Abstract
Patients with heart failure (HF) take many medications to manage their HF and comorbidities, and 20–50% experience depression. Depressed individuals with more complex medication regimens may be at greater risk for poor adherence. The aim of this study was to assess depressive symptoms as a moderator of the relationship between medication regimen complexity and medication adherence in an observational study of patients with HF. In hierarchical linear regression with the final sample of 299, the interaction of medication regimen complexity and depressive symptoms predicted medication adherence, p <.05. For individuals with higher levels of depressive symptoms [1 standard deviation (SD) above the mean], more regimen complexity was associated with lower adherence. For individuals with low (1 SD below the mean) or average levels of depressive symptoms, regimen complexity was unrelated to medication adherence. Care management strategies, including pillboxes and caregiver involvement, may be valuable in HF patients with depression.
Keywords: Heart failure, Cardiovascular diseases, Depressive symptoms, Medication adherence, Self-care
Introduction
Heart failure (HF) is extremely debilitating and has a major impact on the mental and physical lives of individuals and their families (Luttik et al., 2005). In HF, poor ventricular function or myocardial damage over time leads to decreased stroke volume, decreased cardiac output, vaso-constriction, sodium and fluid retention, and further stress on the ventricular wall that results in worsened ventricular function (Jackson et al., 2000). Adequate self-management is complex and includes dietary sodium restriction, increase or maintenance of physical activity levels, symptom monitoring, fluid restrictions (for some patients), and managing a complex system of numerous medications (Luttik et al., 2005).
Medication adherence is the extent to which a patient follows his or her provider’s recommendations about their day-to-day treatment such as timing, dosage, and frequency of medication administration (Bosworth et al., 2011; Osterberg & Blaschke, 2005). HF patients who adhere to recommendations of evidence-based pharmacotherapy regimens demonstrate improved outcomes compared to individuals who do not persist with the recommended regimen (Gislason et al., 2007). Following hospital discharge, there is a continuing decrease in adherence to cardiovascular disease (CVD) medications (e.g., statins, β-blockers; Ho et al., 2009). In HF, estimates of medication adherence range from 40 to 60%, though previous estimates have ranged from 10 to 93% (Wu et al., 2008). Importantly, adherence under 88% is associated with increased risk of mortality in CVD (Wu et al., 2009). The hazard ratio for time to first event for patients achieving ≤88% adherence is 2.2 by dose count (i.e., the percentage of prescribed number of doses that were taken during a 3-month period) and 3.2 by dose day (i.e., the percentage of days the correct number of doses were taken), where time to first event is defined as emergency room visits for HF symptoms worsening, cardiac hospitalizations, or mortality (Wu et al., 2009). However, the literature on medication adherence contains conflicting results as most articles dichotomize adherence using empirically unsupported cutpoints (Wu et al., 2009).
Complex medication regimens (including having to take a medication two, three, or four times daily compared to once) are associated with nonadherence when measured based on the proportion of times someone takes a medication, the proportion of days the correct number of doses were taken, and the proportion of doses taken at the correct time (Coleman et al., 2012a). When the most stringent adherence definition, achieving a high proportion of doses taken at the correct time, was used in one study, the adjusted weighted mean percentage adherence rates were −26.7, −39.0, and −54.2% lower than regimens dosed once daily (for regiments dosed 2-times, 3-times, and 4-times, respectively; Coleman et al., 2012a). Adherence is higher for once-daily medications than for medications prescribed to be administered multiple times daily in patients with chronic diseases (Coleman et al., 2012a). Individuals with HF typically are prescribed several medications daily; some need to be taken multiple times daily or come with complex instructions. Improper dosing (e.g., overdosing with or without toxic effects) and skipping doses impacts the effectiveness of the medications and may lead to adverse side effects.
Dunbar-Jacob et al. (2003) found an inverse relationship between adherence and daily dosing frequency: adherence declined as doses per day increased in their sample of adults with CVD and at least one comorbid condition. Coleman et al. (2012b) found adherence (according to electronic monitoring) to be higher for once-daily medications than medications taken two, three, or four times daily. Moreover, an analysis of 1,077,474 CVD patients revealed medication possession ratios (the percentage of needed doses that the patient has in his/her possession) were higher for medication prescribed once-daily than medication prescribed twice-daily (Bae et al., 2012). Riegel et al. (2012) found that taking two or more medication doses per day predicted steep declines in objectively measured medication adherence in a sample of patients with HF. The relationship between medication adherence and medication regimen complexity has been documented in other conditions including Parkinson’s disease (Malek & Grosset, 2015) and human immunodeficiency virus (HIV) (Hinkin et al., 2002). Most patients are optimally successful when the regimen is as simple as possible (Haynes et al., 2002; McDonald et al., 2002; Richter et al., 2003).
No differences in adherence between once- versus twice-daily carvedilol were reported in individuals with HF using Medication Event Monitoring System (MEMS; MWV Healthcare, Richmond VA; Udelson et al., 2009); the authors noted this may have been due to high baseline adherence and measurement reactivity. It also suggests that the relationship between medication regimen complexity and adherence may be more complex; other factors, such as depressive symptoms, may demonstrate a synergistic effect in this relationship. Udelson et al. (2009) recommend a once daily regimen when possible for the reasons of simplicity, safety, fewer side effects, and the trend that individuals with HF acquire more medications over time, which ultimately further complicates their already complex regimens; however, this is not always possible with HF medication management.
