adequate signal-to-noise. PET data were histogrammed into one static frame and subsequently reconstructed using ordered-subset expectation maximization (OSEM) of three dimensions followed by the maximum a posteriori algorithm, and CT attenuation and scatter correction were applied based on the NEMA NU 4 image-quality parameters (27).
All PET and CT images were co-registered. Image data were analyzed using the General Analysis tools provided by Siemens Inveon Research Workplace (Siemens Medical Solutions). Data were identically window/leveled and scaled according to each animal's decay corrected injection activity. Based on the PET and CT images, a reference volume of interest (VOI) was drawn around each tumor and a separate background tissue VOI was drawn on muscle and liver. VOI thresholding within the reference tumor VOI was adjusted to include all signal greater than sixty percent of the maximum signal. Data were reported as percent injected dose normalized by the mass of the tissue VOI (%ID/gtissue), with the assumption that all tissue density is akin to water (1g/mL). Data were then averaged within pre- and post-treatment groups and normalized to background tissue values.
Statistical Analyses
Comparison of group means was performed using a t-test or Mann-Whitney U test (for non-normally distributed data) where indicated (GraphPad Prism software, v5.01). T-tests were used for comparison of gene expression data between cell lines, specifically without correction for multiple comparisons. For all comparisons, P values equal to or less than 0.05 were considered statistically significant.
Results
Androgen deprivation increases androgen receptor expression
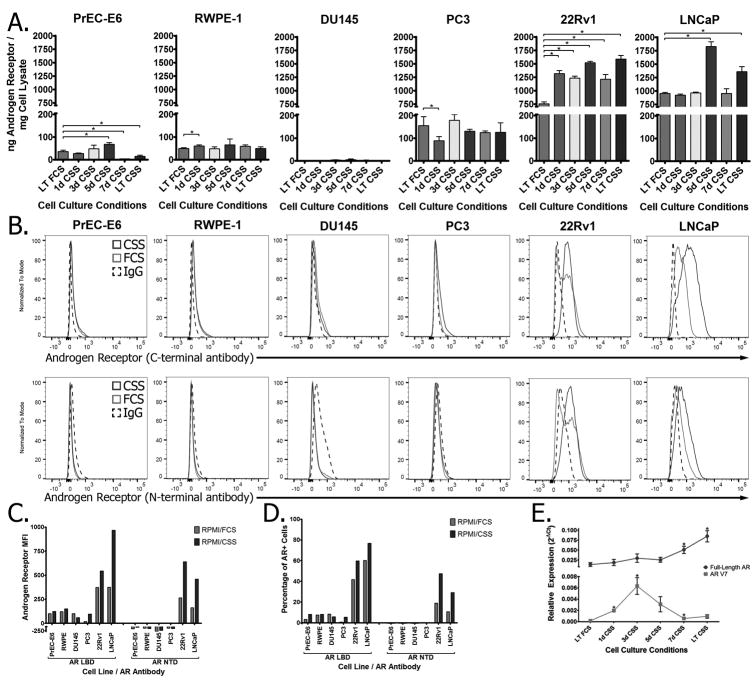
We have previously shown that T cells specific for epitopes derived from the AR LBD can recognize and lyse prostate tumor cells that express the AR (17). We hypothesized that increasing AR expression in these tumor cells by means of androgen deprivation would enhance AR-specific T-cell responses to these tumor cells. To test this, a panel of six prostate cell lines (two immortalized prostate epithelial lines, two androgen-independent prostate cancer lines, and two androgen-dependent prostate cancer lines) were cultured for short (one to seven days) or extended periods (greater than six months) in androgen-deprived medium and analyzed for AR expression. Androgen deprivation resulted in an increase in AR protein expression in androgen-dependent prostate tumor cells as measured by quantitative ELISA (Fig. 1A) as well as by intracellular staining using antibodies directed against both the ligand-binding domain as well as the amino-terminal domain (Fig. 1B, with the amplitude and frequency of AR expression quantified in Fig. 1C and 1D, respectively). Analysis of 22Rv1 cells (which express AR-V7, an LBD-loss splice variant) showed that androgen deprivation led to increasing expression in full-length AR as well as a transient increase in AR-V7 (Fig. 1E), with no detectable expression of AR-V1, AR567es, or other splice variants.
Figure 1. Androgen deprivation increases expression of the androgen receptor in prostate cancer cell lines.
Prostate cell lines (immortalized human epithelial lines: RWPE-1 and PrEC-E6; androgen-independent prostate cancer cell lines: DU-145 and PC-3; and androgen-dependent prostate cancer cell lines: LNCaP and 22Rv1) cultured in either androgen-replete (FCS) or androgen-deprived (CSS) medium for one to seven days (1d-7d) or for greater than six months (long-term: LT) were (A) analyzed for androgen receptor protein expression by quantitative ELISA. (B)Long-term FCS (light grey) or CSS (dark grey) cultured cell lines were analyzed for AR expression by intracellular staining using antibodies specific for the ligand-binding domain (top panels) or amino-terminal domain (lower panels), and quantified for (C) amplitude of AR expression and(D) frequency of AR+ cells. (E) RNA quantified from 22RV1/CSS cells cultured for different periods of time were analyzed for the presence of full-length (dark grey) or AR-V7 (light grey) AR transcripts. In all panels, * indicates P <0.05 by Studentt-test and data is representative of at least two independent experiments.
