Abstract
Decreases in circulating hsCRP have been associated with increased physical activity and exercise training, although the ability of exercise interventions to reduce hsCRP and which individuals benefit the most remains unclear. This meta-analysis evaluates the ability of exercise to reduce hsCRP levels in healthy individuals and in individuals with heart disease. A systematic review and meta-analysis was conducted that included exercise interventions trials from 1995 to 2012. Forty-three studies were included in the final analysis for a total of 3575 participants. Exercise interventions significantly reduced hsCRP (standardized mean difference −0.53 mg/L; 95% CI, −0.74 to −0.33). Results of sub-analysis revealed no significant difference in reductions in hsCRP between healthy adults and those with heart disease (p = .20). Heterogeneity between studies could not be attributed to age, gender, intervention length, intervention type, or inclusion of diet modification. Exercise interventions reduced hsCRP levels in adults irrespective of the presence of heart disease.
Keywords: Coronary disease, Exercise, Heart disease, Inflammation, Biological marker c-reactive protein
Introduction
Circulating levels of high-sensitivity c-reactive protein (hsCRP) is a modifiable risk factor for cardiovascular disease (CVD).1,2 hsCRP was found to be an independent predictor of CVD in a cohort study of 15,792 adults, ages 45–64 years, designed to identify whether hsCRP levels in middle-aged adults was associated with future risk of CVD.3 The authors went on to state that hsCRP may be a good early predictor of CVD even when other traditional risk factors, such as high levels of low-density lipoprotein C (LDL-C), are not present. In another cohort of 3345 German men, the authors found that hsCRP levels were associated with risk of CVD independent of the Framington Risk Score (FRS), and enhanced the prognostic value of the FRS in persons with intermediate risk for CVD.4
Decreases in circulating hsCRP have been associated with lifestyle changes including changes in diet5–8 as well as increased physical activity9–14 and exercise training.15–20 Although accumulating evidence supports the clinical use of hsCRP measurements in healthy individuals to direct preventative treatment regimens, questions remain as to whether exercise interventions reliably reduce hsCRP and which individuals are most likely benefit from programs aimed at reducing circulating levels of hsCRP. Epidemiological studies in patients diagnosed with heart disease as well as in healthy individuals have shown that increased physical activity is associated with decreased levels of hsCRP as well as lower risk of heart disease14,21–28; but, these studies are retrospective in nature and depend on self-report measures of physical activity. Controlled studies in patients with diagnosed heart disease have shown that exercise training is associated with reduced circulating hsCRP29; yet, results in healthy people have been inconclusive.20,30–34 The most recent meta-analysis suggested that hsCRP is not lowered in healthy adults enrolled in an aerobic exercise program (M ± SEM = −0.11 ± 0.14 mg/L, 95% CI: −0.39 to 0.17 mg/L), but at that time only 5 clinical trials met the inclusion criteria.30 A recent meta-analysis in persons diagnosed with heart disease revealed that an exercise intervention is associated with lower hsCRP levels (Standardized Mean Difference (SDM) = −0.345, 95% CI: −0.444 to −0.246); but the meta-analysis included studies that were pre/post-design with no control group.29
There are several explanations for the inconsistent results observed in studies examining the potential of exercise to lower hsCRP. Gender differences in hsCRP levels have been observed, with higher hsCRP levels being associated with intima-media-thickness, a measure of early artherosclerosis, in women but not in men.35 Additionally, decreases in hsCRP level due to an exercise intervention have been observed in obese individuals with glucose intolerance in the absence of cardiovascular disease.36 There is research suggesting that increasing fitness is not associated with lowered hsCRP, but lowered hsCRP following exercise intervention can be explained by resultant reductions in weight.37 Finally, baseline hsCRP may be elevated in patients who have recently experienced a cardiac event,38 and post exercise levels of hsCRP may be elevated in the period directly following intense exercise.39
The primary goals of this meta-analysis were to determine if exercise reduces hsCRP in healthy adults and in individuals with heart disease, and to determine if the reduction is significantly different between groups. A secondary goal of this study was to determine factors that may lead to variance in the intervention effect not explained by chance (i.e., study heterogeneity). The following factors were reviewed: age, gender, intervention duration, intervention type, inclusion of diet in the intervention, timing of blood draws, and presence of risks associated with metabolic syndrome.
Methods
Data sources
Studies for this systematic review and meta-analysis were retrieved through computerized literature searches of PubMED and The Cochrane Central Register of Controlled Trials as well as cross-referencing of review articles and retrieved studies. Keywords used in the search included exercise, physical activity, c-reactive protein, coronary heart disease, cardiovascular disease, inflammation, clinical trials, and adults. The study results were reported using the PRISMA framework, with the exception of the abstract.
Inclusion criteria
Studies performed between the years of 1995 and 2012 were included in the systematic review and meta-analysis if they met the following criteria: 1) randomized and non-randomized trials; 2) exercise intervention ≥4 weeks but ≤3 years; 3) assessment of hsCRP at baseline and following the last exercise session; 4) human subjects >18 years of age; 5) inclusion of control group that did not receive an exercise intervention; 6) studies of healthy adults and/or adults with ischemic heart disease and heart failure without other significant disease processes; and 7) English language studies published in scientific journals. The earliest search date was set at 1995 due to the availability of reliable assays to assess hsCRP levels in blood serum.40 The time frame for the exercise interventions was chosen based on similar inclusion criteria used in a prior meta-analysis of randomized trials in healthy adults.30 Study selection did not include articles in foreign-languages due to concerns regarding translation of results, and it was decided to only include scientific journal articles to ensure inclusion of quality studies.
Data abstraction
An electronic spreadsheet was created to record data from the studies reviewed. Categories that were coded included study characteristics (e.g., source and date), subject characteristics (e.g., age, gender, and health status), exercise training program characteristics (e.g., duration and frequency), change in hsCRP (mg/L), and hsCRP assessment procedure (e.g., time before and after exercise). Subject health status data included abstraction of indicators of metabolic syndrome in accordance with International Diabetes Foundation’s (IDF) consensus statement on metabolic syndrome,41 which defines metabolic syndrome as having central obesity (BMI ≥ 30 kg/m2) and at least two of the following: 1) raised tri-glycerides (≥150 mg/dL or treatment for such), 2) reduced high-density lipoprotein (HDL) cholesterol (<40 mg/dL in males, <50 mg/dL in females, or treatment for such), 3) high blood pressure (systolic ≥130, diastolic ≥85, or treatment for such), and 4) raised fasting glucose (≥100 mg/dL or diagnosis of type II diabetes). A literature review was conducted by the research assistant. Data were abstracted and eligibility was determined by two reviewers. Discrepancies in the data were resolved by consensus.
Statistical analysis
For each study included in the meta-analysis, the mean difference in hsCRP was calculated by subtracting the change difference in the control group from the change difference in the intervention group. A random effects model was used to estimate the standard mean difference in change from baseline for hsCRP. The random effects model was chosen because of its ability to statistically control for heterogeneity as well as to provide for wider 95% confidence intervals (CI) than the fixed-effects model when significant heterogeneity is expected. Ninety-five percent CI were used to establish statistical significance of the results. If the results did not cross zero, they were considered to be significant. Heterogeneity was also examined using the I2-statistic, which is a measure of intervention effect due to known differences in study design.42
Analyses of interaction effects (moderators) for several a priori explanatory variables were conducted. The interaction effects examined included age, gender, duration of exercise intervention, and type of exercise intervention. A subgroup analysis to determine difference in effect for healthy adults versus adults with cardiac disease was also conducted. A one-way, random effects ANOVA model was used to estimate the standard error and variance for each group and to test whether these means were different between groups. It was assumed that the variance among each group was different. Prior to performing the analysis, the moderators were categorized. Interaction effects due to presence of factors related to metabolic syndrome and timing of blood draws was not conducted due to insufficient information within the individual studies.
