Abstract
Tumor cells frequently overexpress heat shock protein 70 (Hsp70) and present it on their cell surface, where it can be recognized by pre‐activated NK cells. In our retrospective study the expression of Hsp70 was determined in relation to tumor‐infiltrating CD56+ NK cells in formalin‐fixed paraffin embedded (FFPE) tumor specimens of patients with SCCHN (N = 145) as potential indicators for survival and disease recurrence. All patients received radical surgery and postoperative cisplatin‐based radiochemotherapy (RCT). In general, Hsp70 expression was stronger, but with variable intensities, in tumor compared to normal tissues. Patients with high Hsp70 expressing tumors (scores 3–4) showed significantly decreased overall survival (OS; p = 0.008), local progression‐free survival (LPFS; p = 0.034) and distant metastases‐free survival (DMFS; p = 0.044), compared to those with low Hsp70 expression (scores 0–2), which remained significant after adjustment for relevant prognostic variables. The adverse prognostic value of a high Hsp70 expression for OS was also observed in patient cohorts with p16‐ (p = 0.001), p53‐ (p = 0.0003) and HPV16 DNA‐negative (p = 0.001) tumors. The absence or low numbers of tumor‐infiltrating CD56+ NK cells also correlated with significantly decreased OS (p = 0.0001), LPFS (p = 0.0009) and DMFS (p = 0.0001). A high Hsp70 expression and low numbers of tumor‐infiltrating NK cells have the highest negative predictive value (p = 0.00004). In summary, a strong Hsp70 expression and low numbers of tumor‐infiltrating NK cells correlate with unfavorable outcome following surgery and RCT in patients with SCCHN, and thus serve as negative prognostic markers.
Keywords: Hsp70, prognostic biomarker, SCCHN, NK cells, IHC, retrospective trial
Short abstract
What's new?
It's difficult to predict how a patient with squamous‐cell carcinoma of the head and neck (SCCHN) will respond to treatment, because every tumor is different. In this study, the authors identified two pre‐treatment measures that were associated with poor prognosis following surgery and RCT: high levels of staining for a protein called Hsp70 in tumor cells, and low numbers of tumor‐infiltrating NK lymphocytes. These measures may thus serve as useful prognostic biomarkers for predicting the response of SCCHN to therapy.
Introduction
The highly conserved, major stress‐inducible Hsp70, also termed HSPA1A, is found in nearly all cellular and subcellular compartments of nucleated cells.1 Hsp70 fulfills a variety of chaperoning functions, such as maintenance of cellular homeostasis2, 3 by assisting folding, maturation and transport of unfolded proteins, and preventing of apoptosis under stressed and non‐stressed conditions.4, 5 Elevated Hsp70 levels are associated with poor prognosis in a variety of tumor entities including osteosarcomas,6, 7 squamous cell carcinoma of the lung,8 lower rectal cancer and hematological diseases.9, 10 Furthermore, tumor in contrast to normal cells, have also been shown to present Hsp70 on their cell surface.11, 12 Membrane localization of Hsp70 on tumor cells is most likely due to a tumor‐specific lipid composition that enables anchorage of Hsp70 in the plasma membrane.13 Depending on its subcellular localization, Hsp70 can fulfill different tasks; on the one hand, it mediates resistance of tumor cells to RCT,14 on the other hand, it serves as a recognition structure for a subtype of natural killer (NK) cells that is able to kill highly aggressive, membrane Hsp70‐positive tumor cells.11, 15 Based on these results, a protocol has been established to activate this NK cell subpopulation by incubating peripheral blood lymphocytes ex vivo with either full length Hsp70 protein, or a peptide derived thereof in combination with low dose IL‐2.16, 17 The immune phenotype of Hsp70 peptide plus IL‐2 activated NK cells has been determined as CD3−CD56brightCD94bright.18 Although CD56bright NK cells are described to secrete pro‐inflammatory cytokines rather than exerting cytotoxicity, these NK cells are able to efficiently eliminate Hsp70 membrane‐positive tumor cells.18 Safety and tolerability of these ex vivo stimulated, autologous NK cells have been demonstrated in a phase I clinical trial.19 Presently a proof‐of‐concept phase II randomized clinical trial is ongoing to study the efficacy of ex vivo Hsp70‐stimulated NK cells in patients with squamous NSCLC in stage IIIA/B after RCT.8, 20
In our study, which is part of the DKTK‐ROG initiative, which aims to identify and validate biomarkers for outcome of RCT in SCCHN,21, 22, 23, 24, 25, 26 we aim to study the role of Hsp70 and tumor‐infiltrating NK cells as prognostic tumor biomarkers. Every year approximately 500,000 new cases are diagnosed worldwide with SCCHN with 4.8% of total cancer incidence and 4.6% cancer mortality.27 Apart from tobacco and alcohol, which are considered as main risk factors for the development of head and neck cancer, infection with human papilloma virus (HPV) has been determined as causally connected to oropharyngeal cancer.28 Due to increasing numbers of HPV infections the incidence for SCCHN is rising especially in younger individuals.29 Patients with locally advanced SCCHN have a 5 year survival rate of 40–60%.27 Given the progress in medicine over the last decades these mortality rates are still not satisfying. Consequently, reliable biomarkers, which are able to predict outcome of therapy at an early time point are urgently needed to stratify patients with respect to prognosis and to guide adaptations for the treatment.
An early event in SCCHN carcinogenesis is related to somatic mutations of the protein p16 (chromosome 9p21) that exerts tumor suppressor function by binding to the cyclin D1 CDK4/CDK6 complex, which results in a G1 arrest.30 Silencing of p16 by homozygous deletion, methylation of the promoter and base pair mutations are associated with a more rapid tumor growth.31 However, with respect to SCCHN the role of p16, as a prognostic marker for outcome of RCT, remains a matter of debate.32
Between 40% and 70% of SCCHN contain mutations in the tumor suppressor gene p53.33 An accumulation of p53, which can be induced not only by mutations, but also by other mechanisms, results in invasive tumor growth and radioresistance.34
Although HPV infection is a risk factor for the development of SCCHN, HPV16 DNA‐positive patients show a better clinical outcome compared to their HPV16 DNA‐free counterparts.35 Part of this effect has been attributed to an HPV16‐induced activation of the immune system. In line with this finding, infiltration of tumors with CD8+ cytotoxic T lymphocytes has been found to be associated with an HPV16 DNA‐positive status and a favorable tumor prognosis.25 In addition, higher numbers of CD56+ tumor‐infiltrating NK cells are associated with better prognosis in oropharyngeal squamous cell carcinoma.36
In our study, we aimed to assess the role of Hsp70 either alone or in relationship with HPV16 DNA, p16 and p53 status, and the infiltration of tumors with CD56+ NK cells, as prognostic markers in patients with SCCHN after surgery and RCT.
Patients and Methods
SCCHN patients
Between 2004 and 2012, patients with histologically confirmed SCCHN of the oro‐, hypopharynx and oral cavity were recruited into the study. Patient characteristics are summarized in Table 1. Apart from pN stage (p = 0.020), none of the other clinical parameters summarized in Table 1 show any significant correlation with the Hsp70 status. All patients were treated with radical surgery and postoperative cisplatin‐based RCT. The tumor bed and regional lymph nodes have been irradiated with a median dose of 50.4 Gy and a boost up to a total dose of 66 Gy to the former tumor area. Assessment of clinical responses and treatment planning were performed by CT, MRI or PET/CT scans. The follow‐up period was at least 24 months.
Table 1.
