Abstract
Aim
To evaluate the effectiveness and tolerability of exenatide once weekly (EQW) compared with basal insulin (BI) among injectable‐drug‐naïve patients with type 2 diabetes mellitus (T2DM) who are elderly or have renal impairment (RI).
Materials and methods
Initiators of EQW and BI with T2DM were identified for the period 2012 to 2015 within a US electronic health record database and matched by propensity score. Matched EQW and BI initiators aged ≥65 years or who had RI were compared. Data on weight, glycated haemoglobin (HbA1c), estimated glomerular filtration rate (eGFR), blood pressure and lipids were obtained at baseline and quarterly (Q1–Q4) or semi‐annually for 1 year after drug initiation. Hypoglycaemia and gastrointestinal symptoms were identified using diagnosis codes and data abstracted from clinical notes.
Results
Among patients aged ≥65 years, HbA1c changed by −0.50 and −0.31 percentage points from baseline to Q4 for EQW and BI initiators, respectively. Weight changed by −1.6 kg among EQW initiators compared with 0.2 kg among BI initiators. Compared with BI initiators, EQW initiators had a 1.45‐fold increased risk of nausea and vomiting. Among patients with RI, HbA1c changed by −0.58 and −0.33 percentage points from baseline to Q4 for EQW and BI initiators, respectively. Weight changed by −1.9 kg for EQW initiators while BI initiators had no change in weight. EQW initiators had a 1.28‐fold increased risk of constipation and diarrhoea compared with BI initiators.
Conclusion
Regardless of age or renal function, the benefits of EQW relative to BI treatment are improved glycaemic control and increased weight loss, which should be weighed against the increased risk of gastrointestinal symptoms.
Keywords: exenatide, basal insulin, cohort study
1. INTRODUCTION
There are 28.9 million people aged ≥20 years living with type 2 diabetes mellitus (T2DM) in the United States, of whom 11.2 million are aged ≥65 years.1 Approximately 20% of adults aged ≥65 years have T2DM. The elderly population includes newly diagnosed patients and patients being treated for many years, patients who are otherwise healthy, patients with other comorbidities, and frail patients. Elderly patients with T2DM are at high risk of the microvascular and macrovascular complications of diabetes, including kidney disease and cardiovascular disease.2, 3
Long‐standing hyperglycaemia is an important risk factor for diabetic nephropathy, and diabetes is the most common cause of chronic kidney disease in the United States. Approximately 20% to 30% of patients with T2DM will develop diabetic nephropathy over time,4 and a substantial proportion of these patients will progress to kidney failure.5 The kidneys play an important role in glycaemic control through gluconeogenesis and tubular reabsorption of glucose.6 Additionally, the kidneys play an important role in the clearance of antihyperglycaemic medications, including glucagon‐like protein‐1 receptor agonists (GLP‐1RAs) and long‐acting basal insulin (BI). Renal impairment (RI) alters glycaemic control, placing patients at high risk of both hyperglycaemia and hypoglycaemia.6 Patients with T2DM and RI, specifically chronic kidney disease, are at increased risk of cardiovascular complications and death.7, 8
The treatment goals for patients with T2DM who are elderly or have RI include the management of hyperglycaemia, prevention of diabetes progression, and avoidance of adverse treatment effects, mainly hypoglycaemia. In these populations, complications of hypoglycaemia include increased risks of hospitalization, cardiovascular events and falls or fractures.5, 8 Elderly patients with T2DM may have RI related to age or T2DM itself, further complicating their treatment options. Intensive treatment for glycaemic control in elderly patients and/or patients with RI may place them at higher risk of hypoglycaemia and its complications.9
In January 2012, the US Food and Drug Administration approved, Bydureon, a once‐weekly form of exenatide (EQW), for the treatment of T2DM. Evidence from clinical trials suggested that EQW improves glucose control compared with twice‐daily exenatide and BI, and is associated with lower occurrence of hypoglycaemia compared with BI.10, 11, 12 Thus, EQW may be a good option for both elderly patients and patients with RI, when an injectable antihyperglycaemic agent is considered. EQW does not require dose titration, as other GLP‐1RAs do, and may have other advantages over insulin, such as the prevention of weight gain and the improvement of blood pressure and lipid profiles.13, 14 Given that elderly patients and patients with RI are typically not well represented in clinical trials, the aim of the present study was to examine the extent to which the benefit of EQW observed in randomized trials translates to these patient groups in a real‐world setting.
2. METHODS
2.1. Data source
The study population was drawn from Optum's Electronic Health Records (EHR) database. The EHR database integrates records from many medical groups and hospitals in the United States to form a broad, de‐identified patient‐level database of healthcare encounters in ordinary clinical practice, and is a geographically diverse representation of >25 000 physicians and 25 million patients. The EHR database captures diagnoses, procedures, medications (prescribed and administered), clinical measures (biometric and laboratory values) and clinical notes (i.e. physician, pathology and radiology) that have been recorded at the time of care.
2.2. Study design and population
We identified injectable‐drug‐naïve patients with T2DM who initiated either EQW or BI between January 2012 and January 2015, with follow‐up through March 2015. Initiators of EQW or BI (insulin glargine or insulin detemir) were identified in the EHR database using National Drug Codes. Eligible patients were required to: be at least aged 18 years on the date of EQW or BI initiation (index date); receive care documented in the EHR database, specifically, at least one outpatient provider visit in the 6 months prior to and including the index date (baseline period); and have at least one diagnosis of T2DM (International Classification of Diseases, ninth revision, Clinical Modification [ICD‐9‐CM] diagnosis codes 250.×0 or 250.×2) during the baseline period. Patients with a prior diagnosis of type 1 diabetes or gestational diabetes, or evidence of an injectable antidiabetic treatment (GLP‐1RA or any insulin) during the baseline period were excluded from the study population.
2.3. Matching
Propensity score matching was implemented in order to achieve balance between EQW and BI initiators with respect to a large number of characteristics.15, 16, 17 Propensity scores were estimated using a logistic regression model that incorporated potential predictors of therapy as the independent variables, and initiation of EQW vs BI as the dependent variable. The potential predictors of therapy included in the propensity score model were demographics, calendar year of EQW or BI initiation, healthcare utilization variables, a priori‐specified confounding and stratification variables (age group, race, glycated haemoglobin [HbA1c], estimated glomerular filtration rate [eGFR] and hypoglycaemia), and empirically identified covariates ascertained from the 100 most prevalent diagnoses, medications and procedures among EQW initiators identified during the baseline period. Clinical observations (ie, body weight, body mass index, systolic and diastolic blood pressure) and laboratory values (ie, HbA1c, serum creatinine, urine albumin/creatinine ratio, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides) were selected from the EHR. For these measures, the last available value occurring in the baseline period was selected to represent status at initiation of therapy. If no value was observed during the baseline period, the value was multiply‐imputed (5 imputations) using baseline covariates, follow‐up values, as available, and the presence or absence of clinical outcomes (eg, microvascular disease, cardiovascular disease and hospitalization) using fully conditional specification methods (FCS).18 eGFR was calculated based on serum creatinine, sex and race variables using the Chronic Kidney Disease Epidemiology Collaboration equation.19, 20 Hypoglycaemia was identified using an algorithm developed by Optum that incorporated both diagnostic codes and natural language processing (NLP) from clinical notes.21, 22
Clinically important variables were identified using univariate c‐statistics and were forced into the propensity score model. Other covariates were allowed to enter the model using a stepwise selection based on a univariate P value for entry (P < .2) and a multivariate P value for remaining in model (P < .3). Each EQW initiator was matched to up to two BI initiators using a greedy matching algorithm. Once an EQW initiator was matched with two BI initiators, the members of the matched set were removed from subsequent matching.23, 24 Covariates included in the propensity score model were balanced across cohorts, and outcome rates observed among EQW and BI initiators were directly compared.
2.4. Subpopulation definitions
Comparisons between the propensity score‐matched EQW and BI initiators were made within 2 subpopulations based on age group (18‐64 years and ≥65 years) and renal function (normal renal function defined as eGFR ≥90.00 mL/min/1.73m2 and RI defined as eGFR <90.00 mL/min/1.73m2).
