Summary
The impacts of rising atmospheric CO2 concentrations on plant disease have received increasing attention, but with little consensus emerging on the direct mechanisms by which CO2 shapes plant immunity. Furthermore, the impact of sub‐ambient CO 2 concentrations, which plants have experienced repeatedly over the past 800 000 yr, has been largely overlooked.
A combination of gene expression analysis, phenotypic characterisation of mutants and mass spectrometry‐based metabolic profiling was used to determine development‐independent effects of sub‐ambient CO 2 (sa CO 2) and elevated CO 2 (eCO 2) on Arabidopsis immunity.
Resistance to the necrotrophic Plectosphaerella cucumerina (Pc) was repressed at sa CO 2 and enhanced at eCO 2. This CO 2‐dependent resistance was associated with priming of jasmonic acid (JA)‐dependent gene expression and required intact JA biosynthesis and signalling. Resistance to the biotrophic oomycete Hyaloperonospora arabidopsidis (Hpa) increased at both eCO 2 and sa CO 2. Although eCO 2 primed salicylic acid (SA)‐dependent gene expression, mutations affecting SA signalling only partially suppressed Hpa resistance at eCO 2, suggesting additional mechanisms are involved. Induced production of intracellular reactive oxygen species (ROS) at sa CO 2 corresponded to a loss of resistance in glycolate oxidase mutants and increased transcription of the peroxisomal catalase gene CAT2, unveiling a mechanism by which photorespiration‐derived ROS determined Hpa resistance at saCO2.
By separating indirect developmental impacts from direct immunological effects, we uncover distinct mechanisms by which CO 2 shapes plant immunity and discuss their evolutionary significance.
Keywords: Arabidopsis, CO2, defence signalling, glycolate oxidase, photorespiration, plant immunity, priming
Introduction
Past and future changes in atmospheric CO2 directly impact plant metabolism (Temme et al., 2015), with feedbacks on resistance to pests and diseases (Strengbom & Reich, 2006; Lake & Wade, 2009; Vaughan et al., 2014; Váry et al., 2015; Zhang et al., 2015; Mhamdi & Noctor, 2016). Although numerous effects of elevated CO2 (e CO2) on disease resistance have been reported, there is little consistency between studies. Some studies report increased disease susceptibility at e CO2 (Lake & Wade, 2009; Vaughan et al., 2014; Váry et al., 2015), while others report no, or stimulatory, effects of e CO2 on disease resistance (Strengbom & Reich, 2006; Riikonen et al., 2008; Pugliese et al., 2012; Zhang et al., 2015; Mhamdi & Noctor, 2016). These discrepancies may arise from differences in e CO2 concentrations, the duration of e CO2 exposure, the method of disease quantification, species‐specific adaptations to CO2 or a combination of all these factors. Furthermore, biotrophic and necrotrophic pathogens are rarely compared within the same study, providing limited information of how distinct components of the plant immune system respond to e CO2. To date, various mechanisms by which CO2 alters disease resistance have been proposed, ranging from changes in leaf nutrition (Strengbom & Reich, 2006), stomatal density (Lake & Wade, 2009) and pathogen‐specific adaptations to altered host metabolism (Váry et al., 2015). Recent evidence suggests a mechanism whereby e CO2 primes pathogen‐induced production of defence regulatory hormones, such as salicylic acid (SA) and jasmonic acid (JA) (Zhang et al., 2015; Mhamdi & Noctor, 2016), which control defences against biotrophic and necrotrophic pathogens, respectively (Thomma et al., 1998). Surprisingly, however, most studies do not take into account the stimulatory effects of CO2 on plant development (Temme et al., 2015), despite evidence that developmental stage can have a profound impact on SA‐dependent and ethylene‐dependent defences (Kus et al., 2002; Shibata et al., 2010).
Knowledge about the effects of sub‐ambient CO2 (saCO2) on plant immunity is limited and may give valuable insights into the evolution of plant defence metabolism at typically low CO2 (below 200 ppm) during glacial periods over the past 800 000 yr (Temme et al., 2015; Galbraith & Eggleston, 2017). While stomatal processes have been implicated in defence at saCO2 (Zhou et al., 2017), the contribution of saCO2 towards post‐invasive plant defence remains unknown. At saCO2, net photosynthetic rate decreases as a consequence of photorespiration, along with increased stomatal conductance, increased foliar nitrogen and lower water use efficiency (Temme et al., 2013; Li et al., 2014). Although it remains unclear whether these changes influence disease resistance, a recent transcriptome study at saCO2 revealed enhanced activity of peroxisomal processes that correlate with changes in expression of defence‐related genes (Li et al., 2014). For instance, peroxisomal metabolism is stimulated at saCO2 (Li et al., 2014), which can boost defence through changes in cellular redox homeostasis (Sørhagen et al., 2013). The photorespiratory machinery is a major source of intracellular hydrogen peroxide (H2O2), which plays an important signalling role in plant defence (Chaouch et al., 2010). This is further highlighted by the CATALASE‐deficient cat2 mutant, which is impaired in scavenging of peroxisomal H2O2 and expresses a constitutive defence phenotype (Chaouch et al., 2010). Therefore, it is plausible that saCO2 influences plant resistance, but the extent, specificity and regulatory mechanisms remain unknown.
