Abstract
BACKGROUND
Maize technologies expressing Bacillus thuringiensis (Bt) insecticidal proteins are widely used in Argentina to control sugarcane borer (Diatraea saccharalis Fabricius). Unexpected D. saccharalis damage was observed to Bt maize events TC1507 (expressing Cry1F) and MON 89034 × MON 88017 (expressing Cry1A.105 and Cry2Ab2) in an isolated area of San Luis Province. Diatraea saccharalis larvae were sampled from MON 89034 × MON 88017 fields in the area to generate a resistant strain (RR), which was subsequently characterized in plant and diet bioassays.
RESULTS
Survivorship of the RR strain was high on TC1507 leaf tissue, intermediate on MON 89034 × MON 88017, and low on MON 810 (expressing Cry1Ab). The RR strain had high resistance to Cry1A.105 (186.74‐fold) and no resistance to Cry2Ab2 in diet bioassays. These results indicate resistance to Cry1F and Cry1A.105 (and likely cross‐resistance between them) but not to Cry1Ab or Cry2Ab2. Resistance to MON 89034 × MON 88017 was functionally recessive. Reviews of grower records suggest that resistance initially evolved to Cry1F, conferring cross‐resistance to Cry1A.105, with low refuge compliance as the primary cause. A mitigation plan was implemented in San Luis that included technology rotation, field monitoring, and grower education on best management practices (BMPs) including refuges.
CONCLUSION
In the affected area, the resistance to Cry1F and Cry1A.105 is being managed effectively through use of MON 89034 × MON 88017 and MON 810 in combination with BMPs, and no spread of resistance to other regions has been observed. © 2017 The Authors. Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: Diatraea saccharalis, maize, Bt proteins, resistance management
1. INTRODUCTION
The sugarcane borer, Diatraea saccharalis (Fabricius) (Lepidoptera: Crambidae), is dispersed across most of South America, Central America, the Caribbean, and the southern USA, infesting a variety of plants including sugarcane, sorghum, maize, wheat, rice and a variety of weeds such as Johnsongrass (Sorghum halepense). In Argentina, D. saccharalis is one of the main insect pests infesting maize.1, 2 Direct damage to maize plants occurs as larvae feed on the leaves and bore into the stem, tunneling and therefore reducing the physical strength of the stalk, which may result in lodging. The mechanical damage to maize stems caused by stalk borers also causes significant disruption of normal plant physiology, including effects on transport and use of nutrients and water.3 Collectively, the damage caused by D. saccharalis to maize plants results in reduced yield potential, broken stalks, and dropped ears.4, 5 Later maize plantings, particularly double‐cropped maize, are likely to present more severe damage inflicted by D. saccharalis.6 As a consequence of the tunneling behavior of this pest, controlling it through insecticidal sprays is challenging7 and frequently ineffective, primarily given the short period between the beginning of the chemical treatment and the moment when the larvae are protected within the maize stem.8, 9, 10 Consequently, the use of maize plants expressing insecticidal proteins from the soil bacterium Bacillus thuringiensis (Bt) was recognized as an effective strategy to manage stalk‐boring lepidopteran pests.11, 12
Since 1998, several Bt maize events have been approved for cultivation in Argentina, including Bt11 and MON 810 (expressing the Cry1Ab protein), TC1507 (expressing Cry1F protein), and MON 89034 (expressing Cry1A.105 and Cry2Ab2 proteins).13 These technologies have been shown to be an effective and environmentally friendly insect management tool to mitigate D. saccharalis damage to maize plants.14, 15 Under these circumstances, the use of maize plants expressing Bt proteins to manage the damage caused by D. saccharalis has been well accepted by growers in Argentina.14, 16
The evolution of resistance to Bt proteins in insect populations through the selection of insects carrying resistance alleles has been identified as the main threat to the sustained use of these technologies.17, 18 The deployment of genetically modified plants expressing one or more Bt proteins at a dose high enough to kill most of the insects heterozygous for the resistance allele (i.e., functionally recessive resistance) in combination with the implementation of a structured refuge area formed by non‐Bt plants19, 20 has been shown to be an effective approach for managing insect resistance.21 Nevertheless, field‐evolved resistance to Bt crops has been documented in Busseola fusca (Fuller) resistant to Cry1Ab maize in South Africa,22 in Spodoptera frugiperda (J.E. Smith) resistant to Cry1F maize in Puerto Rico and Brazil and to Cry1Ab in Brazil,23, 24, 25 in Pectinophora gossypiella (Saunders) resistant to Cry1Ac cotton in India,26 and in Diabrotica virgifera virgifera LeConte resistant to Cry3Bb1 maize in the USA.27 Furthermore, cross‐resistance among current commercially available Cry1 proteins was documented in S. frugiperda 28 and Helicoverpa zea (Boddie).29
In Argentina, unexpected damage caused by D. saccharalis to maize plants expressing Bt proteins (Cry1F, Cry1A.105 and Cry2Ab2) was detected in San Luis Province,30 a semiarid region where intensive agriculture and maize production are relatively recent as a consequence of the adoption of center‐pivot irrigation. In response, a comprehensive resistance mitigation plan was implemented. In this paper, we demonstrate that the unexpected damage in San Luis reflected resistance to Cry1F and Cry1A.105 and characterize that resistance, and describe the mitigation plan implemented in this region.
2. MATERIALS AND METHODS
2.1. Insect rearing and field collections
Two field populations of D. saccharalis were sampled from different regions across the traditional maize production area in the Province of San Luis, Argentina (Table 1; Fig. 1A). A D. saccharalis population sampled from non‐Bt maize plants in La Toma was coded VM, while insects sampled in La Candelaria from MON 89034 × MON 88017 maize plants with unexpected damage caused by D. saccharalis were coded RR. Insects from the RR population were assumed to carry alleles for resistance to at least one of the Bt proteins expressed in MON 89034 (Cry1A.105 and Cry2Ab2). MON 88017 contains the Cry3Bb1 gene for corn rootworm control and is not active against D. saccharalis. A susceptible D. saccharalis colony (SS), sampled from a commercial non‐Bt maize field in Pergamino (Buenos Aires Province) (Table 1; Fig. 1A) and maintained at AgIdea S.A. Laboratory (Pergamino, Buenos Aires Province) on non‐Bt maize material for five generations, was used as a reference in bioassays. Approximately 300 larvae were collected in each sampling area and kept on plant material. Adults were maintained in oviposition cages. To maintain selection pressure, larvae from the RR population were fed with fresh MON 89034 × MON 88017 maize leaf tissue while insects from the SS and VM populations were reared on fresh non‐Bt maize leaf tissue. Colonies of the field populations (VM and RR) were reared for at least two generations in the laboratory until bioassays were carried out.
