ABSTRACT
Particulate matter (PM) exposure during in utero life may entail adverse health outcomes in later-life. Air pollution's adverse effects are known to alter gene expression profiles, which can be regulated by microRNAs (miRNAs). We investigate the potential influence of air pollution exposure in prenatal life on placental miRNA expression. Within the framework of the ENVIRONAGE birth cohort, we measured the expression of six candidate miRNAs in placental tissue from 210 mother-newborn pairs by qRT-PCR. Trimester-specific PM2.5 exposure levels were estimated for each mother's home address using a spatiotemporal model. Multiple regression models were used to study miRNA expression and in utero exposure to PM2.5 over various time windows during pregnancy. The placental expression of miR-21 (−33.7%, 95% CI: −53.2 to −6.2, P = 0.022), miR-146a (−30.9%, 95% CI: −48.0 to −8.1, P = 0.012) and miR-222 (−25.4%, 95% CI: −43.0 to −2.4, P = 0.034) was inversely associated with PM2.5 exposure during the 2nd trimester of pregnancy, while placental expression of miR-20a and miR-21 was positively associated with 1st trimester exposure. Tumor suppressor phosphatase and tensin homolog (PTEN) was identified as a common target of the miRNAs significantly associated with PM exposure. Placental PTEN expression was strongly and positively associated (+59.6% per 5 µg/m³ increment, 95% CI: 26.9 to 100.7, P < 0.0001) with 3rd trimester PM2.5 exposure. Further research is required to establish the role these early miRNA and mRNA expression changes might play in PM-induced health effects. We provide molecular evidence showing that in utero PM2.5 exposure affects miRNAs expression as well as its downstream target PTEN.
KEYWORDS: miRNAs, placenta, air pollution, expression analysis, particulate matter
Abbreviations
- miRNA
microRNA
- PM
Particulate matter
- PM2.5
Particulate matter with diameter less than 2.5 μm
- NO2
Nitrogen dioxide
- CI
Confidence interval
- BMI
Body mass index
- FDR
False discovery rate
- qRT-PCR
Quantitative real-time polymerase chain reaction
- RS
Reporter assay
- qP
qPCR
- WB
Western blot
- IP
Immunoprecipitation
- MA
Microarrays
- Pr
Proteomics
- NGS
Next generation sequencing
- IQR
Interquartile range
- CCND1
Cyclin D1
- CDKN1A/B
Cyclin-dependent kinase inhibitor 1A/B
- TGF/TGFBR2
Transforming growth factor / Transforming growth factor beta receptor II
- STAT3/5
Signal transducer and activator of transcription 3/5
- E2F1
E2F transcription factor 1
- KIT
v-kit hardy-zuckerman 4 feline sarcoma viral oncogene homolog
- HIF1A
Hypoxia inducible factor 1, alpha subunit
- PTEN
Phosphatase and tensin homolog
- APAF1
Apoptotic peptidase activating factor 1
- CDC25A
Cell Division cycle 25A
- BCL2
B-cell CLL/lymphoma 2
- TLR
Toll-like receptor
- TRAF6
Tumor necrosis factor (TNF) receptor-associated factor 6
- NF-kB/ NFKB1
Nuclear factor of kappa light polypeptide gene enhancer in B-cells/1
- IRAK1
Interleukin-1 receptor-associated kinase 1
- ETS1
V-Ets avian erythroblastosis virus E26 oncogene homolog 1
- FOS
FBJ Murine osteosarcoma viral oncogene homolog
- MMP1
Matrix metallopeptidase 1
- FOXO3
Forkhead box O3
- PI3K/AKT
Phosphatidylinositol-3-kinase/Protein kinase B
- VEGF
Vascular endothelial growth factor
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- IPO8
Importine 8
- POLR2A
Polymerase (RNA) II (DNA Directed) Polypeptide A
- UBC
Ubiquitin C
- TERT
Telomerase reverse transcriptase
- TERC
Telomerase RNA template
Introduction
Particulate matter (PM) is an airborne mixture of solid particles and liquid droplets [1], of which fine particles with a diameter less than 2.5 µm (PM2.5) can be inhaled deeply into the lungs. This leads to the generation of oxidative stress and the induction of inflammation [2,3]. PM2.5 exposure contributes to the initiation and progression of various diseases affecting the respiratory and cardiovascular system [4–7]. According to Barker's hypothesis, early life perturbations are crucial for the development of disease later in life [8,9]. Exposure to ambient PM2.5 pollution during pregnancy is significantly associated with increased risk of low birth weight at term in mother-child cohorts of 12 European countries [10] and preterm birth in a very large cohort of singleton pregnancies (≥20 weeks of gestation) from three states of the USA [11].
Prenatal PM exposure has been shown to affect placental weight [12], function and morphology [12,13], and gene expression [14,15]. These processes affect fetal programming and could thereby increase the risk of disease later in life [16].
MicroRNAs (miRNAs) are single-stranded small non-coding RNAs of approximately 22 nucleotides that play a key role in the regulation of gene expression at the posttranscriptional level in many cellular processes, including proliferation and apoptosis, which could lead to diseases such as cancer [17–21]. Notably, miRNAs are able to regulate up to 30% of the human genome [22], where one single miRNA can affect the expression of hundreds of genes, whereas one gene can be targeted by many miRNAs [23].
In healthy adults, the blood-leukocyte expression of miR-146a and miR-222 was found inversely associated with air pollution exposure [24], while miR-21 and miR-222 expression was significantly increased in steel plant workers after exposure to metal-rich PM [25]. Inhalation of ozone was shown to disrupt miRNA expression profiles in human induced-sputum samples and network analysis of the 10 miRNAs with significantly increased expression levels revealed an association with diverse biological processes, including inflammatory and immune response signaling [26].
Interestingly, exposure to environmental agents induces altered miRNA expression patterns both in placental cell lines [27] and cord blood [28], which could potentially contribute to adverse fetal development and health outcomes later in life. Maccani et al. [29] showed that maternal smoking during pregnancy was inversely associated with placental expression of miR-16, miR-21 and miR-146a. Therefore, placenta could be used as an appropriate target organ to assess the impact of air pollution on miRNA expression in the early-life environment.
To date, the potential modulation of placental miRNA expression in association with prenatal exposure to air pollution has not been investigated. For this purpose, six candidate miRNAs, namely miR-16, -20a, -21, -34a, -146a and -222, related to important cellular processes [30] were selected, based on a systematic review [31]. miR-16 and miR-21 are involved in cell cycle, proliferation, and apoptosis [29,32–37]. miR-146a has been described as a regulator of inflammation [29,38]. miR-20a, miR-34a, and miR-222 function in angiogenesis [39–42]. Maternal exposure to air pollution has been suggested to adversely affect pregnancy by inducing oxidative stress and inflammation [5], which may result in impaired placental angiogenesis [12].
In the current study, we investigate whether in utero exposure to particulate matter and nitrogen dioxide during different periods of gestation is associated with placental expression of six candidate miRNAs. We hypothesize that in utero PM exposure might induce epigenetic alterations at the placental miRNA level. To assess whether any miRNA expression alterations could have a functional effect, we also measured expression of a downstream mRNA target.
Results
Characteristics of the study population and air pollution exposure
In the present study, 210 mother-newborns pairs with a mean age of 29.5 years (±4.3) and mean pre-gestational BMI of 24.1 kg/m2 (±4.8) were included. As shown in Table 1, 70% of women never smoked, 14.8% smoked during pregnancy (current-smokers), and the remaining 15.2% quit smoking at the start of pregnancy (past-smokers). For approximately half of the mothers, the newborn was their first child, and 56.2% of the mothers were highly educated. One hundred and fifteen (54.8%) newborns were girls, had a mean gestational age of 39.2 weeks (±1.3), and an average birth weight of 3,395 g (±427); 190 (90.5%) of the newborns had European-Caucasian ethnicity. The frequencies of conception of pregnancies were approximately equally distributed into the four seasons, with the highest rate (29.1%) observed in summer. The average apparent temperature during the 3rd trimester of pregnancy was divided into quartiles of the distribution.
Table 1.
Characteristics of mother-newborn pairs.
| Mean ± SD / Frequency (%) |
||
|---|---|---|
| Characteristics | Original study (n = 210) | Validation study (n = 181) |
| Maternal | ||
| Age, years | 29.5 ± 4.3 | 29.4 ± 4.2 |
| Pre-gestational BMI, kg/m2 | 24.1 ± 4.8 | 24.3 ± 5.0 |
| Smoking status | ||
| Never-smoker | 147 (70.0) | 129 (71.3) |
| Past-smoker | 32 (15.2) | 27 (14.9) |
| Current- smoker | 31 (14.8) | 25 (13.8) |
| Parity | ||
| 1 | 106 (50.5) | 89 (49.2) |
| 2 | 84 (40.0) | 74 (40.9) |
| ≥3 | 20 (9.5) | 18 (9.9) |
| Education | ||
| Low | 23 (10.9) | 20 (11.1) |
| Middle | 69 (32.9) | 59 (32.6) |
| High | 118 (56.2) | 102 (56.3) |
| Newborn | ||
| Gender | ||
| Female | 115 (54.8) | 102 (56.3) |
| Gestational age, weeks | 39.2 ± 1.3 | 39.1 ± 1.3 |
| Birth weight, g | 3,395 ± 427 | 3,384 ± 429 |
| Ethnicity | ||
| European-Caucasian | 190 (90.5) | 163 (90.1) |
| Non-European | 20 (9.5) | 18 (9.9) |
| Other | ||
| Apparent Temperature, oC | ||
| Third trimester (quartiles) | ||
| < Q1 | 53 (25.2) | 46 (25.4) |
| ≥ Q1 and < Q2 | 52 (27.8) | 45 (24.9) |
| ≥ Q2 and < Q3 | 52 (27.8) | 44 (24.3) |
| ≥ Q3 | 53 (25.2) | 46 (25.4) |
| Seasonality (at conception) | ||
| Winter | 57 (27.1) | 53 (29.3) |
| Spring | 50 (23.8) | 38 (21.0) |
| Summer | 61 (29.1) | 55 (30.4) |
| Fall | 42 (20.0) | 35 (19.3) |
The mean outdoor exposures to PM2.5 and NO2 averaged for each of the three trimesters of pregnancy are presented in Table 2.