There is an extensive body of research demonstrating the negative effects of depression on adherence across multiple disease and conditions including diabetes (Gonzalez et al., 2007; Kilbourne et al., 2005; Lin et al., 2004; Osborne & Egede, 2012), chronic obstructive pulmonary disease (Albrecht et al., 2016), systemic lupus erythematosus (Julian et al., 2009), breast cancer (Mausbach et al., 2015) and those undergoing hemodialysis or kidney transplant (Cukor et al., 2009). According to self-report, depression is associated with medication nonadherence (Gehi et al., 2005), and depressed patients are 1.76 times more likely to be non-adherent to medications than non-depressed patients (according to a meta-analysis of 31 studies with 18,245 total participants; Grenard et al., 2011). Depressed patients are 3 times more likely to be noncompliant with overall medical treatment recommendations than nondepressed patients (DiMatteo et al., 2000). About 20–50% of individuals with HF have depressive symptoms or major depressive disorder (Freedland et al., 2003; Jiang et al., 2001). In a study of over 800 individuals with HF, over 33% met criteria for major depression during post-myocardial infarction (MI) hospitalization according to a diagnostic interview (Powell et al., 2005). The exact mechanism for the relationship between depression and medication adherence is unknown. Given the high rates of depression in this disease and the potential for tremendous consequences on outcomes (e.g., mortality), the relationship of depression on adherence in HF deserves close examination.
A landmark study assessing medication adherence and depression tested whether improvements in depressive symptoms preceded improved adherence to aspirin over three months in 172 adults with acute coronary syndrome (Rieckmann et al., 2006a). Adherence was measured using MEMS and an 80% adherence cutoff; depression was assessed with the Beck Depression Inventory (BDI; 2006a). Severely depressed patients were 3.7 times more likely to be nonadherent after controlling for potential confounders (Rieckmann et al., 2006a). Adherence increased in patients whose depression improved and decreased in patients whose depression worsened (Rieckmann et al., 2006a). Patients with transient symptoms of depression adhered better than those with persistent symptoms (Rieckmann et al., 2006b). Although depression has deleterious effects on adherence, improvements in depressive symptoms produced remarkable improvements in adherence (Rieckmann et al., 2006b). Additionally, improvements in depressive symptoms are associated with improvements in medication adherence as well as secondary preventive behaviors in patients with acute coronary syndrome, decompensated HF, or arrhythmia (Bauer et al., 2012). This phenomenon is similarly observed in HIV/acquired immunodeficiency syndrome (AIDS) patients prescribed antiretroviral therapy: the odds of a person adhering to their antiretroviral medication regimen were 83% better when the patient was treated for depression according to a large meta-analysis of 12,243 persons living with HIV/AIDS across 29 studies (Sin & DiMatteo, 2014). However, depression does not fully explain adherence rates, suggesting other factors influence this relationship.
A more recent randomized controlled trial of cognitive behavioral therapy for adherence and depression in type 2 diabetes demonstrates tremendous promise for patients with a similarly serious and complex disease. Compared to patients receiving treatment as usual, those who received the cognitive behavioral therapy intervention achieved adherence rates an average of 20.7% points higher according to measurement via electronic pill cap (Safren et al., 2014). Participants that received the treatment were also more adherent to self-monitoring blood glucose (30.2% points) according to blood glucometer downloads, had lower depression scores, and .72 points lower hemoglobin A1c than controls; these benefits were maintained at 1 year post-intervention (Safren et al., 2014). This multi-faceted approach that targeted depressive symptoms and health behaviors in tandem may be necessary for triggering improvement in self-management of complicated, chronic diseases.
Depressed patients with more complex regimens exhibit poorer medication adherence than depressed patients with simpler regimens. While greater medication regimen complexity may be linked with poorer adherence (Coleman et al., 2012a, b; Dunbar-Jacob et al., 2003), the magnitude of this effect may be greater in individuals with depressive symptoms (Ansell, 2008). For individuals who are cognitively intact, motivated to adhere, and are mentally well, medication regimen complexity may not dramatically affect adherence. Complicated regimens may act as a barrier to adherence in individuals with depressive symptoms. The purpose of the present study was to examine whether depressive symptoms moderated the relationship between medication regimen complexity and medication adherence in adults with systolic HF. We hypothesized that higher medication regimen complexity would predict lower medication adherence, and that depressive symptoms would moderate this relationship where increased depressive symptoms would exert a deleterious effect.
Method
Kent State University, Summa Health System’s Akron City Hospital, and Case Western Reserve University’s University Hospital institutional review boards previously approved the protocol for this study. Participants were recruited through two cardiology services, informational letters, and advertisements. The present study is a part of the Heart Adherence, Behavior, and Cognition (ABC) trial, a longitudinal observational study examining the effects of medication adherence and cognition on outcomes in HF (R01 HL096710-01A1; ClinicalTrials.gov identifier: NCT01461629).
Participants were provided with full verbal and written descriptions of the study’s procedures and were given the opportunity to have questions or concerns addressed by research staff. Participants enrolled in the study granted the researchers permission to gather information from their medical records. Research staff obtained the following information from the clinical database: diagnosis of systolic heart failure, gender, and age. All other information was collected through research assistant-administered tests or self-report in the participants’ home or hospital’s center for clinical trials.