The quality of the studies was assessed using a previously developed 5-point scale that has been shown to be both reliable and valid.43 The scale ranges from 0 to 5 with higher scores representing greater study quality.
In cases where the standard deviation for the change in hsCRP was not reported, it was imputed using the following formula44:
where “Corr” is the correlation coefficient between the standard deviations for the baseline and the standard deviations for the final measures of hsCRP in both the experimental and control groups derived from studies with known standard deviation for change. Corr for both the experimental and control groups were calculated as follows:
The correlation coefficients were averaged to obtain a Corr equal to 0.80. The averaged Corr was included in the equation to impute the standard deviation for hsCRP.
Descriptive statistics were presented as mean ± SD. Primary and secondary outcomes were presented as standardized mean difference (SMD) with 95% CI as well as mean difference (MD) with 95% CI. Hedge’s g statistic was used as the standardized mean difference effect size since it accounts for variance in size of the studies by pooling weighted standard deviations.45
The Egger regression approach was used to examine the sensitivity of changed to hsCRP due to publication bias.46 An α level of <0.05 was used to determine if significant publication bias existed.
All analyses were conducted using Review Manager 5.1 (The Cochrane Corporation, Copenhagen, Denmark).
Results
Study characteristics
Healthy adults
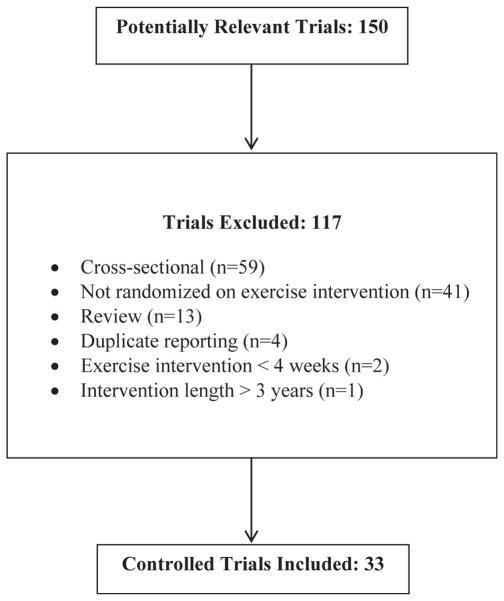
Characteristics of the included studies can be found in Table 1. Of the 150 studies reviewed, 33 studies were included in the final analysis. A description of the review process, including reasons for exclusion, is located in Fig. 1. Nineteen of the studies were performed in the United States, 3 each in Korea and the United Kingdom, 2 each in Canada and Iran, and 1 each in Australia, Brazil, New Zealand, and Portugal. The results for a total of 3575 participants (1918 exercisers and 1657 controls) were pooled and analyzed. Total study size ranged from a low of 14 participants to a high of 349 participants. Mean study quality was 3 out of a possible 5 points (Range = 0–5). Results of the Egger regression test indicated absence of publication bias (p = 0.26).
Table 1.
Characteristics for studies including healthy individuals.
| Reference | Total N Males/females | Age Mean (SD) | Participant characteristics | Intervention type | Intervention length | Significance Control vs. exercise |
|---|---|---|---|---|---|---|
| Arikawa et al. 201147 | 319 0/319 |
25.2 (3.5) | Pre-menopausal, sedentary females | Aerobic | 16-weeks | p = 0.04 |
| Arsenault et al. 200948 | 267 0/267 |
57.3 (6.6) | Post-menopausal, overweight, sedentary females | Aerobic | 6-months | NS |
| Bijeh et al. 201249 | 19 0/19 |
42.3 (3.3) | Sedentary females | Aerobic | 6-months | NS |
| Camhi et al. 201050 | 278 149/125 |
52.9 (6.6) | Dyslipidemic, sedentary males and post-menopausal females | Aerobic | 12-months | p = 0.02 in women with metabolic syndrome; NS for men and women w/out metabolic syndrome |
| Campbell et al. (a) 200851 | 202 102/100 |
55.2 (6.7) | Sedentary males and females | Aerobic | 12-months | NS |
| Campbell et al. (b) 200952 | 115 0/115 |
60.7 (6.9) | Post-menopausal, sedentary females | Aerobic | 3- and 12-months | p = 0.01 |
| Church et al. 201053 | 137 19/118 |
49.7 (10.9) | Sedentary males and females with hsCRP >2 mg/dL and <10 mg/dL | Aerobic | 4-months | NS |
| Donges et al. 201054 | 102 45/57 |
NR | Sedentary males and females | Aerobic or resistance | 10-weeks | p ≤ 0.05 |
| Fontana et al. 200755 | 46 17/29 |
56.7 (2.9) | Sedentary males and females who received healthy living literature | NR | 12-months | p = 0.02 for diet + exercise intervention; NS for exercise only intervention |
| Friedenreich et al. 201156 | 320 0/320 |
60.9 (5.6) | Post-menopausal, sedentary females | Aerobic | 6- and 12-months | p = 0.005 |
| Gray et al. 200957 | 48 11/37 |
49.7 (8.8) | Sedentary males and females | Aerobic | 12-weeks | NS |
| Hammett et al. 200458 | 61 27/34 |
67.0 (5.0) | Sedentary males and post-menopausal females | Aerobic | 6-months | NS |
| Hewitt et al. 200859 | 20 NR |
41.4 (8.0) | Sedentary males and females | Aerobic | 12-weeks | NS |
| Huffman et al. 200660 | 193 104/89 |
52.8 (6.4) | Overweight, sedentary males and post-menopausal females | Aerobic | 6-months | NS |
| Imayama et al. 201261 | 320 0/320 |
57.9 (4.6) | Sedentary females | Aerobic | 12-months | p ≤ 0.001 |
| Jae et al. 200662 | 47 35/12 |
49.7 (6.9) | Sedentary males and females who received diet literature at first visit | Aerobic | 3-months | p < 0.05 |
| Johanssen et al. 201263 | 339 0/339 |
57.3 (6.4) | Hypertensive, post-menopausal, overweight, sedentary females | Aerobic | 6-months | NS |
| Lee et al. 201264 | 22 NR |
40.5 (4.6) | Pre-menopausal, overweight, sedentary females | Aerobic | 14-weeks | NS |
| Libardi et al. 201142 | 36 36/0 |
48.7 (5.3) | Sedentary males | Aerobic and resistance or resistance only | 16-weeks | NS |
| Marcell et al. 200565 | 51 20/31 |
45.3 (8.3) | Sedentary males and females | Aerobic | 16-weeks | NS |
| Martins et al. 201066 | 63 25/38 |
76.0 (8.0) | Sedentary males and females | Aerobic or resistance | 16-weeks | p < 0.05 |
| Mason et al. 201267 | 294 0/294 |
58.0 (5.2) | Sedentary, post-menopausal females | Aerobic | 12-months | NS |
| Murphy et al. 200668 | 20 NR |
41.5 (9.3) | Sedentary males and females | Aerobic | 8-weeks | NS |
| Nicklas et al. (a) 200469 | 201 NR |
69.0 (6.0) | Elderly, overweight, sedentary males and females | Aerobic and resistance | 18-months | p = 0.01 |
| Nicklas et al. (b) 200870 | 369 118/251 |
76.6 (4.3) | Elderly, sedentary males and females enrolled in a healthy living class 1× per month | Aerobic, strength, balance, and flexibility | 12-months | NS |
| Oh et al. 201171 | 52 0/52 |
63.4 (8.5) | Sedentary females with metabolic syndrome | Aerobic and strength | 6-months | p = 0.029 |
| Phillips et al. 201272 | 23 0/23 |
65.6 (2.6) | Obese, sedentary females attending a health and stretching class 1× per week | Resistance | 12-weeks | p < 0.05 |
| Pil-Byung et al. 201173 | 30 30/0 |
23.5 (0.4) | Sedentary males and females who watched a healthy video | Aerobic | 8-weeks | p < 0.05 |
| Stewart et al. (a) 200774 | 60 30/30 |
N/aa | Physically active males and females stratified by age | Aerobic and resistance | 12-weeks | p < 0.01 |
| Stewart et al. (b) 201037 | 421 0/421 |
57.3 (6.4) | Hypertensive, post-menopausal, overweight, sedentary females | Aerobic | 6-months | NS |
| Stoutenberg et al. 201275 | 43 43/0 |
NR | Sedentary males | Aerobic | 12- and16-weeks | NS |
| Vatani et al. 201176 | 30 30/0 |
20.5 (1.1) | Sedentary males | Resistance | 6-weeks | p ≤ 0.05 |
| Villareal et al. 200677 | 27 9/18 |
69.7 (4.6) | Elderly, obese, sedentary males and females | Aerobic, strength, balance and flexibility | 6-months | p < 0.01 |
NS – not significant; NR – not reported.
