Abstract
Aims
To assess the cost‐effectiveness of a two‐component intervention designed to increase attendance at the NHS Stop Smoking Services (SSSs) in England.
Design
Cost‐effectiveness analysis alongside a randomized controlled trial (Start2quit).
Setting
NHS SSS and general practices in England.
Participants
The study comprised 4384 smokers aged 16 years or more identified from medical records in 99 participating practices, who were motivated to quit and had not attended the SSS in the previous 12 months.
Intervention and comparator
Intervention was a personalized and tailored letter sent from the general practitioner (GP) and a personal invitation and appointment to attend a taster session providing information about SSS. Control was a standard generic letter from the GP advertising SSS and asking smokers to contact the service to make an appointment.
Measurements
Costs measured from an NHS/personal social services perspective, estimated health gains in quality‐adjusted life‐years (QALYs) measured with EQ‐5D and incremental cost per QALY gained during both 6 months and a life‐time horizon.
Findings
During the trial period, the adjusted mean difference in costs was £92 [95% confidence interval (CI) = –£32 to –£216) and the adjusted mean difference in QALY gains was 0.002 (95% CI = –0.001 to 0.004). This generates an incremental cost per QALY gained of £59 401. The probability that the tailored letter and taster session is more cost‐effective than the generic letter at 6 months is never above 50%. In contrast, the discounted life‐time health‐care cost was lower in the intervention group, while the life‐time QALY gains were significantly higher. The probability that the intervention is more cost‐effective is more than 83% using a £20 000–30 000 per QALY‐gained decision‐making threshold.
Conclusions
An intervention designed to increase attendance at the NHS Stop Smoking Services (tailored letter and taster session in the services) appears less likely to be cost‐effective than a generic letter in the short term, but is likely to become more cost‐effective than the generic letter during the long term.
Keywords: Cost‐effectiveness, personal tailored risk information, randomized controlled trial, smoking cessation, stop smoking services, uptake of service
Introduction
Smoking is the greatest avoidable cause of mortality and morbidity, and a major public health problem in the United Kingdom 1, 2. Half of smokers will die prematurely due to smoking‐related disease, such as lung cancer, chronic obstructive pulmonary disease (COPD) or coronary heart disease (CHD), and lose an average of 8 years of life 3. The prevalence of smoking in the United Kingdom has dropped from 45% in the 1960s to 19% in 2013; however, the reduction has slowed during the past 5 years 4. The NHS spends more than £5 billion a year on treating smoking‐related diseases, and the societal cost of smoking is approximately £14 billion a year when loss of productivity and economic output due to smoking‐related illness and premature death are taken into account 5, 6.
Government‐funded specialist smoking cessation services, now known as the NHS Stop Smoking Services (SSSs), were established by Primary Care Trusts (PCTs) throughout England in 2000 7. The SSSs provide free, tailored support to all smokers willing to quit, providing a combination of recommended stop smoking pharmacotherapies [nicotine replacement therapy (NRT), bupropion and varenicline] and behavioural support (e.g. group or one‐to‐one support). Among smokers who set a quit date through the SSSs, 48% had quit successfully (self‐reported 2 weeks abstinence at 4 weeks after the designated quit rate), and 70% of these quitters had their results confirmed by expired carbon monoxide (CO) verification 8, 9. However, despite the relatively high quit rate, at least in the short term, smokers are not taking full advantage of the services. The proportion of smokers in England using the SSSs in 2011 was only 4.1% 10. In view of the recent cuts to smoking cessation budgets and the decline of services in England, new approaches are needed and opportunities to tackle smoking within the NHS maximized 11. A large randomized controlled trial (RCT) (Start2quit trial: ISRCTN 76561916) was conducted to test the effectiveness and cost‐effectiveness of a two‐component intervention designed to increase attendance at the SSS in England. In a companion paper, we report the findings on clinical effectiveness 12. This report presents a cost‐effectiveness analysis carried out alongside the Start2quit trial to assess the value for money of the intervention.
Methods
Randomized controlled trial
The Start2quit trial was a pragmatic two‐arm RCT of a two‐component intervention. The study recruited 4384 smokers aged 16 years or more identified from medical records in 99 participating practices, who were motivated to quit and had not attended the SSS in the previous 12 months. A total of 1748 participants were randomized to the control group and received a standard generic letter from the general practitioner (GP) advertising the local SSS and asking the smoker to contact the service to make an appointment to see an adviser. Smokers allocated to the intervention group (n = 2636) received a brief personalized tailored letter sent from the GP using information obtained from a baseline questionnaire and from medical records; they also received a personal invitation and appointment to attend a ‘Come and Try it’ taster session to find out more about the services, with the session run by advisers from the local SSS. One participant in the intervention group withdrew from the study, leaving 4383 participants analysed. Full details of the design of the Start2quit trial have been reported in the published study protocol 13.
Resource use and costs
Costs were estimated from an NHS/Personal Social Services (PSS) perspective to reflect the English NHS decision‐making framework 14. We assessed all costs in UK pounds sterling (£) at 2012–13 prices or adjusted them accordingly using the Hospital and Community Health Services (HCHS) pay and price inflation index 15.
Data on the use of resources were collected at the level of individual participants. We recorded resource use associated with the delivery of the trial interventions, including staff time spent in delivering treatment, consumables (such as postage and printing) and resources required for training the advisers in the delivery of the taster sessions. We also collected participants' use during the previous 6 months of health and social care services using a comprehensive service use questionnaire at both baseline and 6‐month follow‐up. The volume of resource use was multiplied by the unit costs to estimate the cost per participant. Table 1 details the key unit costs, together with their sources 15, 16, 17, 18, 19.
Table 1.