Table 2.
Characteristics of particulate air pollution exposure. Averaged for each mother-newborn pair during the different time windows during pregnancy.
| Air pollutant (µg/m3) | Mean ± SD | IQR | 10th Percentile | 90th Percentile |
|---|---|---|---|---|
| Original study (n = 210) | ||||
| PM2.5 | ||||
| Trimester 1 (1-13 w) | 15.99 ± 5.29 | 8.08 | 10.22 | 24.65 |
| Trimester 2 (14-26 w) | 16.38 ± 5.06 | 8.19 | 10.39 | 23.00 |
| Trimester 3 (27-delivery) | 16.74 ± 5.82 | 9.43 | 10.07 | 25.54 |
| NO2 | ||||
| Trimester 1 (1-13 w) | 19.97 ± 5.86 | 9.09 | 12.74 | 28.06 |
| Trimester 2 (14-26 w) | 20.69 ± 6.04 | 7.95 | 12.98 | 29.10 |
| Trimester 3 (27-delivery) | 20.91 ± 6.46 | 8.49 | 12.74 | 29.35 |
| Validation study (n = 181) | ||||
| PM2.5 | ||||
| Trimester 1 (1-13 w) | 16.12 ± 5.32 | 8.13 | 10.28 | 24.87 |
| Trimester 2 (14-26 w) | 16.49 ± 5.07 | 8.33 | 10.59 | 23.04 |
| Trimester 3 (27-delivery) | 16.93 ± 5.98 | 10.07 | 10.08 | 25.77 |
| NO2 | ||||
| Trimester 1 (1-13 w) | 20.18 ± 5.85 | 9.23 | 12.79 | 28.09 |
| Trimester 2 (14-26 w) | 20.85 ± 6.24 | 8.60 | 13.01 | 29.72 |
| Trimester 3 (27-delivery) | 20.99 ± 6.64 | 8.28 | 12.69 | 29.84 |
Association of miRNAs with exposure to air pollution
Figure 1 shows the change in placental miRNA expression in association with exposure across the three trimesters of pregnancy for PM2.5 and NO2.
Figure 1.
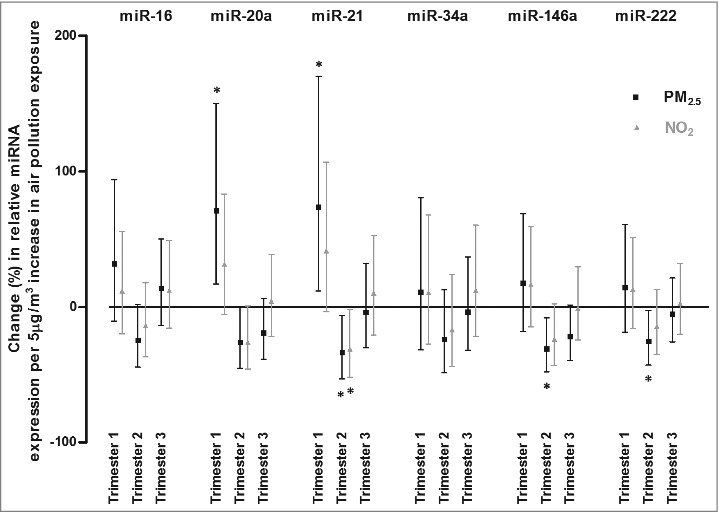
Associations of relative miRNA expression with in utero exposure to air pollution. Associations are presented as percentage changes in relative miRNA (miR-16, miR-20a, miR-21, miR-34a, miR-146a, and miR-222) expression across the three trimesters of pregnancy, for each 5 µg/m3 increase in PM2.5 exposure (black square) and in NO2 exposure (grey triangle). Estimates were adjusted for newborn's gender, gestational age (weeks) and ethnicity (European, non-European), maternal age (years), pre-gestational BMI (kg/m2), smoking status (never-, past- or current-smoker), educational status (low, middle or high), parity (1, 2, or ≥3), seasonality at conception and apparent temperature (during the third trimester). Asterisk (*) indicates statistically significant (P < 0.05).
PM2.5 exposure during the 2nd trimester of gestation was most significantly associated with miRNA expression changes. Placental miR-16 (−24.7%, 95% CI: −44.4 to 2.1, P = 0.069), miR-20a (−26.0%, 95% CI: −45.2 to 0.0, P = 0.052), miR-21 (−33.7%, 95% CI: −53.2 to −6.2, P = 0.022), miR-146a (−30.9%, 95% CI: −48.0 to −8.1, P = 0.012), and miR-222 (−25.4%, 95% CI: −43.0 to −2.4, P = 0.034) expression were inversely associated with PM2.5 exposure during the 2nd trimester of pregnancy. Additionally, miR-146a expression was inversely associated (−21.8%, 95% CI: −39.7 to 1.5, P = 0.066) with 3rd trimester exposure. We found positive associations between 1st trimester particulate air pollution exposure and placental expression of miR-20a (+70.9%, 95% CI: 16.7 to 150.3, P = 0.007) and miR-21 (+73.7%, 95% CI: 11.7 to 170.1, P = 0.015). All estimates were calculated for an increase in PM2.5 exposure of 5 µg/m3.
We obtained similar findings for NO2 exposure, miR-20a (−26.2%, 95% CI: −46.0 to 0.9, P = 0.058), miR-21 (−31.3%, 95% CI: −51.9 to −1.6, P = 0.042), and miR-146a (−23.8%, 95% CI: −43.3 to 2.3, P = 0.072) were inversely associated with NO2 exposure during the 2nd trimester, while a positive association was observed for the placental expression of miR-21 (+41.4%, 95% CI: −3.4 to 106.9, P = 0.076) at term with NO2 exposure during the 1st trimester of pregnancy. Estimates were calculated for an increase in NO2 exposure of 5 µg/m3.
miRNA target prediction and pathway analysis
A list with putative predicted targets compiled from mirTarBase and DIANA-TarBase with all relevant information about their function and methods used for target validation is provided in Table 3.
Table 3.
In silico putative mRNA targets for placental miRNAs under study. For each miRNA, the mRNA targets (n = 15), description, function and the experimentally validated methods are indicated.
| miRNAs | Target mRNAs | Description | Function | Validated methods |
|---|---|---|---|---|
| miR-16 | CCNE1 | Cyclin E1 | Cell cycle | RS, WB, qP, NGS[b], MA[a,b], IP[a] |
| BCL2 | B-cell CLL/lymphoma 2 | Apoptosis | RS, WB, qP, MA, NGS[b], IP[a] | |
| ARL2 | ADP-ribosylation factor-like 2 | Cell cycle | RS, WB, qP[b], MA[a,b], IP[a] | |
| HMGA1 | High mobility group AT-hook 1 | Controls many cellular processes | RS, WB, qP, NGS[b], IP[a] | |
| CDK6 | Cyclin-dependent kinase 6 | Cell cycle | RS, WB, qP, NGS[b], IP[a] | |
| CCND1 | Cyclin D1 | Cell cycle | RS, WB, qP, NGS[b], IP[a] | |
| CCND3 | Cyclin D3 | Cell cycle | RS, WB, qP[b], IP[a] | |
| CHUK | Conserved Helix-Loop-Helix Ubiquitous Kinase | NF-kappa-B signaling pathway | RS[b], WB, qP[a,b], IP[a] | |
| RECK | Reversion-Inducing-Cysteine-Rich Protein With Kazal Motifs | Suppressor of tumorigenicity | RS, WB, qP, NGS[b], IP[a] | |
| CAPRIN1 | Cell cycle associated protein 1 | Synaptic plasticity in neurons & cell proliferation | RS, WB, qP[b], IP[a] | |
| PPM1D | Protein phosphatase, Mg+2/Mn+2dependent, 1D | Cell cycle | RS, WB, qP[b], IP[a] | |
| HMGA2 | High Mobility Group AT-Hook 2 | Cell cycle | RS, WB, qP[b], IP[a] | |
| FGFR1 | Fibroblast Growth Factor Receptor 1 | Controls many cellular processes | RS, WB, qP[b], IP[a] | |
| ZYX | Zyxin | Signal transduction | RS, WB, qP[b], IP[a] | |
| VEGFA | Vascular endothelial growth factor A | Angiogenesis & endothelial cell growth | RS, WB, qP[a,b], NGS[b] | |
| miR-20a | TGFBR2 | Transforming growth factor, beta receptor II | Controls many cellular processes | RS[a,b], WB, qP, MA,NGS[b], IP[a] |
| E2F1 | E2F transcription factor 1 | Cell cycle & DNA replication | RS, WB, qP, MA, NGS[b], IP[a] | |
| CDKN1A | Cyclin-Dependent Kinase Inhibitor 1A | Cell cycle | RS, WB, qP[a,b], NGS[b], IP[a] | |
| STAT3 | Signal Transducer And Activator Of Transcription 3 (Acute-Phase Response Factor) | JAK-STAT signaling cascade | RS, WB, qP, MA, NGS[b], IP[a] | |
| LIMK1 | LIM Domain Kinase 1 | Regulation of actin filament dynamics & signal transduction | RS, WB, qP, MA[b], IP[a] | |
| DUSP2 | Dual Specificity Phosphatase 2 | Regulates mitogenic signal transduction | RS, WB, qP, NGS[b], IP[a] | |
| BMPR2 | Bone morphogenetic protein receptor, type II (serine/threonine kinase) | Endochondral bone formation & embryogenesis | RS, WB, qP, NGS[b], IP[a] | |
| APP | Amyloid beta (A4) precursor protein | Neurite growth, neuronal adhesion & axonogenesis | RS, WB, qP[b], IP[a] | |
| RUNX1 | Runt-related transcription factor 1 | Development of normal hematopoiesis | RS, WB, qP[b], IP[a] | |
| MAP3K5 | Mitogen-Activated Protein Kinase Kinase Kinase 5 | In cascades of cellular responses | RS, WB, qP[b], IP[a] | |
| HIF1A | Hypoxia Inducible Factor 1, Alpha Subunit | Energy metabolism, angiogenesis, apoptosis | RS, WB, qP, NGS[b] | |
| BNIP2 | BCL2/adenovirus E1B 19kDa interacting protein 2 | Suppression of cell death | WB, qP, NGS[b], IP[a] | |
| CCND1 | Cyclin D1 | Cell cycle | RS[a,b], WB, qP, NGS[b] | |
| PTEN | Phosphatase and tensin homolog | Tumor suppressor | RS[a,b], WB, qP, NGS[b] | |