Demographic and clinical characteristics of SCCHN patients
| Total | |||||
|---|---|---|---|---|---|
| Hsp70 scores 0–2 | % | Hsp70 scores 3–4 | % | p‐Value | |
| Gender | 0.922 | ||||
| Male | 65 | 44.8 | 57 | 39.3 | |
| Female | 12 | 8.3 | 11 | 7.6 | |
| Age (mean ± SD) | 56.3 ± 10.3 | 58.1 ± 7.7 | 0.232 | ||
| Tumor site | 0.318 | ||||
| Oropharynx | 44 | 30.3 | 43 | 29.7 | |
| Hypopharynx | 9 | 6.2 | 11 | 7.6 | |
| Oral cavity | 24 | 16.6 | 14 | 9.6 | |
| pT stage | 0.960 | ||||
| 1–2 | 49 | 33.7 | 43 | 29.7 | |
| 3–4 | 28 | 19.3 | 25 | 17.3 | |
| pN stage | 0.020* | ||||
| 0–1 | 24 | 16.6 | 10 | 6.8 | |
| 2–3 | 53 | 36.5 | 58 | 40.1 | |
| Grading | 0.771 | ||||
| 1 | 2 | 1.3 | 2 | 1.3 | |
| 2 | 41 | 28.3 | 33 | 22.8 | |
| 3 | 34 | 23.5 | 33 | 22.8 | |
| Resection margin (ECE) | 0.717 | ||||
| R0 | 43 | 29.7 | 40 | 27.5 | |
| R1 | 34 | 23.4 | 28 | 19.4 | |
| HPV16 DNA | 0.277 | ||||
| Positive | 25 | 17.3 | 28 | 19.3 | |
| Negative | 52 | 35.8 | 40 | 27.6 | |
| p16 | 0.271 | ||||
| Positive | 26 | 17.9 | 29 | 20.1 | |
| Negative | 51 | 35.2 | 39 | 26.8 | |
| p53 | 0.055 | ||||
| Positive | 37 | 25.6 | 22 | 15.1 | |
| Negative | 40 | 27.5 | 46 | 31.8 | |
| Smoking history | 0.570 | ||||
| Yes | 68 | 46.9 | 62 | 42.7 | |
| No | 9 | 6.2 | 6 | 4.2 | |
| CD56+ NK cell infiltration | |||||
| Low | 15 | 28.8 | 13 | 28.3 | |
| High | 37 | 71.2 | 33 | 71.7 |
Bold values marked with * present p‐values <0.05 and are considered as statistically significant.
Formalin‐fixed, paraffin‐embedded (FFPE) sections were obtained from surgical specimen of the tumor from the oro‐, hypopharynx or oral cavity. The oral cavity includes the subsites tongue, floor of mouth, palate and gingival/buccal regions and the oropharynx includes base of the tongue and tonsils. Since this was a multicentre study, the patients were encoded only for the three main anatomic sites oral cavity, oropharynx and hypopharynx. All specimens were centrally acquired together with clinical data, radiotherapy plans, diagnostic images in the DKTK RadPlanBio Platform at the partner site Dresden. A total of 145 patient tumors in UICC stage IVa,b were analyzed for their expression intensity of Hsp70, and the presence of infiltrating CD56+ NK cells was analyzed in 114 tumor sections (intraepithelial, stroma, tumor border) by immunohistochemistry (IHC). The total number of patients in the original study was 221,26 however, serial FFPE sections for Hsp70 staining and NK cell infiltration were available only from 5 (Dresden, Essen, Frankfurt, Munich, Tübingen) DKTK partner sites (Table 1). The multicentre trial was approved by the ethical committees of all 8 DKTK partner sites.
IHC staining and scoring of Hsp70 intensity in tumor sections
Briefly, serial FFPE sections (4 µm) of SCCHN tumor patients (N = 145) were prepared and heated by microwaving for 30 min in target retrieval buffer (DAKO, Wientheid, Germany, cat# S1699) to unmask antibody epitopes. Non‐specific binding was blocked by incubation in protein blocking solution (5% v/v rabbit serum/antibody diluent (REAL antibody diluent, DAKO cat# S2022) for 60 min. Sections were washed in PBS (Sigma‐Aldrich, St Louis, USA) after each step. After an overnight incubation at 4°C with the mouse monoclonal antibody cmHsp70.1 (multimmune GmbH, Munich, Germany; dilution 1:500 in PBS/1% BSA) and another washing step, sections were incubated with Envision+ System HRP‐labelled anti‐mouse polymer (DAKO cat# K4001), followed by a 3,3‐diaminobenzidine (DAB+) chromogen (DAKO cat# K3468) reaction, which was restricted to exactly 4 min for all staining procedures. Nuclei were counterstained with hematoxylin and eosin (H&E). Then sections were embedded in Eukitt (Sigma cat# 03989) mounting medium. Appropriate quality control and quality assurance procedures were implemented including positive (FaDu) and negative (surrounding tissue) controls run with each assay.
According to the staining intensities tumor sections were scored into the following categories: very weak (score 0), weak (score 1), intermediate (score 2), strong (score 3) and very strong (score 4). Since all nucleated cells contain Hsp70, a very weak staining pattern (score 0) was also observed in surrounding normal tissues. IHC scoring for Hsp70 intensity was performed by three independent, trained evaluators (SS, GM, NT). All scores were blinded to the outcome status for each tumor specimen. At least 5 non‐overlapping representative tumor fields were evaluated for each section.
IHC staining and scoring of the number of tumor‐infiltrating CD56+ NK cells
For the detection of tumor‐infiltrating CD56+ NK cells the mouse monoclonal antibody directed against all isoforms of CD56 (NCAM, clone 1B6 Novocastra, Newcastle upon Tyne, UK) was used. After antigen retrieval, as described above, staining was performed using standardized DAKO Envision FLEX Peroxidase Blocking reagent (DAKO cat# K800) and the antibody directed against CD56 for 120 min at room temperature. After washing, sections (N = 114) were incubated with Envision+ System HRP‐labelled anti‐mouse polymer (DAKO cat# K4001), followed by a DAB+ chromogen (DAKO cat# K3468) reaction. H&E staining was performed for a visual distinction of the tumor and its microenvironment. All sections were embedded in Eukitt (Sigma cat# 03989) mounting medium.
The number of infiltrating CD56+ NK cells was determined by counting of at least five different compartments of the tumor and its microenvironment within an area of at least 0.1 mm2 per slide. The counting of the NK cells was performed at a magnification of ×400. The NK cell counts were determined blinded by three–independent researchers (SS, GM, NT).
The total numbers within one area of interest ranged from 0 to 2,000 NK cells within the tumor and its microenvironment. The median number of infiltrating NK cells in an individual area was calculated. The cut‐off point of 50 NK cells was chosen based on visual inspection of all sections. In sections with <50 events the localization of infiltrating NK cells was disperse, whereas sections with >50 events showed a more dense localization of NK cells.
HPV16, p16, p53 analysis
The analysis of the HPV16 status as well as the p16 and p53 staining was performed centrally at the DKTK partner site Dresden. The HPV analysis and genotyping were performed by using the LCD‐Assay HPV‐array HPV 3.5 Kit (Chiron GmbH) after extraction of the genomic DNA, as described previously.28 IHC staining for p16 was performed with the CINtec Immunohistology Kit (Roche). A p16‐positive phenotype was defined if at least 70% of the sample was stained positively as determined by two‐independent researchers.28
Statistics
Distributions of categorical variables within the groups of patients with low and high Hsp70 scores are described by absolute and relative frequencies. For comparisons of these distributions chi‐squared tests were performed. For patients‐ age at diagnosis means and standard deviations are given. Comparisons of the mean were conducted using a two‐sample t‐test.
Overall survival (OS), distant metastases‐free survival (DMFS) and local progression‐free survival (LPFS) were calculated from the start of RCT to the day of death. Kaplan–Meier curves are presented for relevant outcomes and groups. Log Rank tests were performed to compare distributions of event times between patients with low and high Hsp70 scores. In addition, univariate Cox regression models were used to determine hazard ratios (HRs) and 95% confidence intervals (CIs). To assess the additional prognostic value of Hsp70 to other prognostic factors,37 multivariable Cox regression models including Hsp70 and relevant prognostic factors were fit to the data for overall survival (OS), local progression‐free survival (LPFS) and distant metastases‐free survival (DMFS). Median follow‐up time was determined using the reverse Kaplan–Meier method for potential follow‐up.38 All statistical tests were performed two‐sided on a significance level of α = 5%. Analyses were performed with statistical software R version 3.3.239 and IBM SPSS Statistics for Windows, version 23 and 24 (IBM Corp, Armonk, NY).
