Abstract
OBJECTIVES
To investigate the association between total urinary polyphenols (TUPs) and total dietary polyphenols (TDPs) and cognitive decline in an older population.
DESIGN
The Invecchiare in Chianti (InCHIANTI) study, a cohort study with 3 years of follow-up.
SETTING
Tuscany, Italy.
PARTICIPANTS
Individuals without dementia aged 65 and older (N = 652).
MEASUREMENTS
TUP and TDP concentrations were analyzed at baseline using the Folin-Ciocalteu assay and a validated food frequency questionnaire, respectively. Cognition was assessed using the Mini-Mental State Examination (MMSE) and Trail-Making Test (TMT) at baseline and after 3 years of follow-up. Substantial cognitive decline was defined as a reduction in MMSE score of three or more points and an increase of at least 29 seconds on the TMT Part A (TMT-A) and 68 seconds on the TMT Part B (TMT-B) (the worst 10% of the distribution of decline) or as test discontinued because of multiple mistakes on the TMT A and B at follow-up.
RESULTS
Higher TUP levels were associated with lower risk of substantial cognitive decline on the MMSE (odds ratio (OR) comparing extreme tertiles = 0.53, 95% confidence interval (CI) = 0.34–0.85, P-trend = .008) and on the TMT-A (OR = 0.52, 95% CI = 0.28–0.96, P-trend = .03), but not on TMT-B in a logistic regression model that adjusted for baseline cognitive score and potential confounding factors. TDP did not affect the development of substantial cognitive decline in either test.
CONCLUSION
High concentrations of polyphenols, a nutritional biomarker of polyphenol intake, were associated with lower risk of substantial cognitive decline in an older population studied over a 3-year period, suggesting a protective effect against cognitive impairment.
Keywords: urinary polyphenols, dietary polyphenols, biomarker, cognitive decline, epidemiology
Cognitive decline in older persons encompasses a number of different conditions, from a physiological age-associated reduction in cognitive function that occurs in almost every aging individual, the cognitive impairment that often accompanies a global deterioration of health and comorbidity, to the accelerated trajectory of cognitive decline that eventually leads to mild cognitive impairment and dementia.1,2
There is increasing evidence suggesting that dietary factors, 3 in particular high long-chain omega-3 fatty acid intake, low saturated fat intake, and high vegetable intake, are related to cognitive performance in older subjects.4 Over the last few years, there has been much scientific and public health interest in polyphenols5 because of their potential beneficial effects against chronic diseases, particularly cardiovascular diseases, type 2 diabetes mellitus, and some cancers, and overall mortality.6–11 These secondary plant metabolites are natural bioactive compounds that have been identified in foods and beverages.12 Several epidemiological studies have suggested that polyphenol-rich diets are positively associated with better cognitive function.13,14 The neuroprotective activity of dietary polyphenols might be due to their antioxidant and anti-inflammatory properties.15
The protective effects of polyphenols against human diseases depend on the quantity consumed and their bioavailability, which largely differs between compounds and between (interindividual variation) and within (intraindividual variation) subjects.16 In this context, a nutritional biomarker of total dietary polyphenols is essential to provide an accurate assessment of polyphenol exposure to evaluate their protective effects.17 Total urinary polyphenol (TUP) concentration determined using the Folin-Ciocalteu assay, preferably analyzed in 24-hour urine samples,18 is considered an objective biomarker of total dietary polyphenol intake (TDP)17,18 and a proxy measure of dietary fruit and vegetable intake.19 To the best of the knowledge of the authors of the current study, there is only one recent cross-sectional study showing that high TUP concentrations, expressed as urinary creatinine, were associated with better scores in immediate verbal memory in older subjects at high vascular risk.20 The objective of the current study was to evaluate these associations from a longitudinal perspective by studying the effect of baseline TUP concentrations, expressed as 24-hour volume; TDP; and the risk of developing cognitive decline over a 3-year period in older participants free of dementia at baseline who were enrolled in the Invecchiare in Chianti (InCHIANTI) study.
METHODS
Study Population
InCHIANTI (www.inchiantistudy.net) is an ongoing, prospective, community-based study designed to evaluate risk factors affecting the loss of mobility in the older population. It was conducted in Bagno in Ripoli and Greve in Chianti, two Italian towns adjacent to the city of Florence (Tuscany, Italy). Further details of the rationale and the study design have been given elsewhere.21 The Italian National Research Council of Aging Ethical Committee approved the study protocol, and all participants provided informed consent to participate.
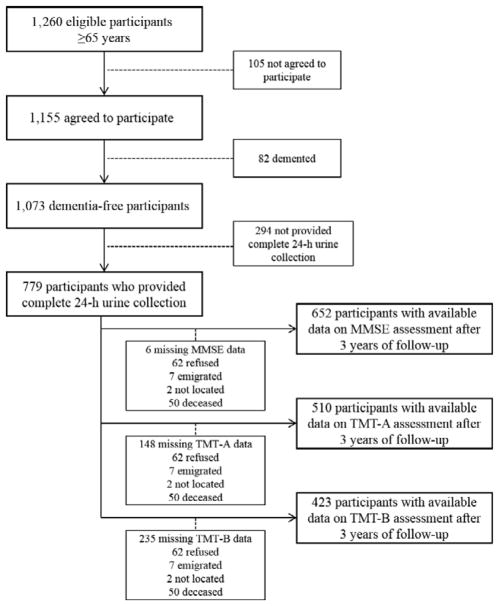
Data were collected at baseline and 3 years after enrollment. Baseline visits occurred between 1998 and 2000, and 3-year assessments took place between 2001 and 2003. The study randomly sampled 1,260 men and women aged 65 and older, of whom 1,155 agreed to participate (participation rate 91.6%). Of these, 82 (7.1%) diagnosed with dementia syndrome at baseline by geriatricians and psychologists with expertise in cognitive impairment were excluded. The final sample for the current analysis included 652 dementia-free adults aged 65 and older who provided complete 24-hour urine collection and completed a cognitive assessment after 3 years of followup. Follow-up data for the Trail-Making Test (TMT) Parts A and B were available for 510 and 423 participants, respectively (Figure 1).