Unit costs (and sources) employed to estimate total costs (in 2012–13 prices).
| Resource item | Unit | Cost | Source |
|---|---|---|---|
| Smoking cessation aids | |||
| NHS Stop Smoking Services | |||
| Group session | Per person per session | £4.6 | 16 |
| Individual session | Per person per session | £17 | 16 |
| Telephone | £5.9 per call at 2008–09 price | £6.4 | 17 |
| Drop‐in | Per person per session | £17 | 16 |
| Couple/family | Per person per session | £8.5 | 16 |
| Other non‐pharmacological smoking cessation aids | |||
| GP visit | 10‐minute brief advice session | £40 | 15 |
| Practice nurse | 10‐minute brief advice session | £7 | 15 |
| Pharmacist | 10‐minute brief advice session | £11 | 15 |
| NHS smoking helpline | £5.9 per call at 2008–09 price | £6.4 | 17 |
| Other smoking helpline | £5.9 per call at 2008–09 price | £6.4 | 17 |
| Pharmacological smoking cessation aids | |||
| Nicotine replacement therapy (NRT) | Per prescription item | £21 | 18 |
| Zyban: bupropion | Per prescription item | £38 | 18 |
| Champix® (Pfizer): varenicline | Per prescription item | £34 | 18 |
| Wider health‐care resource use | |||
| GP visit | Visit (average 11.7 minutes) | £37 | 15 |
| Practice nurse visit | Visit (average 15.5 minutes) | £11 | 19 |
| Day case | Finished consultant episode | £693 | 19 |
| In‐patient (cost per night) | Per bed night | £542 | 19 |
| Out‐patient attendance | Visit | £108 | 19 |
| Accident and emergency attendance | Visit | £114 | 19 |
NHS = National Health Service; GP = general practice.
Outcome measures
The primary health outcome for the cost‐effectiveness analysis was assessed in terms of quality‐adjusted life‐years (QALYs). QALY is a generic health outcome measure that is recommended by the National Institute for Health and Care Excellence (NICE) for economic evaluation 14. Compared to clinical outcome measures, such as quit rate, which vary between studies in terms of length of time quit, length of follow‐up and means of measurement, the QALY permits comparisons between different health‐care programmes 20. NICE has specified an explicit decision‐making threshold range for what should be considered cost‐effective; namely, if an intervention has an incremental cost of less than £20 000–30 000 per additional QALY gained, while there is no similar threshold given in quit rates. In addition, the use of QALYs also allows us to compare the short‐ and long‐term benefits from the trial interventions.
We measured participants' health states using a generic measure of health status; namely, the EQ‐5D questionnaire at baseline and 6‐month follow‐up 21. The EQ‐5D scores were converted to utilities using the UK population tariff, and QALYs were calculated using the area under the curve approach 22, 23, 24. The secondary outcomes were the proportion of participants entering the SSS during a period of 6 months and the proportion of participants quitting smoking at the 6‐month follow‐up.
Cost‐effectiveness analysis
A full cost‐effectiveness analysis (CEA) was conducted on an ‘intention‐to‐treat’ (ITT) basis, where all participants are analysed as randomized. Incremental cost‐effectiveness ratios (ICERs) were used to combine differential mean costs and the effects associated with the two trial groups in a single measure to which a decision rule for cost‐effectiveness can be applied 20:
where E represents the effects and C represents the costs of the intervention, measured in monetary units, and the subscripts ‘I’ and ‘C’ refer to the intervention and control arm, respectively.
Missing data were imputed using Rubin's multiple imputation (MI) method 25, 26, 27. Chained imputation using predictive mean matching over 50 imputations was undertaken to estimate cost and EQ‐5D data items when they were missing. The following independent covariates were specified for the imputation model: intervention group, resource use data and baseline EQ‐5 D score, smoking abstinence and participant characteristics such as age and gender.
To account for the uncertainty due to sampling variation in cost‐effectiveness, a non‐parametric bootstrap re‐sampling technique was employed to obtain confidence intervals for the ICERs by generating 5000 iterations of the mean cost and QALYs for each trial group. 28, 29, 30, 31. A total of 50 imputations were generated to ensure efficient and reproducible estimates. Cost‐effectiveness acceptability curves (CEACs) were plotted based on the outcomes of the bootstrap iterations to show the probability of the intervention being more cost‐effective than the control over a range of a decision‐maker's willingness‐to‐pay (WTP) thresholds 32. All the analyses were conducted with Stata version 13.0 (StataCorp, College Station, TX, USA). Statistical significance was accepted at P < 0.05 in each of the analyses.
Long‐term costs and outcome predictions
Numerous studies have demonstrated that the majority of benefits of smoking cessation are gained from the reduced risk of developing smoking‐related diseases, and the reduced health‐care costs and improved health‐related quality of life it brings over a longer period 3, 33. These benefits may not be evident until later in life, therefore long‐term cost‐effectiveness of the two‐component personalized intervention compared with control was estimated in addition to the short‐term within‐trail analysis. We estimated the life‐time health‐care cost savings and QALYs associated with the two trial interventions using the results of two high‐quality published studies 34, 35.
The life‐time health‐care cost for smokers and quitters are derived from a Markov model built by Ali and colleagues 34. The model used a comprehensive modelling framework to represent the clinical pathways and their consequences associated with smoking and smoking cessation. Costs of smoking are defined by the World Health Organization as the difference between health care or other costs that actually occur due to smoking and the costs that would have occurred had there been no smoking 36. This model estimated health‐care cost savings due to the changing risk of clinical conditions that are known have significant health and economic consequences for smokers and quitters, such as lung cancer, chronic obstructive pulmonary disease (COPD), myocardial infarction and stroke. In the absence of estimated life‐time QALY gains in this model, we combined and utilized another English study by Vogl and colleagues which reported age‐ and gender‐specific EQ‐5D values according to smoking status 35. Relevant life‐time cost savings and QALY gains were attached to the participants by age, gender and smoking status to estimate life‐time outcomes associated with the two interventions. Future costs and health outcomes were discounted at 3.5% per annum 14. The same non‐parametric bootstrap method was used to derive long‐term CEACs to express the probability that the intervention is cost‐effective as a function of the willingness‐to‐pay thresholds during a life‐time horizon.
Sensitivity analysis
In order to explore the potential impact of missing data on the estimated intervention effects and costs, a sensitivity analysis was undertaken to repeat the cost‐effectiveness analysis using complete cases, whereby the results were analysed only for those participants who had both complete cost and outcome data.
Patient involvement
The interests of all parties and the views of the public have been represented fully in the conduct of this study from the design stage onwards. A past successful user of the Camden SSS was invited onto the Trial Management Group as a patient representative and has been fully involved at all Trial Management Group (TMG) meetings. She contributed to the design of both parts of the intervention, to the conduct of the trial and collection of data and was particularly helpful with suggestions of how to maximize response rate to the follow‐up. This patient representative also added greatly to the discussion of the results and of the practical implications of this method of recruitment to the SSS.