Table 1.
Collection site information and total number of Diatraea saccharalis viable adults obtained in the laboratory from larvae and pupae of field populations
| Insect population | Collection site | Province | Latitude | Longitude | n a | Date |
|---|---|---|---|---|---|---|
| RR | La Candelaria | San Luis | 31°59′40.70′′S | 65°51′09.09′′W | 180 | August 2014 |
| VM | La Toma | San Luis | 33°04′59.34′′S | 65°32′03.46′′W | 181 | August 2014 |
| SSb | Pergamino | Buenos Aires | 33°47′05.58′′S | 60°28′20.27′′W | 781 | April 2013 |
More than 300 individuals (larvae and pupae) were collected at the La Candelaria and La Toma collection sites. These values indicate the number of individuals that reached the adult stage.
Individuals of the susceptible (SS) colony were reared for five generations in the laboratory before the bioassays were carried out.
Figure 1.
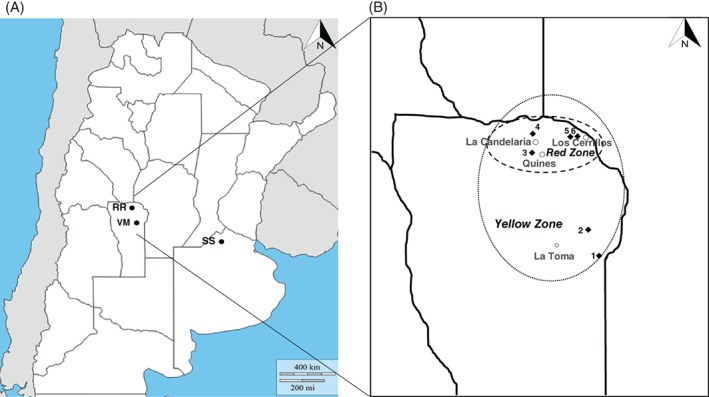
Distribution of field populations of Diatraea saccharalis (RR, VM and SS) used to characterize resistance in Argentina (A) and fields (1–6) evaluated in 2012/2013 season to define the baseline level of damage in San Luis Province (B).
2.2. Characterization of resistance in D. saccharalis
The efficacy of Bt maize in controlling larvae of the field‐collected populations was evaluated first in leaf disc bioassays. Plants of commercially available Bt maize products (TC1507, MON 89034 × MON 88017 and MON 810) and non‐Bt maize checks were grown in an open field plot. The youngest completely expanded leaf was individually removed from plants of each type when they reached phenological stages V5 and/or VT. After leaf removal, the expression of the Bt proteins was confirmed using QuickStix for Cry1A and Cry2A (Envirologix Inc., Portland, ME, USA). Leaf discs 1.5 cm in diameter were cut using a hole punch and placed on a mixture of water–agar at 2.0% (1.5 mL cell–1) in 128‐well bioassay trays (BIO‐BA‐128; CD International, Pitman, NJ, USA). The experimental design was completely randomized with four replicates per Bt maize technology (32 neonates per replicate) for each of the tested phenological stages of maize (V5 and VT). Trays were incubated in an environmental chamber maintained at 26 ± 1 °C, 70% relative humidity (RH), and a 14 h: 10 h (light:dark) photoperiod. Larval mortality and instar of survivors were recorded at 4 days after infestation. Any replicate in which control (non‐Bt) mortality exceeded 20% was discarded. Data were transformed prior to statistical analysis to meet the assumption of normality. Mortality data obtained from laboratory bioassays (x) were transformed into (and then compared by analysis of variance (ANOVA) using infostat statistical software.31
To characterize the level of resistance, larvae from the RR, VM, and SS populations were used in diet‐incorporation bioassays with eight to nine concentrations of Cry1A.105 (0.1–320 µg mL−1 diet) or Cry2Ab2 (0.175–180 µg mL−1 diet). Purified Cry1A.105 protein was provided by Monsanto Argentina at a concentration of ≈0.96 mg mL−1 active Cry1A.105 and stored in a freezer at −80 ± 5 °C. After thawing, the Cry1A.105 protein was diluted in buffer consisting of 25 mm CAPS (3‐cyclohexyl‐aminopropanesulfonic acid), pH ≈ 10.3, 1 mm benzamidine‐HCL, 0.1 mm EDTA (ethylenediaminetetraacetic acid), and 0.2 mm DTT (dithiothreitol). Corn leaf powder containing 7 mg g−1 Cry2Ab2 was diluted in high‐performance liquid chromatography (HPLC) water and used in the bioassays. These protein solutions were incorporated into artificial diet (Sugarcane Borer diet; Southland Products Inc., Lake Village, AR, USA), when the diet temperature reached 55 °C. A 1.5‐mL aliquot of diet containing the protein was poured into each well of 128‐well trays (BIO‐BA‐128; CD International). Trays were sealed with self‐adhesive plastic sheets (BIO‐CV‐16; CD International) that allowed gas exchange with the external environment and then placed in an environmental chamber maintained at 26 ± 1 °C, 70% RH, with a 14 h: 10 h (light:dark) photoperiod. The experimental design was completely randomized, with four replicates per concentration and 32 larvae per replicate at each concentration. Larval mortality and instar of survivors were recorded 7 days after infestation. Concentration–mortality data were analyzed by probit analysis to estimate the concentration that either kills or prevents 50% of larvae from molting to second instar (MIC50) and the respective 95% confidence interval (CI) using jmp 9 Version 10 software.32
To assess the inheritance of the resistance trait, strains with the proper genetic background were developed through the mass cross of females from the RR population with males from the SS population and vice versa, generating two heterozygous (S♂R♀ and S♀R♂) strains. Larvae from these strains, along with RR and SS, were used in diet‐incorporation bioassays with a diagnostic concentration of Cry1A.105 protein (20 µg mL−1 diet) based on the MIC95 determined for the SS strain. Bioassays and data analysis were generally performed as described above. The experimental design was completely randomized, with 10 replicates and 32 larvae per replicate. Larval mortality and instar of survivors were recorded 7 days after infestation. In addition, offspring of each cross was tested on leaf disc bioassays using leaf material from MON 89034 × MON 88017 and non‐Bt maize. The youngest completely expanded leaf was individually removed from plants of each material when they reached phenological stages V8 to V10, and leaf disc assays were performed as described above. The experimental design was completely randomized with four replicates per Bt maize technology (16 neonates per replicate). Larval mortality and instar of survivors was recorded at 4 days after infestation. Data were transformed prior to statistical analysis to meet the assumption of normality. Mortality data obtained from laboratory bioassays (x) were transformed into (variables were then compared by ANOVA using infostat statistical software.31