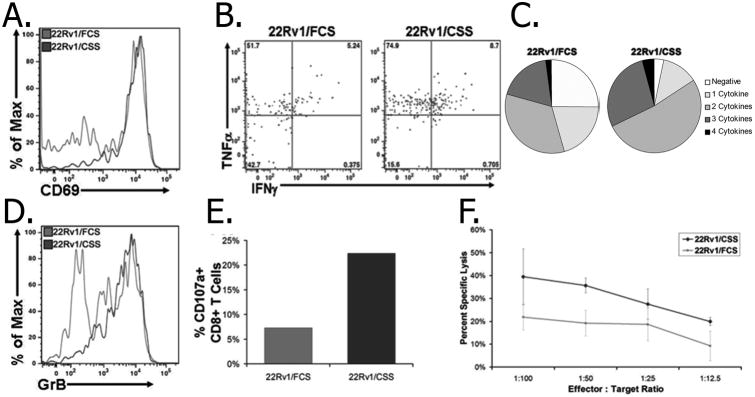
To determine whether this increase in AR expression following androgen deprivation resulted in enhanced AR-specific T-cell effector function against these tumor cells, 22Rv1 cells were first transfected to express HLA-A2 as a model MHC molecule, and one for which AR-restricted epitopes have been identified (17). After generating this cell line, increased AR protein and RNA expression following androgen-deprivation observed in the parental cell lines was confirmed in these HLA-A2-expressing lines (Supplementary Fig. S1A-B). These 22Rv1/FCS and 22Rv1/CSS cells were then incubated with T-cell lines specific for the HLA-A2-restricted AR805 epitope. T cells cultured with the 22Rv1/CSS cell line had higher T-cell activation (as measured by CD69 expression - Fig. 2A), as well as increased expression of Th1 cytokines (Fig. 2B), including CD8+ T cells with polyfunctional cytokine expression (Fig. 2C), compared to T cells that had been stimulated with 22Rv1 cells cultured under androgen-replete conditions. Co-culture with 22Rv1/CSS cells also resulted in higher expression of granzyme B (Fig. 2D), the degranulation marker CD107a (Fig. 2E), as well as increased cytotoxicity (Fig. 2F) compared to co-culture with 22Rv1/FCS cells. Similar studies using splenocytes from HLA-A2 transgenic mice that were directly immunized with another HLA-A2 restricted epitope, AR811, replicated these results in terms of increased cytokine expression, T-cell activation, and a trend to increased cytotoxicity when cultured with androgen-deprived HLA-A2-expressing 22Rv1 cells (Supplementary Fig. S2). The recognition of either cell line was MHC class I restricted, as blockade of HLA-A2 abrogated release of IFNγ in response to stimulation with either cell line by ELISPOT (Supplementary Fig. S2E).
Figure 2. AR-specific T cells have increased recognition and lysis of androgen-deprived tumor cells.
AR805-specific human T-cell cultures were incubated with HLA-A2-expressing 22Rv1/FCS or 22Rv1/CSS cells, and measured for (A) surface expression of CD69, (B) intracellular cytokine expression of IFNγ and/or TNFα, with polyfunctional cytokine expression quantified in (C). Cytolytic and degranulation activity of AR-specific T cells was measured by (D) intracellular granzyme B expression, (E) surface CD107a expression, and (F) tumor cell cytotoxicity.
To further explore immune effects of androgen deprivation on these cell lines, we evaluated 22Rv1 cells, cultured in complete or androgen-deprived media, for changes in mRNA expression among 2568 genes (Fig. 3). Although AR expression was upregulated, there were no changes in expression of MHC class I, or genes directly associated with antigen processing or presentation. Similarly, no difference in HLA-A2 surface expression was detected by flow cytometry (Supplementary Fig. S1C). We observed increases in expression of hsp70 and HLA-E following androgen deprivation, as well as a decrease in HVEM (Fig. 3C-D). No differences were observed in expression of PD-L1 or PD-L2, a result confirmed by flow cytometry (Supplementary Fig. S1D). Androgen antagonists can make prostate cancer cells more susceptible to CTL lysis by down-regulating expression of the anti-apoptotic NAIP gene (20).However, no difference in NAIP gene expression was observed following testosterone depletion alone (Fig. 3D). Consequently, we conclude that the increased recognition of prostate tumor cells by AR-specific CD8+ T cells was predominantly due to increased expression of the AR leading to increased presentation by MHC class I.
Figure 3. Androgen deprivation leads to gene changes in 22Rv1 prostate tumor cells.
22Rv1 cultured in either androgen-replete (FCS, F) or androgen-deprived (CSS, C) medium for greater than six months were analyzed for mRNA gene expression differences (HTG EdgeSeq). (A) relative mRNA quantities for all genes significantly upregulated (left) or downregulated (right) in androgen-deprived medium compared with full medium. Heatmap shows relative expression per gene between FCS and CSS groups (green=low expression, red = high expression), and ranked top to bottom by fold change for all significantly differentially expressed genes. (B) The gene ontology subsets and (C) specific genes associated with immune function.(D)Data for selected genes associated with immune recognition and antigen presentation.* indicates P<0.05 by Studentt-test. (HVEM – herpesvirus entry mediator (TNFRSF14); TAP – transporter associated with antigen processing; CALR – calreticulin; NAIP – baculoviral IAP repeat-containing protein 1).