2.5. Outcome definitions
Change in HbA1c, body weight, blood pressure and lipids (total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides) from baseline were the outcomes measuring treatment effectiveness. These outcomes were summarized in standard intervals over the first year after EQW or BI initiation. HbA1c and body weight were summarized quarterly (3‐month intervals), and blood pressure and lipid profiles were summarized semi‐annually (6‐month intervals). The interval value was taken as the mean of values occurring within an interval. If no value was observed in the interval, the value was multiply‐imputed (5 imputations) using the fully conditional specification method.18 Parameter estimates and associated variance (SEs) were determined within imputed datasets, and pooled (averaged) into a single set of statistics (SAS PROC MIANALYZE) that reflect the uncertainty in parameter estimates within and between all imputations.25
The occurrence of hypoglycaemia and gastrointestinal symptoms (nausea, vomiting, diarrhoea, constipation), and change in renal function from baseline were measures of treatment tolerability. Hypoglycaemia and gastrointestinal symptoms were identified by using both ICD‐9‐CM diagnosis codes within structured fields and NLP clinical notes.21 The ICD‐9‐CM diagnosis codes used to identify hypoglycaemia were based on a modified algorithm described by Ginde et al.26 Gastrointestinal symptoms were identified using ICD‐9‐CM diagnosis codes: 536.2, persistent vomiting; 787.01, nausea and vomiting; 787.02, nausea alone; 787.03, vomiting alone; 787.91, diarrhoea; 564.5, functional diarrhoea; and 564.0×, constipation. NLP identifies sentiment terms (denial, affirmation) of the event in the clinical notes that allows a determination of whether the provider denied or affirmed the occurrence of an event. Events identified using both ICD‐9‐CM diagnosis codes and NLP on the same day in an outpatient setting or within 7 days during a continuous inpatient stay were counted as a single event.
Renal function was evaluated using eGFR summarized in standard quarterly intervals over the first year after initiation of EQW or BI treatment. Again, the interval value was taken as the mean of values occurring within an interval or was multiply‐imputed if no value was available. Pooled estimates of effect were calculated.
2.6. Analysis plan
Each patient was followed from the index date until the earliest occurrence of a new event (counted separately for each event), disenrolment from the EHR system, or end of the study follow‐up period (March 31, 2015). All follow‐up time was attributed to the therapy initiated (EQW or BI) on the index date. The number of patients observed and person‐time of observation were used to calculate the proportion of and rates of outcome occurrence, respectively.
Changes in HbA1c, body weight, blood pressure and lipids were calculated as the absolute difference between the measurements taken in the baseline period and in each standard follow‐up interval. Distributions of changes across each measure were summarized with the mean, mean of absolute differences, or the frequency of measures that were collapsed into a categorical metric. For each measurement, the estimate and its 95% confidence interval (CI) are presented. Comparing EQW and BI initiators, non‐overlapping 95% CIs indicated a significant difference that was unlikely to be explained by chance.
The frequency and proportion of hypoglycaemia and gastrointestinal symptom events among EQW and BI initiators were tabulated during follow‐up. We calculated event incidence rates (IRs) and 95% CIs, using person‐time censored at first event during follow‐up. Cohorts were compared using a relative rate (RR) estimate and its 95% CI. RR estimates with 95% CIs not including the value 1 indicated significant differences in event IR between EQW and BI initiators that were unlikely to be explained by chance.
2.7. Ethics
The study protocol was approved by a central institutional review board and Privacy Board. The study was conducted according to guidelines for Good Pharmacoepidemiology Practice.27
3. RESULTS
We identified 2075 EQW initiators and 73 610 BI initiators meeting study eligibility criteria. The analysis included 2008 EQW initiators matched to 4016 BI initiators by propensity score. Of the matched initiators, 495 (25%) EQW initiators and 952 (24%) BI initiators were aged ≥65 years. eGFR values were available for 1946 (97%) EQW initiators and 3903 (97%) BI initiators. Of these, 1006 (52%) EQW initiators and 2001 (51%) BI initiators had RI. Initiators were followed for an average of 1.5 person‐years. Among the subgroups of patients aged ≥65 years and with RI, EQW and BI initiators were similar to each other with respect to baseline characteristics, including mean HbA1c values, body weight, systolic and diastolic blood pressure, lipids and eGFR (Table 1).
Table 1.
Baseline characteristics for propensity score‐matched cohorts of injectable‐drug‐naive exenatide once weekly and basal insulin initiators, by age and renal function
| Variable | Age group | Renal functiona | ||||||
|---|---|---|---|---|---|---|---|---|
| Age 18‐64 years | ≥65 years | Normal function | Any RI | |||||
| EQW, n (%) | BI, n (%) | EQW, n (%) | BI, n (%) | EQW, n (%) | BI, n (%) | EQW, n (%) | BI, n (%) | |
| Number of patients | 1513 | 3064 | 495 | 952 | 940 | 1902 | 1006 | 2001 |
| Age group | ||||||||
| ≤ 34 years | 65 (4.3) | 136 (4.4) | 53 (5.6) | 110 (5.8) | 9 (0.9) | 18 (0.9) | ||
| 35‐44 years | 215 (14.2) | 433 (14.1) | 170 (18.1) | 331 (17.4) | 35 (3.5) | 88 (4.4) | ||
| 45‐54 years | 540 (35.7) | 1079 (35.2) | 330 (35.1) | 668 (35.1) | 194 (19.3) | 379 (18.9) | ||
| 55‐64 years | 693 (45.8) | 1416 (46.2) | 302 (32.1) | 641 (33.7) | 368 (36.6) | 740 (37.0) | ||
| 65‐74 years | 421 (85.1) | 815 (85.6) | 81 (8.6) | 148 (7.8) | 331 (32.9) | 647 (32.3) | ||
| ≥75 years | 74 (14.9) | 137 (14.4) | 4 (0.4) | 4 (0.2) | 69 (6.9) | 129 (6.4) | ||
| Sex | ||||||||
| Men | 723 (47.8) | 1503 (49.1) | 280 (56.6) | 476 (50.0) | 449 (47.8) | 938 (49.3) | 524 (52.1) | 991 (49.5) |
| Women | 790 (52.2) | 1561 (50.9) | 215 (43.4) | 476 (50.0) | 491 (52.2) | 964 (50.7) | 482 (47.9) | 1010 (50.5) |