2.3. Field performance of MON 89034 × MON 88017
In the 2012/2013 crop season, in response to the report of unexpected damage on MON 89034 × MON 88017 maize plants in the northeast region of San Luis Province in Argentina, a scouting program was put in place to identify any spatial pattern associated with the occurrence and severity of damage. To cover the maize cropping area in that region, maize fields were chosen at random from 100 km southward and 50 km eastward of the reported case near La Candelaria in San Luis Province, Argentina (Fig. 1B). Maize plants were evaluated for holes (indicating the penetration of D. saccharalis larva into the stem) and length of tunneling. These measurements were taken on MON 810, MON 89034 × MON 88017, and TC1507 plants (between 10 and 30 plants per sample). In addition, growers in the affected region were interviewed to understand the possible causes of resistance. Information was gathered on adoption of Bt maize hybrids in recent years, level of compliance with refuge recommendations, management practices adopted by growers, and the history of damage by D. saccharalis on plant hosts other than maize. In the 2013/2014 and 2014/2015 seasons, a more systematic approach was applied to assess and follow up on the level of damage by D. saccharalis after implementation of a resistance mitigation plan in the affected region. Trials were planted in the area following local recommendations for early and late planting dates. In 2013/2014, five trials were established. In 2014/2015, three trials were implemented. Each trial consisted of strips six to eight rows wide and 100–200 m long. The entries in the trials were NK603 (non‐Bt), MON 810 × NK603, and MON 89034 × MON 88017. All entries were in the same genetic background (maize hybrid DK72‐10). The plots were managed following typical agronomical practices used by local farmers. At phenological stage R6, three replicates of 20 consecutive plants were evaluated for the number of plants with tunnels ≥5 cm in total length.
Data were analyzed separately for each location within each season. For each treatment, the proportion of damaged plants was computed separately for each replicate as the number of plants with at least 5 cm tunneling divided by the number of plants evaluated (20 in most cases). To test for statistical differences between treatments, the proportion of damaged plants (p) in each treatment was arcsine transformed to (and pairwise comparison of treatments made using ANOVA (specifically, using the lm function in r followed by the glht function from the r package multcomp).33
3. RESULTS AND DISCUSSION
3.1. Characterization of resistance in D. saccharalis
The mortality of the field‐derived RR strain of D. saccharalis was significantly lower than that of the field‐derived population from non‐Bt maize (VM) and the susceptible laboratory reference strain (SS) when exposed to leaf tissue of TC1507 (expressing the Cry1F protein) and MON 89034 × MON 88017 (expressing the Cry1A.105 and Cry2Ab2 proteins) at the V5 and VT plant growth stages (P < 0.05) (Table 2). No significant difference was detected between the mortality of the RR strain on TC1507 and MON 89034 × MON 88017 leaf tissue collected at the V5 stage; however, mortality of the RR strain was significantly lower on TC1507 leaf tissue relative to the response on MON 89034 × MON 88017 tissue when both were collected at the VT stage (P < 0.05) (Table 2). The mortality of the field‐derived RR strain was significantly higher on MON 810 leaf tissue (expressing the Cry1Ab protein) than on TC1507 or MON 89034 × MON 88017, all collected at VT (P < 0.05) (Table 2).
Table 2.
Percent mortality of Diatraea saccharalis (mean ± standard error) on leaf discs of TC1507, MON 89034 × MON 88017, MON 810, and non‐Bt maize
| Host plant | D. saccharalis straina | ||
|---|---|---|---|
| RR | VM | SS | |
| Maize plants at V5 stage | |||
| Non‐Bt maize | 10.16 ± 3.46 Aa | 11.72 ± 2.67 Aa | 10.16 ± 2.34 Aa |
| TC1507 | 16.41 ± 9.49 Aa | 96.88 ± 1.28 Cb | 92.97 ± 3.46 Bb |
| MON 89034 × MON 88017 | 31.25 ± 9.29 Aa | 80.47 ± 1.97 Bb | 98.44 ± 1.56 Bb |
| Maize plants at VT stage | |||
| Non‐Bt maize | 8.59 ± 1.56 Aa | 7.03 ± 1.97 Aa | 14.06 ± 1.56 Aa |
| TC1507 | 16.41 ± 9.49 Aa | 92.19 ± 3.25 Bb | 92.97 ± 3.46 Bb |
| MON 89034 × MON 88017 | 49.22 ± 6.80 Ba | 93.75 ± 2.21 Bb | 97.66 ± 0.78 Cb |
| MON 810 | 94.53 ± 1.97 Ca | 80.47 ± 7.90 Ba | 97.66 ± 0.78 Ca |
A separate ANOVA was conducted for treatments within each plant stage (V5 and VT). Values followed by the same letter are not significantly different [least significant difference (LSD) t‐test; P > 0.05]. Capital letters indicate comparisons between host plants within each strain (within columns). Differences between strains within each host plant (within rows) are indicated with lowercase letters.