Results
Hsp70 staining intensities in FFPE sections of SCCHN
An incubation of SCCHN tumor sections and the surrounding tissue with cmHsp70.1 monoclonal antibody (mAb) revealed significant differences in the staining intensity. The surrounding epithelial tissue in close proximity to the tumor (Fig. 1 b) always showed a very weak staining intensity (score 0), which is comparable to that of normal head and neck epithelial tissue (Fig. 1 a). Representative FFPE tumor sections of different patients stained with cmHsp70.1 mAb are shown in Figures 1 b–1 f. According to their Hsp70 expression, tumors were scored into groups with very weak to weak (scores 0–1; Figs. 1 b and 1 c), intermediate (score 2; Fig. 1 d), strong (score 3; Fig. 1 e) and very strong (score 4; Fig. 1 f) staining intensity. As an internal reference FFPE sections of xenograft FaDu tumors were co‐stained with each staining procedure (Fig. 1 g). The percentage of tumor sections with a specific staining scores (0–4) are summarized in Figure 1 h.
Figure 1.
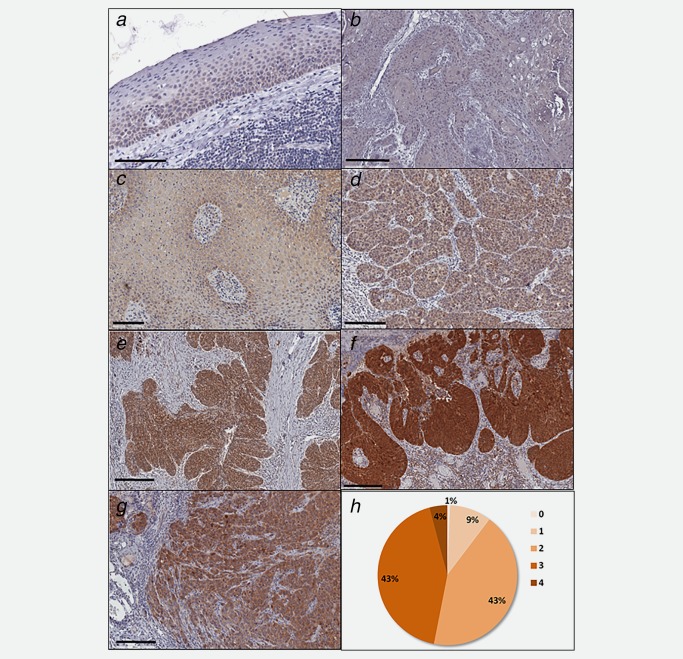
Representative views of the Hsp70 expression in normal epithelial tissue of the head and neck (a: score 0), and SCCHN tissue with very weak (b: score 0), weak (c: score 1), intermediate (d: score 2), strong (e: score 3) and very strong (f: score 4) staining intensity. As an internal control the staining intensity of a xenograft FaDu tumor is shown (g). The distribution of the different staining scores (0–4) in all 145 tumor sections is represented in (h).
Correlation of the Hsp70 staining intensity with OS, LPFS and DMFS
To determine the prognostic value of the Hsp70 expression in relation to OS different Hsp70 staining scores ranging from very weak (0) to very strong (4) have been compared. As summarized in Figure 2 a, increasing Hsp70 scores are associated with an increased mortality. Regarding a dichotomous variable, SCCHN with high Hsp70 staining intensities (scores 3–4, black line) exhibited a significantly lower overall survival (OS; HR = 2.26, p = 0.008; Fig. 2 b, Table 2), local progression‐free survival (LPFS; HR = 1.84, p = 0.034; Table 3) and distant metastases‐free (DMFS; HR = 1.76, p = 0.044; Fig. 2 c, Table 4) than patients with low Hsp70 tumor staining intensity (scores 0–2, gray line). Patients at risk at the different time‐points after start of therapy are shown below each graph. Multivariate analyses of Hsp70 staining intensities after adjustment for other relevant prognostic parameters with OS (Table 2), LPFS (Table 3) and DMFS (Table 4) revealed similar significant results.
Figure 2.
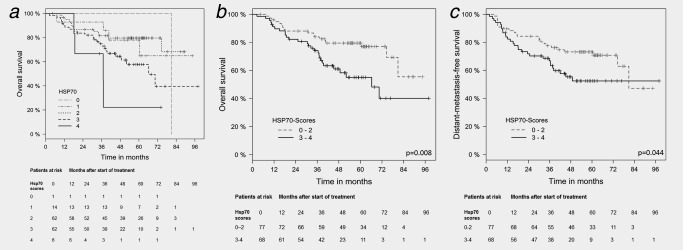
Kaplan–Meier analysis of the prognostic value of Hsp70 expression (scores 0–4) with overall survival (OS) and distant metastases‐free survival (DMFS). (a) Increasing Hsp70 expression (scores 0–4) in FFPE tumor sections of SCCHN patients (N = 145) is associated with sequentially decreased OS. Patients at risk with Hsp70 scores 0–4 at the different time‐points after start of therapy ranging from 0 to 96 months are indicated below. (b) Significant correlation of a low (scores 0–2; N = 77) vs. high (scores 3–4; N = 68) Hsp70 expression in FFPE tumor sections of SCCHN patients with OS. Patients at risk with Hsp70 scores 0–2 vs. 3–4 at different time‐points after start of therapy ranging from 0 to 96 months are indicated below the graph. (c) Significant correlation of a low (scores 0–2) vs. high (scores 3–4) Hsp70 expression in FFPE tumor sections of SCCHN patients with DMFS. Patients at risk with Hsp70 scores 0–2 vs. 3–4 at different time‐points after start of therapy ranging from 0 to 96 months are indicated below the graph.
Table 2.
Univariate and multivariate analysis of Hsp70 and other relevant variables as prognostic factors for overall survival (OS)
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | CIlow | CIupp | p‐Value | HR | CIlow | CIupp | p‐Value | |
| Hsp70 (scores 3–4 vs. 0–2) | 2.26 | 1.24 | 4.12 | 0.008* | 3.62 | 1.89 | 6.92 | 0.0001* |
| Age | 0.98 | 0.96 | 1.01 | 0.232 | ||||
| Gender (female vs. male) | 1.24 | 0.70 | 2.20 | 0.454 | ||||
| Tumor site | 0.001* | 0.072 | ||||||
| Oropharynx vs. oral cavity | 0.40 | 0.24 | 0.66 | 0.0004* | 0.66 | 0.33 | 1.33 | 0.244 |
| Hypopharynx vs. oral cavity | 0.33 | 0.15 | 0.73 | 0.006* | 0.32 | 0.12 | 0.86 | 0.023* |
| pT stage (3–4 vs. 1–2) | 2.20 | 1.37 | 3.52 | 0.001* | 2.00 | 1.09 | 3.65 | 0.024* |
| pN stage (2–3 vs. 0–1) | 1.09 | 0.63 | 1.89 | 0.747 | ||||
| Grading (3 vs. 1–2) | 0.79 | 0.49 | 1.28 | 0.341 | ||||
| Resection margin (ECE) | 1.72 | 1.06 | 2.80 | 0.030* | 2.53 | 1.28 | 4.99 | 0.008* |
| HPV16 DNA (pos vs. neg) | 0.33 | 0.17 | 0.62 | 0.0007* | 0.24 | 0.08 | 0.71 | 0.010* |
| p16 (pos vs. neg) | 0.39 | 0.22 | 0.70 | 0.002* | 0.51 | 0.20 | 1.29 | 0.155 |
| p53 (pos vs. neg) | 1.67 | 1.04 | 2.67 | 0.033* | 0.69 | 0.36 | 1.33 | 0.265 |
| Smoking history (yes vs. no) | 0.80 | 0.54 | 1.17 | 0.250 | ||||
Abbreviations: HR, hazard ratio; CIlow/upp, lower and upper 95% confidence interval.