Androgen deprivation increases AR expression in Myc-CaP tumor cells
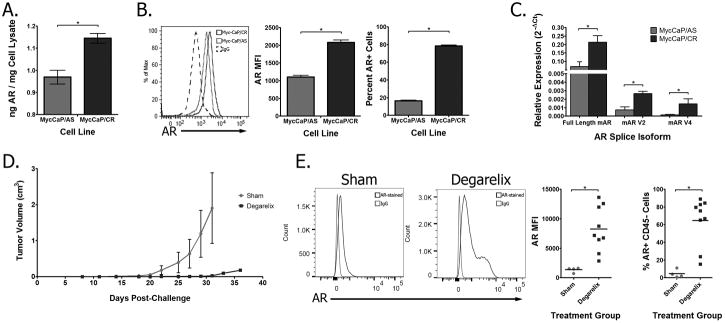
We have used the TRAMP mouse model to study the impact of vaccines targeting AR on tumor development and progression (18). However, androgen deprivation in TRAMP mice, and many other murine prostate tumor models, results in AR loss and the development of neuroendocrine tumors (28). Consequently, we sought to evaluate other models more representative of human prostate cancer that continue to express AR following androgen deprivation. One such model is the Myc-CaP cell line, which mimics the human disease in that it maintains AR expression following castration (21). To confirm this, we studied androgen-sensitive Myc-CaP cells (Myc-CaP/AS, generated from untreated FVB mice), and castration-resistant Myc-CaP cells (Myc-CaP/CR, generated from serial passaging of the Myc-CaP/AS cell line through castrated mice). Similar to what was observed in the human prostate cancer cell lines, the Myc-CaP/CR cell line had increased full-length AR expression as assessed by both quantitative ELISA (Fig. 4A) and intracellular staining compared to the Myc-CaP/AS cell line (Fig. 4B). Although analysis of RNA transcripts showed an increase in the murine AR splice variants mAR-V2 and mAR-V4, these splice variants were less abundantly expressed than the full-length AR (Fig. 4C). To study the expression of AR in vivo, FVB mice were inoculated with Myc-CaP/AS cells, and then given either a sham treatment or castration by administration of a GnRH antagonist (degarelix). Animals were followed for tumor growth (Fig. 4D), and recurrent tumors were collected and CD45- cells were analyzed for AR expression by intracellular staining. Tumors that recurred following androgen deprivation had increased AR expression, both in terms of frequency of CD45- cells with detectable expression of the AR, as well as the amplitude of AR expression within these cells (Fig. 4E).
Figure 4. Androgen deprivation increases AR expression in Myc-CaP tumor cells in vitro and in vivo.
Androgen-sensitive (Myc-CaP/AS) and castrate-resistant (Myc-CaP/CR) cells were analyzed for AR protein expression by (A) quantitative ELISA and(B) intracellular staining (quantified for amplitude and frequency of expression in side panels). (C)RNA samples from Myc-CaP/AS and Myc-CaP/CR cells were analyzed for expression of full-length or splice variants mAR V2 or mAR V4 by quantitative PCR. (D) Myc-CaP/AS tumor-bearing FVB mice with palpable tumors were treated with degarelix (n=4) or sham-treatment (n=3) and followed for tumor growth. Results are representative of two independent studies. (E), recurrent tumors were collected and analyzed for AR expression by intracellular staining using an antibody directed against the ligand-binding domain (amplitude and frequency quantified in side panels). In all panels, * indicates P<0.05 by Studentt-test.
Immunization against AR delayed tumor growth
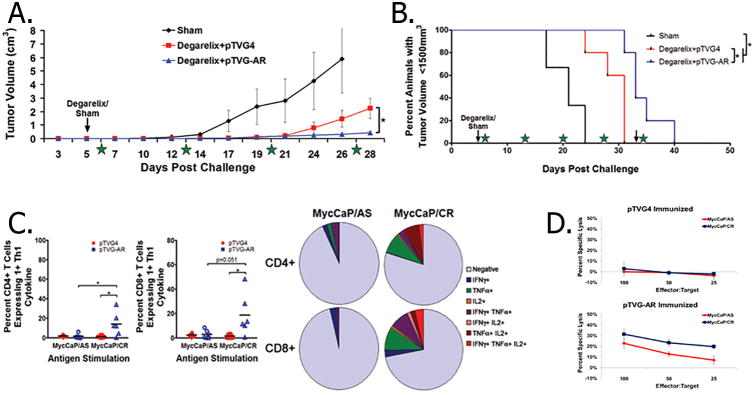
The observation that increased AR expression following androgen-deprivation enhanced AR-specific T-cell responses against tumor cells led us to hypothesize that androgen deprivation in combination with AR-targeted vaccination might delay or prevent the outgrowth of castration-resistant tumors by targeting cells overexpressing AR. Mice were implanted with Myc-CaP/AS tumors, and mice with established tumors were given either a sham treatment or degarelix. Mice treated with degarelix were then randomized to immunization with either a DNA vaccine encoding the AR LBD (pTVG-AR), or an empty vector control (pTVG4). The combination treatment with degarelix and pTVG-AR was found to delay tumor growth compared to treatment with degarelix and control vaccine (Fig. 5A-B). The delays in tumor growth, either with or without androgen deprivation, were mediated by the generation of CD8+ T cells, as CD8 depletion abrogated these antitumor effects following immunization with pTVG-AR, but not the control vaccine (Supplementary Fig. S3). When animals were evaluated for evidence of immune responses against the Myc-CaP/AS or Myc-CaP/CR cell lines, animals immunized with pTVG-AR were found to have increased immune responses against the castration-resistant cell line, both in terms of cytokine expression (Fig. 5C) as well as cytotoxicity (Fig. 5D). In parallel studies, we found that immunization of Myc-CaP/AS-bearing mice with pTVG-AR resulted in an increased number of tumor-infiltrating CD3+ T cells, and this was further increased when vaccination was combined with degarelix treatment (Supplementary Fig. S4).