| Race/Ethnicity | ||||||||
| White | 1198 (79.2) | 2449 (79.9) | 432 (87.3) | 828 (87.0) | 744 (79.1) | 1548 (81.4) | 886 (88.1) | 1729 (86.4) |
| Black/African American | 123 (8.1) | 254 (8.3) | 28 (5.7) | 49 (5.1) | 95 (10.1) | 171 (9.0) | 56 (5.6) | 132 (6.6) |
| Hispanic | 81 (5.4) | 194 (6.3) | 15 (3.0) | 26 (2.7) | 58 (6.2) | 134 (7.0) | 38 (3.8) | 86 (4.3) |
| Asian | 29 (1.9) | 51 (1.7) | 9 (1.8) | 11 (1.2) | 28 (3.0) | 34 (1.8) | 10 (1.0) | 28 (1.4) |
| Multiple races | 30 (2.0) | 27 (0.9) | 1 (0.2) | 14 (1.5) | 15 (1.6) | 15 (0.8) | 16 (1.6) | 26 (1.3) |
| Unknown race | 52 (3.4) | 89 (2.9) | 10 (2.0) | 24 (2.5) | –– | –– | ||
| Region | ||||||||
| Northeast | 54 (3.6) | 127 (4.1) | 23 (4.6) | 28 (2.9) | 32 (3.4) | 82 (4.3) | 43 (4.3) | 72 (3.6) |
| Midwest | 594 (39.3) | 1166 (38.1) | 178 (36.0) | 373 (39.2) | 374 (39.8) | 736 (38.7) | 383 (38.1) | 762 (38.1) |
| South | 545 (36.0) | 1025 (33.5) | 142 (28.7) | 314 (33.0) | 316 (33.6) | 616 (32.4) | 354 (35.2) | 701 (35.0) |
| West | 156 (10.3) | 371 (12.1) | 96 (19.4) | 143 (15.0) | 111 (11.8) | 256 (13.5) | 125 (12.4) | 225 (11.2) |
| Other/Unknown | 164 (10.8) | 375 (12.2) | 56 (11.3) | 94 (9.9) | 107 (11.4) | 212 (11.1) | 101 (10.0) | 241 (12.0) |
| Outpatient physician visits | ||||||||
| 0 | –– | 1 (0.0) | –– | –– | –– | 1 (0.1) | ||
| 1‐2 | 620 (41.0) | 1303 (42.5) | 166 (33.5) | 386 (40.5) | 371 (39.5) | 788 (41.4) | 388 (38.6) | 836 (41.8) |
| 3‐4 | 535 (35.4) | 1020 (33.3) | 154 (31.1) | 288 (30.3) | 339 (36.1) | 652 (34.3) | 325 (32.3) | 623 (31.1) |
| 5‐6 | 192 (12.7) | 418 (13.6) | 89 (18.0) | 138 (14.5) | 114 (12.1) | 257 (13.5) | 160 (15.9) | 291 (14.5) |
| ≥7 | 166 (11.0) | 322 (10.5) | 86 (17.4) | 140 (14.7) | 116 (12.3) | 204 (10.7) | 133 (13.2) | 251 (12.5) |
| Emergency department visits | ||||||||
| 0 | 1408 (93.1) | 2856 (93.2) | 460 (92.9) | 894 (93.9) | 868 (92.3) | 1758 (92.4) | 940 (93.4) | 1881 (94.0) |
| ≥1 | 105 (6.9) | 208 (6.8) | 35 (7.1) | 58 (6.1) | 72 (7.7) | 144 (7.6) | 66 (6.6) | 120 (6.0) |
| Hospitalizations | ||||||||
| 0 | 1464 (96.8) | 2986 (97.5) | 480 (97.0) | 926 (97.3) | 913 (97.1) | 1850 (97.3) | 969 (96.3) | 1950 (97.5) |
| ≥1 | 49 (3.2) | 78 (2.5) | 15 (3.0) | 26 (2.7) | 27 (2.9) | 52 (2.7) | 37 (3.7) | 51 (2.5) |
| 3‐digit diagnostic codes | ||||||||
| 1‐3 | 119 (7.9) | 247 (8.1) | 30 (6.1) | 59 (6.2) | 77 (8.2) | 157 (8.3) | 64 (6.4) | 135 (6.7) |
| 4‐6 | 380 (25.1) | 714 (23.3) | 78 (15.8) | 179 (18.8) | 244 (26.0) | 451 (23.7) | 197 (19.6) | 407 (20.3) |
| 7‐9 | 359 (23.7) | 732 (23.9) | 82 (16.6) | 201 (21.1) | 213 (22.7) | 467 (24.6) | 210 (20.9) | 441 (22.0) |
| ≥10 | 655 (43.3) | 1371 (44.7) | 305 (61.6) | 513 (53.9) | 406 (43.2) | 827 (43.5) | 535 (53.2) | 1018 (50.9) |
| Prescriptions at baseline | ||||||||
| 1‐3 | 213 (14.1) | 448 (14.6) | 70 (14.1) | 149 (15.7) | 129 (13.7) | 260 (13.7) | 135 (13.4) | 317 (15.8) |
| 4‐6 | 359 (23.7) | 696 (22.7) | 94 (19.0) | 206 (21.6) | 224 (23.8) | 459 (24.1) | 216 (21.5) | 416 (20.8) |
| 7‐9 | 386 (25.5) | 726 (23.7) | 100 (20.2) | 208 (21.8) | 245 (26.1) | 459 (24.1) | 227 (22.6) | 442 (22.1) |
| ≥10 | 555 (36.7) | 1194 (39.0) | 231 (46.7) | 389 (40.9) | 342 (36.4) | 724 (38.1) | 428 (42.5) | 826 (41.3) |
| Antidiabetic drug classes | ||||||||
| 0 | 372 (24.6) | 710 (23.2) | 135 (27.3) | 240 (25.2) | 231 (24.6) | 392 (20.6) | 260 (25.8) | 536 (26.8) |
| 1 | 448 (29.6) | 906 (29.6) | 135 (27.3) | 280 (29.4) | 275 (29.3) | 566 (29.8) | 296 (29.4) | 585 (29.2) |
| 2 | 437 (28.9) | 904 (29.5) | 131 (26.5) | 266 (27.9) | 274 (29.1) | 593 (31.2) | 280 (27.8) | 542 (27.1) |
| ≥3 | 256 (16.9) | 544 (17.8) | 94 (19.0) | 166 (17.4) | 160 (17.0) | 351 (18.5) | 170 (16.9) | 338 (16.9) |
| Procedures at baseline | ||||||||
| 0‐9 | 478 (31.6) | 937 (30.6) | 113 (22.8) | 231 (24.3) | 281 (29.9) | 545 (28.7) | 282 (28.0) | 579 (28.9) |
| 10‐19 | 511 (33.8) | 1091 (35.6) | 147 (29.7) | 288 (30.3) | 322 (34.3) | 687 (36.1) | 315 (31.3) | 652 (32.6) |
| 20‐29 | 287 (19.0) | 570 (18.6) | 113 (22.8) | 221 (23.2) | 178 (18.9) | 374 (19.7) | 213 (21.2) | 395 (19.7) |
| ≥30 | 237 (15.7) | 466 (15.2) | 122 (24.6) | 212 (22.3) | 159 (16.9) | 296 (15.6) | 196 (19.5) | 375 (18.7) |
| Ever smoker | ||||||||
| No | 330 (21.8) | 665 (21.7) | 94 (19.0) | 184 (19.3) | 198 (21.1) | 403 (21.2) | 213 (21.2) | 423 (21.1) |
| Yes | 1183 (78.2) | 2399 (78.3) | 401 (81.0) | 768 (80.7) | 742 (78.9) | 1499 (78.8) | 793 (78.8) | 1578 (78.9) |
| Alcohol abuse | ||||||||
| No | 1506 (99.5) | 3058 (99.8) | 492 (99.4) | 949 (99.7) | 934 (99.4) | 1898 (99.8) | 1002 (99.6) | 1996 (99.8) |
| Yes | 7 (0.5) | 6 (0.2) | 3 (0.6) | 3 (0.3) | 6 (0.6) | 4 (0.2) | 4 (0.4) | 5 (0.2) |
| BMI group b | ||||||||
| Underweight/Normal weight | 19 (1.3) | 48 (1.6) | 13 (2.6) | 25 (2.6) | 18 (1.9) | 41 (2.2) | 13 (1.3) | 32 (1.6) |
| Overweight | 165 (10.9) | 339 (11.1) | 111 (22.4) | 164 (17.2) | 113 (12.0) | 234 (12.3) | 153 (15.2) | 247 (12.3) |
| Obese | 841 (55.6) | 1652 (53.9) | 267 (53.9) | 567 (59.6) | 515 (54.8) | 1030 (54.2) | 560 (55.7) | 1125 (56.2) |
| Morbidly obese | 488 (32.3) | 1025 (33.5) | 104 (21.0) | 196 (20.6) | 294 (31.3) | 597 (31.4) | 280 (27.8) | 597 (29.8) |
| HbA1c | ||||||||
| ≤ 7% | 281 (18.6) | 520 (17.0) | 144 (29.1) | 269 (28.3) | 164 (17.4) | 301 (15.8) | 255 (25.3) | 480 (24.0) |
| 7.1‐9% | 749 (49.5) | 1416 (46.2) | 251 (50.7) | 520 (54.6) | 464 (49.4) | 828 (43.5) | 504 (50.1) | 1036 (51.8) |
| > 9% | 483 (31.9) | 1128 (36.8) | 100 (20.2) | 163 (17.1) | 312 (33.2) | 773 (40.6) | 247 (24.6) | 485 (24.2) |
| Hypertension present | 1032 (68.2) | 2102 (68.6) | 407 (82.2) | 788 (82.8) | 612 (65.1) | 1254 (65.9) | 783 (77.8) | 1562 (78.1) |
| Hypoglycaemia present | 65 (4.3) | 131 (4.3) | 26 (5.3) | 44 (4.6) | 44 (4.7) | 78 (4.1) | 45 (4.5) | 90 (4.5) |
| Diabetic complication present | 275 (18.2) | 571 (18.6) | 132 (26.7) | 257 (27.0) | 156 (16.6) | 336 (17.7) | 239 (23.8) | 470 (23.5) |
| Sulphonylurea treatment present | 561 (37.1) | 1224 (40.1) | 202 (40.8) | 398 (41.8) | 337 (36.5) | 771 (41.2) | 400 (39.1) | 800 (39.4) |
| Renal function c | ||||||||