The difference in survivorship of the RR strain on MON 89034 × MON 88017 leaf tissue relative to the SS strain was characterized using diet‐incorporation bioassays with Cry1A.105 and Cry2Ab2 proteins. The results showed a high level of resistance of the RR strain to the Cry1A.105 protein relative to the SS strain (186.74‐fold; Table 3). No relevant resistance (1.51‐fold) was observed in the RR strain to Cry2Ab2 relative to the SS strain (Table 3). The lower level of susceptibility of the SS strain to Cry2Ab2 relative to the field‐derived susceptible VM strain may indicate greater vigor of the SS strain under laboratory conditions and/or natural variation in susceptibility to this protein among D. saccharalis populations (Table 3).
Table 3.
Concentration–response (MIC50; µg mL−1) of Diatraea saccharalis in diet‐incorporation bioassays with purified Cry1A.105 and Cry2Ab2 proteins
| D. saccharalis strain | n | Slope ± SE | MIC50 (95% CI)a | χ2 (df)b | Resistance ratioc |
|---|---|---|---|---|---|
| Tests with Cry1A.105 protein | |||||
| RR | 767 | 1.34 ± 0.09 | 57.89 (42.16–83.66) | 93.95 (6) | 186.74 |
| VM | 768 | 2.12 ± 0.14 | 0.48 (0.39–0.59) | 55.30 (6) | 1.55 |
| SS | 768 | 1.61 ± 0.11 | 0.31 (0.23–0.40) | 46.89 (6) | – |
| Tests with Cry2Ab2 protein | |||||
| RR | 672 | 2.29 ± 0.25 | 116.36 (89.04–116.41) | 25.80 (5) | 1.51 |
| VM | 637 | 2.04 ± 0.19 | 29.01 (23.42–36.29) | 116.15 (5) | 0.38 |
| SS | 668 | 1.54 ± 0.17 | 77.29 (56.50–115.74) | 39.97 (5) | – |
MIC50: concentration that inhibits molting to second instar in 50% of individuals after 7 days.
P < 0.0001 in the goodness‐of‐fit test.
Resistance ratio = (MIC50 of indicated strain)/(MIC50 of SS strain).
Our results from leaf tissue and diet bioassays indicated significant resistance to (and likely cross‐resistance between) Cry1F and Cry1A.105, but not to Cry1Ab when expressed in MON 810 plants or Cry2Ab2, in D. saccharalis from La Candelaria in San Luis (Table 2). Based on the gene sequences, the overall amino acid sequence identity between Cry1A.105 and Cry1F is 90.0%, which would explain cross‐resistance between Cry1F and Cry1A.105.34 The potential for cross‐resistance between Cry1Ab/Ac, Cry1A.105 and Cry1F proteins in Lepidoptera through the alteration of shared binding sites was demonstrated by Hernández‐Rodríguez et al.35 However, the bioassays in the present study indicated little or no cross‐resistance between Cry1F and Cry1Ab as expressed in the corn events TC1507 and MON 810, respectively (Table 2). Results obtained with Spodoptera frugiperda,28 Helicoverpa zea,29 Ostrinia nubilalis 35 and Ostrinia furnacalis 36 showed that moderate levels of cross‐resistance among Cry1F, Cry1A.105, and Cry1Ab are likely to be present. Cry2Ab2 has a distinct mode of action from that of Cry1F and Cry1A proteins37 and therefore cross‐resistance between Cry2Ab2 and Cry1F or Cry1A is unlikely.28, 29, 35, 37, 38, 39
The RR strain was used in reciprocal crosses with the SS strain to generate two heterozygous strains (S♂R♀ and S♀R♂). In the leaf disc test, mortality of the RR strain was moderate when fed on MON 89034 × MON 88017 plants relative to non‐Bt plants (Table 4). The other D. saccharalis strains tested (SS, S♂R♀ and S♀R♂) had complete to almost complete mortality on MON 89034 × MON 88017 leaf tissue, indicating that the resistance trait in the RR strain is likely to be functionally recessive and autosomal (not sex linked) (Table 4). MON 89034 × MON 88017 expresses the Cry1A.105 and Cry2Ab2 proteins and is commercially available in Argentina. The fact that the resistance trait in the RR strain is likely to be functionally recessive to the commercial product supports the importance of refuges to manage resistance in D. saccharalis to Bt pyramids expressing the Cry1A.105 and Cry2Ab2 proteins in Argentina. All four strains tested (SS, S♂R♀, S♀R♂ and RR) showed high survival rates (>80%) on non‐Bt maize leaf tissue.
Table 4.
Percent mortality of Diatraea saccharalis (mean ± SE) strains on leaf discs of MON 89034 × MON 88017 maize and non‐Bt isoline, and in Cry1A.105 protein bioassays
| Type of bioassay | D. saccharalis straina | |||
|---|---|---|---|---|
| RR | R♀S♂ | S♀R♂ | SS | |
| Leaf disc bioassays | ||||
| Non‐Bt maize | 7.81 ± 3.93 Bab | 18.33 ± 1.67 Bb | 4.69 ± 2.99 Ba | 4.79 ± 1.60 Ba |
| MON 89034 × MON 88017 | 62.50 ± 7.65Aa | 93.33 ± 6.67 Ab | 100.00 ± 0.00 Ab | 100.00 ± 0.00 Ab |
| Protein bioassays | ||||
| 20 µg of Cry1A.105 / mL diet | 55.63 ± 8.24a | 89.93 ± 4.65bc | 85.94 ± 7.38b | 99.51 ± 0.27c |
A separate ANOVA was conducted for treatments within each type of bioassay (leaf disc and protein). Values followed by the same letters are not significantly different (LSD t‐test; P > 0.05). Capital letters indicate comparisons between host plants within each strain (within columns). Differences between strains within each host plant and in response to 20 µg of Cry1A.105 mL–1 diet (within rows) are indicated with lowercase letters.