Figure 5. Androgen deprivation combined with immunization using pTVG-AR delayed the recurrence of castrate-resistant Myc-CaP tumors.
Myc-CaP/AS tumor-bearing mice with palpable tumors were given a sham-treatment (n=3) or degarelix along with immunization every two weeks with pTVG-AR (n=5) or empty vector (n=5) and followed for tumor growth (A, tumor volumes; B, Kaplan-Meier curve). Stars represent times of immunization. Results are representative of three independent studies. Splenocytes from androgen-deprived animals immunized with pTVG4 or pTVG-AR were cultured with Myc-CaP/AS or Myc-CaP/CR cells and assessed for CD4+ and CD8+ T-cell intracellular cytokine expression (C, with polyfunctional expression quantified in side pie charts) and (D) cytotoxicity.
ADT combined with immunization against AR decreased tumor growth
As an additional model of human prostate cancer, we utilized PbCre PTENfl/fl mice, in which prostate-specific expression of the Cre recombinase drives deletion of the PTEN tumor suppressor and autochthonous prostate tumor development. The PTEN-CaP8 cell line (derived from an autochthonous PbCre PTENfl/fl tumor) was cultured in androgen-replete or androgen-deprived medium. Androgen-deprivation resulted in more AR protein expression, similar to the human and Myc-CaP prostate cancer cells (Fig. 6A-B). Twenty-week old PbCre+PTENfl/fl mice were then given either a sham treatment or degarelix, in combination with pTVG-AR vaccine or vector control. To non-invasively monitor tumor growth, as well as to randomize animals prior to treatment, we utilized microPET/CT imaging, employing as a radiotracer 124I-CLR1404, a radioiodinated alkylphosphocholine analog that has selective tumor uptake in more than 95% of malignant models (29). Animals were intravenously administered 124I-CLR1404 and subsequently PET/CT scanned within one week prior to initiation and completion of therapy (Fig. 6C), and imaging results were analyzed for mean and maximum tumor uptake. Analysis of tumors pre-treatment showed no difference between mean and maximum tumor uptake (Fig. 6D-E). Although some animals with large tumors died prior to the last imaging session (hence not all animals underwent post-treatment imaging), androgen deprivation resulted in decreased 124I-CLR1404 mean and maximum tumor uptake (Fig. 6F-G). No difference in %ID/gmean or %ID/gmax was detected post-treatment between animals receiving ADT and control vaccine versus animals receiving ADT and AR-targeted vaccine. Animals treated with degarelix and pTVG-AR had smaller tumor volumes, as measured during necropsy and determined by genitourinary complex weight, compared to animals receiving degarelix and pTVG4 (Fig. 6H).
Figure 6. Androgen deprivation increases AR expression in PTEN-deficient tumors, and immunization with pTVG-AR delays the development of castrate-resistant prostate tumors in Pten-/- mice.

PTEN-CaP8 tumor cells cultured in androgen-replete (FCS) or androgen-deprived (CSS) medium were analyzed for AR expression by (A) intracellular staining (quantified for amplitude and expression in side panels) and (B) quantitative ELISA. Twenty-week old PbCre+ PTENfl/fl mice were given a sham treatment (n=9), or degarelix along with immunization every two weeks with pTVG4 (n=13) or pTVG-AR (n=13), for five months. (C) One week prior to initiation and completion of treatment, animals were administered with 124I-CLR1404 and PET/CT scanned 96hr post intravenous injection (PET/CT images pre- and post-treatment). Signal greater than sixty percent of the max PET signal was used to calculate the mean and max percent injected dose (%ID/gtissue) for tumor, and was normalized to background muscle uptake. Pre-treatment (D) meanand (E) maximum 124I-CLR1404 uptake were used for randomization of treatment groups. (F) Changes in %ID/gmean and (G) %ID/gmean pre- to post-treatment were calculated (mean values shown by solid horizontal bars). (H) genitourinary complex masses were collected during necropsy. * indicates P<0.05 by Studentt-test (A, B) and Mann-Whitney U test (D toH).
Discussion
Immunotherapeutic approaches have shown evidence of biological and clinical benefit in preclinical and early stage clinical studies. Rational combinations of these therapies, and combinations with standard treatment modalities, could support more effective treatment regimens. Here we sought to take advantage of the immunostimulatory and antitumor benefits of androgen deprivation and to capitalize on one of the means of resistance to ADT by immunologically targeting the increased expression of AR in tumor cells following ADT. We showed that androgen deprivation results in increased full-length AR expression that persists over time, and that this increased AR expression is associated with these cells being better targets for AR-specific T cells. Furthermore, we showed that a DNA vaccine encoding the AR LBD enhanced immune responses that recognized and lysed castrate-resistant prostate cancer cells, and delayed the recurrence of castrate-resistant disease when combined with ADT. This suggests that, in combination with ADT, a vaccine targeting the AR may produce better results than other antigen-specific vaccines, perhaps because it targets a mechanism of resistance that drives castrate-resistant tumor growth.