| Normal function | 847 (58.0) | 1737 (58.4) | 77 (15.9) | 136 (14.7) | 940 (100.0) | 1902 (100.0) | ||
| Mild impairment | 529 (36.2) | 1027 (34.5) | 243 (50.1) | 501 (54.0) | – | – | 733 (72.9) | 1455 (72.7) |
| Moderate or severe impairmentd | 85 (5.8) | 211 (7.1) | 165 (34.0) | 291 (31.4) | – | – | 273 (27.1) | 546 (27.3) |
| Moderate | 85 (5.8) | 189 (6.4) | 159 (32.8) | 258 (27.8) | – | – | 267 (26.5) | 491 (24.5) |
| Severe | –– | 22 (0.7) | 6 (1.2) | 33 (3.6) | – | – | 6 (0.6) | 55 (2.8) |
| EQW | BI | EQW | BI | EQW | BI | EQW | BI | |
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| HbA1c, % | 8.4 (0.04) | 8.7 (0.04) | 7.9 (0.07) | 7.9 (0.05) | 8.5 (0.06) | 8.8 (0.05) | 8.1 (0.05) | 8.2 (0.04) |
| BMI, kg/m2 | 37.5 (0.19) | 37.7 (0.14) | 35.0 (0.31) | 35.2 (0.22) | 37.3 (0.26) | 37.2 (0.19) | 36.6 (0.23) | 37.1 (0.18) |
| Weight, kg | 109.4 (0.62) | 110.2 (0.44) | 101.9 (0.98) | 100.4 (0.67) | 108.5 (0.83) | 108.9 (0.61) | 107 (0.74) | 107.1 (0.56) |
| SBP, mm Hg | 128.0 (0.36) | 129.0 (0.27) | 130.0 (0.67) | 131.2 (0.49) | 128.1 (0.47) | 128.9 (0.36) | 128.9 (0.48) | 130.1 (0.37) |
| DBP, mm Hg | 77.8 (0.23) | 77.6 (0.17) | 73.3 (0.41) | 72.7 (0.30) | 78.1 (0.29) | 77.7 (0.21) | 75.3 (0.29) | 75.2 (0.23) |
| Albumin/creatinine ratio, μg/mg | 37.6 (2.25) | 44.7 (1.64) | 48.1 (4.02) | 50.1 (3.36) | 36.6 (2.59) | 43.1 (1.97) | 43.7 (3.62) | 48.4 (2.47) |
| Serum creatinine, mg/dL | 0.9 (0.01) | 0.9 (0.01) | 1.0 (0.02) | 1.1 (0.01) | 0.7 (0.00) | 0.7 (0.00) | 1.1 (0.01) | 1.1 (0.01) |
| eGFR, mL/min/1.73m2 | 91.3 (0.54) | 91.1 (0.47) | 68.9 (1.02) | 68.5 (0.69) | 104.4 (0.37) | 104.8 (0.29) | 68.2 (0.56) | 67.3 (0.38) |
| Alanine aminotransferase, U/I | 36.9 (0.76) | 35.8 (0.52) | 30 (0.87) | 28.2 (0.64) | 37.4 (1.02) | 36.7 (0.70) | 33.0 (0.76) | 31.2 (0.54) |
| Aspartate aminotransferase, U/I | 27.3 (0.52) | 27.3 (0.37) | 24.7 (0.72) | 24.3 (0.45) | 27.4 (0.72) | 28 (0.53) | 25.8 (0.53) | 25.2 (0.35) |
| HDL cholesterol, mg/dL | 42.1 (0.35) | 42 (0.23) | 44.2 (0.58) | 44.1 (0.42) | 42.7 (0.46) | 42.5 (0.31) | 42.5 (0.41) | 42.5 (0.28) |
| LDL cholesterol, mg/dL | 94.1 (0.91) | 95.4 (0.68) | 84.4 (1.43) | 86.2 (1.22) | 95.2 (1.14) | 96.1 (0.89) | 88.3 (1.17) | 90.1 (0.86) |
| Total cholesterol, mg/dL | 173.7 (1.19) | 174.7 (0.82) | 161.1 (1.83) | 162.6 (1.56) | 174.9 (1.45) | 174.8 (1.09) | 166.9 (1.35) | 168.4 (0.97) |
| Triglycerides, mg/dL | 203.9 (3.99) | 202.3 (2.33) | 177.3 (4.98) | 177.8 (3.62) | 201.4 (4.92) | 196.6 (2.97) | 195.3 (3.66) | 196.5 (3.02) |
| Haematocrit, % | 41.4 (0.15) | 41.1 (0.11) | 40.5 (0.36) | 39.8 (0.20) | 41.6 (0.18) | 41.4 (0.13) | 40.7 (0.21) | 40.1 (0.14) |
| Haemoglobin, g/dL | 13.8 (0.05) | 13.7 (0.04) | 13.4 (0.12) | 13.2 (0.06) | 13.9 (0.06) | 13.9 (0.05) | 13.6 (0.07) | 13.3 (0.05) |
| Red blood cell count, cells ×106/uL | 4.7 (0.02) | 4.7 (0.01) | 4.5 (0.04) | 4.4 (0.02) | 4.8 (0.02) | 4.7 (0.01) | 4.6 (0.02) | 4.5 (0.02) |
| White blood cells count, cells ×103/uL | 7.8 (0.07) | 7.8 (0.05) | 7.4 (0.12) | 7.6 (0.10) | 7.7 (0.09) | 7.8 (0.06) | 7.7 (0.09) | 7.7 (0.09) |
| Platelet count ×103/uL | 250.4 (2.18) | 246.2 (1.51) | 229.7 (3.22) | 232.9 (2.85) | 251.5 (3.19) | 247.4 (2.24) | 238.9 (2.27) | 238.4 (1.90) |
BI, basal insulin; BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; EQW, exenatide once weekly; HbA1c, glycated haemoglobin; SBP, systolic blood pressure.
Renal Function was assessed by eGFR; eGFR could not be calculated for 151 patients who did not have race information (18‐64 years: EQW (n = 52), BI (n = 89); ≥65 years: EQW (n = 10), BI (n = 24), normal = eGFR ≥90 mL/min/1.73m2; Any RI = eGFR <90 mL/min/1.73m2.
BMI group: underweight/normal = BMI < 24 kg/m2; overweight = 25 kg/m2 < BMI < 3329 kg/m2; obese = 30 kg/m2 < BMI < 39 kg/m2; morbidly obese = BMI > 40 kg/m2.
Renal function: normal = eGFR ≥90 mL/min/1.73m2; mild impairment = 60 mL/min/1.73m2 ≤ eGFR <90 mL/min/1.73m2; moderate/severe impairment = eGFR <60 mL/min/1.73m2.
Moderate and severe impairment populations were combined in this study for matching and analyses; this included patients with both moderate impairment = 30 mL/min/1.73m2 ≤ eGFR <60 mL/min/1.73m2 and patients with severe impairment = eGFR <30 mL/min/1.73m2 measured at baseline.
Among patients aged ≥65 years, EQW initiators had greater declines in HbA1c from baseline to all calendar quarter intervals in the first year of follow‐up, compared with BI initiators (Figure 1A). These differences are compatible with chance findings. The absolute decline in HbA1c from baseline to the first calendar quarter (Q1) was −0.36 percentage points (95% CI −0.53, −0.19) among EQW initiators and −0.17 (95% CI −0.27, −0.07) percentage points among BI initiators. In the last calendar quarter (Q4) the absolute HbA1c decline from baseline was −0.50 percentage points (95% CI −0.67, −0.32) among EQW initiators compared with −0.31 percentage points (95% CI −0.41, −0.21) among BI initiators. EQW initiators had significantly greater declines in body weight from baseline to all calendar quarter intervals compared with BI initiators (Figure 1B). The absolute decline in body weight from baseline to Q4 was −1.6 kg (95% CI −2.26, −0.96) among EQW initiators compared with −0.2 kg (95% CI −0.74, 0.26) among BI initiators (Figure 1B). Blood pressure, lipid profiles and eGFR remained stable from baseline through the first year of follow‐up for both EQW and BI initiators (Table 2, Figure 3A). The IR of nausea and vomiting was 1.45 times higher among EQW initiators compared with BI initiators (95% CI 1.09, 1.91) and the IR of any gastrointestinal symptoms was 1.32 times higher among EQW initiators compared with BI initiators (95% CI 1.08, 1.63; Table 3). Specifically, the IR of nausea among EQW initiators was 119.9 per 1000 person‐years (95% CI 94.2, 150.0) compared with 80.5 per 1000 person‐years (95% CI 66.0, 97.2) among BI initiators (RR 1.5, 95% CI 1.1, 2.0). The IR of vomiting was 69.1 per 1000 person‐years (95% CI 50.6, 92.2) among EQW initiators and 52.1 per 1000 person‐years among BI initiators (95% CI 40.8, 65.7) a difference that was compatible with a chance finding. The IRs of hypoglycaemia, and diarrhoea and constipation were similar between the initiator groups.