Participants
The parent study (HeartABC) enrolled a total of 372 participants. The present study includes 299 older adults who were enrolled in the Heart ABC study and had complete data on variables of interest (see analytic plan for additional details). Participants were recruited from both inpatient services and the associated outpatient cardiology clinic of two health systems in northeast Ohio. They were eligible for participation if they were between 50 and 85 years of age, English-speaking, had a history of systolic HF class II or III for at least 3 months confirmed by medical chart review within 12 months of study enrollment, and lived within 30 miles of either study site. The individual was also only eligible if they were willing to allow research staff into their home for the study visits and to allow research staff to install a telehealth device in their home. Exclusion criteria included class IV HF, a history of neurological disorder that produces cognitive impairment (e.g., stroke, Alzheimer’s disease, severe head injury producing loss of consciousness of 10 minutes or more), history of significant psychological problems (e.g., schizophrenia, substance abuse within past five years), developmental disability that interferes with daily living, renal failure requiring dialysis, history of untreated sleep apnea (including not using prescribed continuous positive airway pressure [CPAP] device, diagnosed through a formal sleep study), history of cardiac surgery within the past 3 months, or terminal illness where the individual was expected to expire within the next 6 months. Additionally, the individual could not have been a telehealth or home healthcare nursing patient through which a professional managed their medication regimen. These criteria were chosen to cultivate a sample that could safely participate and to exclude patients with a priori reasons to have poor adherence due to substantial cognitive impairment.
Measures
Medication regimen complexity
The Medication Regimen Complexity Index (MRCI; George et al., 2004), a validated, self-report measure, was collected at baseline. This 65-item measure assesses the number of drugs in the regimen, dosage frequency, special instructions for medications, and type of medication (George et al., 2004). These factors were weighted and summed to quantify the overall complexity of the complete medication regimen with higher scores indicating more complex regimens. The MRCI offers an advantageous assessment of the medication regimen given that individuals with cardiovascular disease often have medication regimens that vary amongst individuals despite similar medications. For example, regimens for blood thinners are often uniquely scheduled for each individual.
Medication adherence
Medication adherence was determined using a protocol developed for the present study. Adherence data were collected using a medication pillbox connected to the participants’ telephone lines in their homes. Through this connection, the box transferred data throughout the day to an online server. The pillbox was set-up in the participant’s home two weeks after baseline and was picked up six weeks after baseline so that the monitoring period was four weeks long. There was a one-week run-in period followed by three weeks of monitoring. The one-week run-in period was designed to catch equipment issues or lack of mastery in using the device before the active data collection period. Additionally, this provided the participant with an adjustment periods during which they could acclimate to including some of their medications from their regimen in the study pillbox with the others remaining in their previously-used system of choice (e.g., individual pill bottles, a weekly plastic pill box). In the event a participant encountered problems with the pillbox, a research assistant provided support over the phone or in the participant’s home if necessary. In order to minimize measurement reactivity, only the last three weeks of monitoring are used in calculations. Common CVD medications were chosen to fill each of the four bins on the pillbox. The types of medications were chosen to fill the pillbox bins were beta-blockers, angiotensin-converting-enzyme inhibitors (ACE), angiotensin II receptor blockers (ARB), diuretics, aspirin, aldosterone antagonists, clopidogrel, and statins. One medication was chosen from each of the preceding classes, with preference for inclusion in ascending order (i.e., a beta blocker was chosen first, then an ACE, then ARB, then diuretic, and only then aspirin if one of the previous types was unavailable). This hierarchy was chosen in consultation with the study cardiologists because most participants were taking one medication from each of the classes in descending order. This hierarchy allowed for medications with complex and simple dosing, timing, and frequency instructions to be included in the pillbox. Additionally, other available pillboxes commonly measure one medication; while it would have been ideal to measure every medication in the regimen, the selected pillbox was superior to other available options. The pillbox was not alarmed; instead, participants were instructed to take their medications from the pillbox immediately before ingesting them as per their typical medication schedule, which they provided to the researchers at the beginning of the study.
Following data collection, the first week of monitoring data (data collected during the one-week run-in) was removed to minimize measurement reactivity. For the final three weeks of monitoring, medication adherence was determined by calculating a ratio of the percent of days that the individual was compliant (i.e., opening the bin the prescribed number of times) with their medication regimen divided by the number of days monitored. Days when participants did not use the pillbox due to reasons such as travel or hospitalization were excluded.
Depression
Depressive symptoms were assessed using the Patient Health Questionnaire (PHQ-9; Kroenke et al., 2001). The PHQ-9 is the depression module of a self-administered diagnostic instrument for common mental disorders (Kroenke et al., 2001). A score of 5, 10, 15, or 20 represents mild, moderate, moderately severe, and severe depression (Kroenke et al., 2001). The PHQ-9 was administered at baseline and again three weeks later. The mean of the two scores represents the individual’s level of depression.
Covariates
The following covariates were included in all analyses to control for their impact on the variables of interest. All covariates were specified a priori because of known relationships with study variables. Covariates included age, race/ethnicity, education, medical comorbidity, cognitive function, social support (MSPSS), and measures of cardiac disease severity. Age, highest achieved education level, and race/ethnicity were gathered from baseline self-report questionnaires. Self-report New York Heart Association (NYHA) functional classification was used to assess HF severity. Following enrollment, a trained research assistant assessed self-reported HF symptoms and documented a NYHA functional classification at the time of study participation. Patients were classified as Class I (no symptoms), Class II (Mild), Class III (Moderate), or Class IV (Severe). Comorbidities were quantified using The Charlson Comorbidity Index (Charlson et al., 1994). The Charlson combines age and comorbidities to estimate the relative risk of death (Charlson et al., 1994). Each one point increase in the age-comorbidity score approximately corresponds to one less decade of life (Charlson et al., 1994). This comorbidity index is preferable to a comorbidity count in this sample because it weights the comorbidities by effects on mortality, where conditions associated with greater reductions in lifespan are given more weight. Cognitive function was determined by the Modified Mini-Mental Status Examination (3MS; Teng & Chui, 1987). The 3MS is a brief cognitive screening tool.