In addition, another past user of the Camden SSS also contributed to the development of both parts of the intervention. Both service users were consulted on the content of the brief personal letter at all stages of development and on the protocol for the taster sessions. Both users also narrated their own experiences of quitting, and these were used to create the video which formed a part of the taster session. A lay report of the results has been prepared for dissemination to all participating SSSs and general practices, who will also distribute the report to all interested study participants.
Results
In the cost‐effectiveness analysis, 4383 participants were analysed. The sample was 51% male, and the mean age was 49 years. Full details of the trial participants and clinical outcomes are given in the accompanying paper 12.
Resource use and costs
Table 2 summarizes the mean [and standard deviation (SD)] cost for the interventions and the subsequent use of health services during the 6‐month follow‐up period. The costs of delivering the trial intervention and the costs associated with the smoking cessation aids were significantly higher in the intervention group. Participants' total cost relies heavily upon their wider health resource use, but there were no significant differences in the use of resources during the 6‐month follow‐up. After adjusting for baseline resource use, the tailored letter and taster sessions cost an overall mean of £92 more per participant than the generic letter, with 95% confidence intervals (CI) crossing zero (−£32 to £216).
Table 2.
Average cost by category and treatment allocation [prices in GBP (£) in 2012–13].
| Intervention group n = 2635 Mean (SD) |
Control group n = 1748 Mean (SD) |
Differencea (£) (95% CI) |
|
|---|---|---|---|
| Intervention cost | £54 (£12) | £0.9 (£1) | £53 (£52 to £53) |
| NHS SSS attendance cost | £11 (£34) | £5 (£23) | £6 (£4 to £8) |
| Other non‐pharmacological cessation aids | £44 (£32) | £40 (£26) | £4 (£2 to £6) |
| Pharmacological cessation aids | £61 (£49) | £50 (£32) | £10 (£8 to £13) |
| Wider health‐care resource use cost | £608 (£2175) | £583 (£1860) | £25 (−£99 to £149) |
| Total cost | £777 (£2176) | £679 (£1860) | £98 (−£26 to £222) |
| Adjusted total costb | £760 (£2039) | £669 (£2059) | £92 (−£32 to £216) |
The numbers may not add up due to rounding. CI = confidence interval; SD = standard deviation; NHS SSS = National Health Service Stop Smoking Services.
Difference = costs for intervention group – costs for control group;
adjusted for baseline cost.
Outcome measures
The primary health economic outcome was QALYs for 6 months. QALYs for the intervention and control were estimated to be 0.382 (SD = 0.046) and 0.380 (SD = 0.046), respectively, after adjusting for the baseline utility scores (Table 3). However, the difference in QALYs between the two trial groups was not significant. Results of the secondary outcome measures are also given in Table 3. In this study, we applied a range of criteria to assess the throughput and success rates of the interventions, and the main outcome for quitting was the 7‐day point prevalent abstinence as validated by salivary cotinine at the 6‐month follow‐up. Both the proportion of people attending the SSS and the quit rates were significantly higher in the intervention group than in the control group.
Table 3.
Outcome measures and incremental cost‐effectiveness ratios (ICERs).
| Intervention group n = 2635 | Control group n = 1748 | Differencea (95% CI) | ICERs | |
|---|---|---|---|---|
|
QALY Mean (SD) |
QALY Mean (SD) | Difference (95% CI) | ICER (cost per QALY gained) (95% CI) | |
| Unadjusted QALYs | 0.381 (0.141) | 0.380 (0.136) | 0.0001 (−0.009 to 0.008) | £ 862 629 (−£742 154 to £1 159 241) |
| Adjusted QALYs (adjusted for baseline cost and EQ‐5D scores) | 0.382 (0.046) | 0.380 (0.046) | 0.002 (−0.001 to 0.004) | £59 401 (−£604 833 to £644 486) |
| Attendance at SSS (n, %) | Attendance at SSS (n, %) | Odds ratio (95% CI) | ICER (cost per additional person attending NHS SSS) (95% CI) | |
| Attendance at SSS | 458 (17.4%) | 158 (9.0%) | 2.12 (1.75 to 2.57) | £627 (£620 to £634) |
| Smoking outcome | Quitter (n, %) | Quitter (n, %) | Odds ratio (95% CI) | ICER (cost per additional quitter) (95% CI) |
| 24‐hour pp abstinence (self‐report) | 445 (16.9%) | 201 (11.5%) | 1.57 (1.31 to 1.88) | £1700 (−£602 to £4001) |
| 7‐day pp abstinence (validated) | 236 (9.0%) | 97 (5.6%) | 1.68 (1.32 to 2.15) | £2689 (−£952 to £6329) |
| 7‐day pp abstinence (self‐report) | 424 (16.1%) | 187 (10.7%) | 1.61 (1.34 to 1.94) | £1699 (−£601 to £3998) |
| 1‐month prolonged abstinence (self‐report) | 357 (13.6%) | 151 (8.6%) | 1.67 (1.36 to 2.04) | £1866 (−£660 to £4392) |
| 3‐month prolonged abstinence (validated) | 150 (5.7%) | 60 (3.4%) | 1.70 (1.25 to 2.31) | £4053 (−£1435 to £9541) |
| 3‐month prolonged abstinence (self‐report) | 240 (9.1%) | 103 (5.9%) | 1.61 (1.26 to 2.04) | £2849 (−£1008 to £6706) |
Difference = costs/effects for the intervention group – costs/effects for the control group; pp = per person. CI = confidence interval; QALY = quality‐adjusted life‐years; NHS SSS = National Health Service Stop Smoking Services.