Although our studies focused on therapies aimed at preventing androgen production, other means of androgen deprivation could support combinatorial therapies. Although many preclinical studies have evaluated the immune effects of ADT by surgical castration (5,6,11), clinical studies have looked at both preventing production of the ligand alone (4) or in combination with direct AR antagonists (3,7,8), all of which have immunomodulatory effects in the tumor microenvironment. Additionally, in vitro evaluation of enzalutamide (an AR antagonist) and abiraterone (a CYP17A1 inhibitor that prevents androgen production) enhanced the immunogenicity of prostate tumors and increased T-cell killing (20). Treatment of prostate tumor cells with enzalutamide led to a decrease in the anti-apoptotic protein NAIP, facilitating lysis of these cells by cytolytic T cells (20). Although we did not observe changes in NAIP following androgen deprivation alone, these findings suggest that combined androgen deprivation approaches could synergize with immune-based treatment. Clinical studies have also shown that enzalutamide combined with pembrolizumab has led to clinical responses in patients with metastatic prostate cancer (30). However, a comparison of orchiectomy with AR antagonist treatment has suggested that agents that directly bind and inhibit the AR may have immunosuppressive effects by inhibiting the priming and effector function of antigen-specific T cells when given prior to immunotherapy (31). To help resolve these data, the effects of anti-androgen therapies on tumor cells must be distinguished from the effects on the immune cell compartment. Testosterone, for example, can inhibit T-cell differentiation and function through increased expression of Ptpn1, a factor involved in T-cell signaling (32). The effects on T-cell signaling pathways should be clarified before newer AR-targeted therapies move into clinical practice. Notably, several newer agents include not only traditional AR antagonists, but also agents that induce AR degradation or inhibit AR activity through interactions outside the ligand-binding domain. Studies seeking to combine these agents with immunotherapeutic interventions are ongoing both in preclinical models as well as clinical trials.
In addition to androgen deprivation, other approved therapeutic treatments for prostate cancer patients, such as radiation therapy, might synergize with AR-directed immunotherapies. Radiotherapy enhances T cell-mediated tumor cell killing (33), and can enhance immune and antitumor responses when combined with immunotherapy both in pre-clinical models (34,35) as well as in patients with solid malignancies (36,37). Radiotherapy also increases AR expression in prostate cancer cell lines, likely as a DNA damage repair response, leading to increased AR signaling in patients treated with radiotherapy (38). This suggests that an AR-directed vaccine could synergize with radiation therapy. Other chemotherapy approaches, which may nonetheless have beneficial immunomodulatory effects (39), may lead to decreased AR expression (40). However, because combined chemohormonal therapy is being used earlier in the treatment course in patients with recurrent disease (41), evaluation of effects of these therapies on AR expression and potential combination with immunotherapeutic approaches warrants evaluation.
Our gene expression studies suggest that there may be other contributions to immune recognition following ADT. After androgen deprivation, human prostate cancer cells showed increased expression of HLA-E, which presents peptides derived from MHC class I molecules to circulating NK cells to inhibit NK-mediated cytotoxicity. A variety of solid malignancies show increased expression of HLA-E (42), which correlates inversely with NK infiltration and effector function as well as disease-free survival (43). These results suggest that ADT may result in suppressed NK-cell function, and that therapies aimed at restoring NK-cell activity may make tumors more susceptible to antitumor immune responses. In addition, ADT led to an increase in Hsp70 gene expression. Hsp70 expression is associated with increased MHC class I expression (44), and may act as a chaperone, facilitating MHC presentation of AR-derived immunogenic epitopes (45). The possible role of Hsp70 in facilitating immune recognition of prostate cancer cells warrants future study. Finally, although androgen deprivation did not cause changes in PD-L1 expression, it did cause a decrease in expression of HVEM. HVEM is a checkpoint ligand that binds to BTLA and CD160 on T cells to suppress T-cell effector function. HVEM expression is associated with decreased lymphocyte infiltration into tumors and a poor prognosis in colorectal cancer (46). The decreased HVEM expression in prostate tumors in response to ADT may further sensitize these tumors to immunotherapeutic interventions.
We explored non-invasive imaging as a means to monitor tumor growth following AR-targeted combination pharmacological and immunotherapy. We used a PET agent, 124I-CLR1404, that is taken up and retained by a broad spectrum of tumor types, partially due to the overexpression of lipid rafts on cancer cell membranes (29). This agent also shows less non-specific uptake in the bladder and abdomen, making it useful to monitor prostate tumor growth. The uptake of this agent is also independent of changes to AR-regulated genes. Although 124I-CLR1404 may be useful for monitoring tumor growth, other radiotracers monitoring AR-regulated genes might provide information about tumor recurrence. These radiotracers include agents targeting PSMA (18F-DCFPyL (47)), or tracers targeting the androgen receptor itself (18F-DHT (48)), both of which have been used in clinical trials. Concurrent use of both radiotracers (one which can be used to measure tumor growth, such as 124I-CLR1404, and another that detects tumors with AR expression) could potentially provide a means to both identify the optimal time to begin AR-directed immunization and monitor the generation of AR antigen-loss variants that would no longer respond to ADT.