Procedure
Informed consent was obtained from all individual participants included in the study prior to any participation. Participants were evaluated at four home visits over the course of 6 weeks. Participants’ depressive symptoms and medication regimen complexity were assessed at baseline. Two weeks after baseline, the pillbox was installed in the participant’s home. Three weeks after baseline, depression was measured again. Six weeks following baseline, at the final study home visit, the pillbox was collected marking the end of the medication adherence data collection period.
Analytic plan
Prior to conducting analyses, missing data were examined. Sixteen participants died during the course of the study, which included a follow-up period after the completed assessment visits. Of the 365 who completed all four visits, 309 had complete medication adherence data. In this study, 15 participants dropped out prior to completing the second assessment, five dropped out prior to the third, and five dropped out prior to the fourth and final in-home assessment; worsening health status was typically noted as the reason for discontinuing participation. Twenty-six experienced equipment failure. Of those with complete medication adherence data, 10 were missing additional data (PHQ-9, multidimensional scales of perceived social support [MSPSS], or MRCI). We used listwise deletion of individuals’ missing data on these variables, yielding a final sample of 299.
Descriptive analyses, such as frequencies, percentages, means and standard deviations, were used to describe the sample of participants. The relationship between PHQ-9 scores, medication regimen complexity, and medication adherence was assessed through moderation analysis using a series of hierarchical multiple regressions. All variables were normally distributed based on standard cutoffs for skewness and kurtosis. There were no violations of the assumptions of regression. Missing data were excluded listwise. The criterion for statistical significance was set at p <.05 for all analyses.
In the first step, covariates including age, race, education, NYHA class, and cognitive function were entered into the model. In the second step, MRCI scores were included in the model. In the third step, PHQ-9 scores were added. In the fourth step, the cross-product of MRCI score by PHQ-9 score was entered. MRCI and PHQ-9 scores were centered prior to creating the interaction term. All statistical analyses were performed using IBM© Statistical Package for the Social Sciences (SPSS©) version 20.0 statistical software (IBM Corporation).
Results
Characteristics of study participants
Sociodemographics
The sociodemographic characteristics of the final sample (N = 299) are presented in Table 1. The majority of participants were married, Caucasian males. Most of the sample reported little to no depressive symptoms (65.2%). Nearly a quarter (23.75%) reported mild depressive symptoms. Moderate and moderately severe to severe symptoms were reported by 7.36% and 3.68%. The two assessments of depression (taken 3 weeks apart) were highly correlated (r = .74, p <.001). On average, participants were prescribed 10 medications, though the number of medications ranged from 2 to 24.
Table 1.
Sociodemographic characteristics of the sample (N = 299)
| M (SD) | Range (min–max) | |
|---|---|---|
| Age (years) | 68.43 (9.65) | 50–85 |
| PHQ-9 total score (raw) | 4.19 (4.48) | 0–26 |
| Medication adherence (%) | 73.54 (24.96) | 0–100 |
| Charlson Comorbidity Index | 3.30 (1.74) | 1–11 |
| 3MS | 92.08 (6.41) | 69–100 |
|
| ||
| n | % of sample | |
|
| ||
| Sex (male) | 179 | 59.9 |
| Race | ||
| Caucasian (non-Hispanic) | 218 | 72.9 |
| African American | 78 | 26.1 |
| Native American or Alaska Native | 2 | 0.7 |
| Asian | 1 | 0.3 |
| Retired | 199 | 66.6 |
| Married | 179 | 59.9 |
| Highest educational level achieved | ||
| 8th grade or less | 5 | 1.7 |
| 9–11th grade | 28 | 9.4 |
| High school | 88 | 29.4 |
| Technical or trade school | 28 | 9.4 |
| Some college | 82 | 27.4 |
| Bachelors degree | 39 | 13.0 |
| Masters degree | 29 | 9.7 |
| NYHA class | ||
| Class I | 27 | 9.0 |
| Class II | 74 | 24.7 |
| Class III | 183 | 61.2 |
| Class IV | 15 | 5.0 |
Medication regimen complexity and medication adherence
Hierarchical multiple linear regression was performed to examine the relationship between MRCI scores and medication adherence after controlling for covariates. In Block 1, the combination of age, race, education, MSPSS, cognitive function, and NYHA class explained 9.7% of the variance in medication adherence, F(7, 291) = 4.38, p <.001. Minority status was associated with lower medication adherence, β = −.13, p < .05. In addition, lower MSPSS significantly predicted lower medication adherence, β = .19, p < .01. In Block 2, following adjustment for age, race, education, NYHA class, MSPSS, and cognitive function, MRCI scores failed to account for additional variance in medication adherence (ΔR2 = .002, ΔF (1, 290) = .77, p = .38).
Depressive symptoms
In Block 3, depressive symptoms did not account for additional variance in medication adherence (ΔR2 = .01, ΔF (1, 289) = 3.01, p = .08). There was a trend demonstrating a negative relationship between depressive symptoms medication adherence, β = −.11, p = .08.