Figure 1.
Flowchart of participants at each stage of the study. MMSE = Mini-Mental State Examination; TMT =Trail-Making Test.
Total Polyphenol Exposure
Twenty-four-hour urine samples were collected at baseline. Urine samples were drawn, processed, and stored at −80°C until analysis. Total urinary polyphenols (TUPs) were analyzed using F-C assay after solid-phase extraction, as described previously.18,19 TUP concentrations were expressed as mg gallic acid equivalents (GAEs)/d.
Food intake (g/d) was assessed using the Italian version of the food frequency questionnaire, developed and validated in the European Prospective Study into Cancer and Nutrition (EPIC) study.22 Briefly, a self-administered quantitative dietary questionnaire, including 236 food items and structured according to courses in a meal characteristic of Italian dietary habits, was used. Portion sizes were estimated using natural units, household measures, and grams or with the aid of a set of photographs. Energy (kcal/d) intake was calculated using an Italian food composition database created for the EPIC study.23 TDPs were estimated using a food composition database on polyphenols 11 compiled from the three most updated U.S. Department of Agriculture databases (flavonoids, isoflavones, and proanthocyanidins)24–26 and the Phenol-Explorer database. 27 TDP intake was calculated as the sum of flavonoids (anthocyanidins, flavonols, flavanones, flavones, flavanols (including flavan-3-ol monomers, theaflavins, and proanthocyanidins), and isoflavones), phenolic acids, lignans, stilbenes, and other polyphenols and expressed as aglycone equivalents (mg/d),27 after the conversion of polyphenol glycoside and ester contents into aglycone contents on the basis of their respective molecular weights.
Cognitive Tests
The Mini-Mental State Examination (MMSE) is a validated method of assessing global cognitive function that is widely used in clinical practice and research and is an effective screening tool for cognitive impairment in older community-dwelling, hospitalized, and institutionalized adults.28,29 MMSE is an 11-question test that evaluates five areas of cognitive function: orientation, registration, attention and calculation, recall, and language. Scores for the MMSE range from 0 to 30, with higher scores indicating better cognitive function. The TMT is a neuropsychological test commonly used in clinical evaluation for the assessment of cognitive abilities such as visual-conceptual and visual-motor tracking, sustained attention, and task alternation abilities.30,31 The TMT consists of two parts administered in sequence: TMT-A and TMT-B. TMT-A is focused on attention, whereas TMT-B is focused on executive function. Worse performance is indicated by longer times, measured in seconds, to complete the TMT-A and B.
Substantial cognitive decline was defined as a decline in MMSE score of three or more points from baseline to 3 years later28,29 and an increase of at least 29 seconds on the TMT-A and 68 seconds on the TMT-B (the worst 10% of the distribution of subtracting baseline from 3-year follow-up scores in seconds) or discontinuation of the test at follow-up, but not at baseline, because of multiple mistakes on the TMT-A and B.32
Other Baseline Covariates
Trained clinicians ascertained diseases based on information from self-reported physician diagnoses, drug treatments, medical history, clinical examinations, and blood tests. Smoking status (current, former, never-smoker), age, sex, education (years of schooling), and body mass index (kg/m2) were reported or measured. Physical activity was measured using a modified standard questionnaire33 and defined as sedentary (completely inactive or performing light-intensity physical activity <2 h/wk), light physical activity (light-intensity physical activity for 2–4 h/wk), and moderate to high physical activity (light-intensity physical activity for >4 h/wk or moderate-intensity physical activity for >1 h/wk). Specific comorbidities considered in this analysis were congestive heart failure, stroke, cancer, and diabetes mellitus. Renal function was calculated using the Cockcroft–Gault formula: (140–age) × weight (×0.85 if female)/(72 × serum creatinine) and classified into a dichotomous variable: normal (≥60 mL/min) and impaired (<60 mL/min).34 Depressive symptoms were assessed using the Center for Epidemiologic Studies Depression Scale (CES-D) and defined as 16 or higher and less than 16.35 Total blood cholesterol was measured using an automated enzymatic method.36
Statistical Analysis
Descriptive statistics were used to describe participants’ characteristics, means and standard deviations (SD) for normal continuous variables, medians and interquartile ranges (IQRs) for variables with asymmetric distribution, and number of participants and percentages for categorical variables. Baseline characteristics were compared across TUP and TDP tertiles using age-adjusted generalized linear models. Spearman correlations were used to explore the relationships between TUP, TDP, and the sum of fruit and vegetable intake. Linear regression models were used to evaluate associations between baseline cognitive performance (MMSE, TMT-A, TMT-B) and TDP and TUP tertiles. For TMT test performance, the inverse score was used; thus, a higher score corresponded to a better result. Logistic regression models were used to calculate odds ratios (ORs) and 95% confidence intervals (CIs) between TUP and TDP concentrations and substantial cognitive decline. In unadjusted models, only baseline cognitive score was controlled for (Model A). In multivariable models, variables that have been identified as potential confounders were adjusted for: sex, age, education, BMI, total energy intake, physical activity, baseline cognitive score, impaired renal function, smoking status, congestive heart failure, cancer, stroke, diabetes mellitus, depressive symptoms, and total blood cholesterol (Model B). TUPs were analyzed as tertiles of the whole cohort according to cutoff points of 126.4 and 175.5 mg GAE/d urine. TDPs were also categorized as tertiles of the whole cohort according cutoff points of 514.2 and 648.4 mg/d aglycones. Tests for linear trend were performed by considering the median of each tertile as an ordinal variable. TUPs and TDPs were also analyzed as log2-transformed continuous variables because they were not normally distributed. Interactions between TDP and TUP concentrations and sex, age, BMI, education, and smoking status were tested by including product terms in fully adjusted logistic regression models. There was no evidence of colinearity. In sensitivity analyses, models were assessed for TDP excluding participants in the top or bottom 1% of the distribution of the ratio of reported total energy intake to estimated energy requirement to minimize the potential effect of over- and under-reporters. All statistical tests were two-tailed and were performed using the SPSS package program version 20.0 (SPSS, Inc., Chicago, IL). Significance was set at P < .05.