Bold values marked with * present p‐values <0.05 and are considered as statistically significant.
Table 3.
Univariate and multivariate analysis of Hsp70 and other relevant variables as prognostic factors for local progression‐free survival (LPFS)
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | CIlow | CIupp | p‐Value | HR | CIlow | CIupp | p‐Value | |
| Hsp70 (scores 3–4 vs. 0–2) | 1.84 | 1.05 | 3.22 | 0.034* | 2.63 | 1.45 | 4.78 | 0.002* |
| Age | 0.98 | 0.96 | 1.01 | 0.180 | ||||
| Gender (female vs. male) | 1.54 | 0.90 | 2.61 | 0.113 | ||||
| Tumor site | <0.001* | 0.088 | ||||||
| Oropharynx vs. oral cavity | 0.39 | 0.24 | 0.64 | 0.0002* | 0.63 | 0.32 | 1.22 | 0.172 |
| Hypopharynx vs. oral cavity | 0.35 | 0.17 | 0.75 | 0.006* | 0.39 | 0.16 | 0.97 | 0.042* |
| pT stage (3–4 vs. 1–2) | 2.23 | 1.42 | 3.52 | 0.0005* | 1.94 | 1.09 | 3.44 | 0.022* |
| pN stage (2–3 vs. 0–1) | 1.10 | 0.65 | 1.87 | 0.730 | ||||
| Grading (3 vs. 1–2) | 0.75 | 0.47 | 1.21 | 0.243 | ||||
| Resection margin (ECE) | 1.60 | 1.00 | 2.54 | 0.050 | 1.97 | 1.05 | 3.70 | 0.035* |
| HPV16 DNA (pos vs. neg) | 0.33 | 0.18 | 0.61 | 0.0004* | 0.32 | 0.12 | 0.88 | 0.028* |
| p16 (pos vs. neg) | 0.38 | 0.22 | 0.67 | 0.0008* | 0.53 | 0.22 | 1.28 | 0.158 |
| p53 (pos vs. neg) | 1.86 | 1.18 | 2.92 | 0.007* | 0.90 | 0.48 | 1.68 | 0.774 |
| Smoking history (yes vs. no) | 0.84 | 0.58 | 1.22 | 0.368 | ||||
Abbreviations: HR, hazard ratio; CIlow/upp, lower and upper 95% confidence interval.
Bold values marked with * present p‐values <0.05 and are considered as statistically significant.
Table 4.
Univariate and multivariate analysis of Hsp70 and other relevant variables as prognostic factors for distant metastases‐free survival (DMFS)
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | CIlow | CIupp | p‐Value | HR | CIlow | CIupp | p‐Value | |
| Hsp70 (scores 3–4 vs. 0–2) | 1.76 | 1.01 | 3.07 | 0.044* | 2.42 | 1.34 | 4.37 | 0.003* |
| Age | 0.99 | 0.97 | 1.02 | 0.443 | ||||
| Gender (female vs. male) | 1.06 | 0.60 | 1.86 | 0.842 | ||||
| Tumor site | 0.001* | 0.629 | ||||||
| Oropharynx vs. oral cavity | 0.40 | 0.25 | 0.65 | 0.0002* | 0.73 | 0.37 | 1.42 | 0.350 |
| Hypopharynx vs. oral cavity | 0.55 | 0.28 | 1.08 | 0.081 | 0.88 | 0.39 | 2.01 | 0.763 |
| pT stage (3–4 vs. 1–2) | 2.19 | 1.39 | 3.42 | 0.0006* | 1.90 | 1.09 | 3.31 | 0.024* |
| pN stage (2–3 vs. 0–1) | 1.26 | 0.73 | 2.14 | 0.425 | ||||
| Grading (3 vs. 1–2) | 0.87 | 0.55 | 1.38 | 0.563 | ||||
| Resection margin (ECE) | 1.89 | 1.18 | 3.01 | 0.008* | 2.51 | 1.33 | 4.75 | 0.005* |
| HPV16 DNA (pos vs. neg) | 0.35 | 0.19 | 0.63 | 0.0005* | 0.35 | 0.13 | 0.97 | 0.044* |
| p16 (pos vs. neg) | 0.36 | 0.20 | 0.63 | 0.0002* | 0.44 | 0.18 | 1.10 | 0.080 |
| p53 (pos vs. neg) | 1.78 | 1.14 | 2.78 | 0.012* | 0.86 | 0.46 | 1.60 | 0.631 |
| Smoking history (yes vs. no) | 0.81 | 0.56 | 1.17 | 0.261 | ||||
Abbreviations: HR, hazard ratio; CIlow/upp, lower and upper 95% confidence interval.
Bold values marked with * present p‐values <0.05 and are considered as statistically significant.
In a subgroup analysis, the staining intensity of Hsp70 within the tumor was also assessed in combination with the p16, p53 and HPV16 status. Patients with p16‐negative (N = 90; p = 0.001) and p53‐negative (N = 59; p = 0.003) tumors presented a significantly better clinical outcome with respect to OS when the Hsp70 tumor staining intensity was low (scores 0–2, gray line) compared to those with a high (scores 3–4, black line) Hsp70 tumor staining intensity (Figs. 3 a and 3 b). With respect to HPV16, virus DNA‐free patients with a low Hsp70 staining intensity (scores 0–2, gray line) also showed a significantly improved overall survival (N = 92; p = 0.001) compared to those patients with a high tumor staining intensity (scores 3–4, black line; Fig. 3 c).
Figure 3.

Kaplan–Meier analysis of the prognostic value of Hsp70 expression (scores 0–2 vs. 3–4) and overall survival (OS) in subgroups of patients with p16‐, p53‐ and HPV16 DNA‐negative tumors. (a) Significant correlation of a low (scores 0–2) vs. high (scores 3–4) Hsp70 expression in FFPE tumor sections of SCCHN patients with p16‐negative tumors (N = 90) and OS. Patients at risk with Hsp70 scores 0–2 vs. 3–4 and a p16‐negative status at different time‐points after start of therapy ranging from 0 to 96 months are indicated below the graph. (b) Significant correlation of a low (scores 0–2) vs. high (scores 3–4) Hsp70 expression in FFPE tumor sections of SCCHN patients with p53‐negative tumors (N = 86) and OS. Patients at risk with Hsp70 scores 0–2 vs. 3–4 and a p53‐negative status at different time‐points after start of therapy ranging from 0 to 84 months are indicated below the graph. (c) Significant correlation of a low (scores 0–2) vs. high (scores 3–4) Hsp70 expression in FFPE tumor sections of SCCHN patients with HPV16 DNA‐negative tumors (N = 92) and OS. Patients at risk with Hsp70 scores 0–2 vs. 3–4 and a HPV16 DNA‐negative status at different time‐points after start of therapy ranging from 0 to 96 months are indicated below the graph.
In summary, a strong Hsp70 staining intensity of the tumor could be identified as an independent negative prognostic factor for OS, freedom of metastases and locoregional control in patients with SCCHN. The negative prognostic value of a strong Hsp70 expression was also observed in patient cohorts with a p16‐, p53‐, HPV16 DNA‐negative tumors that are known to have an adverse prognosis.
Infiltration of CD56+ NK cells in FFPE sections of SCCHN
Since membrane Hsp70 serves as a target for activated NK cells,17 showing a high expression density of the NK marker CD56,40 a CD56‐specific antibody was used to determine the amount of tumor‐infiltrating NK cells. A representative view of tumors with high (upper graph) and low (lower graph) NK cell infiltration is shown in Figure 4 a. White arrows mark the localization of either singular or multiple CD56+ NK cell infiltrations within the tumor and its microenvironment. The data shown in Figure 4 b and Table 5 clearly indicate that low numbers of CD56+ tumor‐infiltrating NK cells have a negative prognostic value for a significantly decreased OS in patients with SCCHN (HR = 0.29, p = 0.0001). Comparable results were obtained for LPFS (HR = 0.35, p = 0.0009) as shown in Table 6. As illustrated in Figure 4 c and Table 7, distant metastases develop significantly earlier in patients with a low tumor‐infiltration of CD56+ NK cells (HR = 0.31, p = 0.0001).