| KIT | v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog | Proto-oncogene | RS, WB, qP, MA[b] | |
| miR-21 | BTG2 | BTG family, member 2 | Cell cycle | RS, WB, qP, MA,NGS[b], IP[a] |
| PDCD4 | Programmed cell death 4 (neoplastic transformation inhibitor) | Inhibits translation initiation | RS, WB, qP, MA,NGS[b], IP[a] | |
| TGFBR2 | Transforming growth factor, beta receptor II | Controls many cellular responses | RS, WB, qP, MA[a,b], IP[a] | |
| NFIB | Nuclear Factor I/B | Transcription & replication | RS, WB, qP[b], IP, MA[a] | |
| CDC25A | Cell Division Cycle 25A | Cell cycle | RS, qP, NGS[b], MA[a,b], IP[a] | |
| RASGRP1 | RAS guanyl releasing protein 1 (calcium and DAG-regulated) | Regulates T- & B-cells development | RS, WB, qP, NGS[b], MA[a,b] | |
| JAG1 | Jagged 1 | Notch signaling- in cell-fate during hematopoiesis, in early & late stages of mammalian cardiovascular development | RS, WB, NGS[b], IP[a] | |
| APAF1 | Apoptotic peptidase activating factor 1 | Activation of CASP3 | RS, WB, qP[b], MA[a,b] | |
| TIMP3 | TIMP metallopeptidase inhibitor 3 | Inhibits matrix metalloproteinases | RS, WB, qP, MA[a,b] | |
| SOX5 | SRY (sex determining region Y)-box 5 | Transcription factor- embryonic development & cell fate | RS, WB, qP, MA[b] | |
| RECK | Reversion-inducing-cysteine-rich protein with kazal motifs | Tumor invasion and metastasis | RS, WB, qP, MA[a,b] | |
| PTEN | Phosphatase and tensin homolog | Tumor suppressor | RS, WB, qP, MA[a,b] | |
| TPM1 | Tropomyosin 1 (alpha) | Ca+2 dependent regulation of striated muscle contraction | RS, WB, qP, MA[b] | |
| BCL2 | B-cell CLL/lymphoma 2 | Apoptosis | RS, WB, qP, NGS[b] | |
| E2F1 | E2F transcription factor 1 | Cell cycle & DNA replication | RS, WB, qP[b] | |
| miR-34a | CDK6 | Cyclin-dependent kinase 6 | Cell cycle | RS, WB, qP, MA[a,b], NGS[b], IP[a] |
| CCNE2 | Cyclin E2 | Cell cycle | RS, WB, qP, NGS[b], MA[a,b], IP[a] | |
| E2F3 | E2F transcription factor 3 | Cell cycle & DNA replication | RS, NGS[b], WB, qP, MA[a,b], IP[a] | |
| CDK4 | Cyclin-dependent kinase 4 | Cell cycle | RS, WB, qP[a,b], MA[b], IP[a] | |
| NOTCH1 | Notch 1 | Variety of developmental processes by controlling cell fate decisions- development | RS, WB, qP[a,b], MA[b], IP[a] | |
| NOTCH2 | Notch 2 | Variety of developmental processes by controlling cell fate decisions- development | RS, WB, qP, MA[a,b], IP[a] | |
| MYC | v-myc avian myelocytomatosis viral oncogene homolog | Cell cycle, apoptosis & cellular transformation | RS, WB, qP, MA, NGS[b] | |
| JAG1 | Jagged 1 | Notch signaling- in cell-fate during hematopoiesis, in early & late stages of mammalian cardiovascular development | RS, WB, qP[a,b], MA[b] | |
| CCND1 | Cyclin D1 | Cell cycle | RS, NGS[b], WB, qP[a,b], | |
| BCL2 | B-cell CLL/lymphoma 2 | Apoptosis | RS, WB, qP[a,b], MA[b] | |
| MYB | V-Myb Avian Myeloblastosis Viral Oncogene Homolog | Hematopoiesis & tumorigenesis | RS, MA[a,b], WB, qP[b] | |
| SIRT1 | Sirtuin 1 | Coordination of several separated cellular functions such as cell cycle, | RS, WB, qP[a,b], MA[a] | |
| HNF4A | Hepatocyte nuclear factor 4, alpha | Development of | RS, WB, MA[a,b], qP[b] | |
| MET | MET Proto-Oncogene, Receptor Tyrosine Kinase | Controls many cellular processes | RS[b], WB, qP, MA[a,b] | |
| MYCN | V-Myc Avian Myelocytomatosis Viral Oncogene Neuroblastoma Derived Homolog | Transcription factor | RS, WB, qP, MA[b] | |
| miR-146a | IRAK1 | Interleukin-1 Receptor-Associated Kinase 1 | Innate immune response | RS, qP, MA[b], WB[a,b], IP[a] |
| PTGS2 | Prostaglandin-Endoperoxide Synthase 2 (Prostaglandin G/H Synthase And Cyclooxygenase) | Inflammatory prostaglandins | RS, WB, qP[b], IP[a] | |
| STAT1 | Signal Transducer And Activator Of Transcription 1, 91kDa | Mediates cellular responses to interferons & cytokines | RS, WB, qP[b], IP[a] | |
| NFKB1 | Nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 | Transcription factor-immune response | RS, WB, qP, MA, NGS[b] | |
| CXCR4 | Chemokine receptor 4 | Maintenance of immune function | RS, qP, MA[b], WB[a,b] | |
| SMAD4 | SMAD family member 4 | TGF-beta signaling | RS, WB, qP[a,b], MA[b] | |
| BRCA1 | Breast cancer 1, early onset | Tumor suppressor & maintains genomic stability | RS, WB, qP, MA[b] | |
| EGFR | Epidermal growth factor receptor | Controls many cellular responses | RS, qP[b], WB[a,b] | |
| TLR2 | Toll-Like Receptor 2 | Innate immune system | RS,WB[b], qP[a,b] | |
| TRAF6 | TNF receptor-associated factor 6, E3 ubiquitin protein ligase | Immune response | RS, WB, qP, MA[b] | |
| TLR4 | Toll-like receptor 4 | Innate immune system | RS, WB, qP, MA[b] | |
| CD40LG | CD40 Ligand | Immune system | RS, WB, qP[b] | |
| CARD10 | Caspase Recruitment Domain Family, Member 10 | Apoptosis | RS, WB, qP[a,b] | |
| NUMB | Numb Homolog (Drosophila) | Cell fates during development & neurogenesis | RS, WB, qP[b] | |
| ELAVL1 | ELAV Like RNA Binding Protein 1 | Variety of biological processes & diseases | RS, WB, qP[b] | |
| miR-222 | CDKN1B | Cyclin-Dependent Kinase Inhibitor 1B | Cell cycle | RS, WB[a,b], qP, MA, NGS[b], IP[a] |
| FOS | FBJ murine osteosarcoma viral oncogene homolog | Signal transduction, cell proliferation & differentiation | RS, WB, qP, NGS[b], IP[a] | |
| TRPS1 | Trichorhinophalangeal syndrome I | Transcriptional repressor | RS, WB, qP, MA[b], IP[a] | |
| ETS1 | V-Ets Avian Erythroblastosis Virus E26 Oncogene Homolog 1 | Transcription factor in wide variety of different cellular processes | RS[a,b], WB, qP[b], IP[a] | |
| KIT | v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog | Proto-oncogene | RS[a,b], WB, qP, MA[b] | |
| SOD2 | Superoxide Dismutase 2, mitochondrial | Binds to O2− | RS, WB, qP, MA[b] | |
| MMP1 | Matrix metallopeptidase 1 | Cleaves collagens | RS, WB, qP, MA[b] | |
| PTEN | Phosphatase and tensin homolog | Tumor suppressor | RS, WB, qP, MA[b] | |
| STAT5A | Signal transducer and activator of transcription 5A | Signal transduction & activation of transcription | RS, WB, qP[b] | |
| FOXO3 | Forkhead box O3 | Transcription factor for apoptosis | RS, WB, qP[b] | |
| CDKN1C | Cyclin-dependent kinase inhibitor 1C | Negative regulator of cell proliferation | RS[a,b], WB, qP[b] | |
| ESR1 | Estrogen receptor 1 | Controls many cellular processes | RS, WB, qP[b] | |
| TMED7 | Transmembrane Emp24 Protein Transport Domain Containing 7 | Vesicular protein trafficking | WB, qP[b], IP[a] | |
| CERS2 | Ceramide Synthase 2 | Regulates cell growth | RS, WB, qP[b] | |
| DKK2 | Dickkopf WNT Signaling Pathway Inhibitor 2 | In embryonic development & Wnt signaling | RS, WB, qP[b] |
Pathway analysis was performed in MetaCore™ by uploading top 15 experimentally validated miRNA targets of miR-20a, miR-21, miR-146a, and miR-222; the 5 most significant enriched pathways for each set of miRNA targets are indicated in Table 4. Within the top significant identified pathways we identified cell cycle- and cancer-related pathways from the putative targets of miR-20a, apoptosis- and cancer-related pathways for miR-21, immune-related pathways for miR-146a, and hematopoiesis-, cell cycle- and immune-related pathways for miR-222.