In addition to increased expression of the full-length AR, we also observed increased expression of AR-V7, a constitutively active splice variant of the AR that lacks the ligand-binding domain and which can drive resistance to AR-targeted pharmacological therapies in castrate-resistant prostate cancer (49). As others have shown (50), we detected a transient increase in AR-V7 expression immediately following androgen deprivation (albeit less than the expression of full-length AR), which was followed by an increase in full-length AR that was maintained over time. Although increased expression of this constitutively active AR splice variant may drive resistance to AR-directed pharmacological agents, the splice variant may have less of an effect when the AR is targeted immunologically: AR-specific T cells should continue to recognize androgen-deprived prostate tumor cells as long as the full length AR is expressed, regardless of the presence of AR splice variants. However, for variants that have completely lost AR expression, immune responses directed against the AR LBD would be ineffective.
In summary, we have shown that increased AR expression in prostate cancer cells following ADT resulted in enhanced recognition and lysis by AR-specific T cells. The combination of ADT and AR-specific immunization in vivo enhanced antitumor T-cell immunity and delayed recurrence of castrate-resistant tumors. These studies provide a rationale for combining ADT with AR-targeted immunization, an approach that is being evaluated in a Phase I clinical trial (NCT02411786).
Supplementary Material
Acknowledgments
The authors thank the UWCCC Flow Cytometry core facility (and NIH small instrument grants 1S10RR025483-01 and 1S100OD018202-01) for technical support, and the UWCCC Small Animal Imaging Facility (and NIH support grant P30 CA014520). We also thank Dr. Ajit Verma for provision of PbCre-PTEN mice, and Dr. Steve Cho, Dr. Laura Johnson, Ellen Wargowski, Dawn Church, and Ashley Weichmann for helpful discussions.
Funding: This work was supported by the Prostate Cancer Foundation Stephen A. Schwarzman PCF Young Investigator Award (BMO), and NIH R01 CA142608, and P30 CA014520.
Abbreviations used
- ADT
androgen-deprivation therapy
- AR
androgen receptor
- AS
androgen sensitive
- CR
castrate-resistant
- CT
computed tomography
- IFN
interferon
- IL
interleukin
- LBD
ligand-binding domain
- PET
positron emission tomography
- PTEN
Phosphatase and tensin homolog
- TNF
tumor necrosis factor
Footnotes
Author Contribution: BMO and MG designed and performed the experiments, analyzed the data, and prepared the manuscript; JS and TS provided support with PbCre-PTEN mouse colony and assistance with experiments; JJ provided technical support with PET/CT imaging studies, data analysis, and assistance with manuscript preparation; LE provided Myc-CaP tumor cell lines; CGD provided Myc-CaP tumor model and helpful discussions, JW provided 124I-CLR1404 radiotracer; DGM performed experiments and supervised the concept, experimental design, data analysis, and manuscript preparation.
Competing Interests: DGM has ownership interest, receives research support, and serves as consultant to Madison Vaccines, Inc., that has licensed material described in this report. JPW is the founder of Cellectar Biosciences which holds the licensing rights to 124I-CLR1404. None of the other authors have relevant potential competing interests.
References
- 1.Colluru VT, Johnson LE, Olson BM, McNeel DG. Preclinical and clinical development of DNA vaccines for prostate cancer. Urologic oncology. 2016;34(4):193–204. doi: 10.1016/j.urolonc.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodge JW, Ardiani A, Farsaci B, Kwilas AR, Gameiro SR. The tipping point for combination therapy: cancer vaccines with radiation, chemotherapy, or targeted small molecule inhibitors. Semin Oncol. 2012;39(3):323–39. doi: 10.1053/j.seminoncol.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mercader M, Bodner BK, Moser MT, Kwon PS, Park ES, Manecke RG, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A. 2001;98(25):14565–70. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morse MD, McNeel DG. Prostate Cancer Patients Treated with Androgen Deprivation Therapy Develop Persistent Changes in Adaptive Immune Responses. Human immunology. 2010;71(5):496–504. doi: 10.1016/j.humimm.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morse MD, McNeel DG. T cells localized to the androgen-deprived prostate are TH1 and TH17 biased. Prostate. 2012;72(11):1239–47. doi: 10.1002/pros.22476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roden AC, Moser MT, Tri SD, Mercader M, Kuntz SM, Dong H, et al. Augmentation of T cell levels and responses induced by androgen deprivation. J Immunol. 2004;173(10):6098–108. doi: 10.4049/jimmunol.173.10.6098. [DOI] [PubMed] [Google Scholar]
- 7.Mercader M, Sengupta S, Bodner BK, Manecke RG, Cosar EF, Moser MT, et al. Early effects of pharmacological androgen deprivation in human prostate cancer. BJU Int. 2007;99(1):60–7. doi: 10.1111/j.1464-410X.2007.06538.x. [DOI] [PubMed] [Google Scholar]