Age of young participants 25.0 ± 4.9 years and age of older participants 71.0 ± 4.0 years.
Fig. 1.
Flow diagram. Flow diagram for the selection for the selection of studies performed in healthy adults.
Adults with CHD
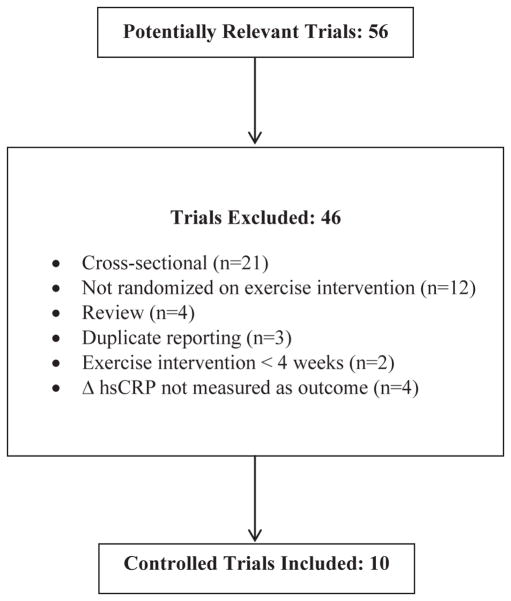
A description of the studies included for review is contained in Table 2. Out of the 56 studies found, 10 studies were included in the final analysis. A description of the review process, including reasons for exclusion, is located in Fig. 2. One trial was performed in each of the following countries: United States, Sweden, Italy, Serbia, Portugal, and China. Two studies came from each of the following countries: Norway and Germany. The results for a total of 827 participants (510 exercisers and 317 controls) were pooled and analyzed. Total study size ranged from 22 participants to 277 participants. Mean study quality was 2 out of a possible 5 points (Range = 0–5). Results of the Egger regression test indicated absence of publication bias (p = 0.86).
Table 2.
Characteristics for studies in individuals with heart disease.
| Reference | Total N Males/females | Age Mean (SD) | Participant characteristics | Intervention type | Intervention length | Significance Control vs. exercise |
|---|---|---|---|---|---|---|
| Astengo et al. 200978 | 56 45/11 |
63.0 (7.8) | Males and females w/stable angina and scheduled for PCI due to advanced CAD | Aerobic and resistance | 8-months | NS |
| Luk et al. 201279 | 64 48/16 |
67.2 (8.5) | Males and females with stable CAD | Aerobic | 8-weeks | NS |
| Milani et al. 200480 | 277 202/75 |
66.3 (11.0) | Males and females post AMI, CABG, or PCI | Aerobic | 12-weeks | p = 0.002 |
| Munk et al. 200981 | 40 33/7 |
59.0 (12.0) | Males and females post PCI w/out prior MI or CABG | Aerobic | 6-months | p = 0.03 |
| Parrinello et al. 200882 | 22 15/7 |
62.7 (4.8) | Sedentary males and females with NYHA class II or III congestive heart failure | Aerobic | 10-weeks | p < 0.05 |
| Rankovic et al. 200983 | 52 29/23 |
60.2 (7.4) | Males and females w/stable CAD | Aerobic | 6-weeks | p < 0.01 |
| Ribeiro et al. 201184 | 38 31/7 |
55.6 (9.3) | Males and females w/CAD following a 1st AMI | Aerobic | 8-weeks | NS |
| Schumacher et al. 200638 | 197 162/35 |
55.0 (8.0) | Males and females w/CAD following an AMI treated w/PCI and/or CABG | Aerobic | 6-months | NS |
| Sixt et al. 200885 | 23 17/6 |
64.0 (6.0) | Males and females w/CAD and impaired glucose tolerance | Aerobic | 4-weeks | |
| Walther et al. 200886 | 66 0/66 |
NR | Males w/stable CAD and eligible for PCI due to a 75% blockage | Aerobic | 24-months | p = 0.03 |
NS – no significance; NR – not reported; CAD – coronary artery disease; PCI – percutaneous intervention; AMI – acute myocardial infarction; CABG – coronary artery bypass graft; NYHA – New York Heart Association.
Fig. 2.
Flow diagram. Flow diagram for the selection of studies performed in persons with heart disease.
Subjects
Healthy adults
Twelve studies were limited to women only, whereas results for men only were reported in 4 studies. The remaining 17 studies evaluated results for both males and females, although two of these studies did a breakdown of the results by gender. Thirteen studies reported that the women were postmenopausal, and two studies specified that the women were premenopausal. Women using hormone replacement therapy were excluded in 8 studies.
One study reported the use of physically active individuals as the control group, whereas the remaining studies indicated that all participants were sedentary. Being overweight (body mass index [BMI] ≥ 25 kg/m2) was an inclusion criterion in seven studies, with two studies including only obese participants (BMI ≥ 30 kg/m2). Participants with hypertension were studied exclusively in 4 studies, whereas another 4 studies excluded hypertensive participants. Two of the studies examined participants with dyslipidemia and one study each examined participants with diabetes mellitus (Type 2) and metabolic syndrome. One study limited participation to subjects with baseline hsCRP levels between 2 mg/L and 10 mg/L. Participants with a history of metabolic disorders were excluded from 13 studies, and a history of smoking in the prior 12-months were excluded from 9 studies.
Adults with CHD
One study reported results for men only, whereas the remaining 9 studies included results for both men and women. Inclusion for 8 of the studies required a diagnosis of stable coronary artery disease (CAD), with 4 of the 8 studies requiring participants to be post-acute myocardial infarction (AMI), percutaneous intervention (PCI), and/or coronary artery bypass graft (CABG) surgery. One of the six studies enrolled participants who were scheduled for PCI, and another of the studies examined patients who would be eligible for PCI due to the presence of 75% blockage, and studied the difference between a group of participants who underwent PCI versus a group of participants who voluntarily participated in a 24-month regimen of aerobic exercise rather than undergoing PCI. In the exercise group, seven patients were removed from the final analysis due to undergoing emergent PCI and/or revascularization for AMI and/or unstable angina. Two of the studies reviewed included patients with heart failure rated as New York Heart Association (NYHA) class II or III.
Assessment of hsCRP
Healthy adults
The average concentration of hsCRP in healthy adults at baseline was 4.6 mg/L ± 4.7 mg/L. Blood samples for hsCRP analysis in 7 studies were drawn 24-h after completion of the last exercise session. One study specified that blood samples were drawn 48-h after the last exercise session, and another specified that blood samples were drawn 2–3 days after the last exercise session. The remaining 24 studies documented blood draws after conclusion of the last exercise session. Fasting blood sampling was conducted in 24 studies, and 14 studies specified that blood sampling was conducted in the AM.