RESULTS
The main characteristics of the study population according to tertiles of TUP and TDP, adjusted for age, are summarized in Table 1. The study included 361 women (55.4%) and 291 men (44.6%), with a mean age of 73. From the lowest to the highest TUP tertiles, participants were younger and more likely to be men. The percentages of participants who experienced substantial cognitive decline on TMT-A over the 3-year follow-up and of those who at baseline had depressive symptoms and cancer progressively decreased with increasing TUP tertiles. Individuals in the highest TUP tertile tended to have a smaller change in MMSE score and were less likely to experience substantial cognitive decline on MMSE than those in the lowest TUP tertile. Moreover, from the lowest to the highest TUP tertiles, participants had a higher energy intake. There were no significant differences across the tertiles of TUP in BMI, education, smoking status, physical activity, fruit and vegetable consumption, total cholesterol, congestive heart failure, or stroke. From the lowest to the highest tertiles of TDP, participants were younger, less likely to be female, and less often current and former smokers and had more education, physical activity, energy dietary intake, and dietary fruit and vegetable consumption and lower total cholesterol. Participants with substantial cognitive decline after 3 years of follow-up were older and more likely to be female, had lower educational achievement and a lower physical activity level, and a higher prevalence of stroke and congestive heart failure than those without cognitive decline. Participants excluded from this study (n = 503) were significantly older (P < .001), took more medications (P = .04), had lower physical activity (P < .001), and were more likely to have renal impairment (P = .006) than those included, whereas there were no significant differences in sex and smoking status (results not shown). TUPs were significantly correlated with fruit and vegetable intake (ρ = 0.141, P < .001) and TDPs (ρ = 0.131, P < .001).
Table 1.
Characteristics of the Study Population According to Tertile of Total Urinary Polyphenols (TUPs) and Total Dietary Polyphenols (TDPs)
| Characteristic | All, N = 652 | TDP | TUP | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Tertile 1, n = 217 | Tertile 2, n = 218 | Tertile 3, n = 217 | P-Valuea | Tertile 1, n = 217 | Tertile 2, n = 218 | Tertile 3, n = 217 | P-Valuea | ||
| Age, mean ± SD | 73.4 (6.4) | 75.0 (7.2) | 73.1 (6.0) | 72.2 (5.6) | <.001 | 74.8 (6.9) | 73.8 (6.5) | 71.8 (5.3) | <.001 |
|
| |||||||||
| Female, n (%) | 361 (55.4) | 148 (68.2) | 120 (55.0) | 93 (42.9) | <.001 | 137 (63.1) | 119 (54.6) | 105 (48.4) | .03 |
|
| |||||||||
| Body mass index, kg/m2, mean ± SD | 27.6 (4.0) | 27.6 (4.2) | 27.5 (3.9) | 27.6 (3.7) | .85 | 27.2 (4.0) | 27.6 (4.0) | 28.0 (3.8) | .35 |
|
| |||||||||
| Education, years, mean ± SD | 5.6 (3.2) | 5.0 (3.0) | 5.7 (3.4) | 6.0 (3.2) | .07 | 5.2 (3.0) | 5.7 (3.8) | 5.8 (2.9) | .54 |
|
| |||||||||
| Physical activity, n (%) | |||||||||
|
| |||||||||
| Sedentary | 106 (16.3) | 49 (22.7) | 36 (16.5) | 21 (9.8) | .003 | 45 (20.8) | 32 (14.8) | 29 (13.4) | .53 |
|
| |||||||||
| Light | 291 (44.8) | 106 (49.1) | 92 (42.2) | 93 (43.3) | 96 (44.4) | 101 (46.8) | 94 (43.3) | ||
|
| |||||||||
| Moderate to high | 252 (38.8) | 61 (28.2) | 90 (41.3) | 101 (47.0) | 75 (34.7) | 83 (38.4) | 94 (43.3) | ||
|
| |||||||||
| Smoking status, n (%) | |||||||||
|
| |||||||||
| Never | 387 (59.4) | 147 (67.7) | 130 (59.6) | 110 (50.7) | .04 | 138 (63.6) | 137 (62.8) | 112 (51.6) | .33 |
|
| |||||||||
| Former | 175 (26.8) | 46 (21.2) | 53 (24.3) | 76 (35.0) | 45 (20.7) | 53 (24.3) | 77 (35.5) | ||
|
| |||||||||
| Current | 90 (13.8) | 24 (11.1) | 35 (16.1) | 31 (14.3) | 34 (15.7) | 28 (12.8) | 28 (12.9) | ||
|
| |||||||||