Table 4.
Functional enrichment analysis for the putative target genes of deregulated miRNAs in association with air pollution exposure (miR-20a, miR-21, miR-146a, and miR-222). The top 5 enriched MetaCore™ pathways, the P-value, FDR (P-value corrected for multiple testing) and the genes present in our dataset and involved in the listed pathways are provided.
| Pathways | P-value | %FDR | Genes involved |
|---|---|---|---|
| miR-20a targets | |||
| Upregulation of MITF in melanoma | 7.24E-07 | 1.00E-04 | E2F1, c-Kit, CDKN1A (p21), HIF1A |
| Cell cycle: Regulation of G1/S transition (part 1) | 9.05E-07 | 1.00E-04 | Cyclin D1, CDKN1A (p21), Cyclin D, TGF-beta receptor type II |
| Transcription Androgen Receptor nuclear signaling | 1.81E-06 | 1.34E-04 | Cyclin D1, CDKN1A (p21), TGF-beta receptor type II, STAT3 |
| IL-6 signaling in multiple myeloma | 3.01E-06 | 1.56E-04 | Cyclin D1, E2F1, CDKN1A (p21), STAT3 |
| Cell cycle: Influence of Ras and Rho proteins on G1/S Transition | 3.52E-06 | 1.56E-04 | Cyclin D1, E2F1, CDKN1A (p21), STAT3 |
| miR-21 targets | |||
| Development: Regulation of epithelial-to-mesenchymal transition (EMT) | 9.98E-07 | 1.45E-04 | Jagged1, TGF-beta receptor type II, Tropomyosin-1, Bcl-2 |
| Apoptosis and survival: p53-dependent apoptosis | 6.02E-06 | 4.36E-04 | E2F1, Apaf-1, Bcl-2 |
| Cell cycle: | 3.21E-04 | 9.37E-03 | CDC25A, E2F1 |
| Mitogenic action of Estradiol / ESR1 (nuclear) in breast cancer | 5.75E-04 | 9.37E-03 | CDC25A, E2F1 |
| Cell cycle: Role of SCF complex in cell cycle regulation | 6.17E-04 | 9.37E-03 | CDC25A, E2F1 |
| miR-146a targets | |||
| Signal transduction: NF-kB activation pathways | 4.91E-12 | 1.32E-09 | TLR2, TRAF6, NF-kB, NF-kB1 (p105), NF-kB1 (p50), IRAK1, TLR4 |
| Immune response: TLR2 and TLR4 signaling pathways | 1.11E-11 | 1.50E-09 | TLR2, TRAF6, NF-kB, NF-kB1 (p105), IRAK1, COX-2 (PTGS2), TLR4 |
| Immune response: Bacterial infections in normal airways | 4.20E-10 | 3.76E-08 | STAT1, TLR2, TRAF6, NF-kB, IRAK1/2, TLR4 |
| Immune response: HSP60 and HSP70/ TLR signaling pathway | 7.71E-10 | 5.18E-08 | TLR2, TRAF6, NF-kB, NF-kB1 (p105), IRAK1/2, TLR4 |
| Immune response: Role of PKR in stress-induced antiviral cell response | 8.17E-08 | 4.40E-06 | STAT1, TLR2, TRAF6, NF-kB, TLR4 |
| miR-222 targets | |||
| Development: c-Kit ligand signaling pathway during hemopoiesis | 2.96E-06 | 6.13E-04 | FOXO3A, c-Kit, CDKN1B (p27KIP1), STAT5 |
| Immune response: MIF-mediated glucocorticoid regulation | 6.45E-06 | 6.68E-04 | ETS1, c-Fos, MMP-1 |
| Cell cycle: ESR1 regulation of G1/S transition | 2.26E-05 | 1.20E-03 | CDKN1B (p27KIP1), c-Fos, ESR1 (nuclear) |
| Immune response: Oncostatin M signaling via MAPK in human cells | 3.20E-05 | 1.20E-03 | c-Fos, MMP-13, MMP-1 |
| Immune response: IL-7 signaling in T lymphocytes | 3.47E-05 | 1.20E-03 | FOXO3A, STAT5A, STAT5 |
The common putative pathways regulated by the top predicted targets (n = 15) of the significant associated miRNAs with PM exposure were identified using the pathway map tool in MetaCore™. The top 10 significant common pathways for all PM-related miRNAs are illustrated in Figure 2. Furthermore, a gene network was generated for the predicted miRNA targets (Figure 3).
Figure 2.
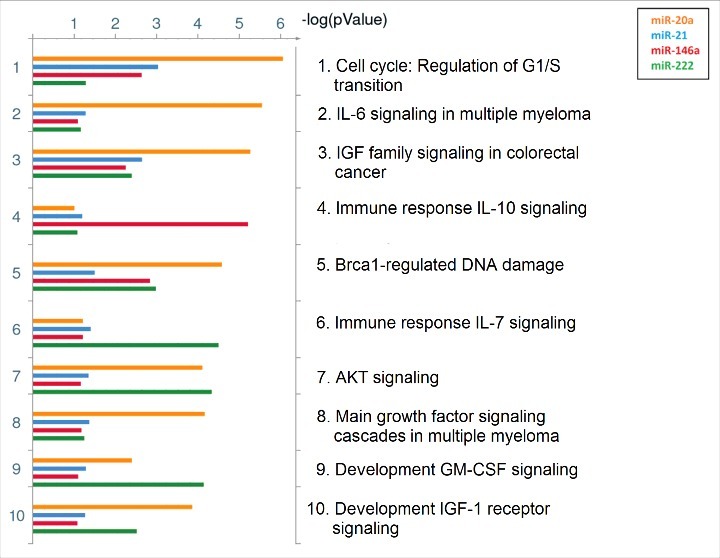
Common putative pathways regulated by identified targets of the significant miRNAs. The top 10 shared pathways for targets of miR-20a, miR-21, miR-146a, and miR-222 are ranked based on their minimum P-value, provided by MetaCore™. Pathways regulated by miR-20a are indicated with orange bars, miR-21 with blue bars, miR-146a with red bars, and miR-222 with green bars. Size of the bars is indicative of the P-value for that respective miRNA.
Figure 3.
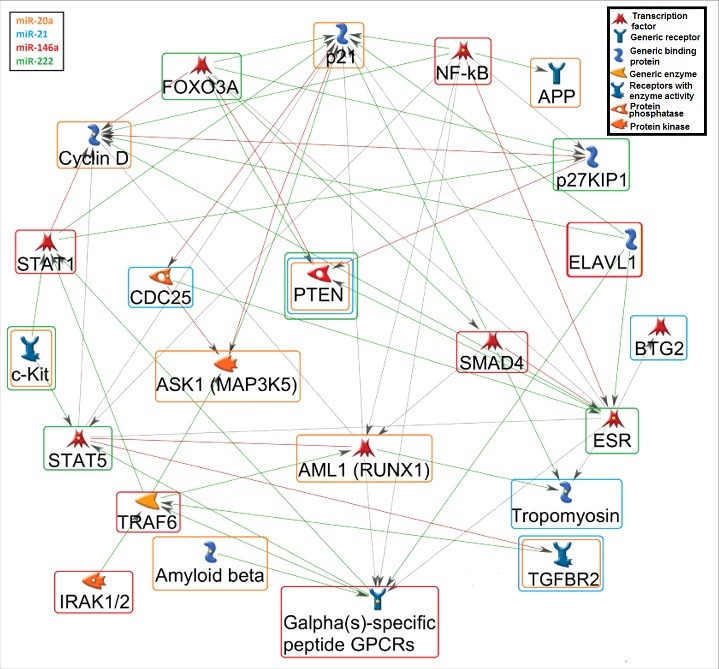
Gene network among the putative miRNA targets. A gene network (MetaCore™) was generated for the potential connections of at least two miRNA-targets. The orange rounded rectangle corresponds to miR-20a, blue to miR-21, red to miR-146a, and green to miR-222 targets. The green arrows show activation, the red arrows indicate inhibition, and the grey arrows are unspecified connections. Details for the genes shown in the figure: AML1 (RUNX1): Runt-related transcription factor 1; APP: Amyloid beta (A4) precursor protein; ASK1 (MAP3K5): Mitogen-Activated Protein Kinase Kinase Kinase 5; BTG2: BTG family, member 2; CCND: Cyclin D; CDC25: Cell Division Cycle 25; CDKN1A (p21): Cyclin-Dependent Kinase Inhibitor 1A; CDKN1B (p21KIP1): Cyclin-dependent kinase inhibitor 1A/B; ELAVL1: ELAV Like RNA Binding Protein 1; ESR: Estrogen receptor; FOXO3A Forkhead box O 3A; GPCRs (CXCR4): Chemokine receptor 4 (G Protein-Coupled Receptors); IRAK1/2: Interleukin-1 Receptor-Associated Kinase 1/2; c-KIT: v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog; NFKB: Nuclear factor of kappa light polypeptide gene enhancer in B-cells; PTEN: Phosphatase and tensin homolog; SMAD4: SMAD family member 4; STAT1/5: Signal Transducer And Activator Of Transcription 1/5; TGFBR2: Transforming growth factor, beta receptor II; TPM1: Tropomyosin; TRAF6: TNF receptor-associated factor 6.
Validation of miRNA target
PTEN is a predicted target for three of the four miRNAs significantly associated with PM exposure in placental tissue (Figure 3). In order to validate this miRNA target, we measured its gene expression (PTEN) by means of qRT-PCR in a subset (n = 181) of our study population. As expected, the placental relative PTEN expression was inversely correlated with the three miRNA candidates: the Pearson correlation coefficients were −0.18 (P = 0.013), −0.27 (P = 0.0003), and −0.25 (P = 0.0007), for miR-20a, miR-21, and miR-222, respectively.