Adults with CHD
The average hsCRP at baseline in adults diagnosed with CHD was 4.3 mg/L ± 4.7 mg/L. All hsCRP measurements were taken after an 8- to 24-h fast. Two of the studies enrolling participants after a coronary event reported that baseline hsCRP levels were assessed after waiting for a period of 16-days in one study and 3–5 weeks in the other study following the event. None of the other studies that enrolled participants after a coronary event reported how long they waited prior to assessing baseline hsCRP levels. All final hsCRP measurements were performed within 24-h after the completion of the exercise intervention.
Intervention programs
Healthy adults
An aerobic exercise intervention was examined in 23 of the studies analyzed, whereas 7 of the studies examined an intervention that included a mix of aerobic, strength, flexibility, and balance exercises. Two studies examined the effect of resistance exercise only on circulating hsCRP. There was one study that did not report the type of exercise intervention.55 The length of the exercise intervention ranged from 6-weeks to 18-months. The length of the individual exercise sessions lasted from 20-min to 90-min with the majority lasting between 45-min and 60-min. The frequency of exercise sessions was 3–6 times per week with the majority specifying 3 sessions per week. Seven studies specified total energy expenditure per week (kilocalorie/kilogram/week [KKW]) rather than times per week with total energy expenditure ranging from 4 KKW to 26 KKW.
The average intensity of the aerobic exercise sessions was approximately 70%–75% of VO2 maximum with a range of 50%–90% of VO2 maximum. For the studies examining resistance training, interventions were established to produce 75%–80% of 1 maximum rep (RM). One study reported results for 55% of 1 RM.
Two studies incorporated a diet program as well as an exercise program into the intervention, whereas another study provided the participants with written diet education at the beginning of the trial. Four studies included a treatment group receiving a diet and exercise intervention as well as an exercise intervention only treatment group. Three studies provided all participants with information regarding healthy living at the beginning of the study, and one study enrolled all participants in a healthy living class that met 1 time per month throughout the duration of the study.
Adults with CHD
Each of the studies reviewed contained an aerobic exercise component, whereas one study added resistance training using bands as well. The shortest intervention length was 4-weeks and the longest was 24-months. The average duration of the exercise intervention for all studies was 148.8 ± 210.8 days. Nine studies reported individual session lengths ranging from 30-min to 55-min, and frequency of exercise was reported for 9 studies with a frequency range of 2–6 times per week. The intensity of aerobic exercise sessions ranged from 65% to 80% maximum heart rate.
Exercise and hsCRP change
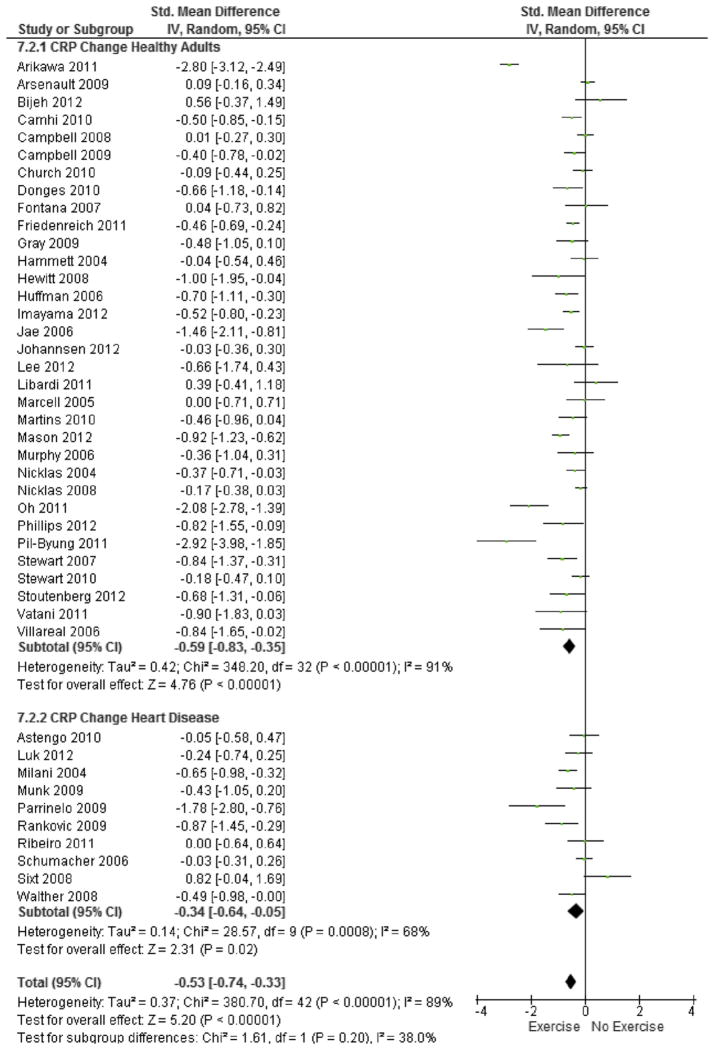
Pooled data from the 43 studies analyzed revealed a significant reduction in hsCRP favoring the exercise intervention group (SMD = −0.53; 95% CI, −0.74 to −0.33; MD = −0.67 mg/L; 95% CI, −0.93 to −0.42; Fig. 3). However, significant heterogeneity was observed between studies (χ2(42, N = 43) = 380.7, p < 0.001, I2 = 89%). Subgroup analysis revealed significant reductions in hsCRP for both healthy adults (SMD = −0.59; 95% CI, −0.83 to −0.35; MD = −0.74 mg/L; 95% CI, −1.05 to −0.44) as well as in adults with cardiovascular disease (SMD = −0.34; 95% CI, −0.64 to −0.05; MD = −0.42 mg/L; 95% CI, −0.81 to −0.06). No significant differences were found in effect size for healthy adults versus those with cardiovascular disease (p = 0.20). A large degree of heterogeneity was observed between studies in healthy adults (I2 = 91%), thus the following variables were examined as possible sources of the observed heterogeneity: gender, age, intervention duration, and intervention type (Table 3). There were no significant interaction effects observed due to the possible sources of heterogeneity analyzed.
Fig. 3.
Forest plot of change in hsCRP due to exercise. Forest plot of change in hsCRP due to exercise in healthy adults as well as individuals with cardiovascular disease. Pooled analysis shows that there is a significant effect of exercise on hsCRP (standardized mean difference −0.53; 95% CI, −0.74 to −0.33). No significant difference in effect sized due to presence of cardiovascular disease was observed (χ2(1,2) = 1.61, p = 0.20).
Table 3.
Subgroup analysis evaluating covariates of interest on the effect size of standardized mean difference in hsCRP.
| Covariate for Δ hsCRP | Effect size (95% CI) | Interaction significance |
|---|---|---|
| Gender | ||
| Males (n = 6) | −0.60 (−1.31, 0.12) | } p = 0.91 |
| Females (n = 14) | −0.64 (−1.09, −0.19) | |
| Age | ||
| <60 years (n = 5) | −1.01 (−2.28, 0.27) | } p = 0.69 |
| ≥60 years (n = 7) | −0.73 (−1.22, −0.24) | |
| Intervention length | ||
| ≤3-months (n = 11) | −0.83 (−1.20, −0.47) | } p = 0.17 |
| 3–6 months (n = 7) | −0.59 (−1.63, 0.46) | |
| 6-months (n = 9) | −0.36 (−0.67, −0.04) | |
| ≥12-months (n = 9) | −0.39 (−0.58, −0.20) | |
| Intervention type | ||
| Aerobic (n = 23) | −0.59 (−0.91, −0.27) | } p = 0.67 |
| Resistance (n = 4) | −0.32 (−0.87, 0.22) | |
| Mixed intervention (n = 6) | −0.63 (−1.13, −0.12) | |
| Inclusion of diet | ||
| Yes (n = 6) | −2.17 (−3.69, −0.65) | } p = 0.09 |
| No (n = 30) | −0.81 (−1.19, −0.43) | |
Discussion
Exercise is associated with a significant reduction in hsCRP for both healthy adults and those with CVD. The pooled effect sizes for each sub group did not differ based on the presence of cardiovascular disease. In a past meta-analysis, it appeared that exercise did not lower hsCRP in adults without CVD.30 This meta-analysis did not support that conclusion. When individual studies were considered, 17 out of the 33 did not report a significant reduction in change in hsCRP in participants enrolled in an exercise intervention compared to controls. Yet, a reduction in hsCRP from baseline due to an exercise intervention was observed in all but one of the studies analyzed. A narrative review could easily reach the conclusion that exercise interventions do not reduce hsCRP levels in healthy adults due to lack of statistical significance between groups, whereas this quantitative review suggests that it does. However, there was substantial heterogeneity between studies in the magnitude of the effect, including when studies enrolling healthy adults or adults with CHD were considered separately; thus the results should be evaluated with caution. Results of the moderator analysis showed that the heterogeneity in effect size for studies enrolling healthy adults was not attributable to age, gender, intervention length, intervention type, or the inclusion of diet modification.