| Energy intake, kcal/d, mean ± SD | 1,921 (549) | 1,608 (437) | 1,918 (456) | 2,237 (558) | <.001 | 1,830 (534) | 1,909 (532) | 2,024 (566) | .02 |
|
| |||||||||
| Dietary fruits and vegetables, g/d, median (IQR) | 436 (323–552) | 309 (245–390) | 445 (375–525) | 576 (470–696) | <.001 | 416 (296–538) | 422 (319–535) | 453 (365–583) | .15 |
|
| |||||||||
| Total dietary polyphenols, mg/d, median (IQR) | 574 (472–701) | 430 (354–470) | 574 (543–610) | 766 (701–855) | <.001 | 551 (452–655) | 562 (457–708) | 601 (518–720) | .12 |
|
| |||||||||
| Total urinary polyphenols, mg gallic acid equivalents/d, median (IQR) | 148 (113–196) | 138 (107–173) | 154 (115–197) | 160 (126–208) | .002 | 103 (82–113) | 148 (138–163) | 218 (196–254) | <.001 |
|
| |||||||||
| Total cholesterol, mg/dL, median (IQR) | 218 (195–247) | 221 (195–247) | 220 (197–251) | 213 (192–243) | .09 | 220 (195–245) | 216 (196–248) | 219 (194–246) | .62 |
|
| |||||||||
| Congestive heart failure, n (%) | 21 (3.2) | 6 (2.8) | 10 (4.6) | 5 (2.3) | .37 | 5 (2.3) | 8 (3.7) | 8 (3.7) | .63 |
|
| |||||||||
| Stroke, n (%) | 32 (4.9) | 11 (5.1) | 14 (6.4) | 7 (3.2) | .31 | 11 (5.1) | 7 (3.2) | 14 (6.5) | .29 |
|
| |||||||||
| Cancer, n (%) | 39 (6.0) | 13 (6.0) | 13 (6.0) | 13 (6.0) | >.99 | 17 (7.8) | 6 (2.8) | 16 (7.4) | .06 |
|
| |||||||||
| Diabetes mellitus, n (%) | 88 (13.5) | 30 (13.8) | 31 (14.2) | 27 (12.4) | .85 | 24 (11.1) | 27 (12.4) | 37 (17.1) | .15 |
|
| |||||||||
| Renal impairment, n (%) | 385 (60.0) | 140 (66.4) | 128 (59.3) | 117 (54.4) | .76 | 145 (67.4) | 128 (60.4) | 112 (52.1) | .44 |
|
| |||||||||
| Depressive symptoms, Center for Epidemiologic Studies Depression Scale score ≥16, n (%) | 97 (14.9) | 35 (16.1) | 31 (14.2) | 31 (14.3) | .97 | 42 (19.4) | 35 (16.1) | 20 (9.2) | .06 |
|
| |||||||||
| Cognitive test scores, mean ± SD | |||||||||
|
| |||||||||
| MMSE | 25.5 (3.0) | 25.0 (3.3) | 25.6 (2.9) | 25.9 (2.5) | .28 | 25.0 (3.4) | 25.6 (2.9) | 25.9 (2.5) | .22 |
|
| |||||||||
| TMT-A | 91.3 (57.2) | 100.9 (63.5) | 89.4 (5.51) | 83.7 (51.4) | .11 | 94.1 (57.8) | 97.4 (61.9) | 82.5 (50.7) | .20 |
|
| |||||||||
| TMT-B | 172.5 (75.1) | 179.4 (76.0) | 171.1 (72.2) | 167.4 (77.2) | .54 | 176.1 (76.5) | 174.8 (76.0) | 166.3 (72.7) | .73 |
|
| |||||||||
| Change in cognitive test scores, mean ± SD | |||||||||
|
| |||||||||
| MMSE | −0.6 (3.7) | −0.9 (4.2) | −0.5 (3.2) | −0.3 (3.5) | .74 | −0.9 (3.7) | −0.9 (4.3) | 0.0 (2.8) | .09 |
|
| |||||||||
| TMT-A | −8.5 (41.1) | −14.8 (45.1) | −5.0 (41.8) | −6.4 (35.9) | .07 | −3.7 (38.3) | −13.5 (48.5) | −8.3 (35.4) | .12 |
|
| |||||||||
| TMT-B | −8.6 (58.3) | −14.4 (58.7) | 0.4 (57.4) | −11.7 (58.6) | .26 | −8.0 (58.2) | −2.6 (50.3) | −14.5 (64.8) | .48 |
|
| |||||||||
| Substantial cognitive decline, n (%)b | |||||||||
|
| |||||||||
| MMSE | 203 (31.1) | 68 (31.3) | 73 (33.5) | 62 (28.6) | .57 | 80 (36.9) | 71 (32.6) | 52 (24.0) | .07 |
|
| |||||||||
| TMT-A | 103 (20.2) | 42 (24.7) | 36 (21.2) | 25 (14.7) | .24 | 46 (27.1) | 33 (19.4) | 24 (14.1) | .04 |
|
| |||||||||
| TMT-B | 221 (52.2) | 73 (51.8) | 78 (55.3) | 70 (49.6) | .57 | 74 (52.5) | 76 (53.9) | 71 (50.4) | .95 |
SD = standard deviation; IQR = interquartile range.
Generalized linear models were adjusted for age.
≥3 points on the Mini-Mental State Examination (MMSE) from baseline to 3 years later and worst 10% of the distribution of subtracting baseline from 3-year follow-up scores in seconds or test discontinued at follow-up for the Trail-Making Test Parts A (TMT-A) and B (TMT-B).
In linear regression models adjusted for potential confounding factors, TUP levels were positively associated with MMSE (β = 0.076, standard error (SE) = 0.243, P = .04) and TMT-A (β = 0.086, SE = 5.174, P = .02) scores but not TMT-B (β = 0.014, SE = 6.835, P = .73). No associations between TDP intake and any of the cognitive tests at baseline were observed.