Figure 1.
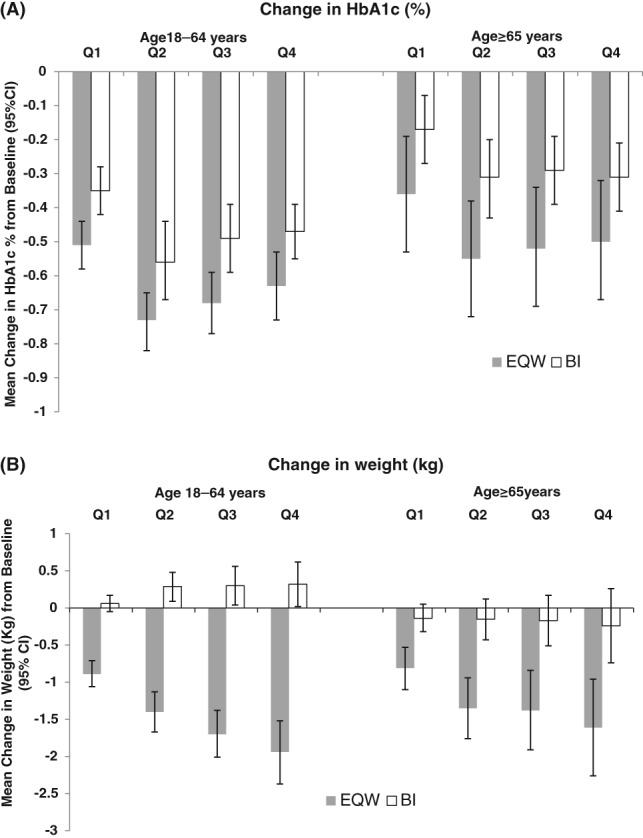
Change in glycated haemoglobin (HbA1c) and weight assessed quarterly (Q1–Q4) in the first year following the initiation of injectable antihyperglycaemic treatment, comparing propensity score matched injectable‐naive exenatide once weekly (EQW) and basal insulin (BI) initiators, by age
Table 2.
Comparison of blood pressure, lipid profile, and renal function between propensity score matched injectable‐naive exenatide once weekly and basal insulin initiators, mean and 95% confidence interval at baseline and semi‐annually (S1, S2) or quarterly (Q1–Q3) in the first year of follow‐up, by age and renal function
| Characteristic | Age group | Renal function | ||||||
|---|---|---|---|---|---|---|---|---|
| 18‐64 years | ≥65 years | Normal function | RI | |||||
| EQW | BI | EQW | BI | EQW | BI | EQW | BI | |
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | |
| SBP, mm Hg | ||||||||
| Baseline | 128.0 (127.3‐128.7) | 129.0 (128.5‐129.6) | 130.0 (128.7‐131.3) | 131.2 (130.2‐132.1) | 128.1 (127.2‐128.9) | 128.8 (128.1‐129.4) | 128.9 (128.0‐129.9) | 130.2 (129.5‐130.9) |
| S1 | 127.5 (126.9‐128.2) | 129.1 (128.6‐129.6) | 129.3 (128.2‐130.5) | 131.9 (131.0‐132.7) | 127.9 (126.9‐128.8) | 128.8 (128.2‐129.5) | 128.3 (127.5‐129.1) | 130.6 (130.0‐131.2) |
| S2 | 128.1 (127.4‐128.9) | 129.6 (129.0‐130.2) | 129.8 (128.4‐131.2) | 131.6 (130.7‐132.5) | 128.6 (127.7‐129.6) | 129.4 (128.7‐130.2) | 128.5 (127.6‐129.5) | 130.8 (130.1‐131.5) |
| DBP, mm Hg | ||||||||
| Baseline | 77.8 (77.4‐78.3) | 77.6 (77.3‐77.9) | 73.3 (72.4‐74.1) | 72.7 (72.1‐73.3) | 78.3 (77.7‐78.9) | 77.8 (77.4‐78.2) | 75.2 (74.6‐75.8) | 75.2 (74.8‐75.6) |
| S1 | 77.2 (76.8‐77.7) | 77.1 (76.8‐77.4) | 73.0 (72.4‐73.7) | 72.6 (72.1‐73.1) | 77.52 (77.0‐78.0) | 77.4 (77.0‐77.8) | 75.1 (74.6‐75.6) | 74.8 (74.4‐75.1) |
| S2 | 77.5 (77.0‐77.9) | 77.1 (76.8‐77.4) | 73.1 (72.2‐74.0) | 72.4 (71.9‐73.0) | 77.99 (77.4‐78.6) | 77.3 (76.8‐77.7) | 75.1 (74.5‐75.7) | 74.7 (74.3‐75.2) |
| Total cholesterol, mg/dL | ||||||||
| Baseline | 173.7 (171.3‐176.0) | 174.7 (173.1‐176.3) | 161.1 (157.5‐164.7) | 162.6 (159.5‐165.8) | 174.7 (171.6‐177.8) | 175.3 (173.1‐177.4) | 167.2 (164.6‐169.8) | 168.2 (166.2‐170.1) |
| S1 | 165.8 (163.3‐168.3) | 169.0 (167.4‐170.5) | 155.3 (151.7‐158.8) | 159.2 (156.2‐162.2) | 166.5 (163.6‐169.5) | 169.0 (167.0‐171.1) | 160.5 (157.1‐163.8) | 164.0 (162.0‐166.0) |
| S2 | 167.1 (164.6‐169.5) | 168.5 (165.8‐171.3) | 158.9 (154.4‐163.5) | 159.0 (155.7‐162.4) | 168.5 (165.5‐171.5) | 169.1 (166.2‐172.0) | 162.3 (159.5‐165.1) | 163.3 (160.6‐166.0) |
| HDL cholesterol, mg/dL | ||||||||
| Baseline | 42.1 (41.4‐42.7) | 42.0 (41.6‐42.5) | 44.2 (43.0‐45.3) | 44.1 (43.3‐44.9) | 42.6 (41.7‐43.4) | 42.4 (41.8‐43.1) | 42.6 (41.9‐43.4) | 42.6 (41.9‐43.3) |
| S1 | 42.1 (41.4‐42.9) | 42.4 (41.9‐42.9) | 44.1 (42.9‐45.3) | 44.4 (43.5‐45.2) | 42.6 (41.7‐43.6) | 42.8 (42.2‐43.4) | 42.6 (41.8‐43.5) | 42.9 (42.3‐43.5) |
| S2 | 43.0 (42.3‐43.7) | 42.7 (42.1‐43.4) | 44.7 (43.5‐46.0) | 44.6 (43.6‐45.5) | 43.6 (42.7‐44.5) | 43.2 (42.5‐43.9) | 43.2 (42.3‐44.1) | 43.1 (42.3‐43.9) |
| LDL cholesterol, mg/dL | ||||||||
| Baseline | 94.1 (92.3‐95.9) | 95.5 (94.1‐96.8) | 84.4 (81.6‐87.2) | 86.2 (83.7‐88.7) | 95.1 (92.8‐97.4) | 96.4 (94.7‐98.0) | 88.5 (86.3‐90.8) | 89.9 (88.2‐91.7) |
| S1 | 89.8 (87.3‐92.2) | 92.5 (90.8‐94.2) | 80.7 (77.8‐83.7) | 84.0 (81.5‐86.5) | 90.7 (88.0‐93.5) | 93.3 (91.2‐95.5) | 84.7 (82.0‐87.3) | 87.6 (85.7‐89.5) |
| S2 | 89.5 (87.7‐91.4) | 91.4 (89.0‐93.8) | 82.9 (79.3‐86.5) | 83.4 (80.6‐86.1) | 91.0 (88.4‐93.6) | 92.5 (89.7‐95.3) | 85.0 (82.8‐87.1) | 86.4 (84.3‐88.6) |
| Triglycerides, mg/dL | ||||||||
| Baseline | 204.0 (195.8‐212.1) | 202.3 (197.8‐206.9) | 177.4 (167.5‐187.2) | 177.8 (170.7‐184.9) | 201.2 (190.0‐212.3) | 197.8 (192.1‐203.5) | 195.7 (188.3‐203.0) | 195.4 (189.6‐201.3) |
| S1 | 180.9 (175.4‐186.5) | 180.7 (174.6‐186.7) | 160.7 (152.9‐168.4) | 163.2 (156.7‐169.6) | 176.5 (168.6‐184.4) | 175.6 (169.6‐181.6) | 176.7 (168.4‐185.0) | 177.4 (170.3‐184.4) |
| S2 | 183.0 (176.0‐190.0) | 182.5 (177.9‐187.0) | 163.4 (154.1‐172.7) | 163.9 (156.7‐171.0) | 178.9 (171.4‐186.4) | 177.7 (171.6‐183.8) | 180.2 (171.5‐189.0) | 178.8 (173.4‐184.1) |
| eGFR, ml/min/1.72 m 2 | ||||||||
| Baseline | 91.3 (90.2‐92.3) | 91.1 (90.1‐92.0) | 68.9 (66.9‐70.9) | 68.5 (67.1‐69.9) | 103.4 (102.5‐104.4) | 103.9 (102.9‐104.9) | 69.7 (68.4‐70.9) | 68.9 (68.1‐69.8) |
| Q1 | 91.0 (89.8‐92.2) | 90.6 (89.6‐91.6) | 69.2 (66.5‐71.8) | 68.4 (67.0‐69.8) | 101.4 (100.2‐102.6) | 101.6 (100.6‐102.6) | 71.2 (69.6‐72.7) | 70.4 (69.5‐71.3) |
| Q2 | 91.0 (89.7‐92.1) | 89.9 (88.9‐91.0) | 69.0 (67.0‐70.9) | 67.8 (66.3‐69.3) | 100.9 (99.8‐101.9) | 100.7 (99.7‐101.7) | 71.6 (70.3‐72.9) | 69.8 (68.7‐70.9) |