One hypothesis for varying results in randomized trials of patients with heart disease is that the timing of baseline hsCRP measurements influences the effect size. Schumacher et al38 reported significant drops in hsCRP in both the exercise intervention group and controls, but no between-group differences in change in hsCRP were observed. Baseline measurements of hsCRP were taken approximately 16-days after a first episode of AMI and were found to be extremely elevated in both the exercise intervention participants and controls (5.91 mg/L and 5.22 mg/L respectively). In this case, the extreme elevation may be due to heart tissue trauma and the reduction in hsCRP may be due to stabilization of the acute phase response rather than an exercise intervention.38 Sixt et al85 also observed reductions hsCRP in both the control and intervention group after 4-weeks, but further examination revealed that the baseline hsCRP level for the participants in the control group was abnormally high. Since the control group consisted of only 10 subjects, an individual with an abnormally high hsCRP value possibly influenced the mean. In the other studies with non-significant results, the baseline levels of hsCRP were normal or mildly increased (range 0.9–1.52 mg/L) in both groups and failed to demonstrate a reduction in hsCRP.78,79,84 Finally, a majority of the studies included in the healthy adults did not report an exact time for conducting the blood draws post intervention. Evidence exists showing exercise increases hsCRP up to 24-h post exercise, but this is in individuals who have performed very strenuous physical activities such as running marathons.87 In a cohort of patients with coronary artery disease, Mouridsen et al39 found that hsCRP increased in the first 5 min following a moderate exercise test, but that this increase was attenuated at 20-h.39 These results indicate that exercise could cause unwanted variation in post intervention hsCRP levels.
An additional hypothesis generated from this meta-analysis is that overweight individuals may be most likely to benefit from an exercise intervention to reduce hsCRP. With respect to obesity, Phillips et al72 found that hsCRP levels were significantly reduced in obese, post-menopausal women who underwent 12-weeks of resistance training. Consistent with this hypothesis, a 34% reduction in hsCRP from baseline was realized in a cohort of obese, older (≥65 years) men and women who underwent an exercise and diet intervention designed to reduce their daily caloric energy by approximately 750 kcal/day.77 Several of the studies stratified the results on various anthropometric measures with change in hsCRP. Arikawa, Thomas, Schmitz, & Kurzer47 found that the observed significant decrease in hsCRP levels due to 16-weeks of aerobic exercise were largely driven by the effects of exercise in obese participants, who had an overall decrease in hsCRP of −4.38 ± 1.4 mg/L versus obese controls who had an increase in hsCRP of 1.44 ± 1.2 mg/L. No significant differences in change in hsCRP were found due to loss of body fat.24 In women, Campbell et al52 found significant reductions in hsCRP due to an aerobic exercise intervention at both 3-months and 12-months that were not realized in non-obese females. Results were similar when stratified by abdominal obesity (waist circumference > 88 cm).52 Church et al53 reported that sedentary men and women who were in the top tertile for weight loss and DEXA-measured body fat experienced the largest decrease in hsCRP due to exercise.62 Similarly, Jae et al62 found that participants in the top two highest quartiles of weight loss had the greatest reduction in hsCRP due to an exercise intervention and Stewart, Earnest, Blair, & Church37 found that participants in the highest quartile for weight loss had the greatest reduction in hsCRP. Women who lost 5% of their body weight due to an exercise and/or exercise plus diet intervention were found to have a significant decrease in hsCRP versus controls.61 In contrast, Marcell, McAuley, Traustodottir, & Reaven65 found no significant change in hsCRP due to exercise before and after stratification by reduction in body fat. Similarly, Campbell et al52 found no difference in change in hsCRP due to exercise when stratified by obesity (BMI > 30 kg/m2) and non-obesity regardless of gender.
Finally, it is interesting to note that inclusion of a modified diet in addition to exercise trended toward an enhanced the decrease in hsCRP from baseline when compared to exercise alone. One reason for this may be a greater propensity to lose weight, as discussed above. Another theory is that what we eat may contribute to a rise in inflammatory response. There is some evidence that low-glycemic load diets are more effective at lowering hsCRP than low-fat diets and high-glycemic load diets.88,89 In a recently funded, randomized trial of 811 overweight and obese adults, hsCRP levels did not change due to changes in diet composition (e.g., fats, carbohydrates, proteins).90 The authors concluded the hsCRP levels were more likely to be associated with amount of adipose tissue.
Potential limitations
The studies analyzed in this meta-analysis utilized many different study designs that may have contributed to the significant amount of heterogeneity observed. For example, differences were noted in the intervention procedures, the length of intervention, and inclusion and exclusion criteria. We were unable to assess heterogeneity due to factors associated with metabolic syndrome or timing of blood draws, thus leaving a question as to the effect of these variables on the overall result of the meta-analysis.
This meta-analysis included studies that were both randomized and non-randomized controlled trials. In addition, the majority of the studies reviewed did not report blinding of study personnel and investigators. Finally, a limited search strategy may have led to incomplete retrieval of studies as well as publication bias. These limitations may have impacted the results observed in this meta-analysis in addition to potentially decreasing overall generalizability.
Conclusions
In conclusion, exercise interventions decreased hsCRP in both healthy adults and those with CVD. The difference in effect size was not significantly different, indicating that exercise in healthy adults may lower future risk of CVD. Exercise interventions may be most beneficial to overweight individuals. Inclusion of a diet modification plan to reduce body fat in addition to physical activity lead to even greater reductions in hsCRP, a hypothesis which requires further study. Additional clinical trials, with enhanced control of potential confounders, are necessary in order to identify best practice for implementing an exercise intervention in both healthy adults as well as in adults with heart disease.
Footnotes
Disclosures: There are no conflicts of interest declared for any of the authors.