In logistic regression models adjusted for baseline cognitive scores, and participants in the highest TUP tertile were significantly less likely than in the lowest to experience substantial cognitive decline according to the MMSE (OR = 0.53, 95% CI = 0.35–0.80, P-trend = .003) and the TMT-A (OR = 0.50, 95% CI = 0.28–0.89, P-trend = .02) (Table 2, Model A). This association remained statistically significant after full adjustment for potential confounders for the MMSE (highest vs lowest tertile OR = 0.53, 95% CI = 0.34–0.85, P-trend = .008) and the TMT-A (OR = 0.52, 95% CI = 0.28–0.96, P-trend = .03). No significant association between TUP concentrations and TMT-B was observed (Table 2, Model B). In fully adjusted models using MMSE, TMT-A, and TMT-B scores, no statistically significant interactions were detected for sex, age, BMI, education, or smoking status.
Table 2.
Logistic Regression Models Describing the Association Between Total Urinary Polyphenol (TUP) Tertile and 3-Year Substantial Cognitive Decline in Older Adults
| Model | MMSE | TMT-A | TMT-B | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Cutoff mg GAE/d | Cases n | OR (95% CI) | Cutoff mg GAE/d | Cases n | OR (95% CI) | Cutoff mg GAE/d | Cases n | OR (95% CI) | |
| Aa | |||||||||
|
| |||||||||
| Tertile 1 | <126.4 | 80 | 1 (reference) | <133.2 | 46 | 1 (reference) | <135.8 | 74 | 1 (reference) |
|
| |||||||||
| Tertile 2 | 126.4–175.5 | 71 | 0.82 (0.55–1.21) | 133.2–183.7 | 33 | 0.56 (0.32–0.97) | 135.8–186.8 | 76 | 0.99 (0.60–1.64) |
|
| |||||||||
| Tertile 3 | >175.5 | 52 | 0.53 (0.35–0.80) | >183.7 | 24 | 0.50 (0.28–0.89) | >186.8 | 71 | 0.90 (0.54–1.49) |
|
| |||||||||
| P-trendb | .003 | .02 | .67 | ||||||
|
| |||||||||
| Continuous (log2) | 203 | 0.69 (0.52–0.92) | 103 | 0.60 (0.41–0.90) | 221 | 1.03 (0.71–1.50) | |||
|
| |||||||||
| Bc | |||||||||
|
| |||||||||
| Tertile 1 | <126.4 | 80 | 1 (reference) | <133.2 | 46 | 1 (reference) | <135.8 | 74 | 1 (reference) |
|
| |||||||||
| Tertile 2 | 126.4–175.5 | 71 | 0.79 (0.51–1.22) | 133.2–183.7 | 33 | 0.55 (0.31–0.99) | 135.8–186.8 | 76 | 1.08 (0.63–1.84) |
|
| |||||||||
| Tertile 3 | >175.5 | 52 | 0.53 (0.34–0.85) | >183.7 | 24 | 0.52 (0.28–0.96) | >186.8 | 71 | 0.95 (0.56–1.62) |
|
| |||||||||
| P-trendb | .008 | .03 | .84 | ||||||
|
| |||||||||
| Continuous (log2) | 203 | 0.71 (0.51–0.97) | 103 | 0.65 (0.42–0.99) | 221 | 1.11 (0.73–1.67) | |||
OR = odds ratio; CI = confidence interval; GAE = gallic acid equivalents.
Substantial cognitive decline was defined as ≥3 points on the Mini-Mental State Examination (MMSE) from baseline to 3 years later and worst 10% of the distribution of subtracting baseline from 3-year follow-up scores in seconds or test discontinued at follow-up for the Trail-Making Test Parts A (TMT-A) and B (TMT-B).
Adjusted for baseline cognitive score only.
Obtained by assigning the median of each tertile as scores.
Adjusted for baseline cognitive score, sex, age, education, body mass index, total energy intake, physical activity, total cholesterol, impaired renal function, smoking status, congestive heart failure, cancer, stroke, diabetes mellitus, and depressive symptoms.
In multivariable logistic models adjusted for all confounders, no statistically significant associations were found between TDP and substantial cognitive decline risk using the MMSE (highest vs lowest tertile OR = 1.08, 95% CI = 0.65–1.78, P-trend = .84), the TMT-A (OR = 0.63, 95% CI = 0.32–1.25, P-trend = .17), or the TMT-B (OR = 1.16, 95% CI = 0.63–2.14, P-trend = .77) (Table S1, Model B). No significant interactions were found between MMSE, TMT-A or TMT-B and sex, age, BMI, education, and smoking status.
Sensitivity analyses were performed by excluding from the analysis participants in the top or the bottom 1% of the distribution of the ratio of total energy intake for TDP. The ORs for the sensitivity analyses were nearly identical to the results based on the whole cohort (data not shown).