| Q3 | 91.0 (89.7‐92.3) | 89.8 (88.6‐90.9) | 68.9 (66.9‐70.9) | 67.6 (65.9‐69.3) | 100.8 (99.5‐102.1) | 100.8 (99.7‐101.8) | 72.0 (70.6‐73.4) | 69.3 (67.9‐70.7) |
| Q4 | 90.6 (89.0‐92.3) | 89.3 (88.1‐90.4) | 68.8 (66.4.‐71.2) | 67.7 (66.0‐69.4) | 100.5 (98.8‐102.2) | 100.0 (99.0‐101.0) | 71.5 (69.8‐73.3) | 69.1 (67.9‐70.3) |
BI, basal insulin; BMI, body mass index; CI, confidence interval; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; EQW, exenatide once weekly; HbA1c, glycated haemoglobin; SBP, systolic blood pressure.
Table 3.
Comparison of the occurrence of hypoglycaemia and gastrointestinal symptoms between propensity score matched injectable‐naive exenatide once weekly and basal insulin initiators, by age and renal function
| Characteristic | N | Number of events (%) | IR per 1000 person‐years (95% CI) | Relative rate (95% CI) | |
|---|---|---|---|---|---|
| Hypoglycaemia | |||||
| 18‐64 years | EQW | 1513 | 101 (6.7) | 46.5 (37.8‐56.5) | 0.75 (0.60‐0.95) |
| BI | 3064 | 262 (8.6) | 61.6 (54.3‐69.5) | Reference | |
| ≥65 years | EQW | 495 | 48 (9.7) | 72.1 (53.2‐95.6) | 0.92 (0.65‐1.29) |
| BI | 952 | 106 (11.1) | 78.5 (64.3‐95.0) | Reference | |
| Nausea and vomiting | |||||
| 18‐64 years | EQW | 1513 | 257 (17.0) | 130.0 (114.6‐146.9) | 1.13 (0.97‐1.32) |
| BI | 3064 | 467 (15.2) | 115.0 (104.8‐126.0) | Reference | |
| ≥65 years | EQW | 495 | 83 (16.8) | 133.8 (106.5‐165.8) | 1.45 (1.09‐1.91) |
| BI | 952 | 123 (12.9) | 92.5 (76.9‐110.3) | Reference | |
| Constipation and diarrhoea | |||||
| 18‐64 years | EQW | 1513 | 257 (17.0) | 130.1 (114.7‐147.0) | 1.11 (0.96‐1.30) |
| BI | 3064 | 470 (15.3) | 116.7 (106.4‐127.8) | Reference | |
| ≥65 years | EQW | 495 | 108 (21.8) | 181.8 (149.2‐219.5) | 1.21 (0.96‐1.54) |
| BI | 952 | 186 (19.5) | 150.0 (129.2‐173.2) | Reference | |
| Any gastrointestinal symptom | |||||
| 18‐64 years | EQW | 1513 | 386 (25.5) | 212.1 (191.5‐234.4) | 1.13 (1.00‐1.28) |
| BI | 3064 | 704 (23.0) | 187.0 (173.4‐201.3) | Reference | |
| ≥65 years | EQW | 495 | 148 (29.9) | 270.1 (228.3‐317.3) | 1.32 (1.08‐1.63) |
| BI | 952 | 242 (25.4) | 203.9 (179.0‐231.3) | Reference | |
| Hypoglycaemia | |||||
| Normal function | EQW | 924 | 60 (6.5) | 44.9 (34.3‐57.8) | 0.75 (0.56‐1.01) |
| BI | 1873 | 159 (8.5) | 60.1 (51.1‐70.1) | Reference | |
| RI | EQW | 1022 | 84 (8.2) | 59.2 (47.2‐73.3) | 0.81 (0.63‐1.05) |
| BI | 2030 | 204 (10.0) | 72.8 (63.2‐83.6) | Reference | |
| Nausea and vomiting | |||||
| Normal function | EQW | 924 | 165 (17.9) | 136.7 (116.7‐159.2) | 1.21 (1.00‐1.46) |
| BI | 1873 | 286 (15.3) | 113.4 (100.6‐127.3) | Reference | |
| RI | EQW | 1022 | 166 (16.2) | 126.3 (107.8‐147.1) | 1.19 (0.98‐1.43) |
| BI | 2030 | 290 (14.3) | 106.6 (94.6‐119.6) | Reference | |
| Constipation and diarrhoea | |||||
| Normal function | EQW | 924 | 166 (18.0) | 139.0 (118.7‐161.9) | 1.28 (1.05‐1.55) |
| BI | 1873 | 273 (14.6) | 109.0 (96.5‐122.7) | Reference | |
| RI | EQW | 1022 | 194 (19.0) | 150.2 (129.8‐172.9) | 1.06 (0.89‐1.26) |
| BI | 2030 | 371 (18.3) | 141.8 (127.8‐157.0) | Reference | |
| Any gastrointestinal symptom | |||||
| Normal function | EQW | 924 | 254 (27.5) | 231.2 (203.6‐261.4) | 1.28 (1.09‐1.49) |
| BI | 1873 | 422 (22.5) | 180.8 (164.0‐198.9) | Reference | |
| RI | EQW | 1022 | 268 (26.2) | 223.9 (197.9‐252.3) | 1.11 (0.95‐1.28) |
| BI | 2030 | 502 (24.7) | 202.3 (185.0‐220.8) | Reference | |
BI, basal insulin; CI, confidence interval; EQW, exenatide once weekly; IR, incidence rate; RI, renal impairment.
Among patients with RI, EQW initiators had significantly greater declines in HbA1c from baseline to all calendar quarter intervals during follow‐up, compared with BI initiators. EQW initiators had a decline of −0.41 percentage points (95% CI −0.52, −0.30) in Q1, compared with −0.21 percentage points (95% CI −0.27, −0.16) among BI initiators. By the end of the first year the declines were −0.58 percentage points (95% CI −0.71, −0.45) vs −0.33 percentages points (95% CI −0.43, −0.23), among EQW and BI initiators, respectively (Figure 2A). EQW initiators had significantly greater declines in body weight from baseline to all calendar quarter intervals during follow‐up compared with BI initiators, who experienced no appreciable weight loss during the year of follow‐up (Figure 2B). Blood pressure, lipid profiles and eGFR remained stable from baseline to all calendar quarter intervals for both EQW and BI initiators (Table 2 and Figure 3B). EQW initiators had a lower risk of hypoglycaemia than BI initiators, which was compatible with a chance finding (RR 0.81, 95% CI 0.63, 1.05; Table 3). Renal function, assessed according to eGFR, remained stable across all four quarters and was similar among EQW and BI initiators in both age groups (Figure 3A) and regardless of RI (Figure 3B).