Cost‐effectiveness analysis
ICERs were used to combine the costs and health benefits in a single measure to assess the incremental cost‐effectiveness of the trial intervention (Table 3). The adjusted mean costs were £92 higher and the adjusted mean QALYs were 0.002 higher in the intervention group compared with the control group. This generates an ICER of £59 401 (95% CI = –£604 833 to 644 486) per QALY gained. Figure 1 shows the cost‐effectiveness plane (CEP) representing 5000 bootstrapped resamples of the difference in costs and difference in QALYs when comparing the intervention group with the control group. The cost‐effectiveness acceptability curve (CEAC) (Fig. 2) illustrates that the probability that the tailored letter and taster session is more cost‐effective than the generic letter at 6 months is well below 50%, over a NICE decision‐making threshold range of £20 000–30 000 per QALY gained. Only at higher WTP thresholds (> £59 401 per QALY) does the intervention become more likely to be cost‐effective compared with the control.
Figure 1.
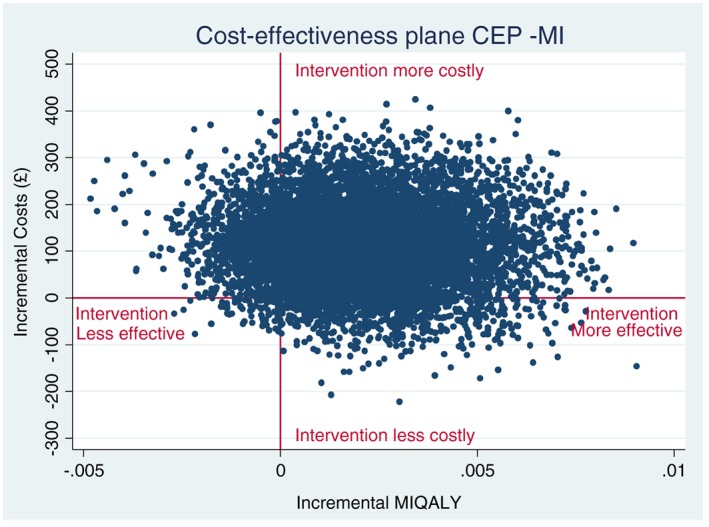
Cost‐effectiveness plane (multiple imputation analysis).
Figure 2.
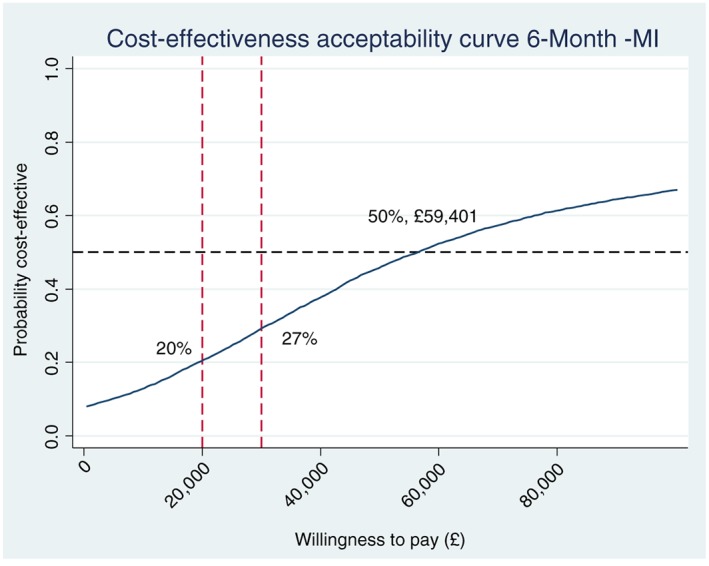
Cost‐effectiveness acceptability curve (multiple imputation analysis).
Table 3 also shows the total costs in relation to the other outcome measures. Four hundred and fifty‐eight (17.4%) smokers in the treatment group and 158 (9.0%) smokers in the control group attended the SSS. The average costs incurred in the intervention group and control group were £54 and 0.9, respectively, resulting in an ICER of £627 per additional attendee to the SSS. The cost per additional quitter ranged from £1699 to 4053 according to the criteria for abstinence. For the main quit rate (biochemical validation of 7‐day abstinence), the corresponding ICER was £2689 (95% CI = –£952 to 6329) per additional quitter. This indicates that if decision‐makers are willing to pay more than £627 to help one more smoker to attend the SSS, or £2689 to generate an additional quitter, the tailored letter and taster session would be the preferred option, otherwise usual care should be adopted.
Long‐term costs and outcome predictions
The cost‐effectiveness analysis was extrapolated to the life‐time time horizon using QALYs as the outcome measure. The life‐time accumulative QALY gains by smoking status, gender and age group, both before and after discounting, are summarized in Table 4. The life‐time horizon was defined as the participant's remaining life‐time between the age they entered the trial and the time they reach the national average life expectancy at birth (81 years in 2013 in the United Kingdom) 37. Overall life‐time health costs due to smoking‐related diseases for both smokers and ex‐smokers were derived from the published economic model and are listed in Table 5 34. Participants in the intervention group were expected to have health‐care cost savings of £210 (before discounting) and £74 (after discounting) during their life‐time compared to those in the control group. At the same time, they have higher life‐time QALY gains of 0.470 (before discounting) and 0.196 (after discounting) than people in the control group. The negative ICERs suggest that, during the participants' life‐time, tailored letters and taster sessions generate more QALY gains at a lower cost, indicating that the intervention is more cost‐effective than the control condition. Figure 3 presents CEACs for long‐term cost‐effectiveness. Using the NICE decision‐making threshold range of £20 000–30 000 per QALY gained, the probability that the intervention was more cost‐effective was 83% before discounting and 86% after discounting in the long term, which suggests that the intervention is a good use of NHS resources.
Table 4.