3.2. Field performance of MON 89034 × MON 88017 and mitigation plans
The data from a first scouting, along with comprehensive inspections on most of the farms in the San Luis region in the 2012/2013 season (Table 5; Fig. 1B), were used to delimit two distinct areas according to the severity of D. saccharalis resistance. Severity was assessed based on the percentage of plants with damage above a threshold of at least 5 cm of tunneling. The area with most damage to MON 89034 × MON 88017 plants (∼11 000 ha) was named the “Red Zone”, where 100% of the plants evaluated met the defined threshold of ≥5 cm of tunneling. An area of approximately ∼30 000 ha surrounding the “Red Zone” and usually cultivated with maize but where the damage detected on MON 89034 × MON 88017 plants in the 2012/2013 season was absent was named the “Yellow Zone”.
Table 5.
Preliminary characterization of the damage caused by Diatraea saccharalis on MON 89034 × MON 88017, TC1507, and MON 810 in the affected region of San Luis Province, 2012/2013 season
| Evaluation sitea | Host plant | % of plants damagedb | Tunneling (cm)c | Latitude | Longitude |
|---|---|---|---|---|---|
| 1 | MON 810 | 0.00 | – | 33°07′51.96′′S | 65°08′12.42′′W |
| 2 | MON 89034 × MON 88017 | 0.00 | – | 32°53′46.02′′S | 65°21′02.34′′W |
| 3 | TC1507 | 37.50 | 6.00 | 32°11′51.18′′S | 65°51′58.68′′W |
| 4 | MON 89034 × MON 88017 | 100.00 | 10.00 | 31°59′40.70′′S | 65°51′09.09′′W |
| 5 | MON 810 | 0.00 | – | 31°58′08.16′′S | 65°34′30.00′′W |
| 6 | MON 89034 × MON 88017 | 7.00 | 5.00 | 31°58′29.82′′S | 65°31′39.36′′W |
See Fig. 1. Evaluation sites 4 and 6 correspond to La Candelaria and to Los Cerrillos, respectively.
Maize plants with ≥5 cm of tunneling.
–, not applicable.
The affected area is an isolated region surrounded by mountain ranges to the southeast (Sierras de San Luis), east and northeast (Sierras de Córdoba or de Comechingones), and west (Sierras Occidentales). It has a hot semi‐arid climate with dry, mild winters, dry, hot summers, a long frost‐free period, a high evapotranspiration rate, and woody shrubs as the native flora. Therefore, it has not been part of the main agricultural area of Argentina. Agriculture became a relevant economic activity in the region after 2005 when pivot irrigation was implemented. Based on interviews with farmers and reviews of their planting records, since the introduction of pivot irrigation, the main crops planted in the Red Zone have been maize, soybean and wheat which together represent approximately 75% of the production, followed by potato, cotton and sorghum. In the case of maize, farmers rapidly adopted Bt hybrids for the control of D. saccharalis and, when new events for the control of S. frugiperda (the main lepidopteran pest in the area) were available in 2008, there was a rapid switch of Bt technologies towards TC1507 and later also MON 89034 × MON 88017, which enabled higher yields for early and late planting dates. The farmers' records show that practically no refuge was implemented for Bt maize during that entire period. According to these records, TC1507 was introduced with 5% of the maize area in the 2008/2009 season (95% was Cry1Ab‐expressing technologies), increasing to 29% of the area in the following season, and reaching 52–53% in 2010/2011 and 2011/2012 as an average in the whole Red Zone. However, in the area around La Candelaria (the epicenter of the resistance problem), the adoption of the single event expressing Cry1F reached almost 90%. In 2011, MON 89034 × MON 88017 was introduced with 8% of the maize area in the region, increasing to 15% in the 2012/2013 season when resistance was reported (data provided by Consorcio Regional de Experimentación Agrícola ‐CREA‐ of the region Traslasierra). Therefore, the observed resistance likely arose as Cry1F resistance (which also conferred cross‐resistance to Cry1A.105) and the primary cause of the resistance evolution was inadequate refuge compliance.
A resistance mitigation plan was implemented in the affected area in parallel with studies to characterize resistance. Given that several Bt corn technologies were impacted, the mitigation plan was developed in a joint effort among companies in the Argentina Seed Association (ASA) and promptly communicated to the relevant governmental agencies. Several actions were triggered to decrease D. saccharalis populations in the affected area and reduce the selection pressure imposed by MON 89034 × MON 88017. The mitigation actions were customized according to the intensity of the resistance issue. In the “Red Zone”, an exhaustive monitoring for D. saccharalis egg masses was conducted over more than 8000 hectares in the first year of the mitigation plan (2013/2014). This field monitoring program was later expanded and currently covers approximately 14 000 ha in San Luis Province. Since the first detection of unexpected damage caused by D. saccharalis to MON 89034 × MON 88017 plants, Monsanto Argentina also has been providing growers in the affected region with insecticides to control this pest. In addition, growers have been recommended to rotate their crops from maize to another crop and/or to use Bt maize hybrids with a different mechanism of action [i.e. MON 810 or Bt11 (expressing Cry1Ab) or MIR162 × Bt11 (expressing Cry1Ab and Vip3Aa20)], thus avoiding the use of the technologies impacted by resistance (MON 89034 and TC1507). Control of volunteer maize plants and weeds capable of hosting D. saccharalis (e.g. Johnsongrass) was recommended as an additional measure to reduce pest pressure. As another component of the resistance mitigation plan, a robust effort to train and educate growers on pest management was initiated in San Luis Province. Growers receive information and training on the requirement to implement structured refuges (i.e. 10% of the total maize planting area to be planted with non‐Bt maize), the best practices to scout their fields and identify unexpected damage on MON 89034 × MON 88017 plants and on other D. saccharalis host crops (e.g. wheat and sorghum), and the proper way to spray insecticides if necessary. Additionally, the National Seed Institute (INASE) issued a Resolution to prevent maize seed production in the area (Res. INASE N° 328/2013). In the “Yellow Zone”, the actions consisted of monitoring for damage on all Bt maize products to determine the spread of resistance into the region as well as enhanced training and monitoring of growers regarding refuge implementation. Approximately 20 000 ha of maize were covered in the first year of the mitigation plan. In the rest of the country, a Communication Program was launched by ASA to educate growers on best management practices (BMPs) applicable to Bt maize and raise awareness of refuge recommendations. In addition to these measures, in the second year of the mitigation plan, given the yield loss caused by the high pressure of S. frugiperda in the previous season, a compromise was established with farmers to use Cry1Ab‐expressing events in early plantings to decrease D. saccharalis pressure with the possibility of using TC1507 and MON 89034 × MON 88017 in late plantings to control S. frugiperda, all with monitoring and chemical control when necessary to reduce the pressure from both pest species.