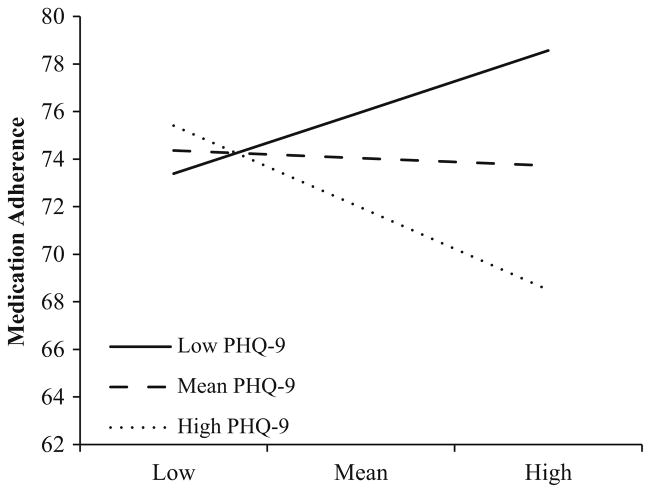
In Block 4, the addition of the MRCI by PHQ-9 interaction term accounted for additional variance in medication adherence (ΔR2 = .015, ΔF (1, 288) = 4.70, p < .05). For individuals with higher depressive symptomology (1 SD above the mean), higher regimen complexity tended to be associated with lower medication adherence, b = −.28, p = .06. Regimen complexity had no significant impact on medication adherence in individuals with no (1 SD below the mean) or average depressive symptoms (Fig. 1). See Table 2 for a full summary of regression analyses.
Fig. 1.
Interaction effect of depressive symptoms as a moderator of medication regimen complexity predicting medication adherence
Table 2.
Medication regimen complexity predicting medication adherence
| b (SE b) | t | β | |
|---|---|---|---|
| Block 1 | |||
| Age | .24 (.16) | 1.49 | .09 |
| Race | −7.5 (3.46)* | −2.17* | −.13* |
| Education | −.59 (.99) | −.59 | −.04 |
| NYHA class | −3.79 (2.01)± | −1.89± | −.04± |
| MSPSS | .32 (.10)** | 3.26** | .19** |
| Charlson | −.49 (.83) | −.58 | −.03 |
| 3MS | .21 (.24) | .88 | .06 |
| R2 | .097 | ||
| F | 4.38*** | ||
| Block 2 | |||
| MRCI | −.11 (.12) | −.88 | −.05 |
| ΔR2 | .002 | ||
| ΔF | .77 | ||
| Block 3 | |||
| PHQ-9 | −.62 (.36)± | −1.74± | −.1± |
| ΔR2 | .01± | ||
| ΔF | 3.01± | ||
| Block 4 | |||
| Interaction | −.06 (.03)* | −2.17* | −.33* |
| ΔR2 | .015* | ||
| ΔF | 4.70* | ||
NYHA New York Heart Association, MSPSS Multidimensional Scale of Perceived Social Support, 3MS Modified Mini-Mental Status Examination, PHQ-9 Patient Health Questionnaire-9, MRCI Medication Regimen Complexity Index total score; Interaction = cross product of PHQ-9 and MRCI
p = .08;
p < 0.05;
p < 0.01;
p < .001
Discussion
This study provided evidence that depressive symptoms moderate the relationship between medication regimen complexity and medication adherence in patients with HF. Specifically, in individuals with higher depressive symptomology, having a more complex medication regimen is associated with lower medication adherence. Conversely, regimen complexity was not related to medication adherence in individuals with little or no depressive symptoms.
This study replicated findings that depressive symptoms are related to poorer medication adherence in patients with CVD (Carney et al., 1995; Rieckmann et al., 2006a, b), but found the results more specifically in patients with HF. Possible explanations for this relationship include fatigue, a symptom of both HF and depression, which likely reduces the energy necessary for HF self-management (Falk et al., 2007). Depression may lower motivation to adhere to the self-management regimen as has been seen in diabetes (Egede & Osborn, 2010).
Our findings extend the previous literature examining complex medication regimens. Other studies have found an inverse relationship between adherence and daily dosing frequency (Dunbar-Jacob et al., 2003). Here, medication regimen complexity varied tremendously. This study is the first to illustrate a moderation of medication regimen complexity in depressive symptoms predicting medication adherence in patients with HF. The present sample had higher adherence (M = 73.57, SD = 24.84) than previous estimates (Wu et al., 2008), but individuals who were nonadherent to CPAP were excluded due to exclusion criteria of the parent study; study-wide exclusion of these “non-adherers” may have inflated adherence rates.
These findings are novel for a number of reasons. To date, this is one of the largest examinations of medication regimen complexity and electronically-measured medication adherence in a HF sample. The study design for data collection (i.e., home visits) allowed for the inclusion of more clinically limited individuals who are typically excluded from studies of HF either through inability to travel to research sites or due to their functioning. This study demonstrated that even mild levels of depressive symptoms negatively influence medication adherence.
The current findings contain limitations. Many individuals with HF are prescribed more than four medications and may differentially adhere to certain medications due to side effects or other factors. The pillbox selected for this study could only measure four medications; future studies should utilize newly available devices that can measure a larger portion of the participant’s medication regimen. Though separating the medications selected for monitoring could have increased the participants’ adherence for the selected medications since they knew they were being monitored, the run-in period was designed to reduce this reactivity. Future studies will ideally monitor all of the medications in the regimen. The MRCI score was calculated based on the complexity of the entire regimen rather than solely on the four monitored medications. Some of the most complex medications (which typically are not in tablet or capsule form and could therefore not be contained in the telemedicine device) were not included in monitoring, so participants with complex regimens may have achieved inflated adherence rates. Though it would increase financial and data collection burden, future studies should monitor adherence to the complete medication regimen with a device capable of monitoring medications not in pill form. The present study did not control for current antidepressant medication use or psychotherapeutic treatment targeting depression; participants were not surveyed about whether or not they were currently engaged in depression treatment. Although others (Rieckmann et al., 2006a, b) have shown that adherence improves with remission of depressive symptoms, we expected that persistent and current depressive symptoms, even if undergoing treatment with an antidepressant, would continue to impact on adherence. Future studies may wish to consider treatment status in their results.