Placental relative PTEN expression was strongly and positively associated with third trimester PM2.5 exposure (+59.6% per 5 µg/m³ increment, 95% CI: 26.9 to 100.7, P < 0.0001) and borderline significantly associated with third trimester NO2 exposure (+25.7% per 5 µg/m³ increment, 95% CI: −0.9 to 59.4, P = 0.061). The detailed estimates of the other trimesters for PTEN and the three regulatory miRNAs are given in Supplementary Figure S1.
Discussion
Epigenetic modifications by miRNAs may provide a plausible link between in utero exposure to particulate air pollution and alterations in gene expression that might lead to disease phenotypes related to fetal programming [16]. The placenta plays a crucial role in transfer of nutrients and oxygen from the mother to the fetus. Therefore, perturbations in the maternal environment can be transmitted to the fetus by changes in placental functions. Since particulate matter exposure has been shown to affect miRNA expression both in animal [43] and human studies [44], it is perceivable that miRNAs could be involved in regulating the in utero response to PM exposure as well. Therefore, we studied the expression of six candidate miRNAs involved in many biological processes (cell proliferation, cell cycle, apoptosis, inflammation, angiogenesis) related to air pollution or environmental stressors exposure [25,29,31]. We found that the placental expression of several candidate miRNAs involved in important biological processes was inversely associated with in utero particulate air pollution exposure, mainly during the second trimester of pregnancy. These molecular epidemiological observations might have important health consequences as downregulation of miR-16 and miR-21 has been shown to be significantly associated with fetal growth restriction [37], a condition which may result in many complications including preeclampsia [45].
Our inverse association between second trimester PM exposure and placental miR-miR-146a expression at birth is consistent with observations in a recent study [46] in which miR-146a expression at birth, among other miRNAs, was found to be associated with placental lead levels. Our observations on PM-induced placental miRNA changes in newborns are parallel to observations in placentas of mothers who smoked during pregnancy. Significant lower miRNAs expression of miR-16, miR-21, and miR-146a, were identified in placentas from smoking mothers [29]. However, in our study population, smoking had no significant effect on studied miRNA expression (data not shown).
Similarly, investigators of the Normative Aging Study showed in 77-year-old men downregulation of blood leukocyte miRNAs expression in miR-21, miR-146a, and miR-222 in association with 7-day average PM and black carbon exposure [24]. However, Bollati et al. [25] observed significantly higher expression of miR-21 and miR-222 pre- and post-exposure in peripheral blood leukocytes from 63 steel factory workers. The differences between the direction of the effect in which PM exposure on the studied miRNAs between our observations in newborns and the Normative Ageing Study [24] might be attributed to the different tissue, duration, magnitude, and/or composition of the PM exposure.
Our study investigated the association between placental expression of miRNA at birth in associations with exposure to ambient particulate air pollution for different time windows of gestation. The sensitivity of the epigenetic system to environmental factors occurs primarily during the period of developmental plasticity, as this is the time point when epigenetic marks undergo critical modifications [47]. Previously, we reported that exposure to particulate air pollution from fertilization up to and including embryo implantation was associated with lower global DNA methylation levels in placental tissue at birth [48]. In the current study, we observed significant associations between lower miRNA expression and PM exposure during the second trimester of pregnancy, which indicates that this is a critical time window for PM-related epigenetic changes at the level of miRNA. For “miR-20a and -21”, we found significant higher placental expression at birth in association with prenatal particulate matter exposure during the 1st trimester, in contrast to an inverse association with PM exposure during the second trimester. It is not uncommon that expression changes are specific to the time window of exposure; for example, miR-9 expression decreased upon ethanol exposure in early development stages in mice and fish [49,50], whereas it increased at later developmental stages in mice and in adult rats [51,52]. It is conceivable that a similar mechanism of action could regulate the response to air pollution exposure for the different trimesters of pregnancy, as each developmental time window has its own hallmark physiological events, regulated by different molecular processes [53].
Recently, miRNA expression has been also shown to be regulated by telomerase reverse transcriptase (TERT) at early stages of miRNA biogenesis. Particularly, suppression of TERT decreased the levels of miRNA expression in human cells, including miR-20a, miR-21, and miR-222 [54]. TERT and telomerase RNA component (TERC) are essential elements of telomerase, a ribonucleoprotein complex responsible for the telomere elongation [55]. Previously, higher maternal residential traffic exposure has been associated with shorter placental telomere length at birth [56], which may be linked to decreased telomerase activity. Hence, our observed placental miRNA alterations could be also mediated by decreased levels of telomerase activity caused by maternal PM exposure during pregnancy.
To further understand the biological function of these miRNAs, we identified their putative targets and performed overrepresentation enrichment analysis on the experimentally validated targets of significantly associated miRNAs with PM exposure. Targets of miR-20a were found to potentially regulate pathways involved mainly in cell cycle [CCND1, CDKN1A(p21), TGFBR2, STAT3] and cancer [E2F1, KIT, CDKN1A(p21), HIF1A]. For miR-21, its putative targets regulated pathways in cancer (PTEN, APAF1), cell cycle (E2F1, CDC25A), and apoptosis (APAF1, BCL2, E2F1). For miR-146a, immune-related pathways (TLR2, TRAF6, NFKB1, IRAK1, TLR4) were predominantly regulated. Lastly, for miR-222, pathways involved in immune responses (ETS1, FOS, MMP1, FOXO3A, STAT5) and hematopoiesis [FOXO3A, KIT, CDKN1B (p27), STAT5] were identified. In addition, cell cycle-related pathway was found to be the most significant among the shared regulated pathways by the putative targets of the significant miRNAs (miR-20a, -21, -146a, and -222) associated with air pollution.
A common putative target of the miR-20a, miR-21, and miR-222 (Figure 3), PTEN, is involved in many key cellular processes by negatively regulating PI3K/AKT pathway involving cell survival, cell cycle, angiogenesis, and metabolism [57]. Interestingly, in a validation experiment, we demonstrated that PTEN expression inversely correlated with miR-20a, miR-21, and miR-222 expression in placental tissue. These findings confirm the miRNA-PTEN co-expression in placental tissue, which is an important criterion for the validation of miRNA targets [58]. The inverse association observed between air pollution exposure and miRNA expression was accompanied by a positive association between air pollution exposure and PTEN expression, as expected. During normal pregnancy, placental PTEN expression decreases with the development of the placenta and as pregnancy progresses [59]. Maccani et al. have reported that downregulation of miR-21 through induction of PTEN in placenta could lead to reduced invasion of maternal decidua, migration and growth of placental cells [37].
Aberrant expression of immune-related target genes, such as TLR4, has been associated with inflammation-induced preterm delivery [60], and the activation of NF-κB with increased oxidative stress resulting in pregnancy complications, e.g., preeclampsia [61]. Low expression of angiogenesis-related genes (MMP2, VEGF, TGF-β) and high expression of apoptosis-related genes (caspases) have been associated with recurrent pregnancy loss [62]. Placental vascular development is a crucial process for fetal development ensuring an optimal blood flow between fetus and mother, and an increased uterine vascular resistance and reduced blood flow have been associated with pregnancy complications and fetal growth retardation [63].
In addition, under normal conditions in early pregnancy, genes regulating cell cycle, differentiation, metabolic process, and angiogenesis are overexpressed, whereas genes involving in metabolic process, stress response, signaling and ion transport are upregulated in late pregnancy [64]. However, in our study, we only measured the miRNA expression at birth; thereby, the regulation of miRNA targets across the different time windows of pregnancy cannot be assessed.
A limitation of this study is that placental tissue is composed of a complex population of cells (syncytiotrophoblasts/cytotrophoblasts, mesenchymal cells, Hofbauer cells, and fibroblasts). To minimize the impact of regional differences we combined 4 fetal samples taken at four standardized sites across the middle region of the placenta (approximately 4 cm away from the umbilical cord) to extract miRNAs. Regardless of this, the placenta might be used as a proxy for epigenetic changes in the fetus, as it is derived from the outer layer of the blastocyst. The organ has a great plasticity to a range of intrauterine conditions and exposures. We cannot answer whether epigenetic alterations in placental tissue affect the fetus in a direct manner or indirectly by adaptations in its function. Secondly, although our results were robust and independent of other studied factors, we cannot eliminate the possibility of residual confounding by some unknown factor that is associated with both miRNA expression and ambient air pollution. Season and apparent temperature were taken into account as epigenetic adaptive changes to season have been reported [65]. Our study was not designed to evaluate temporal changes of miRNA expression during pregnancy and may be hampered by the fact that assays of term placentas may not reflect in vivo miRNA expression patterns occurring earlier at critical points of development.
In conclusion, we observed significant associations between PM exposure and miRNA (miR-20a, miR-21, miR-146a, and miR-222) and mRNA (PTEN) expression. The second trimester was identified as the most significantly affected time window for the analyzed miRNAs. The potential regulation of immune-, cell cycle-, and angiogenesis-related pathways could underlie the observed miRNA expression changes due to early life exposure to particulate matter.
Materials and methods
Study population
The protocols of the ENVIRONAGE (ENVIRonmental influence ON AGEing) birth cohort are approved by the Ethics Committees of the University of Hasselt and the South-East-Limburg hospital (ZOL). Participating mothers provided written informed consent when they arrived at the hospital for delivery, and completed study questionnaires in the postnatal ward after delivery to provide detailed information on maternal age, pre-gestational BMI, maternal education, occupation, smoking status, alcohol consumption, place of residence, use of medication, parity, and newborn's ethnicity. Past-smokers were defined as those who had quit smoking before pregnancy. Smokers continued smoking during pregnancy. Ethnicity was classified based on the native country of the newborn's grandparents as European-Caucasian (when two or more grandparents were European) or non-European (when at least three grandparents were of non-European origin). We asked women whether they occasionally consumed alcohol during pregnancy. Maternal education was coded as low (no diploma or primary school), middle (high school), or high (college or university degree).