Cumulative life‐time QALY gains by gender and age group.
| Cumulative life‐time QALY gains by gender and age group (before discounting) | |||||||
|---|---|---|---|---|---|---|---|
| Life‐time QALY gain (male) | 16–24 | 25–34 | 35–44 | 45–54 | 55–64 | 65–74 | 75–79 |
| Ex‐occasional smokera | 65.603 | 46.986 | 37.555 | 28.379 | 19.675 | 11.574 | 3.706 |
| Ex‐regular smoker | 64.914 | 46.461 | 37.155 | 28.097 | 19.501 | 11.481 | 3.679 |
| Light smoker | 64.196 | 46.010 | 36.744 | 27.742 | 19.216 | 11.299 | 3.615 |
| Moderate smoker | 63.341 | 45.433 | 36.267 | 27.368 | 18.946 | 11.131 | 3.556 |
| Heavy smoker | 61.915 | 44.463 | 35.492 | 26.764 | 18.505 | 10.858 | 3.463 |
| Life‐time QALY gain (female) | 16–24 | 25–34 | 35–44 | 45–54 | 55–64 | 65–74 | 75–83 |
| Ex‐occasional smoker | 49.664 | 45.974 | 36.868 | 27.877 | 19.284 | 11.354 | 3.540 |
| Ex‐regular smoker | 49.002 | 45.369 | 36.381 | 27.509 | 19.030 | 11.203 | 3.494 |
| Light smoker | 48.622 | 44.997 | 36.059 | 27.245 | 18.827 | 11.079 | 3.448 |
| Moderate smoker | 48.006 | 44.425 | 35.590 | 26.874 | 18.557 | 10.909 | 3.389 |
| Heavy smoker | 46.874 | 43.377 | 34.747 | 26.225 | 18.095 | 10.629 | 3.293 |
| Cumulative life‐time QALY gains by gender and age group (after discounting) | |||||||
|---|---|---|---|---|---|---|---|
| Discounted life‐time QALY gain (male) | 16–24 | 25–34 | 35–44 | 45–54 | 55–64 | 65–74 | 75–79 |
| Ex‐occasional smoker | 22.421 | 20.530 | 18.032 | 14.924 | 11.165 | 6.370 | 3.347 |
| Ex‐regular smoker | 22.137 | 20.287 | 17.832 | 14.776 | 11.067 | 6.320 | 3.322 |
| Light smoker | 21.992 | 20.117 | 17.648 | 14.589 | 10.903 | 6.218 | 3.264 |
| Moderate smoker | 21.732 | 19.874 | 17.424 | 14.394 | 10.749 | 6.124 | 3.211 |
| Heavy smoker | 21.274 | 19.463 | 17.060 | 14.078 | 10.497 | 5.971 | 3.127 |
| Discounted life‐time QALY gain (female) | 16–24 | 25–34 | 35–44 | 45–54 | 55–64 | 65–74 | 75–83 |
| Ex‐occasional smoker | 22.201 | 20.558 | 18.379 | 15.590 | 12.267 | 8.062 | 5.385 |
| Ex‐regular smoker | 21.901 | 20.288 | 18.136 | 15.385 | 12.105 | 7.956 | 5.315 |
| Light smoker | 21.762 | 20.132 | 17.980 | 15.234 | 11.973 | 7.863 | 5.246 |
| Moderate smoker | 21.497 | 19.885 | 17.749 | 15.026 | 11.797 | 7.737 | 5.156 |
| Heavy smoker | 20.997 | 19.423 | 17.332 | 14.661 | 11.499 | 7.531 | 5.010 |
Ex‐regular smokers: people who used to smoke sometimes but have now quit;
light smokers: smokers who smoke fewer than 10 cigarettes a day;
moderate smokers: smokers who smoke between 10 and 19 cigarettes a day;
heavy smokers: smokers who smoke 20 or more cigarettes a day.
Ex‐occasional smokers: people who have smoked only once or twice. QALY = quality‐adjusted life‐years.
Table 5.
Long‐term cost‐effectiveness results.
| Before discounting | Discounted | |||
|---|---|---|---|---|
| Intervention group (n = 2635) | Control group (n = 1748) | Intervention group (n = 2635) | Control group (n = 1748) | |
| Life‐time cost (mean, SD) | £19 390 (£2776) | £19 601 (£2787) | £5775 (£1109) | £5848 (£1114) |
| Cost differencea | –£210 (−£432 to £11) | –£74 (£–162 to £15) | ||
| Life‐time QALY gains (mean, SD) | 27.009 (11.894) | 26.539 (11.943) | 13.974 (4.424) | 13.778 (4.442) |
| QALY differencea | 0.470 (−0.478 to 1.419) | 0.196 (−0.157 to 0.549) | ||
| ICER (cost per QALY gained) (95% CI) | –£447 (95% CI = –£4368 to £3646) | –£376 (95% CI = –£3881 to £3207) | ||
Difference = costs/effects for the intervention group – costs/effects for the control group. QALY = quality‐adjusted life‐years; SD = standard deviation; ICER = incremental cost‐effectiveness ratios; CI = confidence interval.
Figure 3.
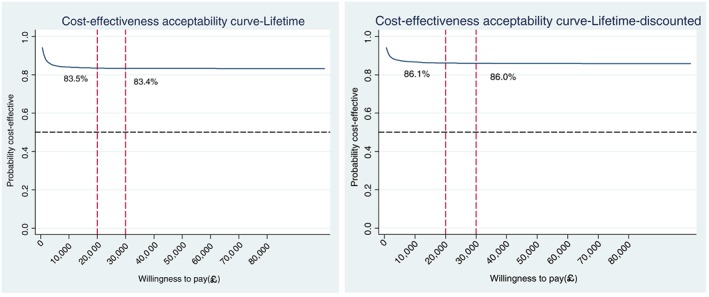
Life‐time cost‐effectiveness acceptability curve (before and after being discounted).
Sensitivity analysis
Sensitivity analysis was carried out to investigate the robustness of the results. Complete cost and cost‐effectiveness data were available for 1667 (63%) of participants in the intervention group and 1108 (63%) of participants in the control group. The results of the complete case analysis were very similar to those from the primary analysis, and results from both analyses are summarized in Table 6. For both trial groups, the average costs and the gains in cost‐effectiveness were slightly higher in the complete cases, and the ICERs decreased slightly. Figure 4 shows the CEAC with QALY as the outcome measure from the complete case analysis. The probability of the intervention being more cost‐effective compared to the control group increased slightly, but the overall conclusion remains the same. The complete case results appeared to be robust compared with the analyses including imputed data.
Table 6.