The results from field trials conducted in the two following seasons, 2013/2014 (Table 6; Fig. 2) and 2014/2015 (Table 7; Fig. 2), indicated that both MON 810 and MON 89034 × MON 88017 effectively reduced the damage caused by D. saccharalis in the affected area. In 2013/2014, MON 810 showed superior protection in the locations with high insect pressure (Los Cerrillos and Las Lomitas) (Table 6). During the 2014/2015 season, three trials were planted with the same isohybrids as in the previous season. The non‐Bt maize hybrid used as a reference material reached the monitoring threshold of 5% plants with ≥5 cm of tunneling at all three sites. The results from the second season (2014/2015), after mitigation actions were implemented in the affected region, were similar to those of the first season; both MON 810 and MON 89034 × MON 88017 effectively reduced the damage caused by D. saccharalis in the affected area relative to non‐Bt maize, but MON 810 offered superior protection (Table 7; Fig. 2).
Table 6.
Percent of plants with unexpected damage (≥5 cm of tunneling) ± SE, and mean tunneling on Bt and non‐Bt maize hybrids by natural infestation of Diatraea saccharalis across sites in San Luis Province, 2013/2014 season
| Location | Entry | Percent of plants with unexpected damagea | Tunneling (cm) |
|---|---|---|---|
| Balde de Quines | Non‐Bt maize | 28.33 ± 5.82 a | 13.77 |
| MON 810 | 0.00 ± 0.00 b | 0.00 | |
| MON 89034 × MON 88017 | 1.67 ± 1.65 b | 8.00 | |
| La Candeleria | Non‐Bt maize | 75.00 ± 5.59 a | 16.44 |
| MON 810 | 8.33 ± 3.57 b | 15.80 | |
| MON 89034 × MON 88017 | 11.67 ± 4.14 b | 8.57 | |
| Las Lomitas | Non‐Bt maize | 98.33 ± 1.65 a | 26.60 |
| MON 810 | 0.00 ± 0.00 c | 0.00 | |
| MON 89034 × MON 88017 | 40.00 ± 7.75 b | 12.91 | |
| Los Cerrillos | Non‐Bt maize | –b | –b |
| MON 810 | 0.00 ± 0.00 c | 0.00 | |
| MON 89034 × MON 88017 | 53.33 ± 6.44 b | 9.97 | |
| San Vicente | Non‐Bt maize | 60.00 ± 7.30 a | 20.52 |
| MON 810 | 0.00 ± 0.00 b | 0.00 | |
| MON 89034 × MON 88017 | 6.67 ± 0.04 b | 9.67 |
Values within a column followed by the same letter are not significantly different (P > 0.05).
–, not assessed.
Figure 2.
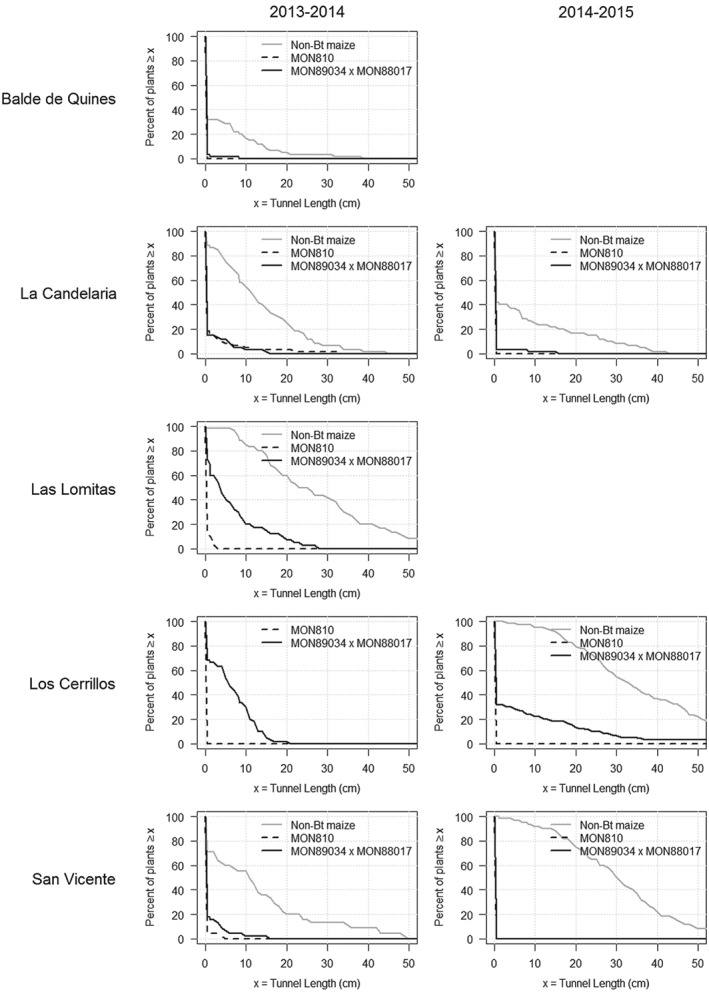
Distribution of tunnel length across plants by trait, region (rows), and season (columns). The x‐axis is tunnel length (x), and the y‐axis is percent of plants with tunnel length ≥ x.
Table 7.