- 8.Gannon PO, Poisson AO, Delvoye N, Lapointe R, Mes-Masson AM, Saad F. Characterization of the intra-prostatic immune cell infiltration in androgen-deprived prostate cancer patients. Journal of immunological methods. 2009;348(1-2):9–17. doi: 10.1016/j.jim.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Shen YC, Kochel C, Francica B, Alme A, Nirschl C, Nirschl T, et al. Combining androgen deprivation with immune checkpoint blockade delays the development of castration resistance in a murine model of prostate cancer. Philadelphia, PA: 2015. [Google Scholar]
- 10.Akins EJ, Moore ML, Tang S, Willingham MC, Tooze JA, Dubey P. In situ vaccination combined with androgen ablation and regulatory T-cell depletion reduces castration-resistant tumor burden in prostate-specific pten knockout mice. Cancer Res. 2010;70(9):3473–82. doi: 10.1158/0008-5472.CAN-09-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drake CG, Doody AD, Mihalyo MA, Huang CT, Kelleher E, Ravi S, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer cell. 2005;7(3):239–49. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koh YT, Gray A, Higgins SA, Hubby B, Kast WM. Androgen ablation augments prostate cancer vaccine immunogenicity only when applied after immunization. Prostate. 2009 doi: 10.1002/pros.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ardiani A, Farsaci B, Rogers CJ, Protter A, Guo Z, King TH, et al. Combination therapy with a second-generation androgen receptor antagonist and a metastasis vaccine improves survival in a spontaneous prostate cancer model. Clin Cancer Res. 2013;19(22):6205–18. doi: 10.1158/1078-0432.CCR-13-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNeel DG, Smith HA, Eickhoff JC, Lang JM, Staab MJ, Wilding G, et al. Phase I trial of tremelimumab in combination with short-term androgen deprivation in patients with PSA-recurrent prostate cancer. Cancer Immunol Immunother. 2012;61(7):1137–47. doi: 10.1007/s00262-011-1193-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Small EJ, Lance RS, Gardner TA, Karsh LI, Fong L, McCoy C, et al. A Randomized Phase II Trial of Sipuleucel-T with Concurrent versus Sequential Abiraterone Acetate plus Prednisone in Metastatic Castration-Resistant Prostate Cancer. Clin Cancer Res. 2015;21(17):3862–9. doi: 10.1158/1078-0432.CCR-15-0079. [DOI] [PubMed] [Google Scholar]
- 16.Madan RA, Gulley JL, Schlom J, Steinberg SM, Liewehr DJ, Dahut WL, et al. Analysis of overall survival in patients with nonmetastatic castration-resistant prostate cancer treated with vaccine, nilutamide, and combination therapy. Clin Cancer Res. 2008;14(14):4526–31. doi: 10.1158/1078-0432.CCR-07-5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olson BM, McNeel DG. CD8+ T cells specific for the androgen receptor are common in patients with prostate cancer and are able to lyse prostate tumor cells. Cancer Immunol Immunother. 2011;60:781–92. doi: 10.1007/s00262-011-0987-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olson BM, Johnson LE, McNeel DG. The androgen receptor: a biologically relevant vaccine target for the treatment of prostate cancer. Cancer Immunol Immunother. 2013;62(3):585–96. doi: 10.1007/s00262-012-1363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gregory CW, Hamil KG, Kim D, Hall SH, Pretlow TG, Mohler JL, et al. Androgen receptor expression in androgen-independent prostate cancer is associated with increased expression of androgen-regulated genes. Cancer Res. 1998;58(24):5718–24. [PubMed] [Google Scholar]
- 20.Ardiani A, Gameiro SR, Kwilas AR, Donahue RN, Hodge JW. Androgen deprivation therapy sensitizes prostate cancer cells to T-cell killing through androgen receptor dependent modulation of the apoptotic pathway. Oncotarget. 2014;5(19):9335–48. doi: 10.18632/oncotarget.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellis L, Lehet K, Ramakrishnan S, Adelaiye R, Pili R. Development of a castrate resistant transplant tumor model of prostate cancer. Prostate. 2012;72(6):587–91. doi: 10.1002/pros.21465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer cell. 2003;4(3):209–21. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 23.Markey MP, Bergseid J, Bosco EE, Stengel K, Xu H, Mayhew CN, et al. Loss of the retinoblastoma tumor suppressor: differential action on transcriptional programs related to cell cycle control and immune function. Oncogene. 2007;26(43):6307–18. doi: 10.1038/sj.onc.1210450. [DOI] [PubMed] [Google Scholar]
- 24.Eason DD, Coppola D, Livingston S, Shepherd AT, Blanck G. Loss of MHC class II inducibility in hyperplastic tissue in Rb-defective mice. Cancer Lett. 2001;171(2):209–14. doi: 10.1016/s0304-3835(01)00603-6. [DOI] [PubMed] [Google Scholar]
- 25.Smith HA, Cronk RJ, Lang JM, McNeel DG. Expression and Immunotherapeutic Targeting of the SSX Family of Cancer-Testis Antigens in Prostate Cancer. Cancer Res. 2011;71:6785–95. doi: 10.1158/0008-5472.CAN-11-2127. [DOI] [PubMed] [Google Scholar]