Placental tissue was collected and deep-frozen within 10 minutes after delivery. Four biopsies at the fetal side of placental villous tissue, shielded by the chorio-amniotic membrane, were obtained, preserved in RNA later overnight at 4oC, and then stored at -20oC. The biopsies were taken at four standardized locations across the middle point of placenta, at approximately 4 cm distance from the umbilical cord.
Our study population (n = 210) within ENVIRONAGE cohort was recruited from September 2011 to January 2014. In 210 placentas, miR-16, miR-20a, miR-21, miR-34a, miR-146a, and miR-222, were measured. To validate the miRNAs putative regulatory role on placental PTEN, we performed a validation study. The transcript of the placental PTEN gene, which is regulated by miR-20a, miR-21, and miR-222, was measured in 181 (86.2%) of the newborns. To clarify the generalizability of the study, we have compared the characteristics of these 210 mother-newborn pairs with the data of the birth register of Flanders (Northern part of Belgium). This register comprises all births from Flanders (n = 648,711) from 1999–2009 [66]. The main characteristics including maternal age, maternal education, parity, ethnicity, and birth weight are in line with the birth register of all births between 1999–2009 in the Northern part of Belgium and therefore our sample of mother-newborn pairs can be considered to be representative for the population in Flanders (Supplementary Table S1).
Air pollution exposure
Air pollution exposure was assessed as described previously [67]. In brief, we interpolated the regional background level of PM2.5 for each mother's residential address using a spatial temporal interpolation method (Kriging) that employs pollution data collected in the official fixed site monitoring network and land cover data retrieved from satellite images (Corine land cover data set) in combination with a dispersion model. The utilized dispersion model was described previously [68,69]. This model chain provides daily PM2.5 values using data from the Belgian telemetric air quality network, combined with information from point sources and line sources which are interpolated to a high resolution receptor grid. In the Flemish region of Belgium, more than 80% of the temporal and spatial variability (R2) could be explained by the interpolation tool [70]. To explore potentially critical exposures during pregnancy, individual mean PM2.5 concentrations (µg/m3) were calculated for various periods, for which the date of conception was estimated based on ultrasound data: each of the three trimesters of pregnancy, with trimesters being defined as: 1–13 weeks (1st trimester), 14–26 weeks (2nd trimester), and 27 weeks to delivery (3rd trimester). Additionally, nitrogen dioxide (NO2) exposure was interpolated using the same methods as PM2.5 exposure. We have complete residential information during and before pregnancy. For those that moved during pregnancy (n = 20, 9.5%), we calculated exposure windows accounting for the address changes during this period.
RNA isolation and DNase treatment
Total RNA and miRNA were isolated from pooled biopsies using the miRNeasy mini kit (Qiagen, KJ Venlo, the Netherlands) according to the manufacturer's protocol. Quality control of the extracted total RNA and miRNA was assessed by spectrophotometry (Nanodrop ND-1000; Isogen Life Science, De Meern, the Netherlands). Sample purity was assessed by calculating the A260/280 and A260/230 ratios. The average (±SD) yield of total RNA per placenta biopsy was 4.4 (±1.2) µg with average A260/280 ratio of 1.96 (±0.03) and average A260/230 ratio of 1.85 (±0.18). DNase treatment was performed on extracted RNA samples according to the manufacturer's instructions (Turbo DNA-free kit, Ambion, Life Technologies, Diegem, Belgium). Extracted RNA was stored at −80 °C until further use. In a pilot experiment, the variability within the four individual biopsies was assessed in a subset of ten placental tissues. The average Cq values of miRNAs (miR-21, miR-222, and RNU6) within the four biopsies of each placenta varied between 2–9% (CV). To reduce interplacental differences, we used pooled samples from 4 placental biopsies.
Reverse transcription and miRNA expression analysis
RNA was reverse transcribed using the Megaplex reverse transcription (RT) stem-loop primer pool A (Applied Biosystems, Foster City, CA), enabling miRNA specific cDNA synthesis of 380 different human miRNAs and small RNA controls, according to the manufacturer's protocol. Briefly, 375 ng total RNA was reverse transcribed as follows: 2 minutes at 16°C, 1 minute at 42°C and 1 minute at 50°C, for 40 cycles (Thermocycler PCR, Techne, Staffordshire, UK). Afterwards, cDNA was stored at −20°C for a maximum of one week until qRT-PCR measurements were performed.
miRNA qRT-PCR analysis was performed using Taqman miRNA assays (Applied Biosystems, Foster City, CA), according to the manufacturer's protocol. All target sequences of the miRNAs and control RNA are available in Supplementary Table S2. An input of 5 ng cDNA was used for PCR reactions, which were run on a 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA), as follows: a polymerase activation for 2 minutes at 50°C, a denaturation step for 10 min at 95°C and an anneal/extension step (40 cycles) for 15 seconds at 95°C and for 1 min at 60°C. For normalization the endogenous control RNU6 was used. In order to minimize the technical variation between the different runs of the same miRNA assay, inter-run calibrators (IRCs) were applied. Expression of candidate miRNAs was studied and Cq values were collected with SDS 2.3 software. Amplification efficiencies were between 90–115% for all assays. The relative miRNA expression was calculated by 2−ΔΔCq method using qBase plus software (Biogazelle, Belgium). Data is presented as relative quantities of target miRNA normalized to endogenous control miRNA. All samples were analyzed in triplicate. Replicates were included when the ΔCq was smaller than 0.5.
miRNA target prediction and pathway analysis
Many in silico prediction tools have been developed to identify putative miRNA-target genes. We utilized miRTarBase [71] and DIANA–TarBase [72] for prediction of targets for those miRNAs that revealed significant associations with in utero air pollution exposure, namely miR-16, miR-20a, miR-21, miR-34a miR-146a, and miR-222. miRTarBase v6.0 includes many miRNA-target interactions, retrieved manually from research articles in literature related to functional studies of human miRNAs [71]. DIANA-TarBase v7.0 identifies miRNA-target interactions which have been highly curated from published experiments [73]. The available experimental evidence on prediction of miRNA targets was used as a determinant for the selection of target genes. We considered reporter assay (RS), qPCR (qP), Western blot (WB), and immunoprecipitation (IP) as strong evidenced assays, while assays included high-throughput analyses such as microarrays (MA), proteomics (Pr), and next generation sequencing (NGS) were considered as less strongly evidenced. The identified putative miRNA targets were ranked based on available strong evidenced assays and, subsequently, the top 15 targets were selected for analysis.
MetaCore™ (Thomson Reuters, New York, USA) was used for pathway analysis. We performed pathway analysis by overrepresentation analysis for each set (n = 15) of predicted miRNAs target genes of miR-20a, miR-21, miR-146a, and miR-222. MiR-16 and miR-34a were excluded from pathway analysis, as we did not observe significant associations for these miRNAs with in utero air pollution exposure. The obtained P-values were corrected for multiple hypotheses testing by applying Benjamini and Hochberg's FDR [74]. Extended lists of enriched pathway maps (n = 50) are provided in Supplementary Table S3. Additionally, we identified the shared pathways regulated by the miRNAs of interest. The list of common pathways (n = 33) regulated by these miRNAs with their P-values and FDR is given in Supplementary Table S4.
miRNA target validation by qRT-PCR
The validation of a common miRNA target was performed in a subset (n = 181, 86.2%). Total RNA (3 μg) were reverse transcribed into cDNA by GoScript Reverse Transcription System (Promega, Madison, WI, USA) using Thermal cycler (TC-5000; Techne, Burlington, NJ, USA). The synthetized cDNA was stored at –20°C for further applications.
qRT-PCR analysis was performed using a 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA), according to the manufacturer's protocol. PTEN, as target gene (primer assay: Hs.PT.58.4416071, RefSeq number: NM_000314), and GAPDH (primer assay: Hs.PT.53a.24391631.gs, RefSeq number: NM_001256799), IPO8 (primer assay: Hs.PT.56a.40532361, RefSeq number: NM_001190995), UBC (primer assay: Hs.PT.39a.22214853, RefSeq number: NM_021009), and POLR2A (primer assay: Hs.PT.56a.25515089, RefSeq number: NM_000937), as reference genes were measured. An input of 6ng of cDNA was added to TaqMan Fast Advanced Master Mix (Life Technologies) and PrimeTimeTM assay (Integrated DNA Technologies, Coralville, IA, USA). The same cycling conditions as previously mentioned were used. Inter-run calibrators and reference genes were used for normalization. The expression of target and reference genes was measured, and the Cq values were collected using SDS 2.3 software. The raw data were processed to normalized relative gene expression by 2−ΔΔCq method using qBase plus software (Biogazelle, Belgium). The amplification efficiencies for all assays were within the acceptable range (90-115%) (data not shown). All samples were analyzed in triplicate and replicates were included when the ΔCq was smaller than 0.5.
Statistical analysis
For database management and statistical analysis, we used SAS software (Version 9.3 SAS Institute, Cary, NC, USA). We tested the normality of the obtained relative quantities of miRNA expression. Because of non-normal distribution the relative miRNA expressions were log-transformed. Categorical data are presented as frequencies (%) or numbers and continuous data as mean (±SD). We performed multiple linear regression to assess the independent associations between placental miRNA expression and in utero exposure to particulate air pollution, while adjusting for maternal age (years), pre-gestational body mass index (BMI) (kg/m2), smoking status (never-smoker, past-smoker, or current-smoker), educational status (low, middle or high), parity (1, 2, or ≥3), and newborn's gender, gestational age (weeks) and ethnicity, seasonality (at conception) and apparent temperature during the 3rd trimester of pregnancy divided into quartiles of the distribution. Using the same model, the association between relative miRNA expression and air pollution exposure was estimated for each trimesters of pregnancy.
Likewise, in a subsequent validation experiment, the relative placental PTEN expression was first log-transformed and then associated with air pollution using the same multiple regression model, as described in the previous paragraph. Pearson correlations between miRNAs of interest and PTEN expression were evaluated.