Percent of plants with unexpected damage (≥5 cm of tunneling) ± SE, and mean tunneling on Bt and non‐Bt maize hybrids by natural infestation of Diatraea saccharalis across sites in San Luis Province, 2014/2015 season
| Location | Entry | Percent of plants with unexpected damagea | Tunneling (cm) |
|---|---|---|---|
| La Candelaria | Non‐Bt maize | 36.67 ± 6.22 a | 19.59 |
| MON 810 | 0.00 ± 0.00 b | 0.00 | |
| MON 89034 × MON 88017 | 3.33 ± 2.32 b | 11.50 | |
| Los Cerrillos | Non‐Bt maize | 98.33 ± 1.17 a | 35.89 |
| MON 810 | 0.00 ± 0.00 c | 0.00 | |
| MON 89034 × MON 88017 | 28.33 ± 4.11 b | 24.76 | |
| San Vicente | Non‐Bt maize | 96.67 ± 2.32 a | 30.91 |
| MON 810 | 0.00 ± 0.00 b | 0.00 | |
| MON 89034 × MON 88017 | 0.00 ± 0.00 b | 0.00 |
Values within a column followed by the same letter are not significantly different (P > 0.05).
On a regional scale, the adoption of effective refuge by farmers in the region was assessed through a growers' survey. The survey results indicated an increase from practically 0% of growers implementing refuges before the incident to 75% and 87% adoption in the “Red Zone” in the 2013/2014 and 2014/2015 seasons, respectively.
At the country level, annual systematic monitoring of unexpected damage caused by D. saccharalis to MON 89034 × MON 88017 which is supported by Monsanto Argentina shows that, to date, the resistant biotype has been confined to the originally affected region in the northeast part of San Luis Province. This positive scenario is primarily the result of the mitigation actions implemented by the seed/biotechnology industry and the farmers in the region, along with governmental measures such as the Resolution mentioned above. Although MON 89034 × MON 88017 continues to bring value to corn growers, it is important to monitor the performance of current Bt technologies outside of the affected region and implement actions to mitigate resistance in D. saccharalis if detected. In addition, the design, development, and release of a next generation of effective Bt maize pyramids combining highly effective and novel mechanisms of action is central to an effective insect resistance management (IRM) strategy in Argentina. Furthermore, understanding and aligning on IRM imperatives (e.g. ensuring refuge seed supply) across the seed industry is fundamental to the effective IRM implementation for Bt crops.
REFERENCES
- 1. Igarzábal D, Fichetti P and Tognelli M, Claves prácticas para la identificación de larvas de Lepidoptera en cultivos de importancia agrícola en Córdoba (Argentina). Gayana Zool 58:99–142 (1994). [Google Scholar]
- 2. Iannone N and Leiva P, Bioecología y control de plagas en el cultivo de maíz. Capítulo 8 in Bases para el manejo del cultivo de maíz. Guillermo Eyherabide Editor. Ediciones INTA; 2015. ISBN: 978‐987‐679‐141‐0. [Online]. Available: http://inta.gob.ar/documentos/bases‐para‐el‐manejo‐del‐cultivo‐de‐maiz [10 May 2017]. [Google Scholar]
- 3. Martin S, Darrah L and Hibbard B, Divergent selection for rind penetrometer resistance and its effects on European corn borer damage and stalk traits in corn. Crop Sci 44:711–717 (2004). [Google Scholar]
- 4. Aragón J, Las principales plagas del cultivo. Métodos de control. CREA: Maíz. Cuaderno de actualización técnica 57:51–61 (1996). [Google Scholar]
- 5. Iannone N, Control químico de Diatraea tecnología que apunta a la alta producción. Revista de tecnología agropecuaria. Divulgación técnica del INTA Pergamino. Vol. VI. Nro. 17. pp. 33–37 (2001). [Google Scholar]
- 6. Carta HG, Ventimiglia LA and Rillo SN, Maíz de segunda pero "de primera". Rev de Téc Agrop INTA Pergamino. Bs. As. Argentina. 14:26–28 (2000). [Google Scholar]
- 7. Iannone N, Couretot A and Cacciamani M, Tecnología de control del barrenador del tallo Diatraea saccharalis Fab. Demostración técnico económica en cultivo de maíz. Revista de tecnología agropecuaria. Divulgación técnica del INTA Pergamino. Vol. VIII Nro. 22. pp. 10–13 (2003).
- 8. Alonso SN and Miguez YFN, El barrenador del tallo del maíz. CREA 109:20–30 (1984). [Google Scholar]
- 9. Farias JR, Costa EC, Guedes JVC, Arbage AP, Neto AB, Bigolin M et al, Managing the sugarcane borer, Diatraea saccharalis, and corn earworm, Helicoverpa zea, using Bt corn and insecticide treatments. J Insect Sci 13:109 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Michelotto MD, Finoto EL, Martins ALM and Duarte AP, Interação entre transgênicos (Bt) e inseticidas no controle de pragas‐chave em híbridos de milho‐safrinha. Arq Inst Biol 78:71–79 (2011). [Google Scholar]
- 11. Huesing J and English L, The impact of Bt crops on the developing world. AgBioForum 7:84–95 (2004). [Google Scholar]
- 12. Hutchison WD, Burkness EC, Mitchell PD, Moon RD, Leslie TW, Fleischer SJ et al, Areawide suppression of European corn borer with Bt maize reaps savings to non‐Bt maize growers. Science 330:222–225 (2010). [DOI] [PubMed] [Google Scholar]
- 13. Dirección de Biotecnología , Secretaría de Valor Agregado, Ministerio de Agroindustria de la República Argentina. [Online]. Available: http://www.minagri.gob.ar/sitio/areas/biotecnologia/ogm/ [27 October 2016]
- 14. Flores F and Parodi B, Maíces Bt: manejo de la resistencia de los insectos blanco y nuevos eventos disponibles. EEA INTA Marcos Juárez, Argentina: (2011). [Online]. Available: http://inta.gob.ar/sites/default/files/script‐tmp‐inta‐_manejo_de_la_resistencia_de_los_insectos_blanco.pdf [27 Oct 2016]. [Google Scholar]
- 15. Trigo EJ, Fifteen years of genetically modified crops in Argentine agriculture. ArgenBio Report, 49 p. (2011). [Google Scholar]
- 16. Fava FD, Imwinkelried JM and Trumper EV, Proyecto Regional de Agricultura Sustentable. Manejo del Barrenador del Tallo del Maíz Diatraea saccharalis (Lepidoptera: Crambidae). Boletín N° 6: 1–4. Ediciones INTA, Centro Regional Córdoba, Argentina. ISSN 1668‐2882 (2004).