Figure 2.
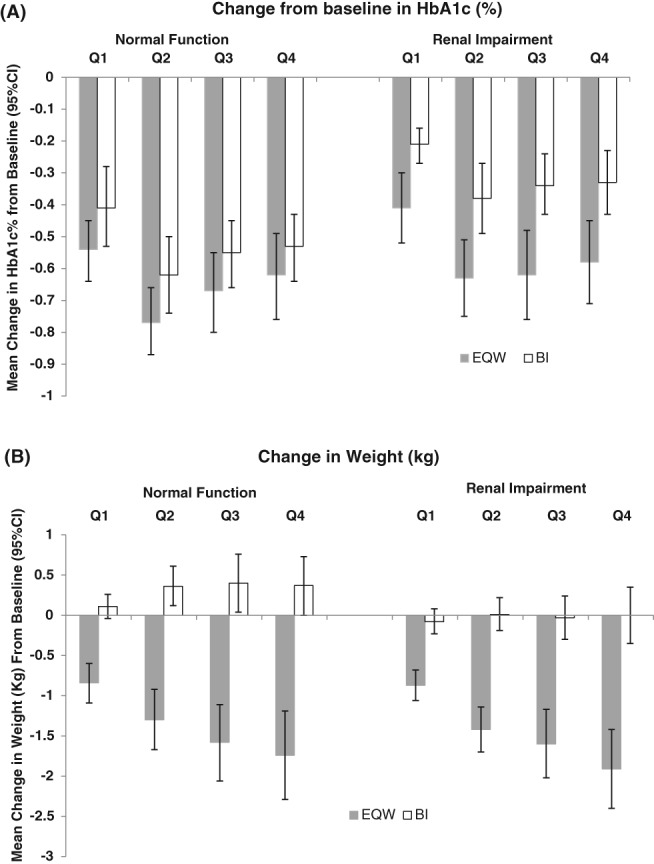
Change in glycated haemoglobin (HbA1c) and weight assessed quarterly (Q1–Q4) in the first year following the initiation of injectable antihyperglycaemic treatment, comparing propensity score‐matched injectable‐drug‐naive exenatide once weekly (EQW) and basal insulin (BI) initiators, by renal function
4. DISCUSSION
We examined the effectiveness and tolerability of EQW compared with BI in patients with T2DM by age group and renal function. The real‐world results of this observational study with 1 year of follow‐up can be compared with the findings of the DURATION 3 trial that compared EQW to insulin glargine, a BI, with follow‐up of up to 84 weeks (1.6 years).11, 28 Both studies showed a greater relative decline in HbA1c and weight among EQW initiators compared with BI initiators, with less hypoglycaemia and more nausea experienced by EQW initiators. Yet, the effects measured are attenuated in the present observational study; this may be related to population differences between the two studies. The observational cohort study included a broader range of patients compared with the population of the DURATION 3 trial; specifically, the cohort study included patients with evidence of glycaemic control at initiation of treatment, patients who initiated EQW or BI as first‐line treatment, elderly patients and patients with severe RI. These populations were not represented in the DURATION 3 trial. Also, while clinical notes in the EHR may capture more mentions of hypoglycaemia and gastrointestinal symptoms, including mild and moderate events that do not require third‐party assistance, the passive capture of these events in the EHR is not comparable to the active recording of these events in a clinical trial. Our algorithm enumerates these events from both structured fields and clinical notes, yet the events recorded in the EHR are probably underestimates of the actual event rates.
While our focus was to assess the effectiveness and tolerability of EQW relative to BI among patients with T2DM who are elderly or have RI, comparing young and elderly patients and patients with and without RI provides the real‐world evidence that the benefits of EQW relative to BI remain apparent, yet are tempered in elderly patients and patients with RI. Specifically, we observed that EQW initiators had numerically larger reductions in HbA1c compared with BI initiators regardless of age group and renal function, with significantly larger reductions observed among EQW initiators than BI initiators with RI. EQW initiators experienced significantly larger reductions in body weight compared with BI initiators among those aged ≥65 years and those with RI. Regardless of age group or renal function, a decreased risk of hypoglycaemia was observed in EQW initiators compared with BI initiators, but the differences were consistent with chance. A 30% greater risk of any gastrointestinal symptoms was observed among EQW initiators compared with BI initiators aged ≥65 years and initiators with normal renal function. These findings are consistent with both clinical trials and real‐world data which have demonstrated improved glycaemic control, greater weight loss, and lower risk of hypoglycaemia with EQW compared with BI use, but an increased occurrence of gastrointestinal symptoms.10, 12, 29, 30 The study findings add to our knowledge about the benefit and risk of EQW compared with BI in patients with T2DM who have RI. Yet, while we analysed all RI together in the present study, the majority of this population included patients with mild RI (EQW: 72.9%; BI: 72.7%); fewer patients had moderate RI (EQW: 26.5%; BI: 24.5%) or severe RI (EQW: 0.6%; BI: 2.8%; Table 1). The reader should be cautioned that the inferences made in this study are generalizable to patients with mild RI, and possibly mild to moderate RI, only. Although EQW is excreted through the kidney, no dose alteration has been recommended for patients with mild or moderate RI.31 EQW is not recommended for patients with severe RI.32
It should be noted that approximately one‐third of the patients aged ≥65 years had moderate or severe RI at baseline, and 40% of the patients with RI were aged ≥65 years. Efficacy and tolerability of EQW and BI treatment in patients with T2DM who are both elderly and have RI was not examined in the present study and may differ from that in patients with only one of these factors.
The present study was based on an analysis of EHR data that has certain inherent limitations because the data are collected for the purpose of clinical patient management, not research. Healthcare encounters with medical providers who do not contract with Optum's EHR would not be observed. Prescription data represent the intent of the provider, but do not indicate that a medication was filled, consumed or that it was taken as prescribed. Unlike data collected in clinical trials, where standardization of clinical and laboratory measures is controlled, the Optum EHR includes real‐world clinical data obtained from multiple medical and laboratory settings used for customary clinical care. For laboratory values in particular, we assume that a value measured for an individual (eg, HbA1c% = 7.2) represents that patient's status at that point in time regardless of the laboratory that produced the result. This assumption is predicated on the fact that in the United States, the Centers for Medicare and Medicaid Services regulate all laboratories that perform testing on humans through the Clinical Laboratory Improvement Amendments (CLIA).33 The purpose of CLIA is to ensure the accuracy and reliability of laboratory testing. Further, in an effort to remove potentially erroneous values in the data, we applied multiple data restriction steps to the clinical and laboratory data, prior to imputation. The steps applied to data as a whole included assuring that only values having the same units were included, trimming of spurious values found in the tails (<1% and >99%), and restricting of values to ranges considered as valid. Lastly, at the individual level, interquartile range trimming was applied to exclude extreme and potentially invalid data from the analysis. Our measures of effectiveness were assessed as the change from baseline of an individual's measures over time. While individuals are likely to have less variability in their own data, admittedly even an individual could have multiple providers or laboratories contributing to the data in their electronic medical record; therefore, while we assume them to be objective measures in most cases, individual laboratory measures (eg, HbA1c and LDL cholesterol) and clinical measures (weight and blood pressure) used to assess effectiveness in this study are subject to variation resulting from use of different instrumentation and technicians producing and recording results. This variation is expected to be random so that it leads to greater variability in the measures than actually exists, resulting in wider CIs for each measured variable, and not systematic in a way that would result in bias for or against EQW.