The effect of air pollutants on miRNA/mRNA expression is presented as percentage of change [change (%) = (10(β*5) – 1)*100] with 95% confidence intervals (CI), for each 5-µg/m3 increment in air pollution exposure at each time window.
Supplementary Material
Funding Statement
This work was supported by the EC | European Research Council (ERC) [grant number ERC-2012-StG 310898]; and Research Foundation Flanders (FWO) [grant number 12D7714N].
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The ENVIRONAGE birth cohort is supported by grants from the European Research Council (ERC-2012-StG 310898) and Flemish Research Council (FWO G073315N). Karen Vrijens is a postdoctoral fellow of the FWO (12D7714N).
References
- [1].Pope CA, Dockery DW. Health effects of fine particulate air pollution: Lines that connect. J Air Waste Manage Assoc. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. PMID:16805397 [DOI] [PubMed] [Google Scholar]
- [2].Yang W, Omaye ST. Air pollutants, oxidative stress and human health. Mutat Res. 2009;674:45–54. doi: 10.1016/j.mrgentox.2008.10.005. PMID:19013537 [DOI] [PubMed] [Google Scholar]
- [3].Ghio AJ, Kim C, Devlin RB. Concentrated ambient air particles induce mild pulmonary inflammation in healthy human volunteers. Am J Respir Crit Care Med. 2000;162:981–988. doi: 10.1164/ajrccm.162.3.9911115. PMID:10988117 [DOI] [PubMed] [Google Scholar]
- [4].Ghio AJ, Huang Y-CT. Exposure to concentrated ambient particles (CAPs): A review. Inhal Toxicol. 2004;16:53–59. doi: 10.1080/08958370490258390. PMID:14744665 [DOI] [PubMed] [Google Scholar]
- [5].Brook RD, Rajagopalan S, Pope CA, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American heart association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. PMID:20458016 [DOI] [PubMed] [Google Scholar]
- [6].Malmström K, Pelkonen AS, Malmberg LP, et al. Lung function, airway remodelling and inflammation in symptomatic infants: outcome at 3 years. Thorax. 2011;66:157–162. doi: 10.1136/thx.2010.139246. PMID:21199817 [DOI] [PubMed] [Google Scholar]
- [7].Blomberg A, Krishna MT, Bocchino V, et al. The inflammatory effects of 2 ppm NO2 on the airways of healthy subjects. Am J Respir Crit Care Med. 1997;156:418–424. doi: 10.1164/ajrccm.156.2.9612042. PMID:9279218 [DOI] [PubMed] [Google Scholar]
- [8].Barker DJP. Developmental origins of adult health and disease. J Epidemiol Commun Health. 2004;58:114–115. doi: 10.1136/jech.58.2.114. PMID:14729887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Alastalo H, von Bonsdorff MB, Räikkönen K, et al. Early life stress and physical and psychosocial functioning in late adulthood. PLoS ONE. 2013;8:e69011. doi: 10.1371/journal.pone.0069011. PMID:23861956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pedersen M, Giorgis-Allemand L, Bernard C, et al. Ambient air pollution and low birthweight: a European cohort study (ESCAPE). Lancet Resp Med. 2013;1:695–704. doi: 10.1016/S2213-2600(13)70192-9. PMID:24429273 [DOI] [PubMed] [Google Scholar]
- [11].Rappazzo KM, Daniels DL, Messer LC, et al. Exposure to fine particulate matter during pregnancy and risk of preterm birth among women in New Jersey, Ohio, and Pennsylvania, 2000–2005. Environ Health Perspect. 2014;122:992–997. doi: 10.1289/ehp.1307456. PMID:24879653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].van den Hooven EH, Pierik FH, de Kluizenaar Y, et al. Air pollution exposure and markers of placental growth and function: The generation R study. Environ Health Perspect. 2012;120:1753–1759. PMID:22922820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Veras MM, Damaceno-Rodrigues NR, Caldini EG, et al. Particulate Urban air pollution affects the functional morphology of mouse placenta. Bio Reprod. 2008;79:578–584. doi: 10.1095/biolreprod.108.069591. PMID:18509159 [DOI] [PubMed] [Google Scholar]
- [14].Saenen ND, Plusquin M, Bijnens E, et al. In Utero fine particle air pollution and placental expression of genes in the brain-derived Neurotrophic factor signaling pathway: An ENVIRONAGE birth cohort study. Environ Health Perspect. 2015. doi: 10.1289/ehp.1408549.PMID:25816123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].de Melo JO, Soto SF, Katayama IA, et al. Inhalation of fine particulate matter during pregnancy increased IL-4 cytokine levels in the fetal portion of the placenta. Toxicol Lett. 2015;232:475–480. doi: 10.1016/j.toxlet.2014.12.001. PMID:25481569 [DOI] [PubMed] [Google Scholar]
- [16].Jansson T, Powell T. Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clin Sci. 2007;113: 1–13. doi: 10.1042/CS20060339. PMID:17536998. [DOI] [PubMed] [Google Scholar]
- [17].Etheridge A, Lee I, Hood L, et al. Extracellular microRNA: A new source of biomarkers. Mutat Res. 2011;717:85–90. doi: 10.1016/j.mrfmmm.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. PMID:15372042 [DOI] [PubMed] [Google Scholar]
- [19].Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/S0092-8674(04)00045-5. PMID:14744438 [DOI] [PubMed] [Google Scholar]
- [20].Wang Y, Lee CGL. MicroRNA and cancer – focus on apoptosis. J Cell Mol Med. 2009;13:12–23. doi: 10.1111/j.1582-4934.2008.00510.x. PMID:19175697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lu M, Zhang Q, Deng M, et al. An analysis of human MicroRNA and disease associations. PLoS ONE. 2008;3:e3420. doi: 10.1371/journal.pone.0003420. PMID:18923704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Felekkis K, Touvana E, Stefanou C, et al. microRNAs: a newly described class of encoded molecules that play a role in health and disease. Hippokratia. 2010;14:236–240. PMID:21311629 [PMC free article] [PubMed] [Google Scholar]
- [23].Wilczynska A, Bushell M. The complexity of miRNA-mediated repression. Cell Death Differ. 2015;22:22–33. doi: 10.1038/cdd.2014.112. PMID:25190144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fossati S, Baccarelli A, Zanobetti A, et al. Ambient particulate air pollution and microRNAs in elderly men. Epidemiology. 2014;25:68–78. doi: 10.1097/EDE.0000000000000026. PMID:24257509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bollati V, Marinelli B, Apostoli P, et al. Exposure to metal-rich particulate matter modifies the expression of candidate Micrornas in peripheral blood leukocytes. Environ Health Perspect. 2010;118:763–768. doi: 10.1289/ehp.0901300. PMID:20061215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fry RC, Rager JE, Bauer R, et al. Air toxics and epigenetic effects: ozone altered microRNAs in the sputum of human subjects. Am J Physiol Lung Cell Mol Physiol 2014;306:L1129–L1137. doi: 10.1152/ajplung.00348.2013. PMID:24771714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Avissar-Whiting M, Veiga K, Uhl K, et al. Bisphenol a exposure leads to specific MicroRNA alterations in placental cells. Reprod Toxicol. 2010;29:401–406. doi: 10.1016/j.reprotox.2010.04.004. PMID:20417706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Herberth G, Bauer M, Gasch M, et al. Maternal and cord blood miR-223 expression associates with prenatal tobacco smoke exposure and low regulatory T-cell numbers. J Allergy Clinical Immunol. 2014;133:543–550.e4. doi: 10.1016/j.jaci.2013.06.036. PMID:23978443 [DOI] [PubMed] [Google Scholar]
- [29].Maccani MA, Avissar-Whiting M, Banister CE, et al. Maternal cigarette smoking during pregnancy is associated with downregulation of miR-16, miR-21, and miR-146a in the placenta. Epigenetics. 2010;5:583–589. doi: 10.4161/epi.5.7.12762. PMID:20647767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rudov A, Balduini W, Carloni S, et al. Involvement of miRNAs in placental alterations mediated by oxidative stress. Oxid Med Cell Longev. 2014;2014:7. doi: 10.1155/2014/103068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Vrijens K, Bollati V, Nawrot TS. MicroRNAs as potential signatures of environmental exposure or effect: A systematic review. Environ Health Perspect. 2015;123:399–411. doi: 10.1289/ehp.1408459. PMID:25616258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhou X, Ren Y, Moore L, et al. Downregulation of miR-21 inhibits EGFR pathway and suppresses the growth of human glioblastoma cells independent of PTEN status. Lab Invest. 2010;90:144–155. doi: 10.1038/labinvest.2009.126. PMID:20048743 [DOI] [PubMed] [Google Scholar]
- [33].Cimmino A, Calin GA, Fabbri M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Nat Acad Sci U S A. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. PMID:16166262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rivas MA, Venturutti L, Huang Y-W, et al. Downregulation of the tumor-suppressor miR-16 via progestin-mediated oncogenic signaling contributes to breast cancer development. Breast Cancer Res: BCR. 2012;14:R77–R. doi: 10.1186/bcr3187. PMID:22583478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Yang Y, Meng H, Peng Q, et al. Downregulation of microRNA-21 expression restrains non-small cell lung cancer cell proliferation and migration through upregulation of programmed cell death 4. Cancer Gene Ther. 2015;22:23–29. doi: 10.1038/cgt.2014.66. PMID:25477028 [DOI] [PubMed] [Google Scholar]
- [36].Ke Y, Zhao W, Xiong J, et al. Downregulation of miR-16 promotes growth and motility by targeting HDGF in non-small cell lung cancer cells. FEBS Lett. 2013;587:3153–3157. doi: 10.1016/j.febslet.2013.08.010. PMID:23954293 [DOI] [PubMed] [Google Scholar]