- 17. Gould F, Sustainability of transgenic insecticidal cultivars: integrating pest genetics and ecology. Annu Rev Entomol 43:701–726 (1998). [DOI] [PubMed] [Google Scholar]
- 18. Tabashnik BE, Brévault T and Carrière Y, Insect resistance to Bt crops: lessons from the first billion acres. Nat Biotechnol 31:510–521 (2013). [DOI] [PubMed] [Google Scholar]
- 19. Roush RT, Managing pests and their resistance to Bacillus thuringiensis: can transgenic crops be better than sprays? Biocontrol Sci Technol 4:501–516 (1994). [Google Scholar]
- 20. Tabashnik BE, Gould F and Carrière Y, Delaying evolution of insect resistance to transgenic crops by decreasing dominance and heritability. J Evol Biol 17:904–912 (2004). [DOI] [PubMed] [Google Scholar]
- 21. Huang F, Andow DA and Buschman L, Success of the high‐dose/refuge resistance management strategy after 15 years of Bt crop use in North America. Entomol Exp Applic 140:1–16 (2011). [Google Scholar]
- 22. Van Rensburg JBJ, First report of field resistance by the stem borer, Busseola fusca (Fuller), to Bt‐transgenic maize. S Afr J Plant Soil 24:147–151 (2007). [Google Scholar]
- 23. Storer NP, Babcock JM, Schlenz M, Meade T, Thompson GD, Bing JW et al, Discovery and characterization of field resistance to Bt maize: Spodoptera frugiperda (Lepidoptera: Noctuidae) in Puerto Rico. J Econ Entomol 103:1031–1038 (2010). [DOI] [PubMed] [Google Scholar]
- 24. Farias JR, Andow DA, Horikoshi RJ, Sorgatto RJ, Fresia P, Santos AC et al, Field‐evolved resistance to Cry1F maize by Spodoptera frugiperda (Lepidoptera: Noctuidae) in Brazil. Crop Prot 64:150–158 (2014). [Google Scholar]
- 25. Omoto C, Bernardi O, Salmeron E, Sorgatto RJ, Dourado PM, Crivellari A et al, Field‐evolved resistance to Cry1Ab maize by Spodoptera frugiperda in Brazil. Pest Manag Sci 72:1727–1736 (2016). [DOI] [PubMed] [Google Scholar]
- 26. Dhurua S and Gujar GT, Field‐evolved resistance to Bt toxin Cry1Ac in the pink bollworm, Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae), from India. Pest Manag Sci 67:898–903 (2011). [DOI] [PubMed] [Google Scholar]
- 27. Gassmann AJ, Petzold‐Maxwell JL, Keweshan RS and Dunbar MW, Field‐evolved resistance to Bt maize by western corn rootworm. PLoS ONE 6:e22629 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bernardi D, Salmeron E, Horikoshi RJ, Bernardi O, Dourado PM, Carvalho RA et al, Cross‐resistance between Cry1 proteins in fall armyworm (Spodoptera frugiperda) may affect the durability of current pyramided Bt maize hybrids in Brazil. PLoS ONE 10:e0140130 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Welch KL, Unnithan GC, Degain BA, Wei J, Zhang J, Li X et al, Cross‐resistance to toxins used in pyramided Bt crops and resistance to Bt sprays in Helicoverpa zea . J Invertebr Pathol 132:149–156 (2015). [DOI] [PubMed] [Google Scholar]
- 30. Trumper EV, Resistencia de insectos a cultivos transgénicos con propiedades insecticidas. Teoría, estado del arte y desafíos para la República Argentina. Agriscientia 31:109–126 (2014). [Google Scholar]
- 31. Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M and Robledo CW, InfoStat versión 2016. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina; [Online]. Available: http://www.infostat.com.ar [Google Scholar]
- 32. SAS Institute , JMP software—Introductory guide version 9.0. SAS Institute, Cary, NC: (2010). [Google Scholar]
- 33.R Development Core Team, R: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria, (2014). [Online]. Available: http://www.r‐project.org [Google Scholar]
- 34. Huang F, Qureshi JA, Meagher RL Jr, Reisig DD, Head GP, Andow DA et al, Cry1F resistance in fall armyworm Spodoptera frugiperda: single gene versus pyramided Bt maize. PLoS ONE 9:e112958 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hernández‐Rodríguez CS, Hernández‐Martínez P, Van Rie J, Escriche B and Ferré J, Shared midgut binding sites for Cry1A.105, Cry1Aa, Cry1Ab, Cry1Ac and Cry1Fa proteins from Bacillus thuringiensis in two important corn pests, Ostrinia nubilalis and Spodoptera frugiperda . PLoS ONE 8:e68164 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang Y, Wang Y, Wang Z, Bravo A, Soberón M and He K, Genetic basis of Cry1F resistance in a laboratory selected Asian corn borer strain and its cross‐resistance to other Bacillus thuringiensis toxins. PLoS ONE 11:e0161189 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Storer NP, Thompson GD and Head GP, Application of pyramided traits against Lepidoptera in insect resistance management for Bt crops. GM Crops Food 3:154–162 (2012). [DOI] [PubMed] [Google Scholar]
- 38. Yang F, Kerns DL, Brown S, Kurtz R, Dennehy T, Braxton B et al, Performance and cross‐crop resistance of Cry1F‐maize selected Spodoptera frugiperda on transgenic Bt cotton: implication for resistance management. Sci Rep 6:28059 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yang F, Kerns DL, Head G, Brown S and Huang F, Susceptibility of Cry1F‐maize resistant, heterozygous, and susceptible Spodoptera frugiperda to Bt proteins used in transgenic cotton. Crop Prot 98:128–135 (2017). [Google Scholar]