Because data are not collected in a systematically unified way, study measures (eg, laboratory results and clinical observations) are available at varying frequency and intervals between patients. To facilitate the use of EHR data for the assessment of measures of efficacy and tolerability, a multiple imputation method was implemented in order to supplement the observed values by imputing values (as needed) within standard intervals of follow‐up. Multiple imputation is founded on the assumption that unobserved variables are missing at random after conditioning on observed covariates. This assumption is more broadly applicable than the assumption that missingness is independent from any covariate, observed or unobserved, the assumption that is necessary for a complete case analysis (ie, restricting to patients without missing data). While multiple imputation reduces the potential for bias by relaxing the assumptions about the nature of the unobserved data, it is possible that patients with observed values are systematically different from those with unobserved values in unmeasured ways.
These findings can inform treatment decisions of prescribers considering an injectable antihyperglycaemic treatment. Specifically, that relative to BI, EQW offers a clinical advantage to patients with T2DM who are elderly or have RI with respect to the likelihood of achieving both glycaemic control and weight loss without an increased risk of hypoglycaemia or worsening renal function. In the elderly, these benefits should be weighed against a modest increase in the risk of gastrointestinal symptoms.
ACKNOWLEDGMENTS
This study was funded by AstraZeneca. We thank Tamar Aroyan, Jillian Jessup and Veena Hoffman (Optum Epidemiology) for their assistance with this manuscript.
Loughlin AM, Qiao Q, Nunes AP, et al. Effectiveness and tolerability of therapy with exenatide once weekly vs basal insulin among injectable‐drug‐naïve elderly or renal impaired patients with type 2 diabetes in the United States. Diabetes Obes Metab. 2018;20:898–909. https://doi.org/10.1111/dom.13175
Funding information AstraZeneca, Grant/Award number: N/A
REFERENCES
- 1. Centers for Disease Control and Prevention . National Diabetes Statistics Report: Estimates of Diabetes and its Burden in the United States, 2014. Altlanta, GA: US Department of Health and Human Services: 2014. [Google Scholar]
- 2. Halter JB, Musi N, McFarland Horne F, et al. Diabetes and cardiovascular disease in older adults: current status and future directions. Diabetes. 2014;63(8):2578‐2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grundy SM, Benjamin IJ, Burke GL, et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100(10):1134‐1146. [DOI] [PubMed] [Google Scholar]
- 4. Butt S, Hall P, Nurko S. Diabetic Nephropathy. http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/nephrology/diabetic‐nephropathy/. Published August 2010. Accessed December 5, 2016.
- 5. Ghaderian SB, Hayati F, Shayanpour S, Beladi Mousavi SS. Diabetes and end‐stage renal disease; a review article on new concepts. J Renal Inj Prev. 2015;4(2):28‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pecoits‐Filho R, Abensur H, Betônico CC, et al. Interactions between kidney disease and diabetes: dangerous liaisons. Diabetol Metab Syndr. 2016;8:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pálsson R, Patel UD. Cardiovascular complications of diabetic kidney disease. Adv Chronic Kidney Dis. 2014;21(3):273‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sarnak MJ, Levey AS, Schoolwerth AC, et al. American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108(17):2154‐2169. [DOI] [PubMed] [Google Scholar]
- 9. Lipska KJ, Ross JS, Miao Y, Shah ND, Lee SJ, Steinman MA. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med. 2015;175(3):356‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open‐label, non‐inferiority study. Lancet. 2008;372(9645):1240‐1250. [DOI] [PubMed] [Google Scholar]
- 11. Diamant M, Van Gaal L, Stranks S, et al. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION‐3): an open‐label randomised trial. Lancet. 2010;375(9733):2234‐2243. [DOI] [PubMed] [Google Scholar]
- 12. Davies M, Heller S, Sreenan S, et al. Once‐weekly exenatide versus once‐ or twice‐daily insulin detemir: randomized, open‐label, clinical trial of efficacy and safety in patients with type 2 diabetes treated with metformin alone or in combination with sulfonylureas. Diabetes Care. 2013;36(5):1368‐1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wysham CH, MacConell LA, Maggs DG, Zhou M, Griffin PS, Trautmann ME. Five‐year efficacy and safety data of exenatide once weekly: long‐term results from the DURATION‐1 randomized clinical trial. Mayo Clin Proc. 2015;90(3):356‐365. [DOI] [PubMed] [Google Scholar]
- 14. Grimm M, Han J, Weaver C, et al. Efficacy, safety, and tolerability of exenatide once weekly in patients with type 2 diabetes mellitus: an integrated analysis of the DURATION trials. Postgrad Med. 2013;125(3):47‐57. [DOI] [PubMed] [Google Scholar]
- 15. Seeger JD, Kurth T, Walker AM. Use of propensity score technique to account for exposure‐related covariates: an example and lesson. Med Care. 2007;45:S143‐S148. [DOI] [PubMed] [Google Scholar]
- 16. Seeger JD, Williams PL, Walker AM. An application of propensity score matching using claims data. Pharmacoepidemiol Drug Saf. 2005;14:465‐476. [DOI] [PubMed] [Google Scholar]
- 17. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41‐50. [Google Scholar]
- 18. Lee KJ, Carlin JB. Multiple imputation for missing data: fully conditional specification versus multivariate normal imputation. Am J Epidemiol. 2010;171:624‐632. [DOI] [PubMed] [Google Scholar]
- 19. Valente MA, Hillege HL, Navis G, et al. The Chronic Kidney Disease Epidemiology Collaboration equation outperforms the Modification of Diet in Renal Disease equation for estimating glomerular filtration rate in chronic systolic heart failure. Eur J Heart Fail. 2014;16:86‐94. [DOI] [PubMed] [Google Scholar]
- 20. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nunes AP, Yang J, Tunceli K, et al. Interim results on the relationship between mild‐moderate and severe hypoglycemia and cardiovascular disease in a cohort of sulfonylurea users. 51st European Association for the Study of Diabetes, Stockholm, Sweden, 2015.
- 22. Nunes AP, Yu S, Kurtyka K, et al. Natural language processing of clinical notes in electronic health records to improve capture of hypoglycemia. 30th International Conference of Pharmacoepidemiology, Taipei, Taiwan, 2014.
- 23. NCSS Statistical Software . Data matching–optimal and greedy. http://www.ncss.com/wp‐content/themes/ncss/pdf/Procedures/NCSS/Data_Matching‐Optimal_and_Greedy.pdf. Accessed September 30, 2014.
- 24. Parsons LS. Reducing bias in a propensity score matched‐pair sample using greedy matching techniques. (2001); In SAS SUGI 26, Paper 214‐26 http://www2.sas.com/proceedings/sugi26/p214-26.pdf. Accessed September 30, 2014.
- 25. SAS Institute Inc . SAS/STAT® 13.1 User's Guide. Chapter 62. The MIANALYE Procedure. Cary, NC: SAS Institute Inc.; 2013. https://support.sas.com/documentation/onlinedoc/stat/131/mianalyze.pdf. Accessed June 22, 2016. [Google Scholar]
- 26. Ginde AA, Blanc PG, Lieberman RM, Camargo CA. Validation of ICD‐9‐CM coding algorithm for improved identification of hypoglycemia visits. BMC Endocr Disord. 2008;8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. The International Society for Pharmacoepidemiology . Guidelines for good pharmacoepidemiology practices (GPP). Pharmacoepidemiol Drug Saf. 2008;17:200‐208. [DOI] [PubMed] [Google Scholar]
- 28. Diamant M, Van Gaal L, Stranks S, et al. Safety and efficacy of once‐weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes over 84 weeks. Diabetes Care. 2012;35(4):683‐689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Loughlin AM, Qiao Q, Johnsson KM, et al. Real World Clinical Outcomes Among Exenatide Once‐Weekly Initiators Compared to Matched Initiators of Basal Insulin. Presented at the American Diabetes Association 76th Scientific Sessions, New Orleans LA, 2016.
- 30. Loughlin AM, Qiao Q, Nunes AP, et al. Effectiveness and tolerability of therapy with exenatide once‐weekly versus basal insulin among injectable‐naive type 2 diabetes patients in a real world setting in the USA. Diabetes Spectr. [In press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Linnebjerg H, Kothare PA, Park S, et al. Effect of renal impairment on the pharmacokinetics of exenatide. Br J Clin Pharmacol. 2007;64(3):317‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bydureon® [package insert] , West Chester, Ohio: Amylin Ohio, LLC.; September 2015.
- 33. Centers for Medicare & Medicaid Services, Clinical Laboratory Improvement Amendments, Web site . https://www.cms.gov/Regulations‐and‐Guidance/Legislation/CLIA/index.html. Published May 04, 2017. Accessed October 15, 2017.