DISCUSSION
In this population-based study of older adults without dementia, high concentrations of TUPs were associated with an approximately 47% lower risk of substantial cognitive decline in global cognitive function (tested using the MMSE) and an approximately 48% lower risk of substantial cognitive decline in attention (measured using the TMT-A) over a 3-year period but not with the TMT-B, which is mainly focused on measuring executive function. No significant association was found between TDP and any cognitive test, as was also observed with all-cause mortality in a previous InCHIANTI study.11 This may be due to the difficulty in assessing TDP intake. Although TUP is a part of polyphenol intake, it also accounts for the bioavailability of polyphenols16 and may therefore be a more-precise measure of true exposure because of the large variability between and within polyphenol absorption and metabolism.17
In a cross-sectional study, total polyphenols expressed according to urinary creatinine normalization were linearly associated with better scores in immediate verbal memory in older adults at high cardiovascular risk.20 In the middle-aged Supplémentation en Vitamines et Minéraux Antioxydants (SU.VI.MAX) cohort, high total polyphenol intake, estimated using six repeated 24-hour dietary recalls, was associated with better language and verbal memory after a follow-up of 13 years.14 Similar to the current associations between TUP or TDP and TMT-B, no significant associations were observed between TDP and executive functioning in the SU.VI.MAX study.14 Furthermore, in other prospective studies, the intake of flavonoids was associated with better cognitive evolution in dementia-free older adults13 and a lower risk of Alzheimer’s disease, the most common form of dementia.37,38 In another prospective study, consumption of fruit and vegetables, and thus antioxidant nutrients (vitamins E and C), was associated with better verbal memory.39
In the present study, TUP levels, but not TDP intake, were inversely associated with cognitive decline over 3 years. Similar findings were observed with TUP levels in the cross-sectional analysis of the PREvención con DIeta MEDiterránea study.20 As far as the authors of the current study know, no cross-sectional studies have focused on the relationship between TDP and cognitive function. In a small subsample of postmenopausal women from the Providing Regional Observations to Study Predictors of Events in the Coronary Tree study, which is one of the Dutch cohorts included in the EPIC study, higher intake of lignans, but not isoflavones, was associated with higher MMSE scores at enrollment,40 whereas in the Lothian Birth Cohort 1936, flavonoid intake was not associated with any of the cognitive tests performed after adjusting for confounding factors at the age of 70.41
The potential mechanisms of the protective effects of polyphenols on cognitive function may be linked to their antioxidant and anti-inflammatory properties. Polyphenols reduce neuronal damage and death from oxidative reactions by inhibiting the generation of reactive oxygen species, lipid peroxidation, apoptosis, protein oxidation, metal chelation, and damage to cellular signaling.15,42–46 The direct interactions within ERK and PI3-kinase/Akt signaling pathways, which have been associated with the maintenance of the number and quality of synaptic connections in critical brain regions, may mediate potential actions of polyphenols. 47 Additional mechanisms are related to the inhibition of neuronal apoptosis activated by neurotoxic species or the disruption of amyloid β aggregation and effects on amyloid precursor protein processing through the inhibition of β-secretase or activation of α-secretase.47
This study has several strengths. First, to the best of the knowledge of the authors, this is the first prospective study to investigate the association between total polyphenols and substantial cognitive decline in an older population. Moreover, urinary polyphenols expressed according to 24-hour volume are an objective biomarker of total phenolic intake18 and a proxy biomarker of fruit and vegetable consumption.19 The main advantage of a nutritional biomarker over a dietary biomarker is that it is a more-precise and more-proximal measure than dietary assessment. 48 Currently, the adapted F-C assay in urine samples is considered a valid biomarker for total polyphenol intake and is a rapid, economic, and easy method to apply to large-scale epidemiological studies. TUP expressed as 24-hour volume is considered the criterion standard for assessing urinary excretion.18 Cognitive function was assessed using the MMSE (the most widely used instrument for measuring the course of cognitive change in older adults over time28,29) and the TMT-A and TMT-B, which are also commonly used in the assessment of psychomotor speed, visuospatial scanning, and executive ability.30,31 Finally, the logistic regression models were adjusted for the most-important confounding variables related to cognitive decline, such as sociodemographic characteristics, health behaviors, and chronic diseases.
Nevertheless, this study has some limitations. First, InCHIANTI was performed in community-dwelling older subjects living in two sites in Tuscany (Italy), so the sample might not be representative of the general Italian population. Second, cognitive decline is a heterogeneous condition, and its underlying cause was not assessed. Third, measurement errors in the dietary questionnaires may have influenced the results. Furthermore, the present study population was aged 65 and older and therefore may be less reliable in recalling food intake than younger subjects, although the food frequency dietary questionnaire was country specific and previously validated for some polyphenol-rich foods, such as fruits, vegetables, tea, coffee, and wine, in a similar population;22 moreover, participants with dementia syndrome at baseline were excluded. In addition, dietary consumption of polyphenols may be underestimated, because the food composition tables for polyphenols were not totally completed, although an extensive common database was used for the current study.11,18
In conclusion, in older adults without dementia, higher TUP concentrations were associated with lower risk of substantial cognitive decline over a 3-year period after adjusting for potential confounders. No significant association was found using TDPs. These findings suggest a protective effect of total polyphenols and, indirectly, of diets rich in polyphenols, against cognitive decline in older adults. Further epidemiological studies and clinical trials are warranted to clarify the potential preventive role of polyphenols and their underlying mechanisms. The identification of factors that reduce or delay cognitive decline is essential to improve the autonomy and quality of life of older people.
Supplementary Material
Acknowledgments
Conflict of Interest: None of the authors report any conflicts of interest. All authors had access to the data and a role in writing the manuscript.
The InCHIANTI study is supported in part by the Italian Ministry of Health and by the U.S. National Institute on Aging (NIA). The authors are grateful for the support granted by Spanish government grants from the Ministry of Economy and Competitiveness (MINECO) and cofunded by the Fondo Europeo de Desarrollo Regional: the CONSOLIDER-INGENIO 2010 program, FUN-C-Food (CSD2007–063), and the JPI HDHL FOODBALL (PCIN-2014-133). We are also grateful for the award of 2014SGR1566 from the Generalitat de Catalunya’s Agency AGAUR. Partially funded by the International Nut and Dried Fruit Council Foundation in collaboration with the Bosch I Gimpera Foundation (FBG307906). M.R. thanks the training abroad MAPFRE Grant. A.C. thanks the PIE-BKC, Campus of International Excellence program of the Spanish Ministry of Education, Culture and Sport. MU-S. thanks the Ramón y Cajal program (RYC-2011–09677) from MINECO and the Fondo Social Europeo.