- 26.Hutcheson J, Bourgo RJ, Balaji U, Ertel A, Witkiewicz AK, Knudsen ES. Retinoblastoma protein potentiates the innate immune response in hepatocytes: significance for hepatocellular carcinoma. Hepatology (Baltimore, Md. 2014;60(4):1231–40. doi: 10.1002/hep.27217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, et al. Integrative genomic profiling of human prostate cancer. Cancer cell. 2010;18(1):11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ku SY, Rosario S, Wang Y, Mu P, Seshadri M, Goodrich ZW, et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science. 2017;355(6320):78–83. doi: 10.1126/science.aah4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sweis RF, Spranger S, Bao R, Paner GP, Stadler WM, Steinberg G, et al. Molecular Drivers of the Non-T-cell-Inflamed Tumor Microenvironment in Urothelial Bladder Cancer. Cancer Immunol Res. 2016;4(7):563–8. doi: 10.1158/2326-6066.CIR-15-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29(15):1949–55. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 31.Pu Y, Xu M, Liang Y, Yang K, Guo Y, Yang X, et al. Androgen receptor antagonists compromise T cell response against prostate cancer leading to early tumor relapse. Science translational medicine. 2016;8(333):333ra47. doi: 10.1126/scitranslmed.aad5659. [DOI] [PubMed] [Google Scholar]
- 32.Kissick HT, Sanda MG, Dunn LK, Pellegrini KL, On ST, Noel JK, et al. Androgens alter T-cell immunity by inhibiting T-helper 1 differentiation. Proc Natl Acad Sci U S A. 2014;111(27):9887–92. doi: 10.1073/pnas.1402468111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gameiro SR, Jammeh ML, Wattenberg MM, Tsang KY, Ferrone S, Hodge JW. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget. 2014;5(2):403–16. doi: 10.18632/oncotarget.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64(12):4328–37. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 35.Harris TJ, Hipkiss EL, Borzillary S, Wada S, Grosso JF, Yen HR, et al. Radiotherapy augments the immune response to prostate cancer in a time-dependent manner. Prostate. 2008;68(12):1319–29. doi: 10.1002/pros.20794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Golden EB, Chhabra A, Chachoua A, Adams S, Donach M, Fenton-Kerimian M, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. The lancet oncology. 2015;16(7):795–803. doi: 10.1016/S1470-2045(15)00054-6. [DOI] [PubMed] [Google Scholar]
- 37.Slovin SF, Higano CS, Hamid O, Tejwani S, Harzstark A, Alumkal JJ, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013;24(7):1813–21. doi: 10.1093/annonc/mdt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spratt DE, Evans MJ, Davis BJ, Doran MG, Lee MX, Shah N, et al. Androgen Receptor Upregulation Mediates Radioresistance after Ionizing Radiation. Cancer Res. 2015;75(22):4688–96. doi: 10.1158/0008-5472.CAN-15-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drake CG, Sharma P, Gerritsen W. Metastatic castration-resistant prostate cancer: new therapies, novel combination strategies and implications for immunotherapy. Oncogene. 2014;33(43):5053–64. doi: 10.1038/onc.2013.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gan L, Chen S, Wang Y, Watahiki A, Bohrer L, Sun Z, et al. Inhibition of the androgen receptor as a novel mechanism of taxol chemotherapy in prostate cancer. Cancer Res. 2009;69(21):8386–94. doi: 10.1158/0008-5472.CAN-09-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. The New England journal of medicine. 2015;373(8):737–46. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palmisano GL, Contardi E, Morabito A, Gargaglione V, Ferrara GB, Pistillo MP. HLA-E surface expression is independent of the availability of HLA class I signal sequence-derived peptides in human tumor cell lines. Human immunology. 2005;66(1):1–12. doi: 10.1016/j.humimm.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 43.Levy EM, Bianchini M, Von Euw EM, Barrio MM, Bravo AI, Furman D, et al. Human leukocyte antigen-E protein is overexpressed in primary human colorectal cancer. Int J Oncol. 2008;32(3):633–41. [PubMed] [Google Scholar]
- 44.Wells AD, Rai SK, Salvato MS, Band H, Malkovsky M. Hsp72-mediated augmentation of MHC class I surface expression and endogenous antigen presentation. International immunology. 1998;10(5):609–17. doi: 10.1093/intimm/10.5.609. [DOI] [PubMed] [Google Scholar]
- 45.Cho JA, Lee YS, Kim SH, Ko JK, Kim CW. MHC independent anti-tumor immune responses induced by Hsp70-enriched exosomes generate tumor regression in murine models. Cancer Lett. 2009;275(2):256–65. doi: 10.1016/j.canlet.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 46.Inoue T, Sho M, Yasuda S, Nishiwada S, Nakamura S, Ueda T, et al. HVEM expression contributes to tumor progression and prognosis in human colorectal cancer. Anticancer research. 2015;35(3):1361–7. [PubMed] [Google Scholar]
- 47.Rowe SP, Macura KJ, Mena E, Blackford AL, Nadal R, Antonarakis ES, et al. PSMA-Based [(18)F]DCFPyL PET/CT Is Superior to Conventional Imaging for Lesion Detection in Patients with Metastatic Prostate Cancer. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging. 2016;18(3):411–9. doi: 10.1007/s11307-016-0957-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beattie BJ, Smith-Jones PM, Jhanwar YS, Schoder H, Schmidtlein CR, Morris MJ, et al. Pharmacokinetic assessment of the uptake of 16beta-18F-fluoro-5alpha-dihydrotestosterone (FDHT) in prostate tumors as measured by PET. J Nucl Med. 2010;51(2):183–92. doi: 10.2967/jnumed.109.066159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. The New England journal of medicine. 2014;371(11):1028–38. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu Z, Chen S, Sowalsky AG, Voznesensky OS, Mostaghel EA, Nelson PS, et al. Rapid induction of androgen receptor splice variants by androgen deprivation in prostate cancer. Clin Cancer Res. 2014;20(6):1590–600. doi: 10.1158/1078-0432.CCR-13-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.