Summary of the cost‐effectiveness results from the multiple imputation analysis versus completed case analysis.
| Multiple imputation analysis | Complete case analysis | |||
|---|---|---|---|---|
| Intervention group (n = 2635) | Control group (n = 1748) | Intervention group (n = 1667) | Control group (n = 1108) | |
| Adjusted total cost (mean, SD) | £760 (£2039) | £669 (£2059) | £851 (£2455) | £724 (£2465) |
| Cost difference (£) (95% CI) | £92 (−£32 to £216) | £127 (−£60 to £314) | ||
| Adjusted QALY at 6 months | 0.382 (0.046) | 0.380 (0.046) | 0.384 (0.052) | 0.382 (0.053) |
| QALY difference (95% CI) (mean, SD) | 0.002 (−0.001 to 0.004) | 0.003 (−0.044 to 0.055) | ||
| ICER (cost per QALY gained) (95% CI) | £59 401 (−£604 833 to £644 486) | £49 842 (−£425 064 to £536 813) | ||
| Intervention cost (mean, SD) | £54 (£12) | £0.9 (£1) | £55 (£13) | £0.9 (£2) |
| Attendance at SSS (n, %) | 458 (17.4%) | 158 (9.0%) | 334 (20.0%) | 102 (9.2%) |
| ICER (cost per additional attendee NHS SSS) (95% CI) | £627 (£620 to £634) | £498 (£491 to £504) | ||
| Validated 7‐day abstinence at 6 months | 236 (8.96%) | 97 (5.55%) | 214 (12.84%) | 87 (7.85%) |
| ICER (cost per additional quitter) (95% CI) | £2689 (−£952 to £6329) | £2552 (−£1199 to £6303) | ||
QALY = quality‐adjusted life‐years; SD = standard deviation; ICER = incremental cost‐effectiveness ratios; CI = confidence interval.
Figure 4.
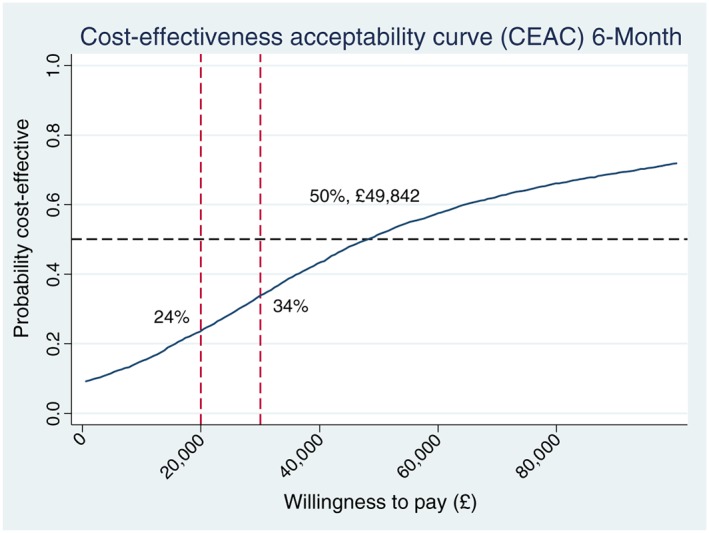
Cost‐effectiveness acceptability curve (complete case analysis).
Discussion
Although smoking prevalence in the United Kingdom has remained unchanged in recent years, the use of NHS Stop Smoking Services has continued to decline sharply 8. The Start2quit trial is a timely study to explore new interventions to increase the take‐up of the SSS, and ultimately to increase the number of successful quit attempts. There are similar studies in the literature investigating the effectiveness of interventions to increase the attendance at the SSS 38, 39 but this is the first study that has estimated the cost‐effectiveness of the intervention to inform smokers about the services.
Our main finding was that, compared with the generic letter, the tailored letter plus the taster session is likely to be more effective but more costly. The within‐trial cost‐effectiveness analysis shows that in the short term, i.e. 6 months, the intervention is less likely to be cost‐effective compared with the control. However, in common with many preventive interventions, the majority of benefits from stopping smoking are gained from the reduced risks of developing smoking‐related diseases, such as lung cancer and COPD, later in life 3, 33. The 6‐month follow‐up of the trial is therefore insufficient to capture the longer‐term health benefits of the intervention 20, 27, 40. The long‐term cost‐effectiveness analysis, which considered the life‐time cost savings and QALY gains from the two interventions, suggests that the tailored letter and taster sessions had a greater than 83% chance of being more cost‐effective compared with the standard generic letter at WTP thresholds, ranging between £20 000 and 30 000 per QALY gained.
The direct intervention costs were, as expected, higher in the tailored letter and taster session group than in the generic letter group (£54 versus 0.9), due to the complex design of the intervention. In the intervention group, 60% of the total £54 cost was spent on sending baseline questionnaires to gather information for generating the tailored letter, while for the control group the baseline questionnaires were used only for research purposes, and this cost was considered as a research cost and hence excluded from the analysis. A potential way to reduce the intervention cost is to use cheaper alternatives, such as e‐mails, to reach smokers to obtain any information needed for the tailored letter. However, the response rates may be lower from e‐mails compared with traditional postal questionnaires and further studies are recommended 41
During the trial period, smokers in the intervention group were more likely to use smoking cessation aids, both from the SSS and from other sources. The mean cost of SSS attendance was more than twice in the intervention group than in the control group (£11 versus 5), which indicates that the intervention greatly increased the usage of the SSS. The use of other pharmacological and non‐pharmacological cessation aids, such as GP, nurse, pharmacist visits and smoking helplines, were also significantly higher in the intervention group, but other, wider, health‐care resource uses not linked directly to smoking cessation were not significantly different between the two groups.
In the long‐term cost‐effectiveness analysis, we took into account only the direct health‐care costs for treating smoking‐related disease, and other indirect costs of smoking, such as productivity losses due to smoking‐related diseases and premature death, costs of accidental fires and second‐hand smoking, were not included 42 Therefore, the intervention could generate more cost savings and become more cost‐effective if we take into account the indirect costs of smoking, given that the quit rate was significantly higher in the intervention group than in the control group.
Strengths and limitations of the study
To the best of our knowledge, the present study is the first full economic evaluation alongside a large RCT to have assessed the cost‐effectiveness of a two‐component intervention designed to increase attendance at the SSS in the United Kingdom. A particular strength of the study is that we assessed the cost‐effectiveness of the intervention both in the short term and during a life‐time horizon. The primary analysis used a full imputed data set, but we also carried out complete case analysis in a sensitivity analysis to test the robustness of the results. The conclusion remained robust after analysis of the statistical uncertainty.