- [37].Maccani MA, Padbury JF, Marsit CJ. miR-16 and miR-21 Expression in the placenta is associated with fetal growth. PLoS ONE. 2011;6:e21210. doi: 10.1371/journal.pone.0021210. PMID:21698265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Baldeón RL, Weigelt K, de Wit H, et al. Decreased serum level of miR-146a as sign of chronic inflammation in Type 2 diabetic patients. PLoS ONE. 2014;9:e115209. doi: 10.1371/journal.pone.0115209. PMID:25500583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res. 2008;79:581–588. doi: 10.1093/cvr/cvn156. PMID:18550634 [DOI] [PubMed] [Google Scholar]
- [40].Wang W, Feng L, Zhang H, et al. Preeclampsia Up-regulates angiogenesis-associated MicroRNA (i.e., miR-17, -20a, and -20b) that target Ephrin-B2 and EPHB4 in human placenta. J Clinical Endocrinol Metab. 2012;97:E1051–E9. doi: 10.1210/jc.2011-3131. PMID:22438230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Umemura K, Ishioka S-i, Endo T, et al. Roles of microRNA-34a in the pathogenesis of placenta accreta. J Obstet Gynaecol Res. 2013;39:67–74. doi: 10.1111/j.1447-0756.2012.01898.x. PMID:22672425 [DOI] [PubMed] [Google Scholar]
- [42].Zhao T, Li J, Chen AF. MicroRNA-34a induces endothelial progenitor cell senescence and impedes its angiogenesis via suppressing silent information regulator 1. Am J Physiol Endocrinol Metab. 2010;299:E110–E116. doi: 10.1152/ajpendo.00192.2010. PMID:20424141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Farraj AK, Hazari MS, Haykal-Coates N, et al. ST Depression, arrhythmia, vagal dominance, and reduced cardiac Micro-RNA in particulate-exposed rats. Am J Respir Cell Mol Biol. 2011;44:185–196. doi: 10.1165/rcmb.2009-0456OC. PMID:20378750 [DOI] [PubMed] [Google Scholar]
- [44].Motta V, Angelici L, Nordio F, et al. Integrative analysis of miRNA and inflammatory gene expression after acute particulate matter exposure. Toxicol Sci. 2013;132:307–316. doi: 10.1093/toxsci/kft013. PMID:23358196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Pineles BL, Romero R, Montenegro D, et al. Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. Am J Obstet Gynecol. 2007;196:261.e1–261.e6. doi: 10.1016/j.ajog.2007.01.008. [DOI] [PubMed] [Google Scholar]
- [46].Li Q, Kappil MA, Li A, et al. Exploring the associations between microRNA expression profiles and environmental pollutants in human placenta from the National Children's Study (NCS). Epigenetics. 2015;10:793–802. doi: 10.1080/15592294.2015.1066960. PMID:26252056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8:253–62. doi: 10.1038/nrg2045. PMID:17363974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Janssen BG, Godderis L, Pieters N, et al. Placental DNA hypomethylation in association with particulate air pollution in early life. Part Fibre Toxicol. 2013;10:22-. doi: 10.1186/1743-8977-10-22. PMID:23742113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sathyan P, Golden HB, Miranda RC. Competing Interactions between Micro-RNAs Determine neural progenitor survival and proliferation after ethanol exposure: evidence from an Ex Vivo model of the fetal cerebral cortical neuroepithelium. J Neurosci. 2007;27:8546–8557. doi: 10.1523/JNEUROSCI.1269-07.2007. PMID:17687032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tal TL, Franzosa JA, Tilton SC, et al. MicroRNAs control neurobehavioral development and function in zebrafish. FASEB J. 2012;26:1452–1461. doi: 10.1096/fj.11-194464. PMID:22253472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wang L-L, Zhang Z, Li Q, et al. Ethanol exposure induces differential microRNA and target gene expression and teratogenic effects which can be suppressed by folic acid supplementation. Human Reprod. 2009;24:562–579. doi: 10.1093/humrep/den439. PMID:19091803 [DOI] [PubMed] [Google Scholar]
- [52].Pietrzykowski AZ, Friesen RM, Martin GE, et al. Post-transcriptional regulation of BK channel splice variant stability by miR-9 underlies neuroadaptation to alcohol. Neuron. 2008;59:274–287. doi: 10.1016/j.neuron.2008.05.032. PMID:18667155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Costantine M. Physiologic and pharmacokinetic changes in pregnancy. Front Pharmacol. 2014;5. doi: 10.3389/fphar.2014.00065. PMID:24772083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lassmann T, Maida Y, Tomaru Y, et al. Telomerase Reverse Transcriptase Regulates microRNAs. Int J Mol Sci. 2015;16:1192–1208. doi: 10.3390/ijms16011192. PMID:25569094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Colgin LM, Reddel RR. Telomere maintenance mechanisms and cellular immortalization. Curr Opin Genet Dev. 1999;9:97–103. doi: 10.1016/S0959-437X(99)80014-8. [DOI] [PubMed] [Google Scholar]
- [56].Bijnens E, Zeegers MP, Gielen M, et al. Lower placental telomere length may be attributed to maternal residential traffic exposure; a twin study. Environ Int. 2015;79:1–7. doi: 10.1016/j.envint.2015.02.008. PMID:25756235 [DOI] [PubMed] [Google Scholar]
- [57].Kitagishi Y, Matsuda S. Redox regulation of tumor suppressor PTEN in cancer and aging. Int J Mol Med. 2013;31:511–515. doi: 10.3892/ijmm.2013.1235. PMID:23313933 [DOI] [PubMed] [Google Scholar]
- [58].Kuhn DE, Martin MM, Feldman DS, et al. Experimental validation of miRNA targets. Methods. 2008;44:47–54. doi: 10.1016/j.ymeth.2007.09.005. PMID:18158132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Tokyol C, Aktepe F, Dilek FH, et al. Comparison of Placental PTEN and β1 integrin expression in early spontaneous abortion, early and late normal pregnancy. Ups J Med Sci. 2008;113:235–242. doi: 10.3109/2000-1967-231. PMID:18509818 [DOI] [PubMed] [Google Scholar]
- [60].Li L, Kang J, Lei W. Role of Toll-like receptor 4 in inflammation-induced preterm delivery. Mol Human Reprod. 2010;16:267–272. doi: 10.1093/molehr/gap106. PMID:19995880 [DOI] [PubMed] [Google Scholar]
- [61].Vaughan JE, Walsh SW. Activation of NF-κB in Placentas of Women with Preeclampsia. Hypertens Pregnancy. 2012;31:243–251. doi: 10.3109/10641955.2011.642436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Choi H-K, Choi BC, Lee S-H, et al. Expression of angiogenesis- and apoptosis-related genes in chorionic villi derived from recurrent pregnancy loss patients. Mol Reprod Dev. 2003;66:24–31. doi: 10.1002/mrd.10331. PMID:12874795 [DOI] [PubMed] [Google Scholar]
- [63].Reynolds LP, Redmer RD. Utero-placental vascular development and placental function. J Anim Sci. 1995;73:1839–1851. doi: 10.2527/1995.7361839x. PMID:7545661 [DOI] [PubMed] [Google Scholar]
- [64].Winn VD, Haimov-Kochman R, Paquet AC, et al. Gene expression profiling of the human maternal-fetal interface reveals dramatic changes between midgestation and term. Endocrinology. 2007;148:1059–1079. doi: 10.1210/en.2006-0683. PMID:17170095 [DOI] [PubMed] [Google Scholar]
- [65].Bind M-A, Zanobetti A, Gasparrini A, et al. Effects of temperature and relative humidity on DNA methylation. Epidemiology. 2014;25:561–569. doi: 10.1097/EDE.0000000000000120. PMID:24809956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Cox B, Martens E, Nemery B, et al. Impact of a stepwise introduction of smoke-free legislation on the rate of preterm births: analysis of routinely collected birth data. BMJ. 2013;346:f441. doi: 10.1136/bmj.f441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Janssen BG, Byun H-M, Gyselaers W, et al. Placental mitochondrial methylation and exposure to airborne particulate matter in the early life environment: An ENVIRONAGE birth cohort study. Epigenetics. 2015;10:536–544. doi: 10.1080/15592294.2015.1048412. PMID:25996590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Lefebvre W, Degrawe B, Beckx C, et al. Presentation and evaluation of an integrated model chain to respond to traffic- and health-related policy questions. Environ Modell Software. 2013;40:160–170. doi: 10.1016/j.envsoft.2012.09.003. [DOI] [Google Scholar]
- [69].Lefebvre W, Vercauteren J, Schrooten L, et al. Validation of the MIMOSA-AURORA-IFDM model chain for policy support: Modeling concentrations of elemental carbon in Flanders. Atmos Environ. 2011;45:6705–6713. doi: 10.1016/j.atmosenv.2011.08.033. [DOI] [Google Scholar]
- [70].Maiheu B VB, Viaene P, De Ridde rK, et al. Identifying the best available large-scale concentration maps for air quality in Belgium. Mol, Belgium: Flemish Institute for Technological Research (VITO); 2013. [Google Scholar]
- [71].Hsu S-D, Tseng Y-T, Shrestha S, et al. miRTarBase update 2014: an information resource for experimentally validated miRNA-target interactions. Nucleic Acids Res. 2014;42:D78–D85. doi: 10.1093/nar/gkt1266. PMID:24304892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Vlachos IS, Kostoulas N, Vergoulis T, et al. DIANA miRPath v.2.0: investigating the combinatorial effect of microRNAs in pathways. Nucleic Acids Res. 2012;40:W498–W504. doi: 10.1093/nar/gks494. PMID:22649059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Vlachos IS, Paraskevopoulou MD, Karagkouni D, et al. DIANA-TarBase v7.0: indexing more than half a million experimentally supported miRNA:mRNA interactions. Nucleic Acids Res. 2014. doi: 10.1093/nar/gku1215. PMID:25416803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Royal Stat Soc Series B. 1995;57:289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