Figure 4.
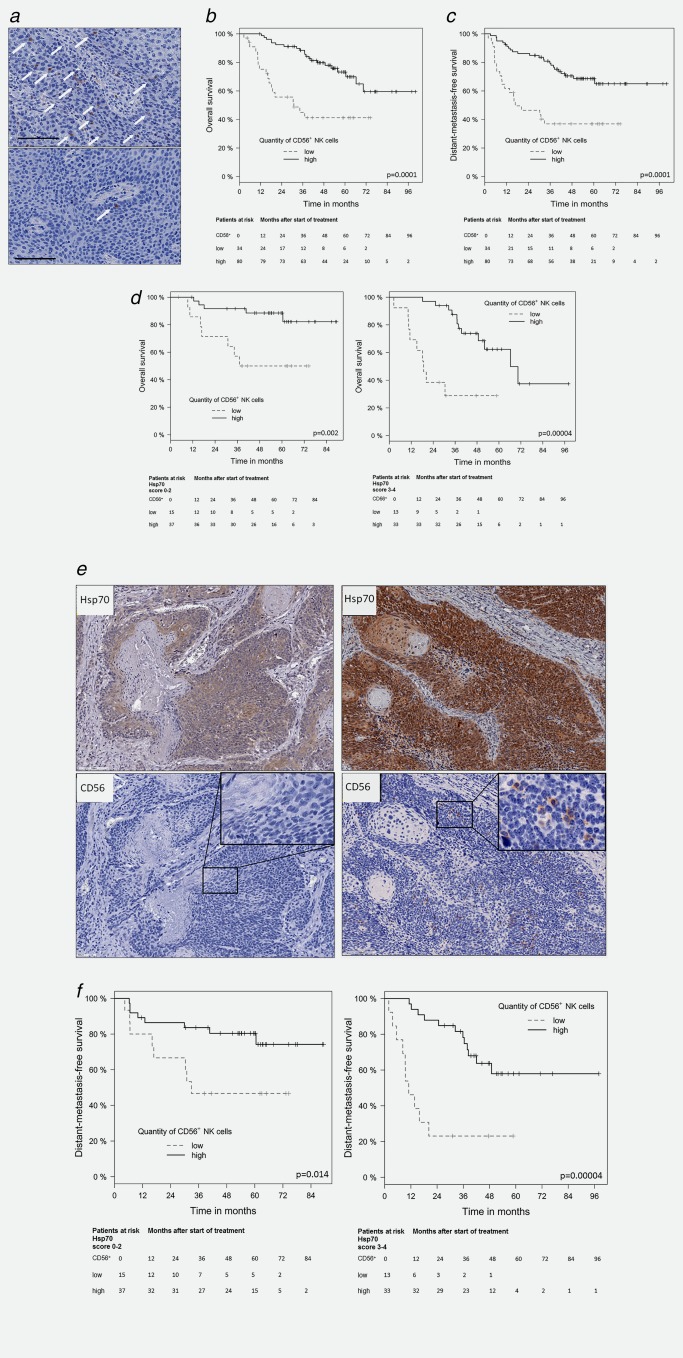
Kaplan–Meier analysis of the prognostic value of infiltrating CD56+ NK cells in FFPE tumor sections of SCCHN patients with overall survival (OS) and distant metastases‐free survival (DMFS). (a) Representative view of SCCHN sections with high (upper graph) and low numbers (lower graph) of infiltrating CD56+ NK cells. Selected singular as well as groups of CD56+ NK cells are marked with white arrows, scale bar, 100 µm. (b) Significant correlation of infiltrating CD56+ NK cells in tumor sections of SCCHN patients (N = 114) and OS. Black line represents high and gray lines represent low numbers of infiltrating CD56+ NK cells. Patients at risk with low and high numbers of infiltrating CD56+ NK cells at different time‐points after start of therapy ranging from 0 to 96 months are indicated below the graph. (c) Significant correlation of infiltrating CD56+ NK cells in tumor sections of SCCHN patients (N = 114) and DMFS. Black line represents high and gray lines represent low numbers of infiltrating CD56+ NK cells. Patients at risk with low and high numbers of infiltrating CD56+ NK cells at different time‐points after start of therapy ranging from 0 to 96 months are indicated below the graph. (d) Significant correlation of infiltrating CD56+ NK cells in tumor sections with low (left graph; scores 0–2) and high Hsp70 expression (right graph, scores of 3–4) and OS. Patients at risk with low and high numbers of infiltrating CD56+ NK cells and an Hsp70 scores of 0–2 vs. 3–4 at different time‐points after start of therapy ranging from 0 to 84 and 0 to 96 months are indicated below the graph are indicated below each graph. (e) Representative views of serial FFPE sections of tumors with an Hsp70 score of 2 (left graph) and a low number of infiltrating NK cells and an Hsp70 score of 4 (right graph) and a high number of infiltrating NK cells. The insets show a 400× magnification. (f) Significant correlation of tumor‐infiltrating CD56+ NK cells in tumor sections with low (left graph; scores of 0–2) vs. high Hsp70 expression (right graph; scores of 3–4) and DMFS. Patients at risk with low and high numbers of infiltrating CD56+ NK cells and an Hsp70 scores of 0–2 vs. 3–4 at different time‐points after start of therapy ranging from 0 to 84 and 0 to 96 months are indicated below the graph are indicated below each graph.
Table 5.
Univariate and multivariate analysis of tumor‐infiltrating CD56+ NK cells and other relevant variables as prognostic factors for overall survival (OS)
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | CIlow | CIupp | p‐Value | HR | CIlow | CIupp | p‐Value | |
| CD56 (high vs. low) | 0.29 | 0.15 | 0.55 | 0.0001* | 0.27 | 0.12 | 0.60 | 0.001* |
| Tumor site | ||||||||
| Oropharynx vs. oral cavity | 0.86 | 0.35 | 2.07 | 0.732 | ||||
| Hypopharynx vs. oral cavity | 0.56 | 0.15 | 2.05 | 0.381 | ||||
| pT stage (3–4 vs. 1–2) | 2.33 | 1.12 | 4.79 | 0.022* | ||||
| Resection margin (ECE) | 2.28 | 0.92 | 5.65 | 0.075 | ||||
| HPV16 DNA (pos vs. neg) | 0.29 | 0.07 | 1.10 | 0.070 | ||||
| p16 (pos vs. neg) | 0.60 | 0.20 | 1.85 | 0.377 | ||||
| p53 (pos vs. neg) | 1.06 | 0.46 | 2.48 | 0.885 | ||||
Abbreviations: HR, hazard ratio; CIlow/upp, lower and upper 95% confidence interval.
Bold values marked with * present p‐values <0.05 and are considered as statistically significant.
Table 6.
Univariate and multivariate analysis of tumor‐infiltrating CD56+ NK cells and other relevant variables as prognostic factors for local progression‐free survival (LPFS)
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | CIlow | CIupp | p‐Value | HR | CIlow | CIupp | p‐Value | |
| CD56 (high vs. low) | 0.35 | 0.19 | 0.65 | 0.0009* | 0.35 | 0.17 | 0.74 | 0.005* |
| Tumor site | ||||||||
| Oropharynx vs. oral cavity | 0.77 | 0.32 | 1.79 | 0.547 | ||||
| Hypopharynx vs. oral cavity | 0.62 | 0.19 | 2.02 | 0.430 | ||||
| pT stage (3–4 vs. 1–2) | 2.26 | 1.13 | 4.53 | 0.021* | ||||
| Resection margin (ECE) | 1.63 | 0.72 | 3.74 | 0.241 | ||||
| HPV16 DNA (pos vs. neg) | 0.38 | 0.11 | 1.33 | 0.130 | ||||
| p16 (pos vs. neg) | 0.68 | 0.24 | 1.95 | 0.477 | ||||
| p53 (pos vs. neg) | 1.39 | 0.61 | 3.15 | 0.437 | ||||
Abbreviations: HR, hazard ratio; CIlow/upp, lower and upper 95% confidence interval.