Despite these limitations, these findings contain several important research and clinical implications. Depressive symptoms and adherence may vary throughout the disease course. Future studies should examine first incidence of depression as a predictor of adherence behavior; for individuals with a longstanding history of depressive symptoms prior to HF onset, self-management may be more difficult due to low self-efficacy, hopelessness, decreased ability to concentrate, and fatigue. Other researchers should examine complex regimens’ influences on the entire HF self-management plan, as well as methods for improving medication adherence and HF self-management. Health-care providers should make every attempt possible to reduce the complexity of the medication regimen (e.g., switching powders to pills, minimizing the number of daily doses) particularly for patients with HF and depressive symptoms. Since this may not be possible for many individuals with HF, particularly those with extensive comorbidities, this group would likely benefit from devising and utilizing adherence-augmenting techniques including weekly pillboxes, calendars, and reminder calls from family and pharmacies. In order to reduce psychosocial distress that may be interfering with medication adherence, providers may wish to target knowledge, beliefs, emotional distress, well-being, behavioral skills, and coping as these appear to be useful targets in type 2 diabetes, which is a similarly complex disease (Gonzalez et al., 2016). Regardless of the medical condition(s) affecting the patient, all interventions targeting medication nonadherence should directly involve the patient in the decision-making process prior to intervention implementation (Krueger et al., 2005) provided the patient has the capacity to make decisions about their care; this is likely necessary for ensuring the intervention’s success. Individuals with HF and comorbidities and with more severe HF are particularly susceptible to lower adherence (Calvin et al., 2012). Individuals with complex comorbidities and increased disease severity may require special attention from providers and caregivers to achieve sufficient medication adherence. As was done with patients in the present study, providers should simplify instructions, educate patients about why a medication is prescribed and how it helps, address fears regarding side effects, ask patients what would help them be more adherent, explain how to handle missed or late doses, ask about adherence during every visit, and reward adherence (American College of Preventative Medicine, 2011).
The present study examined the influence of depressive symptoms on the relationship between complex medication regimens and medication adherence in patients with HF. Nearly one-third of the present sample demonstrated at least mild depressive symptoms as indicated by PHQ-9 scores. Depressive symptoms moderated the relationship between medication regimen complexity and medication adherence: having depressive symptoms was associated with complex medication regimens exerting a negative effect on medication adherence. Healthcare providers should simplify self-management guidelines including medication schedules as much as possible and educate patients about adherence strategies, particularly for patients with HF and depression. Simplified medication regimens complemented with numerous adherence aids as a part of a larger streamlined self-management plan could ultimately improve quality of life and longevity among HF patients with depressive symptoms.
Acknowledgments
This research was funded by generous grant support from the National Heart, Lung, and Blood Institute at the National Institute of Health (R01 HL096710-01A1 and T32 5T32HL076134-10). The authors wish to acknowledge all of the participants and study staff that contributed to the present work being possible.
Footnotes
Compliance with ethical standards
Conflict of interest Carly M. Goldstein, Emily C. Gathright, John Gunstad, Mary A. Dolansky, Joseph D. Redle, Richard Josephson, Shirley M. Moore, and Joel W. Hughes declare that they have no conflict of interest.
Human and animal rights and Informed consent All procedures followed were in accordance with ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
References
- Albrecht JS, Park Y, Hur P, Huang T, Harris I, Netzer G, et al. Adherence to maintenance medications among older adults with chronic obstructive pulmonary disease: The role of depression. Annals of the American Thoracic Society. 2016;13:1497–1504. doi: 10.1513/AnnalsATS.201602-136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Preventative Medicine. Medication adherence—improving health outcomes: A resource from the American College of Preventative Medicine. 2011 Retrieved from http://c.ymcdn.com/sites/www.acpm.org/resource/resmgr/timetools-files/adherencetimetool.pdf.