Author Contributions: Rabassa, Cherubini, Andres-Lacueva: research design. Rabassa, Urpi-Sarda, Andres-Lacueva: samples analyses. Rabassa, Zamora-Ros: statistical analysis. Rabassa: drafting the manuscript. Cherubini, Zamora-Ros, Andres-Lacueva: critical revision. Bandinelli, Ferrucci: study concept, coordination. All authors read and approved the final manuscript.
Sponsor’s Role: None.
Footnotes
Additional Supporting Information may be found in the online version of this article:
Table S1. Logistic regression models describing the association between total dietary polyphenols (TDP) tertiles and 3-year substantial cognitive decline in older subjects.
Please note: Wiley-Blackwell is not responsible for the content, accuracy, errors, or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Albert MS. Changes in cognition. Neurobiol Aging. 2011;32(Suppl 1):S58–S63. doi: 10.1016/j.neurobiolaging.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillette Guyonnet S, Abellan Van Kan G, Andrieu S, et al. IANA task force on nutrition and cognitive decline with aging. J Nutr Health Aging. 2007;11:132–152. [PubMed] [Google Scholar]
- 4.Daviglus ML, Bell CC, Berrettini W, et al. National Institutes of Health State-of-the-Science Conference statement: Preventing Alzheimer disease and cognitive decline. Ann Intern Med. 2010;153:176–181. doi: 10.7326/0003-4819-153-3-201008030-00260. [DOI] [PubMed] [Google Scholar]
- 5.Albarracin SL, Stab B, Casas Z, et al. Effects of natural antioxidants in neurodegenerative disease. Nutr Neurosci. 2012;15:1–9. doi: 10.1179/1476830511Y.0000000028. [DOI] [PubMed] [Google Scholar]
- 6.Cassidy A, Mukamal KJ, Liu L, et al. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation. 2013;127:188–196. doi: 10.1161/CIRCULATIONAHA.112.122408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Dam RM, Naidoo N, Landberg R. Dietary flavonoids and the development of type 2 diabetes and cardiovascular diseases: Review of recent findings. Curr Opin Lipidol. 2013;24:25–33. doi: 10.1097/MOL.0b013e32835bcdff. [DOI] [PubMed] [Google Scholar]
- 8.Geybels MS, Verhage BAJ, Arts IC, et al. Dietary flavonoid intake, black tea consumption, and risk of overall and advanced stage prostate cancer. Am J Epidemiol. 2013;177:1388–1398. doi: 10.1093/aje/kws419. [DOI] [PubMed] [Google Scholar]
- 9.Zamora-Ros R, Jimenez C, Cleries R, et al. Dietary flavonoid and lignan intake and mortality in a Spanish cohort. Epidemiology. 2013;24:726–733. doi: 10.1097/EDE.0b013e31829d5902. [DOI] [PubMed] [Google Scholar]
- 10.Zamora-Ros R, Forouhi NG, Sharp SJ, et al. The association between dietary flavonoid and lignan intakes and incident type 2 diabetes in European populations: The EPIC-InterAct Study. Diabetes Care. 2013;36:3961–3970. doi: 10.2337/dc13-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zamora-Ros R, Rabassa M, Cherubini A, et al. High concentrations of a urinary biomarker of polyphenol intake are associated with decreased mortality in older adults. J Nutr. 2013;143:1445–1450. doi: 10.3945/jn.113.177121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez-Jimenez J, Fezeu L, Touvier M, et al. Dietary intake of 337 polyphenols in French adults. Am J Clin Nutr. 2011;93:1220–1228. doi: 10.3945/ajcn.110.007096. [DOI] [PubMed] [Google Scholar]
- 13.Letenneur L, Proust-Lima C, Le Gouge A, et al. Flavonoid intake and cognitive decline over a 10-year period. Am J Epidemiol. 2007;165:1364–1371. doi: 10.1093/aje/kwm036. [DOI] [PubMed] [Google Scholar]
- 14.Kesse-Guyot E, Fezeu L, Andreeva VA, et al. Total and specific polyphenol intakes in midlife are associated with cognitive function measured 13 years later. J Nutr. 2012;142:76–83. doi: 10.3945/jn.111.144428. [DOI] [PubMed] [Google Scholar]
- 15.Vauzour D. Dietary polyphenols as modulators of brain functions: Biological actions and molecular mechanisms underpinning their beneficial effects. Oxid Med Cell Longev. 2012;2012:914273. doi: 10.1155/2012/914273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landete JM. Updated knowledge about polyphenols: Functions, bioavailability, metabolism, and health. Crit Rev Food Sci. 2012;52:936–948. doi: 10.1080/10408398.2010.513779. [DOI] [PubMed] [Google Scholar]
- 17.Zamora-Ros R, Rabassa M, Llorach R, et al. Application of dietary phenolic biomarkers in epidemiology: Past, present, and future. J Agric Food Chem. 2012;60:6648–6657. doi: 10.1021/jf204742e. [DOI] [PubMed] [Google Scholar]
- 18.Zamora-Ros R, Rabassa M, Cherubini A, et al. Comparison of 24-h volume and creatinine-corrected total urinary polyphenol as a biomarker of total dietary polyphenols in the Invecchiare InCHIANTI study. Anal Chim Acta. 2011;704:110–115. doi: 10.1016/j.aca.2011.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medina-Remon A, Barrionuevo-Gonzalez A, Zamora-Ros R, et al. Rapid Folin-Ciocalteu method using microtiter 96-well plate cartridges for solid phase extraction to assess urinary total phenolic compounds, as a biomarker of total polyphenols intake. Anal Chim Acta. 2009;634:54–60. doi: 10.1016/j.aca.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Valls-Pedret C, Lamuela-Raventos RM, Medina-Remon A, et al. Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. J Alzheimers Dis. 2012;29:773–782. doi: 10.3233/JAD-2012-111799. [DOI] [PubMed] [Google Scholar]