Bold values marked with * present p‐values <0.05 and are considered as statistically significant.
Table 7.
Univariate and multivariate analysis of tumor‐infiltrating CD56+ NK cells and other relevant variables as prognostic factors for distant metastases‐free survival (DMFS)
| Univariate | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | CIlow | CIupp | p‐Value | HR | CIlow | CIupp | p‐Value | |
| CD56 (high vs. low) | 0.31 | 0.17 | 0.57 | 0.0001 | 0.27 | 0.13 | 0.55 | 0.0004* |
| Tumor site | ||||||||
| Oropharynx vs. oral cavity | 0.75 | 0.33 | 1.70 | 0.489 | ||||
| Hypopharynx vs. oral cavity | 2.43 | 0.76 | 7.74 | 0.135 | ||||
| pT stage (3–4 vs. 1–2) | 2.06 | 1.05 | 4.06 | 0.036* | ||||
| Resection margin (ECE) | 2.35 | 1.02 | 5.42 | 0.044* | ||||
| HPV16 DNA (pos vs. neg) | 0.57 | 0.17 | 1.95 | 0.370 | ||||
| p16 (pos vs. neg) | 0.49 | 0.17 | 1.40 | 0.184 | ||||
| p53 (pos vs. neg) | 1.66 | 0.73 | 3.75 | 0.227 | ||||
Abbreviations: HR, hazard ratio; CIlow/upp, lower and upper 95% confidence interval.
Bold values marked with * present p‐values <0.05 and are considered as statistically significant.
Similar results are found in subgroup analysis of tumors with low (scores 0–2) and high Hsp70 expression (scores 3–4). As shown in Figure 4 d, low numbers of CD56+ tumor‐infiltrating NK cells in tumor sections with low (left graph; scores 0–2, p = 0.002) and high (right graph; scores 3–4, p = 0.00004) Hsp70 expression correlate with significantly decreased OS. Representative views of serial sections of tumors with low (score 2, upper left graph) and high (score 4, upper right graph) Hsp70 expression and a low (lower left graph) and high (lower right graph) NK cell infiltration are illustrated in Figure 4 e.
Low numbers of tumor‐infiltrating CD56+ NK cells also correlate with a significantly shorter DMFS, as shown in Figure 4 f. The combination of a high Hsp70 expression (scores 3–4) and a low NK cell infiltration shows better negative prognostic value (p = 0.0004; Fig. 4 f, right graph) than a low Hsp70 expression (scores 0–2) and a low NK cell infiltration (p = 0.014; Fig. 4 f, left graph) although both values were statistical significant.
Univariate and multivariate analysis of tumor‐infiltrating CD56+ NK cells in combination with OS (Table 5), LPFS (Table 6) and DMFS (Table 7) revealed that both markers Hsp70 and NK cells have prognostic value.
Analysis of the prognostic value of the CD56/Hsp70 marker combination in patients with differential HPV16 status revealed that a high NK cell infiltration positively correlated with an improved OS in HPV16 DNA‐negative (N = 92, p = 0.004; Fig. 5 a), but not in HPV16 DNA‐positive (N = 53; Fig. 5 b) patient cohort, irrespective of the Hsp70 status. The lack of statistical significance in the HPV16 DNA‐positive patient cohort might be explained by the low number of patients per subgroup.
Figure 5.
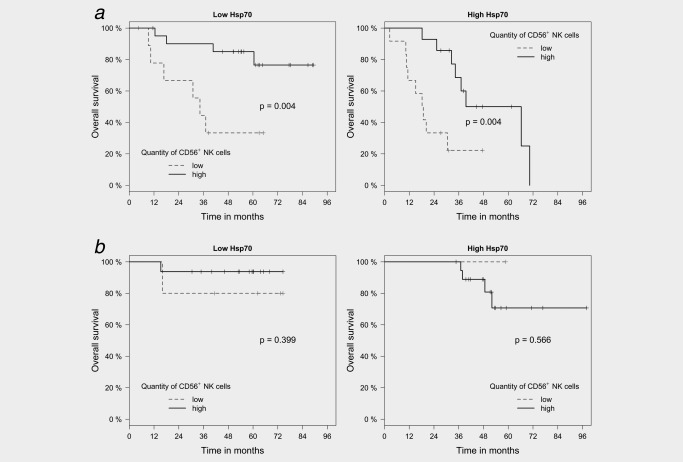
Kaplan–Meier analysis of the prognostic value of the CD56+ NK cell/Hsp70 marker combination in FFPE tumor sections of HPV16 DNA‐negative (N = 92) and ‐positive (N = 53) SCCHN patients with overall survival (OS). (a) Significant correlation of infiltrating CD56+ NK cells in tumor sections of HPV16 DNA‐negative SCCHN patients (N = 92) and OS in sections with a low (scores 0–2, p = 0.004) and high (scores 3–4, p = 0.004) Hsp70 expression. Black line represents high and gray lines represent low numbers of infiltrating CD56+ NK cells. (b) No significant correlation of infiltrating CD56+ NK cells in tumor sections of HPV16 DNA‐positive SCCHN patients (N = 53) and OS in sections with a low (scores 0–2) and high (scores 3–4) Hsp70 expression. Black line represents high and gray lines represent low numbers of infiltrating CD56+ NK cells.
Discussion
The search for tumor‐specific biomarkers that are frequently overexpressed in malignantly transformed cells resulted in the identification of the highly conserved, major stress‐inducible Hsp70.41 Apart from elevated cytosolic expression levels and in contrast to normal cells, tumor cells frequently present Hsp70 on their cell surface.11, 12, 42 It is well accepted that high cytosolic43 and membrane14 expression levels of Hsp70 are associated with resistance to therapy and thus might increase tumorigenesis. Previous work has indicated that Hsp70 is also expressed on the membrane of SCCHN.44 In our study, we addressed the question of the role of cytosolic Hsp70 as a prognostic tumor marker in patients with SCCHN after surgery and postoperative radiotherapy.
To directly compare the Hsp70 staining intensity in different tumors, FFPE sections with identical thickness (4 µm) have to be stained using the exact same staining protocol together with an internal reference. A comparison of the Hsp70 expression in all SCCHN sections following this procedure revealed major differences in the Hsp70 staining intensity ranging from very weak to very strong. Our data suggest that an accumulation of Hsp70 within the tumor tissue positively correlates with a decreased OS, PFS and DMFS after surgery and RCT. Therefore, a high Hsp70 expression serves as a negative prognostic marker for patients with SCCHN. This negative prognostic value of Hsp70 is in line with reports demonstrating anti‐apoptotic and pro‐tumorigenic activities for Hsp70.43, 45
Every nucleated cell type expresses Hsp70 at low levels in the cytosol, but nearly all tumor types show a significantly enhanced Hsp70 expression. Therefore, normal tissues as well as the tumor microenvironment cannot be expected to be completely negative for Hsp70.
A comparison of membrane‐bound and cytosolic Hsp70 levels in tumor cells revealed that approximately 70–90% of the total Hsp70 is residing in the cytosol.41 Together with the finding that cmHsp70.1 mAb42 detects both, membrane‐bound and cytosolic Hsp70, it is not possible to distinguish both localizations in FFPE sections.