However, our study had several limitations. The primary outcome measure used in the study was QALYs measured by EQ‐5D, which is a widely applied generic instrument for measuring the quality of life. However, it may not be able to capture all aspects of quality of life changes for smokers, especially in the short term. The adjusted QALY difference between the two groups was very small within the trial period [0.002 QALYs (95% CI = –0.001 to 0.004)], which resulted in very high ICERs at the 6‐month point [£59 401 per additional QALY gained (95% CI = –£604 833 to 644 486)]. The high ICERs led subsequently to the conclusion that the intervention is not cost‐effective in the short term, because it far exceeded the NICE WTP threshold (£30 000 per QALY gained).
In the long‐term cost‐effectiveness analysis, all quitters were assumed to remain abstinent from smoking after the intervention. Due to the complexity of smoking behaviour the risk of relapse for quitters is very high but, conversely, many smokers may achieve quitting smoking spontaneously, i.e. self‐initiated smoking cessation without interventions 43 Therefore, further studies, such as RCTs with longer follow‐up periods or the use of models that consider the changes in smoking behaviour, are needed.
In conclusion, using the within‐trial data, the use of tailored letters and taster sessions is a more costly and more effective intervention compared with the standard generic letter, but is unlikely to be cost‐effective during the short term. However, quitting smoking yields far greater health‐care cost savings and health benefits during the long‐term through the reduced risk of developing smoking‐related diseases. The long‐term results indicate that during a life‐time horizon the tailored letter and taster sessions become more effective and less costly, and this intervention has a great probability of being more cost‐effective at more than 86% using the £20 000–30 000 per QALY gained decision‐making threshold.
Declaration of interests
None.
Acknowledgements
This project is funded by the Health Technology Assessment programme (HTA), which is part of the National Institute for Health Research (NIHR) (Project no. 08/58/02). This body provides funding to independent research investigating the effectiveness of different health care treatments in the NHS. It identifies the most important questions that the NHS needs to answer and commissions the most important research. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the HTA Programme, NIHR, NHS or the Department of Health. We would like to thank all members of the Research Team, in particular Leanne Gardner, Dimitra Kale and Molly Sweeney Magee, who managed the recruitment of participants and data collection and provided administrative support to the trial. The successful completion of the study would not have been possible without the support and input from the local UK PRCNs and CLRNs, the local collaborators and service managers, of the participating Stop Smoking Services, Practice Managers and administrative staff at all of the participating General Practices. We also acknowledge the support of the Trial Steering Committee and user representative Sue Dowd, who sat on both the Trial Management group and the Trial Steering Committee.
Wu, Q. , Gilbert, H. , Nazareth, I. , Sutton, S. , Morris, R. , Petersen, I. , Galton, S. , and Parrott, S. (2018) Cost‐effectiveness of personal tailored risk information and taster sessions to increase the uptake of the NHS stop smoking services: the Start2quit randomized controlled trial. Addiction, 113: 708–718. doi: 10.1111/add.14086.
References
- 1. Action on Smoking and Health (ASH) . Smoking Statistics: Illness and Death. London: ASH; 2015. [Google Scholar]
- 2. Britton J. ABC of Smoking Cessation. Malden, MA: BMJ Books; 2004. [Google Scholar]
- 3. Doll R., Peto R., Boreham J., Sutherland I. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ 2004; 328: 1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Action on Smoking and Health (ASH) . Smoking Statistics: Who Smokes and How Much. London; ASH; 2014. [Google Scholar]
- 5. Nash R., Featherstone H. Cough Up: Balancing Tobacco Income and Costs in Society. London: Policy Exchange; 2010. [Google Scholar]
- 6. Allender S., Balakrishnan R., Scarborough P., Webster P., Rayner M. The burden of smoking‐related ill health in the UK. Tob Control 2009; 18: 262–267. [DOI] [PubMed] [Google Scholar]
- 7. McNeill A., Raw M., Whybrow J., Bailey P. A national strategy for smoking cessation treatment in England. Addiction 2005; 100: 1–11. [DOI] [PubMed] [Google Scholar]
- 8. Health and Social Care Information Centre . Statistics on NHS Stop Smoking Services in England 1 April 2013 to 31 March 2014. Final Report 2014. Available at: http://digital.nhs.uk/catalogue/PUB14610 (accessed 15 February 2016) (Archived at http://www.webcitation.org/6v1Mfohke on 16 November 2017).
- 9. Dobbie F., Hiscock R., Leonardi‐Bee J., Murray S., Shahab L., Aveyard P. et al Evaluating long‐term outcomes of NHS stop smoking services (ELONS): a prospective cohort study. Health Technol Assess 2015; 19: 1–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. West R., Brown J. Smoking and Smoking Cessation in England in 2011: Findings From the Smoking Toolkit Study 2012. Available at: http://www.smokinginengland.info/latest-statistics/ (accessed 15 February 2016) (Archived at http://www.webcitation.org/6v1SZUtnh on 16 November 2017).
- 11. Cancer Research UK/Action on Smoking and Health (ASH) . Cutting Down: The Reality of Budget Cuts to Local Tobacco Control. A report by Action on Smoking and Health commissioned by Cancer Research UK. 2016. Available at: https://www.cancerresearchuk.org/sites/default/files/local_authority_survey_2016_report_cruk_finalfinal.pdf (accessed 15 February 2016) (Archived at http://www.webcitation.org/6v1Tz98u6 on 16 November 2017).
- 12. Gilbert H., Sutton S., Morris R., Petersen I., Galton S., Wu Q. et al Effectiveness of personal tailored risk information and taster sessions to increase the uptake of smoking cessation services: randomized controlled trial (Start2quit). Lancet 2016; 389: 823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gilbert H., Sutton S., Morris R., Parrot S., Galton S., Nazareth I. Evaluating the effectiveness of using personal tailored risk information and taster sessions to increase the uptake of smoking cessation services: study protocol for a randomised controlled trial. Trials 2012; 13: 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guide to the Methods of Technology Appraisal. London: National Institute for Health and Care Excellence (NICE), NICE; 2013. [PubMed] [Google Scholar]
- 15. Curtis L. Unit Costs of Health and Social Care 2013. Personal Social Services Research Unit: Kent; 2013. [Google Scholar]
- 16. National Institute for Health and Care Excellence (NICE) . Smoking Cessation Services: Costing Report. London: NICE; 2008. [Google Scholar]
- 17. Wu Q., Parrott S., Godfrey C., Gilbert H., Nazareth I., Leurent B. et al Cost‐effectiveness of computer‐tailored smoking cessation advice in primary care: a randomized trial (ESCAPE). Nicotine Tob Res 2014; 16: 270–278. [DOI] [PubMed] [Google Scholar]
- 18. Health and Social Care Information Centre . Statistics on NHS Stop Smoking Services in England April 2014 to March 2015. Final Report 2015. Available at: http://digital.nhs.uk/catalogue/PUB18002 (accessed 15 February 2016) (Archived at http://www.webcitation.org/6v1U8yCyi on 16 November 2017).