References
- 1.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107:391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 2.Sabatine MS, Morrow DA, Jablonski KA. Prognostic significance of the Centers for Disease Control/American Heart Association high-sensitivity c-reactive protein cut points for cardiovascular and other outcomes in patients with stable coronary artery disease. Circulation. 2007;115:1528–1536. doi: 10.1161/CIRCULATIONAHA.106.649939. [DOI] [PubMed] [Google Scholar]
- 3.Ballantyne CM, Houri J, Notarbartolo A, Melani L, Lipka L. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double blind trial. Circulation. 2003;107:2409–2415. doi: 10.1161/01.CIR.0000068312.21969.C8. [DOI] [PubMed] [Google Scholar]
- 4.Koenig W, Lowel H, Baumert J, Meisinger C. C-reactive protein modulates risk prediction based the on Framingham score: implications for future risk assessment: results from a large cohort study in southern Germany. Circulation. 2004;109:1349–1353. doi: 10.1161/01.CIR.0000120707.98922.E3. [DOI] [PubMed] [Google Scholar]
- 5.Tchernof A, Nolan A, Sites CK, Ades PA, Poehlman ET. Weight loss reduces c-reactive protein levels in obese postmenopausal women. Circulation. 2002;105:564–569. doi: 10.1161/hc0502.103331. [DOI] [PubMed] [Google Scholar]
- 6.Esposito K, Pontillo A, Di Palo C. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women. A randomized trial. JAMA. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 7.Seshadri P, Iqbal N, Stern L. A randomized study comparing the effects of a low-carbohydrate diet and a conventional diet on lipoprotein subfractions and c-reactive protein levels in patients with severe obesity. Am J Med. 2004;117:398–405. doi: 10.1016/j.amjmed.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 8.O’Brien KD, Brehm BJ, Seeley RJ. Diet-induced weight loss is associated with decreases in plasma serum amyloid A and c-reactive protein independent of dietary macronutrient composition in obese subjects. J Clin Endocrinol Metab. 2005;90:2244–2249. doi: 10.1210/jc.2004-1011. [DOI] [PubMed] [Google Scholar]
- 9.Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Rimm EB. Leisure time physical activity and reduced levels of obesity-related inflammatory markers. Obes Res. 2003;11:1055–1064. doi: 10.1038/oby.2003.145. [DOI] [PubMed] [Google Scholar]
- 10.Niu K, Hozawa A, Fujita K. Influence of leisure-time physical activity on the relationship between c-reactive protein and hypertension in a community-based elderly population of Japan: the Tsurugaya Project. Hypertens Res. 2005;28:747–754. doi: 10.1291/hypres.28.747. [DOI] [PubMed] [Google Scholar]
- 11.Panagiotakos DB, Pitsavos C, Chrysohoou C, Kavouras S, Stefanadis C. The associations between leisure-time activity and inflammatory and coagulation markers related to cardiovascular disease: the ATTICA study. Prev Med. 2005;40:432–437. doi: 10.1016/j.ypmed.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Kamijo T, Murakami M. Regular physical exercise improves physical motor functions and biochemical markers in middle-age and elderly women. J Phys Act Health. 2009;6:55–62. doi: 10.1123/jpah.6.1.55. [DOI] [PubMed] [Google Scholar]
- 13.LeCheminant J, Tucker L, Russell K. Physical activity and c-reactive protein levels: the confounding role of body fat. J Phys Act Health. 2011;8:481–487. doi: 10.1123/jpah.8.4.481. [DOI] [PubMed] [Google Scholar]
- 14.Loprinzi P, Cardinal B, Crespo C. Objectively measured physical activity and c-reactive protein: National Health and Nutrition Examination Survey 2003–2004. Scand J Med Sci Sports. 2011:1–7. doi: 10.1111/j.1600-0838.2011.01356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammett CJK, Prapavessis H, Baldi C. Effects of exercise training on 5 inflammatory markers associated with cardiovascular risk. Am Heart J. 2006;151:367.e7–367.e16. doi: 10.1016/j.ahj.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Kondo T, Kobayashi I, Murakami M. Effect of exercise on circulating adipokine levels in obese young women. Endocr J. 2006;53:189–195. doi: 10.1507/endocrj.53.189. [DOI] [PubMed] [Google Scholar]
- 17.De Salles BF, Simao R, Fleck SJ, Dias I, Kraemer-Aguiar LG, Bouskela E. Effects of resistance training on cytokines. Int J Sports Med. 2010;31:441–450. doi: 10.1055/s-0030-1251994. [DOI] [PubMed] [Google Scholar]
- 18.Ahmadi N, Eshaghian S, Huizenga R, Sosnin K, Ebrahimi R, Siegel R. Effects of intense exercise and moderate caloric restriction on cardiovascular risk factors and inflammation. Am J Med. 2011;124:978–982. doi: 10.1016/j.amjmed.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 19.Daray LA, Henagan TM, Zanovec M. Endurance and resistance training lowers c-reactive protein in young, healthy females. Appl Physiol Nutr Metab. 2011;36:660–670. doi: 10.1139/h11-077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lavie CJ, Church TS, Milani RV, Earnest CP. Impact of physical activity, cardio-respiratory fitness, and exercise training on markers of inflammation. J Cardiopulm Rehabil Prev. 2011;31:137–145. doi: 10.1097/HCR.0b013e3182122827. [DOI] [PubMed] [Google Scholar]
- 21.Geffken DF, Cushman M, Burke GL, Polak JF, Sakkinen PA. Association between physical activity and markers of inflammation in a healthy elderly population. Am J Epidemiol. 2001;153:242–250. doi: 10.1093/aje/153.3.242. [DOI] [PubMed] [Google Scholar]
- 22.Abramson JL, Vaccarino V. Relationship between physical activity and inflammation among apparently healthy middle-aged and older U.S. adults. Arch Intern Med. 2002;162:1286–1292. doi: 10.1001/archinte.162.11.1286. [DOI] [PubMed] [Google Scholar]
- 23.Ford ES. Does exercise reduce inflammation? Physical activity and c-reactive protein among U.S. adults. Epidemiology. 2002;13:561–568. doi: 10.1097/00001648-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Colbert LH, Visser M, Simonsick EM, Tracy RP, Newman AB. Physical activity, exercise, and inflammatory markers in older adults: findings from the health, aging, and body composition study. J Am Geriatr Soc. 2004;52:1098–1104. doi: 10.1111/j.1532-5415.2004.52307.x. [DOI] [PubMed] [Google Scholar]
- 25.Mora S, Lee I, Buring JE, Ridker PM. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. JAMA. 2006;295:1412–1419. doi: 10.1001/jama.295.12.1412. [DOI] [PubMed] [Google Scholar]
- 26.Arkabartabartoori M, Lean MEJ, Hankey CR. The associations between current recommendation for physical activity and cardiovascular risks associated with obesity. Eur J Clin Nutr. 2008;62:1–9. doi: 10.1038/sj.ejcn.1602693. [DOI] [PubMed] [Google Scholar]
- 27.Nazmi A, Oliveira IO, Victora CG. Correlates of c-reactive protein levels in young adults: a population-based cohort study of 3827 subjects in Brazil. Braz J Med Biol Res. 2008;41:357–367. doi: 10.1590/s0100-879x2008000500003. [DOI] [PubMed] [Google Scholar]
- 28.Hamer M, Sabia S, Batty GD, Shipley MJ, Tabak AG. Physical activity and in-flammatory markers over 10 years: Follow-up of men and women from the Whitehall II cohort study. Circulation. 2012;126:928–933. doi: 10.1161/CIRCULATIONAHA.112.103879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swardfager W, Herrmann N, Cornish S. Exercise intervention and inflammatory markers in coronary artery disease: a meta-analysis. Am Heart J. 2012;163:666–676. doi: 10.1016/j.ahj.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 30.Kelley GA, Kelley KS. Effects of aerobic exercise on c-reactive protein, body composition, and maximum oxygen consumption in adults: a meta-analysis of randomized controlled trials. Metabolism. 2006;55:1500–1507. doi: 10.1016/j.metabol.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 31.Plaisance EP, Grandjean PW. Physical activity and high-sensitivity c-reactive protein. Sports Med. 2006;36:443–458. doi: 10.2165/00007256-200636050-00006. [DOI] [PubMed] [Google Scholar]