- Ansell BJ. Not getting to goal: The clinical costs of noncompliance. Journal of Managed Care Pharmacy. 2008;14:9–15. doi: 10.18553/jmcp.2008.14.S6-B.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae J, Dobesh P, Klepser D, Anderson J, Zagar A, McCollam P, et al. Adherence and dosing frequency of common medications for cardiovascular patients. American Journal of Managed Care. 2012;18:139–146. [PubMed] [Google Scholar]
- Bauer LK, Caro MA, Beach SR, Mastromauro CA, Lenihan E, Januzzi JL, et al. Effects of depression and anxiety improvement on adherence to medication and health behaviors in recently hospitalized cardiac patients. American Journal of Cardiology. 2012;109:1266–1271. doi: 10.1016/j.amjcard.2011.12.017. [DOI] [PubMed] [Google Scholar]
- Bosworth H, Granger B, Mendys P, Brindis R, Burkholder R, Czajkowski S, et al. Medication adherence: A call for action. American Heart Journal. 2011;162:412–424. doi: 10.1016/j.ahj.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvin JE, Shanbhag S, Avery E, Kane J, Richardson D, Powell L. Adherence to evidence-based guidelines for heart failure in physicians and their patients: Lessons from the Heart Failure Adherence Retention Trial (HART) Congestive Heart Failure. 2012;18:73–78. doi: 10.1111/j.1751-7133.2011.00263.x. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Eisen SA, Rich MW, Jaffe AS. Major depression and medication adherence in elderly patients with coronary artery disease. Health Psychology. 1995;14:88–90. doi: 10.1037/0278-6133.14.1.88. [DOI] [PubMed] [Google Scholar]
- Charlson M, Szatrowski T, Peterson J, Gold J. Validation of a combined comorbidity index. Journal of Clinical Epidemiology. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- Coleman CI, Limone B, Sobieraj DM, Lee S, Roberts MS, Kaur R, et al. Dosing frequency and medication adherence in chronic disease. Journal of Managed Care Pharmacy. 2012a;18:527–539. doi: 10.18553/jmcp.2012.18.7.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman CI, Roberts MS, Sobieraj DM, Lee S, Alam T, Kaur R. Effect of dosing frequency on chronic cardiovascular disease medication adherence. Current Medical Research and Opinion. 2012b;28:669–680. doi: 10.1185/03007995.2012.677419. [DOI] [PubMed] [Google Scholar]
- Cukor D, Rosenthal DS, Jindal RM, Brown CD, Kimmel PL. Depression is an important contributor to low medication adherence in hemodialyzed patients and transplant recipients. Kidney International. 2009;75:1223–1229. doi: 10.1038/ki.2009.51. [DOI] [PubMed] [Google Scholar]
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effect of anxiety and depression on patient adherence. Archives of Internal Medicine. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- Dunbar-Jacob J, Bohachick P, Mortimer MK, Sereika SM, Foley SM. Medication adherence in persons with cardiovascular disease. Journal of Cardiovascular Nursing. 2003;18:209–218. doi: 10.1097/00005082-200307000-00006. [DOI] [PubMed] [Google Scholar]
- Egede LE, Osborn CY. Role of motivation in the relationship between depression, self-care, and glycemic control in adults with type 2 diabetes. The Diabetes Educator. 2010;36:276–283. doi: 10.1177/0145721710361389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk K, Swedberg K, Gaston-Johansson F, Ekman I. Fatigue is a prevalent and severe symptom associated with uncertainty and sense of coherence in patients with chronic heart failure. European Journal of Cardiovascular Nursing. 2007;6:99–104. doi: 10.1016/j.ejcnurse.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Freedland KE, Rich MW, Skala JA, Carney RM, Dávila-Román VG, Jaffe AS. Prevalence of depression in hospitalized patients with congestive heart failure. Psychosomatic Medicine. 2003;65:119–128. doi: 10.1097/01.psy.0000038938.67401.85. [DOI] [PubMed] [Google Scholar]
- Gehi A, Haas D, Pipkin S, Whooley MA. Depression and medication adherence in outpatients with coronary heart disease: Findings from the Heart and Soul Study. Archives of Internal Medicine. 2005;165:2508–2513. doi: 10.1001/archinte.165.21.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J, Phun YT, Bailey MJ, Kong D, Stewart K. Development and validation of the medication regimen complexity index. The Annals of Pharmacotherapy. 2004;38:1369–1376. doi: 10.1345/aph.1D479. [DOI] [PubMed] [Google Scholar]
- Gislason GH, Rasmussen JN, Abildstrom SZ, Schramm TK, Hansen ML, Buch P, et al. Persistent use of evidence-based pharmacotherapy in heart failure is associated with improved outcomes. Circulation. 2007;116:737–744. doi: 10.1161/CIRCULATIONAHA.106.669101. [DOI] [PubMed] [Google Scholar]
- Gonzalez JS, Safren SA, Cagliero E, Wexler DJ, Delahanty L, Wittenberg E, et al. Depression, self-care, and medication adherence in type 2 diabetes: Relationships across the full range of symptom severity. Diabetes Care. 2007;30:2222–2227. doi: 10.2337/dc07-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JS, Tanenbaum ML, Commissariat PV. Psychosocial factors in medication adherence and diabetes self-management: Implications for research and practice. American Psychologist. 2016;71:539–551. doi: 10.1037/a0040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenard JL, Munjas BA, Adams JL, Suttorp M, Maglione M, McGlynn EA, et al. Depression and medication adherence in the treatment of chronic diseases in the United States: A meta-analysis. Journal of General Internal Medicine. 2011;26:1175–1182. doi: 10.1007/s11606-011-1704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes RB, McDonald HP, Garg AX. Helping patients follow prescribed treatment: Clinical applications. Journal of the American Medical Association. 2002;288:2880–2883. doi: 10.1001/jama.288.22.2880. [DOI] [PubMed] [Google Scholar]