- 19. Department of Health . Reference costs 2012–13; 2013. Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/261154/nhs_reference_costs_2012-13_acc.pdf (accessed 3 August 2015) (Archived at http://www.webcitation.org/6v1WNwNSs on 16 November 2017).
- 20. The EuroQol Group . EuroQol—a new facility for the measurement of health‐related quality of life. The EuroQol Group. Health Policy 1990; 16: 199–208. [DOI] [PubMed] [Google Scholar]
- 21. Dolan P. Modeling valuations for EuroQol health states. Med Care 1997; 35: 1095. [DOI] [PubMed] [Google Scholar]
- 22. Drummond M., Sculpher M., Torrance G. W., O'Brien B. J., Stoddart G. L. Methods for the Economic Evaluation of Health Care Programmes, 3rd edn. Oxford: Oxford University Press; 2005, p. 21. [Google Scholar]
- 23. Dolan P., Gudex C., Kind P., Williams A. A Social Tariff for EuroQol: Results from a UK General Population Survey. York: Centre for Health Economics; 1995. [Google Scholar]
- 24. Richardson G., Manca A. Calculation of quality adjusted life years in the published literature: a review of methodology and transparency. Health Econ 2004; 13: 1203–1210. [DOI] [PubMed] [Google Scholar]
- 25. Drummond M. F., Jefferson T. O. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ economic evaluation working party. BMJ 1996; 313: 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coyle D., Davies L., Drummond M. F. Trials and tribulations. Emerging issues in designing economic evaluations alongside clinical trials. Int J Technol Assess Health Care 1998; 14: 135–144. [DOI] [PubMed] [Google Scholar]
- 27. Petrou S., Gray A. Economic evaluation alongside randomised controlled trials: design, conduct, analysis, and reporting. BMJ 2011; 342: d1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Efron B., Tibshirani R. An Introduction to the Bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- 29. Chaudhary M. A., Stearns S. C. Estimating confidence intervals for cost‐effectiveness ratios: an example from a randomized trial. Stat Med 1996; 15: 1447–1458. [DOI] [PubMed] [Google Scholar]
- 30. Willan A. R., O'Brien B. J. Confidence intervals for cost‐effectiveness ratios: an application of Fieller's theorem. Health Econ 1996; 5: 297–305. [DOI] [PubMed] [Google Scholar]
- 31. Severens J. L., De Boo T. M., Konst E. M. Uncertainty of incremental cost‐effectiveness ratios. A comparison of Fieller and bootstrap confidence intervals. Int J Technol Assess Health Care 1999; 15: 608–614. [PubMed] [Google Scholar]
- 32. Fenwick E., Claxton K., Sculpher M. Representing uncertainty: the role of cost‐effectiveness acceptability curves. Health Econ 2001; 10: 779–787. [DOI] [PubMed] [Google Scholar]
- 33. Rasmussen S. R., Prescott E., Sorensen T. I., Sogaard J. The total lifetime costs of smoking. Eur J Public Health 2004; 14: 95–100. [DOI] [PubMed] [Google Scholar]
- 34. Ali S., Godfrey C. A., Parrott S. Economic Model of Adult Smoking Related Costs and Consequences for England: London: Public Health Research Consortium; 2011.
- 35. Vogl M., Wenig C. M., Leidl R., Pokhrel S. Smoking and health‐related quality of life in English general population: implications for economic evaluations. BMC Public Health 2012; 12: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. World Health Organization (WHO) . Economics of Tobacco Toolkit: Assessment of the Economic Costs of Smoking. Geneva: World Health Organization; 2011. Available at: http://apps.who.int/iris/bitstream/10665/44596/1/9789241501576_eng.pdf (accessed 3 August 2015) (Archived at http://www.webcitation.org/6v1VG4Beg on 16 November 2017). [Google Scholar]
- 37. World Health Organization (WHO) . Global Health Observatory Data Repository: Life Expectancy, Data By Country. Geneva, Switzerland: WHO; 2015. Available at: http://apps.who.int/gho/data/node.main.688?lang=en (accessed 3 August 2015) (Archived at http://www.webcitation.org/6v1UuEHdQ on 16 November 2017). [Google Scholar]
- 38. Murray R. L., Coleman T., Antoniak M., Stocks J., Fergus A., Britton J. et al The effect of proactively identifying smokers and offering smoking cessation support in primary care populations: a cluster‐randomized trial. Addiction 2008; 103: 998–1006; discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 39. Lichtenstein E., Hollis J. Patient referral to a smoking cessation program: who follows through? J Fam Pract 1992; 34: 739–744. [PubMed] [Google Scholar]
- 40. Hlatky M. A., Owens D. K., Sanders G. D. Cost‐effectiveness as an outcome in randomized clinical trials. Clin Trials 2006; 3: 543–551. [DOI] [PubMed] [Google Scholar]
- 41. Pit S. W., Vo T., Pyakurel S. The effectiveness of recruitment strategies on general practitioner's survey response rates—a systematic review. BMC Med Res Methodol 2014; 14: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Action on Smoking and Health (ASH) . The Economics of Tobacco. London: ASH; 2015. [Google Scholar]
- 43. Woolacott N. F., Jones L., Forbes C. A., Mather L. C., Sowden A. J., Song F. J. et al The clinical effectiveness and cost‐effectiveness of bupropion and nicotine replacement therapy for smoking cessation: a systematic review and economic evaluation. Health Technol Assess 2002; 6: 1–245. [DOI] [PubMed] [Google Scholar]