- 21.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: Bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 22.Pisani P, Faggiano F, Krogh V, et al. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol. 1997;26(Suppl 1):S152–S160. doi: 10.1093/ije/26.suppl_1.s152. [DOI] [PubMed] [Google Scholar]
- 23.Salvini S. A food composition database for epidemiological studies in Italy. Cancer Lett. 1997;114:299–300. doi: 10.1016/s0304-3835(97)04686-7. [DOI] [PubMed] [Google Scholar]
- 24.U.S. Department of Agriculture, Agricultural Research Service. USDA database for the flavonoid content of selected foods, release 3. Nutrient Data Laboratory; 2011. [Google Scholar]
- 25.U.S. Department of Agriculture, Agricultural Research Service. USDA database for the isoflavone content of selected foods, release 2.0. Nutrient Data Laboratory; 2008. [Google Scholar]
- 26.U.S. Department of Agriculture, Agricultural Research Service. USDA database for the proanthocyanidin content of selected foods. Nutrient Data Laboratory; 2004. [Google Scholar]
- 27.Neveu V, Perez-Jimenez J, Vos F, et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database (Oxford) 2010;2010:bap024. doi: 10.1093/database/bap024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell AJ. A meta-analysis of the accuracy of the Mini-Mental State Examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res. 2009;43:411–431. doi: 10.1016/j.jpsychires.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’ A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 30.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 31.Reitan RM. Trail Making Test: Manual for Administration and Scoring. Tuscon, AZ: Reitan Neuropsychology Laboratory; 1992. [Google Scholar]
- 32.Llewellyn DJ, Lang IA, Langa KM, et al. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med. 2010;170:1135–1141. doi: 10.1001/archinternmed.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: Classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 35.Radloff LS. The Center for Epidemiologic Studies Depression Scale: A self report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 36.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 37.Engelhart MJ, Geerlings MI, Ruitenberg A, et al. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA. 2002;287:3223–3229. doi: 10.1001/jama.287.24.3223. [DOI] [PubMed] [Google Scholar]
- 38.Beking K, Vieira A. Flavonoid intake and disability-adjusted life years due to Alzheimer’s and related dementias: A population-based study involving twenty-three developed countries. Public Health Nutr. 2010;13:1403–1409. doi: 10.1017/S1368980009992990. [DOI] [PubMed] [Google Scholar]
- 39.Peneau S, Galan P, Jeandel C, et al. Fruit and vegetable intake and cognitive function in the SU.VI.MAX 2 prospective study. Am J Clin Nutr. 2011;94:1295–1303. doi: 10.3945/ajcn.111.014712. [DOI] [PubMed] [Google Scholar]
- 40.Franco OH, Burger H, Lebrun CEI, et al. Higher dietary intake of lignans is associated with better cognitive performance in postmenopausal women. J Nutr. 2005;135:1190–1195. doi: 10.1093/jn/135.5.1190. [DOI] [PubMed] [Google Scholar]
- 41.Butchart C, Kyle J, McNeill G, et al. Flavonoid intake in relation to cognitive function in later life in the Lothian Birth Cohort 1936. Br J Nutr. 2011;106:141–148. doi: 10.1017/S0007114510005738. [DOI] [PubMed] [Google Scholar]
- 42.Rossi L, Mazzitelli S, Arciello M, et al. Benefits from dietary polyphenols for brain aging and Alzheimer’s disease. Neurochem Res. 2008;33:2390–2400. doi: 10.1007/s11064-008-9696-7. [DOI] [PubMed] [Google Scholar]
- 43.Joseph J, Cole G, Head E, et al. Nutrition, brain aging, and neurodegeneration. J Neurosci. 2009;29:12795–12801. doi: 10.1523/JNEUROSCI.3520-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gonzalez-Gallego J, Garcia-Mediavilla MV, Sanchez-Campos S, et al. Fruit polyphenols, immunity and inflammation. Br JNutr. 2010;104(Suppl 3):S15–S27. doi: 10.1017/S0007114510003910. [DOI] [PubMed] [Google Scholar]
- 45.Spencer JP, Vafeiadou K, Williams RJ, et al. Neuroinflammation: Modulation by flavonoids and mechanisms of action. Mol Aspects Med. 2012;33:83–97. doi: 10.1016/j.mam.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 46.Schaffer S, Asseburg H, Kuntz S, et al. Effects of polyphenols on brain ageing and Alzheimer’s disease: Focus on mitochondria. Mol Neurobiol. 2012;46:161–178. doi: 10.1007/s12035-012-8282-9. [DOI] [PubMed] [Google Scholar]
- 47.Williams RJ, Spencer JP. Flavonoids, cognition, and dementia: Actions, mechanisms, and potential therapeutic utility for Alzheimer disease. Free Radical Biol Med. 2012;52:35–45. doi: 10.1016/j.freeradbiomed.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 48.Jenab M, Slimani N, Bictash M, et al. Biomarkers in nutritional epidemiology: Applications, needs and new horizons. Hum Genet. 2009;125:507–525. doi: 10.1007/s00439-009-0662-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.