A number of studies indicated that a HPV16 DNA‐positive status is associated with favorable outcome,46 although HPV16 has been determined as a co‐founding factor that can initiate SCCHN. This finding might be related to the immunostimulatory activity of the virus to induce anti‐tumor‐specific immune responses. However, a positive correlation of HPV16 has also been found between CTLA‐4 expression, Treg infiltration and HPV16 DNA positivity, which might exert immunosuppressive effects.47 Therefore, in combination with RCT blocking of immune checkpoint inhibitors might be beneficial to overcome immunosuppression in SCCHN. Apart from an involvement of the immune system additional factors such as smaller tumor size, lower amount of CD44+/CD98+ tumor stem cells,48 higher sensitivity to RCT,26 less tumor hypoxia, (E6)‐induced inhibition of p53,49, 50 younger patient age and less toxin (tobacco, alcohol) intake are also involved in a favorable outcome.26, 36
We also could demonstrate that in patients with HPV16 DNA‐free tumors the Hsp70 staining intensity still could separate two subgroups with respect to outcome. Patients with HPV16‐negative tumors and low Hsp70 staining intensity (scores 0–2) showed a significantly improved OS compared to those with a high Hsp70 staining intensity (scores 3–4).
With respect to the p16 and p53 status,30, 31, 32 patients with p16‐ (N = 90) and p53‐negative SCCHN tumors presented a significantly worse clinical outcome when the Hsp70 tumor staining intensity was high. Therefore, we assume that Hsp70 in addition to p16 and p53 might provide additional prognostic value.
Depending on the subcellular, membrane‐bound and extracellular localization Hsp70 mediates different functions. However, it is worth mentioning that the membrane status of Hsp70 cannot be determined by IHC, since most tumor cells show a very strong Hsp70 cytosolic staining41 that does not allow to detect the faint membrane staining pattern in FFPE sections. Despite these limitations, we addressed the question whether a strong cytosolic Hsp70 expression might affect infiltration of CD56+ NK cells into the tumor tissue and whether a strong infiltration of NK cells might also be associated with favorable clinical outcome, as previously shown for CD8+ cytotoxic lymphocytes.25
A correlation of OS with high numbers of infiltrating NK cells supports the hypothesis that apart from the adaptive also the innate immune system plays an important role in the outcome of surgery and RCT in SCCHN.44 Indeed, patients with high numbers of tumor‐infiltrating CD56+ NK cells show superior OS.36 However, overall life expectancy is significantly lower in the group of patients bearing tumors with high cytosolic Hsp70 levels. This might be explained by high intracellular Hsp70 levels that impair NK cell and RCT‐mediated tumor cell apoptosis by inhibiting lysosomal permeabilization.45 The anti‐apoptotic effect of intracellular Hsp70 might overrule beneficial effects mediated by tumor‐infiltrating NK cells.
A high number of tumor‐infiltrating NK cells results not only in an improved OS but also in DMFS. This finding might be due to the fact that the Hsp70 membrane density is often stronger on metastases compared to primary tumors,51 and thus might serve as a target for the cytolytic attack by NK cells. In contrast to intracellular Hsp70, membrane‐bound and extracellular residing Hsp70 have been found to stimulate anti‐tumor immunity.52 Since normal cells do not present Hsp70 on their cell surface, membrane‐bound Hsp70 can serve as a tumor‐selective target for Hsp70 peptide‐activated NK cells.16, 17 Extracellular Hsp70 released by viable tumor cells53 that chaperones tumor‐derived peptides might be able to stimulate the cytolytic activity of CD8+ T lymphocytes. A previous study of our group revealed that a high membrane expression of Hsp70 on primary tumor cells correlated with high soluble Hsp70 levels in patients with SSCHN.54 These data are in line with the finding that high cytosolic Hsp70 levels are associated with a high membrane Hsp70 expression and high soluble Hsp70 levels in primary glioblastoma.55 In an ongoing prospective study extracellular, membrane‐bound and cytosolic Hsp70 levels will be assessed in SCCHN patients by using the lipHsp70 ELISA,53 flow cytometry and IHC together with the immunophenotype of peripheral blood lymphocytes. Our study will allow a better understanding of the role of Hsp70 as a diagnostic marker and its interaction with the immune system.
References
- 1. Kampinga HH, Craig EA. The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat Rev Mol Cell Biol 2010;11:579–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hartl FU, Bracher A, Hayer‐Hartl M. Molecular chaperones in protein folding and proteostasis. Nature 2011;475:324–32. [DOI] [PubMed] [Google Scholar]
- 3. Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci 2005;62:670–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beere HM, Wolf BB, Cain K, et al. Heat‐shock protein 70 inhibits apoptosis by preventing recruitment of procaspase‐9 to the Apaf‐1 apoptosome. Nat Cell Biol 2000;2:469–75. [DOI] [PubMed] [Google Scholar]
- 5. Gabai VL, Sherman MY. Invited review: interplay between molecular chaperones and signaling pathways in survival of heat shock. J Appl Physiol 2002;92:1743–8. [DOI] [PubMed] [Google Scholar]
- 6. Uozaki H, Ishida T, Kakiuchi C, et al. Expression of heat shock proteins in osteosarcoma and its relationship to prognosis. Pathol Res Pract 2000;196:665–73. [DOI] [PubMed] [Google Scholar]
- 7. Asling J, Morrison J, Mutsaers AJ. Targeting HSP70 and GRP78 in canine osteosarcoma cells in combination with doxorubicin chemotherapy. Cell Stress Chaper 2016;21:1065–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gunther S, Ostheimer C, Stangl S, et al. Correlation of Hsp70 serum levels with gross tumor volume and composition of lymphocyte subpopulations in patients with squamous cell and adeno non‐small cell lung cancer. Front Immunol 2015;6:556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pfister K, Radons J, Busch R, et al. Patient survival by Hsp70 membrane phenotype: association with different routes of metastasis. Cancer 2007;110:926–35. [DOI] [PubMed] [Google Scholar]
- 10. Steiner K, Graf M, Hecht K, et al. High HSP70‐membrane expression on leukemic cells from patients with acute myeloid leukemia is associated with a worse prognosis. Leukemia 2006;20:2076–9. [DOI] [PubMed] [Google Scholar]
- 11. Multhoff G, Botzler C, Jennen L, et al. Heat shock protein 72 on tumor cells: a recognition structure for natural killer cells. J Immunol 1997;158:4341–50. [PubMed] [Google Scholar]
- 12. Multhoff G, Botzler C, Wiesnet M, et al. A stress‐inducible 72‐kDa heat‐shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer 1995;61:272–9. [DOI] [PubMed] [Google Scholar]
- 13. Gehrmann M, Liebisch G, Schmitz G, et al. Tumor‐specific Hsp70 plasma membrane localization is enabled by the glycosphingolipid Gb3. PLoS One 2008;3:e1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Murakami N, Kühnel A, Schmid TE, et al. Role of membrane Hsp70 in radiation sensitivity of tumor cells. Radiat Oncol 2015;10:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Multhoff G, Mizzen L, Winchester CC, et al. Heat shock protein 70 (Hsp70) stimulates proliferation and cytolytic activity of natural killer cells. Exp Hematol 1999;27:1627–36. [DOI] [PubMed] [Google Scholar]
- 16. Multhoff G, Pfister K, Botzler C, et al. Adoptive transfer of human natural killer cells in mice with severe combined immunodeficiency inhibits growth of Hsp70‐expressing tumors. Int J Cancer 2000;88:791–7. [DOI] [PubMed] [Google Scholar]
- 17. Multhoff G, Pfister K, Gehrmann M, et al. A 14‐mer Hsp70 peptide stimulates natural killer (NK) cell activity. Cell Stress Chaper 2001;6:337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Romagnani C, Juelke K, Falco M, et al. CD56bright/CD16‐ killer Ig‐like receptor‐NK cells display longer telomeres and acquire features of CD56dim NK cells upon activation. J Immunol 2007;178:4947–55. [DOI] [PubMed] [Google Scholar]