- 32.Wilund K. Is the anti-inflammatory effect of regular exercise responsible for reduced cardiac disease? Clin Sci. 2007;112:543–555. doi: 10.1042/CS20060368. [DOI] [PubMed] [Google Scholar]
- 33.Puglisi MJ, Fernandez ML. Modulation of c-reactive protein, tumor necrosis factor-α, and adiponectin by diet, exercise, and weight loss. J Nutr. 2008;138:2293–2296. doi: 10.3945/jn.108.097188. [DOI] [PubMed] [Google Scholar]
- 34.Michigan A, Johnson TV, Master VA. Review of the relationship between c-reactive protein and exercise. Mol Diag Ther. 2011;15:265–275. doi: 10.1007/BF03256418. [DOI] [PubMed] [Google Scholar]
- 35.Sander K, Shulze Horn C, Briesenick C, Sander D. High sensitivity c-reactive protein is independently associated with early carotid artery progression in women but not in men. Stroke. 2007;38:2881–2886. doi: 10.1161/STROKEAHA.106.481531. [DOI] [PubMed] [Google Scholar]
- 36.Andersson J, Boman K, Jansson J, Nilsson T, Lindahl B. Effect of intensive lifestyle intervention on c-reactive protein in subjects with impaired glucose tolerance and obesity. Results from a randomized controlled trial with 5-year followup. Biomarkers. 2008;13:671–679. doi: 10.1080/13547500802661266. [DOI] [PubMed] [Google Scholar]
- 37.Stewart LK, Earnest CP, Blair SN, Church TS. Effects of different doses of physical activity on c-reactive protein among women. Med Sci Sports Exerc. 2010;42:701–707. doi: 10.1249/MSS.0b013e3181c03a2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schumacher A, Peersen K, Sommervoll L, Seljeflot I, Arnesen H, Otterstad JE. Physical performance is associated with markers of vascular inflammation in patients with coronary heart disease. Eur J Cardiovasc Prev Rehabil. 2006;13:356–362. doi: 10.1097/01.hjr.0000188244.54287.96. [DOI] [PubMed] [Google Scholar]
- 39.Mouridsen MR, Nielsen OW, Carlsen CM, Mattsson M, Ruwald MH. High-sensitivity c-reactive protein and exercise-induced changes in subjects suspected of coronary artery disease. J Inflamm Res. 2014;7:45–55. doi: 10.2147/JIR.S54360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ridker PM. High-sensitivity c-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001;103:1813–1818. doi: 10.1161/01.cir.103.13.1813. [DOI] [PubMed] [Google Scholar]
- 41.Alberti G, Zimmet P, Shaw J, Grundy F. The IDF Consensus Worldwide Definition of Metabolic Syndrome. 2006 [Google Scholar]
- 42.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistencies in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jadad AR, Moore RA, Carroll D. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 44.Higgins JPT, Greene S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. Updated March 2011. Available from: www.cochrane-handbook.org. [Google Scholar]
- 45.Hedges LV, Olkin I, editors. Statistical Methods for Meta-analysis. San Diego (Calif): Academic Press; 1985. [Google Scholar]
- 46.Egger M, Davey Smith G, Schneider M. Bias in meta-analysis detected by a simple graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arikawa AY, Thomas W, Schmitz KH, Kurzer MS. Sixteen weeks of exercise reduces c-reactive protein levels in young women. Med Sci Sports Exerc. 2011;43:1002–1009. doi: 10.1249/MSS.0b013e3182059eda. [DOI] [PubMed] [Google Scholar]
- 48.Arsenault BJ, Cote M, Cartier A. Effect of exercise training on cardiometabolic risk markers among sedentary, but metabolically healthy overweight or obese postmenopausal women with elevated blood pressure. Atherosclerosis. 2009;207:1–9. doi: 10.1016/j.atherosclerosis.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bijeh N, Hosseini SR, Hejazi K. The effect of aerobic exercise on serum c-reactive protein and leptin levels in untrained middle-aged women. Iran J Public Health. 2012;41:36–41. [PMC free article] [PubMed] [Google Scholar]
- 50.Camhi SM, Stefanick ML, Ridker PM, Young DR. Changes in c-reactive protein from low-fat diet and/or physical activity in men and women with and without metabolic syndrome. Metabolism. 2010;59:54–61. doi: 10.1016/j.metabol.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campbell KL, Campbell PT, Ulrich CM. No reduction in c-reactive protein following a 12-month randomized controlled trial of exercise in men and women. Cancer Epidemiol Biomarkers Prev. 2008;17:1714–1718. doi: 10.1158/1055-9965.EPI-08-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Campbell PT, Campbell KL, Wener MH. A yearlong exercise intervention decreases CRP among obese postmenopausal women. Med Sci Sports Exerc. 2009;41:1533–1539. doi: 10.1249/MSS.0b013e31819c7feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Church TS, Earnest CP, Thompson AM. Exercise without weight loss does not reduce c-reactive protein: the INFLAME study. Med Sci Sports Exerc. 2010;42:708–716. doi: 10.1249/MSS.0b013e3181c03a43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Donges CE, Duffield R, Drinkwater EJ. Effects of resistance or aerobic exercise training on interleukin-6, c-reactive protein, and body composition. Med Sci Sports Exerc. 2010;42:304–313. doi: 10.1249/MSS.0b013e3181b117ca. [DOI] [PubMed] [Google Scholar]
- 55.Fontana L, Villareal DT, Weiss EP. Calorie restriction or exercise: effects on coronary heart disease risk factors. A randomized controlled trial. Am J Physiol Endocrinol Metabol. 2007;293:e197–e202. doi: 10.1152/ajpendo.00102.2007. [DOI] [PubMed] [Google Scholar]
- 56.Friedenreich CM, Neilson HK, Woolcott CG. Inflammatory marker changes in a yearlong randomized exercise intervention trial among postmenopausal women. Cancer Prev Res. 2011;5:98–108. doi: 10.1158/1940-6207.CAPR-11-0369. [DOI] [PubMed] [Google Scholar]
- 57.Gray SR, Baker G, Wright A, Fitzsimons CF, Mutrie N, Nimmo MA. The effect of a 12-week walking intervention on markers of insulin resistance and systemic inflammation. Prev Med. 2009;48:39–44. doi: 10.1016/j.ypmed.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 58.Hammett CJK, Oxenham HC, Baldi JC. Effect of 6-months exercise training on c-reactive protein levels in healthy elderly subjects. J Am Coll Cardiol. 2004;44:2411–2413. doi: 10.1016/j.jacc.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 59.Hewitt JA, Whyte GP, Moreton M, van Someren KA, Levine TS. The effects of a graduated aerobic exercise programme on cardiovascular disease risk factors in the NHS workplace: a randomized controlled trial. J Occup Med Toxicol. 2008;3:1–10. doi: 10.1186/1745-6673-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huffman KM, Samsa GP, Slentz CA. Response of high-sensitivity c-reactive protein to exercise training in an at-risk population. Am Heart J. 2006;152:793–800. doi: 10.1016/j.ahj.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 61.Imayama I, Ulrich CM, Alfano CM. Effects of a caloric restriction weight loss diet and exercise on inflammatory biomarkers in overweight/obese post-menopausal women: a randomized controlled trial. Cancer Res. 2012;72:2314–2326. doi: 10.1158/0008-5472.CAN-11-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jae S, Fernhall B, Heffernan KS. Effects of lifestyle modifications on c-reactive protein: contribution of weight loss and improved aerobic capacity. Metabolism. 2006;55:825–831. doi: 10.1016/j.metabol.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 63.Johanssen NM, Swift DL, Johnson WD. Effect of different doses of aerobic exercise on total white blood cell (WBC) and WBC subfraction number in post-menopausal women: results from DREW. PLos One. 2012;7:e31319. doi: 10.1371/journal.pone.0031319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee M, Park K, Kim D, Choi S, Kim H. Effects of high-intensity exercise training on body composition, abdominal fat loss, and cardiorespiratory fitness in middle-aged Korean females. Appl Physiol Nutr Metabol. 2012;37:1019–1027. doi: 10.1139/h2012-084. [DOI] [PubMed] [Google Scholar]