- Hinkin CH, Castellon SA, Durvasula RS, Hardy DJ, Lam MN, Mason KI, et al. Medication adherence among HIV+ adults: Effects of cognitive dysfunction and regimen complexity. Neurology. 2002;59:1944–1950. doi: 10.1212/01.wnl.0000038347.48137.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: Its importance in cardiovascular outcomes. Circulation. 2009;119:3028–3035. doi: 10.1161/CIRCULATION.108.768986. [DOI] [PubMed] [Google Scholar]
- Jackson G, Gibbs CR, Davies MK, Lip GYH. ABC of heart failure pathophysiology. British Medical Journal. 2000;320:167–170. doi: 10.1136/bmj.320.7228.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Alexander J, Christopher E, Kuchibhatla M, Gaulden LH, Cuffe MS, et al. Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Archives of Internal Medicine. 2001;161:1849–1856. doi: 10.1001/archinte.161.15.1849. [DOI] [PubMed] [Google Scholar]
- Julian LJ, Yelin E, Yazdany J, Panopalis P, Trupin L, Criswell LA, et al. Depression, medication adherence, and service utilization in systemic lupus erythematosus. Arthritis Care & Research. 2009;61:240–246. doi: 10.1002/art24236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbourne AM, Reynolds CF, Good CB, Sereika SM, Justice AC, Fine M. How does depression influence diabetes medication adherence in older patients? American Journal of Geriatric Psychiatry. 2005;13:202–210. doi: 10.1176/appi.ajgp.13.3.202. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9. Journal of General Internal Medicine. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger KP, Berger BA, Felkey B. Medication adherence and persistence: A comprehensive review. Advances in Therapy. 2005;22:313–356. doi: 10.1007/BF02850081. [DOI] [PubMed] [Google Scholar]
- Lin EHB, Katon W, von Korff M, Rutter C, Simon GE, Oliver M, et al. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care. 2004;27:2154–2160. doi: 10.2337/diacare.27.9.2154. [DOI] [PubMed] [Google Scholar]
- Luttik ML, Jaarsma T, Moser D, Sanderman R, van Veldhuisen DJ. The importance and impact of social support on outcomes in patients with heart failure: An overview of the literature. Journal of Cardiovascular Nursing. 2005;20:162–169. doi: 10.1097/00005082-200505000-00007. [DOI] [PubMed] [Google Scholar]
- Malek N, Grosset DG. Medication adherence in patients with Parkinson’s disease. CNS Drugs. 2015;29:47–53. doi: 10.1007/s40263-014-0220-0. [DOI] [PubMed] [Google Scholar]
- Mausbach BT, Schwab RB, Irwin SA. Depression as a predictor of adherence to adjuvant endocrine therapy (AET) in women with breast cancer: A systematic review and meta-analysis. Breast Cancer Research and Treatment. 2015;152:239–246. doi: 10.1007/s10549-015-3471-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: Scientific review. Journal of the American Medical Association. 2002;28:2868–2879. doi: 10.1001/jama.288.22.2868. [DOI] [PubMed] [Google Scholar]
- Osborn CY, Egede LE. The relationship between depressive symptoms and medication non-adherence in type 2 diabetes: The role of social support. General Hospital Psychiatry. 2012;34:249–253. doi: 10.1016/j.genhosppsych.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterberg L, Blaschke T. Adherence to medication. New England Journal of Medicine. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- Powell LH, Catellier D, Freedland KE, Burg MM, Woods SL, Bittner V, et al. Depression and heart failure in patients with a new myocardial infarction. American Heart Journal. 2005;149:851–855. doi: 10.1016/j.ahj.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Richter A, Anton SE, Koch P, Dennett SL. The impact of reducing dose frequency on health outcomes. Clinical Therapeutics. 2003;25:2307–2335. doi: 10.1016/s0149-2918(03)80222-9. [DOI] [PubMed] [Google Scholar]
- Rieckmann N, Gerin W, Kronish IM, Burg MM, Chaplin WF, Kong G, et al. Course of depressive symptoms and medication adherence after acute coronary syndromes. Journal of the American College of Cardiology. 2006a;48:2218–2222. doi: 10.1016/j.jacc.2006.07.063. [DOI] [PubMed] [Google Scholar]
- Rieckmann N, Kronish IM, Haas D, Gerin W, Chaplin WF, Burg MM, et al. Persistent depressive symptoms lower aspirin adherence after acute coronary syndromes. American Heart Journal. 2006b;152:922–927. doi: 10.1016/j.ahj.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Riegel B, Lee C, Ratcliffe S, De Geest S, Potashnik S, Patey M, et al. Predictors of objectively measured medication nonadherence in adults with heart failure. Circulation Heart Failure. 2012;5:430–436. doi: 10.1161/CIRCHEARTFAILURE.111.965152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safren SA, Gonzalez JF, Wexler DJ, Psaros C, Delahanty LM, Blashill AJ, et al. Diabetes Care. 2014;37:625–633. doi: 10.2337/dc13-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin NL, DiMatteo MR. Depression treatment enhances adherence to antiretroviral therapy: A meta-analysis. Annals of Behavioral Medicine. 2014;47:259–269. doi: 10.1007/s12160-013-9559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng E, Chui H. The Modified Mini-Mental State Examination (3MS) Canadian Journal of Psychiatry. 1987;41:114–121. [PubMed] [Google Scholar]
- Udelson JE, Pressler SJ, Sackner-Bernstein J, Massaro J, Ordronneau P, Lukas MA, et al. Adherence with once daily versus twice daily carvedilol in patients with heart failure: The compliance and quality of life study comparing once-daily controlled-release Carvedilol CR and twice-daily immediate-release Carvedilol IR in patients with heart failure (CASPER) trial. Journal of Cardiac Failure. 2009;15:385–393. doi: 10.1016/j.cardfail.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Wu JR, Moser DK, de Jong MJ, Rayens MK, Chung ML, Riegel B, et al. Defining an evidence-based cutpoint for medication adherence in heart failure. American Heart Journal. 2009;157:285–291. doi: 10.1016/j.ahj.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JR, Moser DK, Lennie TA, Burkhart PV. Medication adherence in patients who have heart failure: A review of the literature. Nursing Clinics of North America. 2008;43:133–153. doi: 10.1016/j.cnur.2007.10.006. [DOI] [PubMed] [Google Scholar]