- 19. Krause SW, Gastpar R, Andreesen R, et al. Treatment of colon and lung cancer patients with ex vivo heat shock protein 70‐peptide‐activated, autologous natural killer cells: a clinical phase I trial. Clin Cancer Res 2004;10:3699–707. [DOI] [PubMed] [Google Scholar]
- 20. Specht HM, Ahrens N, Blankenstein C, et al. Heat shock protein 70 (Hsp70) peptide activated natural killer (NK) cells for the treatment of patients with non‐small cell lung cancer (NSCLC) after radiochemotherapy (RCTx) – from preclinical studies to a clinical phase II trial. Front Immunol 2015;6:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Balermpas P, Rödel F, Krause M, et al. The PD‐1/PD‐L1 axis and human papilloma virus in patients with head and neck cancer after adjuvant chemoradiotherapy: a multicentre study of the German Cancer Consortium Radiation Oncology Group (DKTK‐ROG). Int J Cancer 2017;141:594–603. [DOI] [PubMed] [Google Scholar]
- 22. Linge A, Lohaus F, Lock S, et al. HPV status, cancer stem cell marker expression, hypoxia gene signatures and tumour volume identify good prognosis subgroups in patients with HNSCC after primary radiochemotherapy: a multicentre retrospective study of the German Cancer Consortium Radiation Oncology Group (DKTK‐ROG). Radiother Oncol 2016;121:364–73. [DOI] [PubMed] [Google Scholar]
- 23. Tinhofer I, Stenzinger A, Eder T, et al. Targeted next‐generation sequencing identifies molecular subgroups in squamous cell carcinoma of the head and neck with distinct outcome after concurrent chemoradiation. Ann Oncol 2016;27:2262–8. [DOI] [PubMed] [Google Scholar]
- 24. Linge A, Lock S, Gudziol V, et al. Low cancer stem cell marker expression and low hypoxia identify good prognosis subgroups in HPV(−) HNSCC after postoperative radiochemotherapy: a multicentre study of the DKTK‐ROG. Clinical Can Res 2016;22:2639–49. [DOI] [PubMed] [Google Scholar]
- 25. Balermpas P, Rodel F, Rodel C, et al. CD8+ tumour‐infiltrating lymphocytes in relation to HPV status and clinical outcome in patients with head and neck cancer after postoperative chemoradiotherapy: a multicentre study of the German cancer consortium radiation oncology group (DKTK‐ROG). Int J Cancer 2016;138:171–81. [DOI] [PubMed] [Google Scholar]
- 26. Lohaus F, Linge A, Tinhofer I, et al. HPV16 DNA status is a strong prognosticator of loco‐regional control after postoperative radiochemotherapy of locally advanced oropharyngeal carcinoma: results from a multicentre explorative study of the German Cancer Consortium Radiation Oncology Group (DKTK‐ROG). Radiother Oncol 2014;113:317–23. [DOI] [PubMed] [Google Scholar]
- 27. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–E86. [DOI] [PubMed] [Google Scholar]
- 28. Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. JCO 2011;29:4294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stein AP, Saha S, Yu M, et al. Prevalence of human papillomavirus in oropharyngeal squamous cell carcinoma in the United States across time. Chem Res Toxicol 2014;27:462–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lai S, El‐Naggar AK. Differential expression of key cell cycle genes (p16/cyclin D1/pRb) in head and neck squamous carcinomas. Lab Invest 1999;79:255–60. [PubMed] [Google Scholar]
- 31. Califano J, Ahrendt SA, Meininger G, et al. Detection of telomerase activity in oral rinses from head and neck squamous cell carcinoma patients. Cancer Res 1996;56:5720–2. [PubMed] [Google Scholar]
- 32. Geisler SA, Olshan AF, Weissler MC, et al. p16 and p53 Protein expression as prognostic indicators of survival and disease recurrence from head and neck cancer. Clin Can Res 2002;8:3445–53. [PubMed] [Google Scholar]
- 33. Boyle JO, Hakim J, Koch W, et al. The incidence of p53 mutations increases with progression of head and neck cancer. Cancer Res 1993;53:4477–80. [PubMed] [Google Scholar]
- 34. Koch WM, Brennan JA, Zahurak M, et al. p53 mutation and locoregional treatment failure in head and neck squamous cell carcinoma. J Natl Cancer Inst 1996;88:1580–6. [DOI] [PubMed] [Google Scholar]
- 35. Wittekindt C, Wagner S, Mayer CS, et al. Basics of tumor development and importance of human papilloma virus (HPV) for head and neck cancer. GMS Curr Top Otorhinolaryngol Head Neck Surg 2012;11:Doc09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wagner S, Wittekindt C, Reuschenbach M, et al. CD56‐positive lymphocyte infiltration in relation to human papillomavirus association and prognostic significance in oropharyngeal squamous cell carcinoma. Int J Cancer 2016;138:2263–73. [DOI] [PubMed] [Google Scholar]
- 37. Schemper M, Smith TL. A note on quantifying follow‐up in studies of failure time. Control Clin Trials 1996;17:343–6. [DOI] [PubMed] [Google Scholar]
- 38. McShane LM, Altman DG, Sauerbrei W, et al. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer 2005;93:387–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Team RC . R: a language and environment for statistical computing. Vienna, Austria: Foundation for Statistical Computing, URL https://wwwR-projectorg, 2015. [Google Scholar]
- 40. Gross C, Schmidt‐Wolf IG, Nagaraj S, et al. Heat shock protein 70‐reactivity is associated with increased cell surface density of CD94/CD56 on primary natural killer cells. Cell Stress Chaper 2003;8:348–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weidle UH, Maisel D, Klostermann S, et al. Intracellular proteins displayed on the surface of tumor cells as targets for therapeutic intervention with antibody‐related agents. Cancer Genomics Proteomics 2011;8:49–63. [PubMed] [Google Scholar]
- 42. Stangl S, Gehrmann M, Riegger J, et al. Targeting membrane heat‐shock protein 70 (Hsp70) on tumors by cmHsp70.1 antibody. Proc Natl Acad Sci USA 2011;108:733–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaper 2005;10:86–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kleinjung T, Arndt O, Feldmann HJ, et al. Hsp70 membrane expression on head and neck cancer biopsy – a target for NK cells. Int J Radiat Oncol Biol Phys 2003;57:820–6. [DOI] [PubMed] [Google Scholar]
- 45. Nylandsted J, Gyrd‐Hansen M, Danielewicz A, et al. Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J Exp Med 2004;200:425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mandal R, Şenbabaoğlu Y, Desrichard A, et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight 2016;1:e89829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rietbergen MM, Martens‐de Kemp SR, Bloemena E, et al. Cancer stem cell enrichment marker CD98: a prognostic factor for survival in patients with human papillomavirus‐positive oropharyngeal cancer. Eur J Cancer 2014;50:765–73. [DOI] [PubMed] [Google Scholar]
- 49. Zhang Y, Xiong Y, Yarbrough WG. ARF promotes MDM2 degradation and stabilizes p53: ARF‐INK4a locus deletion impairs both the Rb and p53 tumor suppression pathways. Cell 1998;92:725–34. [DOI] [PubMed] [Google Scholar]
- 50. Agrawal N, Frederick MJ, Pickering CR, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science 2011;333:1154–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Botzler C, Schmidt J, Luz A, et al. Differential Hsp70 plasma membrane expression on primary human tumors and metastases in mice with severe combined immunodeficiency. Int J Cancer 1998;77:942–8. [DOI] [PubMed] [Google Scholar]
- 52. Horvath I, Multhoff G, Sonnleitner A, et al. Membrane‐associated stress proteins: more than simply chaperones. Biochim Biophys Acta 2008;1778:1653–64. [DOI] [PubMed] [Google Scholar]
- 53. Breuninger S, Erl J, Knape C, et al. Quantitative analysis of liposomal heat shock protein 70 (Hsp70) in the blood of tumor patients using an novel LipHsp70 ELISA. Clin Cell Immunol 2014;5:2–10. [Google Scholar]
- 54. Gehrmann M, Specht HM, Bayer C, et al. Hsp70 – a biomarker for tumor detection and monitoring of outcome of radiation therapy in patients with squamous cell carcinoma of the head and neck. Radiat Oncol 2014;9:131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Thorsteinsdottir J, Stangl S, Fu P, et al. Overexpression of cytosolic, plasma membrane bound and extracellular Hsp70 in primary glioblastomas. J Neurooncol 2017;135:443–52. [DOI] [PubMed] [Google Scholar]