- 65.Marcell TJ, McAuley KA, Traustadottir T, Reaven PD. Exercise training is not associated with improved levels of c-reactive protein and adiponectin. Metabolism. 2005;54:533–541. doi: 10.1016/j.metabol.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 66.Martins RA, Verissimo MT, Silva M, Cumming SP, Teixeira AM. Effects of aerobic and strength-based training on metabolic health indicators in older adults. Lipids Health Dis. 2010;9:1–6. doi: 10.1186/1476-511X-9-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mason C, Foster-Schubert KE, Imayama I. History of weight cycling does not impede future weight loss or metabolic improvements in postmenopausal women. Metabolism. 2012 Jun; doi: 10.1016/j.metabol.2012.06.012. http://dx.doi.org/10.1016/j.metabol.2012.06.012 (E-pub ahead of print) [DOI] [PMC free article] [PubMed]
- 68.Murphy MH, Murtagh EM, Boreham CAG, Hare LG, Nevill AM. The effect of a worksite based walking programme on cardiovascular risk in previously sedentary civil workers. BMC Public Health. 2006;6:1–8. doi: 10.1186/1471-2458-6-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nicklas BJ, Ambrosius W, Messier SP. Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: a randomized controlled clinical trial. Am J Clin Nutr. 2004;79:544–551. doi: 10.1093/ajcn/79.4.544. [DOI] [PubMed] [Google Scholar]
- 70.Nicklas BJ, Hsu F, Brinkley TJ. Exercise training and plasma c-reactive protein and interleukin-6 in elderly people. J Am Geriatr Soc. 2008;56:2045–2052. doi: 10.1111/j.1532-5415.2008.01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oh E, Bang S, Kim S. Therapeutic lifestyle modification program reduces plasma levels of the chemokines CRP and MCP-1 in subjects with metabolic syndrome. Biol Res Nurs. 2011;15:48–55. doi: 10.1177/1099800411416637. [DOI] [PubMed] [Google Scholar]
- 72.Phillips MD, Patrizi RM, Cheek DJ, Wooten JS, Barbee JJ, Mitchell JB. Resistance training reduces subclinical inflammation in obese, postmenopausal women. Med Sci Sports Exerc. 2012;44:2099–2110. doi: 10.1249/MSS.0b013e3182644984. [DOI] [PubMed] [Google Scholar]
- 73.Pil-Byung C, Shin-Hwan Y, Il-Gyu K. Effects of exercise program on appetite regulating hormones, inflammatory mediators, lipid profiles, and body composition in healthy men. J Sports Med Phys Fit. 2011;51:654–663. [PubMed] [Google Scholar]
- 74.Stewart LK, Flynn MG, Campbell WW. The influence of exercise training on inflammatory cytokines and c-reactive protein. Med Sci Sports Exerc. 2007;39:1714–1719. doi: 10.1249/mss.0b013e31811ece1c. [DOI] [PubMed] [Google Scholar]
- 75.Stoutenberg M, Kressler J, Chen GL. Aerobic training does not alter CRP in apparently healthy, untrained men. J Sports Med Phys Fit. 2012;52:53–62. [PubMed] [Google Scholar]
- 76.Vatani D, Ahmadi S, Dehrashid K, Gharibi F. Changes in cardiovascular risk factors and inflammatory markers of young, healthy men after six weeks of moderate or high intensity resistance training. J Sports Med Phys Fit. 2012;51:695–700. [PubMed] [Google Scholar]
- 77.Villereal DT, Miller BV, Banks M, Fontana L, Sinacore DR, Klein S. Effect of lifestyle intervention on metabolic coronary heart disease risk factors in obese older adults. Am J Clin Nutr. 2006;84:1317–1323. doi: 10.1093/ajcn/84.6.1317. [DOI] [PubMed] [Google Scholar]
- 78.Astengo M, Dahl A, Karlsson T, Mattsson-Hulten L, Wiklund O, Wennerblom B. Physical training after percutaneous coronary intervention in patients with stable angina: effects on working capacity, metabolism, and markers of inflammation. Eur J Cardiovasc Prev Rehabil. 2009;17:349–354. doi: 10.1097/HJR.0b013e3283336c8d. [DOI] [PubMed] [Google Scholar]
- 79.Luk T, Dai Y, Siu C. Effect of exercise training on vascular endothelial function in patients with stable coronary artery disease: a randomized controlled trial. Eur J Prev Cardiol. 2012;19:830–839. doi: 10.1177/1741826711415679. [DOI] [PubMed] [Google Scholar]
- 80.Milani RV, Lavie CJ, Mehra MR. Reduction in c-reactive protein through cardiac rehabilitation and exercise training. J Am Coll Cardiol. 2004;43:1056–1061. doi: 10.1016/j.jacc.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 81.Munk PS, Staal EM, Butt N, Isaksen K, Larsen A. High-intensity interval training may reduce in-stent restenosis following percutaneous coronary intervention with stent implantation: a randomized controlled trial evaluating the relationship to endothelial function and inflammation. Am Heart J. 2009;158:734–741. doi: 10.1016/j.ahj.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 82.Parrinello G, Torres D, Paterna S, Di Pasquale P, Trapanese C, Licata G. Short-term walking physical training and changes in body hydration status, B-type natriuretic peptide and c-reactive protein levels in compensated congestive heart failure. Int J Cardiol. 2008;144:97–100. doi: 10.1016/j.ijcard.2008.12.130. [DOI] [PubMed] [Google Scholar]
- 83.Rankovic G, Milicic B, Savic T, Dindic B, Mancev C, Pesic G. Effects of physical exercise on inflammatory parameters and risk for repeated acute coronary syndrome in patients with ischemic heart disease. Vojnosanit Pregl. 2009;66:44–48. doi: 10.2298/vsp0901044r. [DOI] [PubMed] [Google Scholar]
- 84.Ribeiro F, Alves AJ, Teixeira M. Exercise training increases interleukin-10 after an acute myocardial infarction: a randomized clinical trial. Int J Sports Med. 2012;33:192–198. doi: 10.1055/s-0031-1297959. [DOI] [PubMed] [Google Scholar]
- 85.Sixt S, Rastan A, Desch S. Exercise training but not rosiglitazone improves endothelial function in prediabetic patients with coronary disease. Eur J Cardiovasc Prev Rehabil. 2008;15:473–478. doi: 10.1097/HJR.0b013e3283002733. [DOI] [PubMed] [Google Scholar]
- 86.Walther C, Mobius-Wrinkler S, Linke A. Regular exercise training compared with percutaneous intervention leads to a reduction of inflammatory markers and cardiovascular events in patients with coronary artery disease. Eur J Cardiovasc Prev Rehabil. 2008;15:107–112. doi: 10.1097/HJR.0b013e3282f29aa6. [DOI] [PubMed] [Google Scholar]
- 87.Kasapis C, Thompson PD. The effects of physical activity on serum c-reactive protein and inflammatory markers. JACC. 2005;45:1563–1569. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 88.Pereira MA, Swain J, Goldfine AB, Rifai N, Ludwig DS. Effects of a low-glycemic load diet on resting energy expenditure and heart disease risk factors during weight loss. JAMA. 2004;292:2482–2490. doi: 10.1001/jama.292.20.2482. [DOI] [PubMed] [Google Scholar]
- 89.Pittas AG, Roberts RB, Das SK, Gilhooly GH, Saltzman E. The effects of the dietary glycemic load on type 2 diabetes risk factors during weight loss. Obesity. 2006;14:2200–2209. doi: 10.1038/oby.2006.258. [DOI] [PubMed] [Google Scholar]
- 90.Nicklas JM, Sacks FM, Smith SR, LeBoff MS, Rood JC. Effect of dietary composition of weight loss diets on high-sensitivity c-reactive protein: the randomized POUNDS LOST trial. Obesity. 2013;21:681–689. doi: 10.1002/oby.20072. [DOI] [PMC free article] [PubMed] [Google Scholar]