In this study, we have examined the direct impacts of saCO2 (200 ppm), aCO2 (400 ppm) and e CO2 (1200 ppm) on plant immunity by eliminating confounding effects of CO2 on plant development. Using a plant development correction, we show that CO2 has differential impacts on resistance against the biotrophic oomycete Hyaloperonospora arabidopsidis (Hpa) and the necrotrophic fungus Plectosphaerella cucumerina (Pc). Subsequent molecular and biochemical characterization of CO2‐dependent resistance phenotypes uncovered differing mechanisms by which CO2 shapes the plant immune system. Apart from priming effects of e CO2 on hormone‐dependent defences, we provide evidence for a critical role of photorespiration in plant defence at saCO2 and discuss possible evolutionary implications.
Materials and Methods
Reagents and chemicals
All chemicals and reagents were purchased from Sigma‐Aldrich unless stated otherwise.
Plant cultivation and growth conditions
Arabidopsis thaliana (L.) Heynh. accession Col‐0 was used as wild‐type plant genotype throughout this study, along with Col‐0 mutant lines npr1‐1 (Cao et al., 1997), sid2‐1 (Wildermuth et al., 2001), jar1‐1 (Staswick, 2002), aos1‐1 (Przybyla et al., 2008), rbohD/F (Torres et al., 2002), gox1‐2 (SALK_051930; Alonso et al., 2003) and haox1‐2 (SALK_022285; Alonso et al., 2003). Plants were cultivated under short‐day conditions (8.5 h 20°C : 15.5 h 18°C, light : dark; 65% relative humidity). Seeds were stratified for 2 d in the dark at 4°C and planted in 60 ml pots, containing a sand : compost mixture (2 : 3). After 7 d of germination, seedlings were thinned to prevent crowding. Plants were cultivated in climate‐ and CO2‐controlled growth cabinets (SGC097.PPX.F; Sanyo Gallenkamp PLC, Leicester, UK) under ambient conditions (aCO2; 400 ppm, i.e. μl l−1), sub‐ambient CO2 (saCO2; 200 ppm) or elevated CO2 (e CO2; 1200 ppm). Growth chambers were supplemented with compressed CO2 (BOC, Guildford, UK) or scrubbed with Sofnolime 797 (AP diving, Helston, UK) to maintain constant CO2 at indicated concentrations.
Plant development correction
Using leaf numbers of 3‐ and 4.5‐wk‐old plants as a proxy of development stage at different CO2 regimes (Boyes et al., 2001), seed germination at saCO2 was started 7 d earlier than at aCO2, whereas seed germination at e CO2 was delayed by 3 d in comparison to aCO2. Development correction (DC) resulted in plants with equal numbers of leaves at all three CO2 concentrations at the day of pathogen inoculation (eight‐leaf stage for Hpa and 18‐leaf stage for Pc; Supporting Information Fig. S1). This experiment was repeated once with comparable results.
Pathogenicity assays
Due to its sensitivity to age‐related resistance (ARR), assays with Hpa (strain WACO9) were conducted with relatively young plants (3 wk old at aCO2, or eight‐leaf stage). Plants were inoculated with 5 × 104 conidiospores ml−1 and left at high humidity. Shoot tissues were collected at 6 or 7 d post‐inoculation (dpi) for trypan blue staining and microscopy analysis of Hpa colonisation, as described previously (Luna et al., 2012). Briefly, levels of Hpa colonisation were assigned to four distinct classes, as illustrated in Fig. S2: (I) no pathogen development; (II) presence of hyphal colonisation; (III) extensive colonisation and presence of conidiophores; and (IV) extensive colonisation and the presence of conidiophores and > 10 oospores. At least 50 leaves from > 15 plants per treatment were used to determine distributions of inoculated leaves across the four Hpa colonization classes. Differences in class distributions between genotype–treatment combinations were analysed for statistical significance, using Fisher's exact tests (R, v.3.1.2). To ensure necrotrophic infection, assays with Pc (strain BMM) were based on droplet inoculation (6 μl, 5 × 106 spores ml−1) on four to six fully expanded leaves of eight plants at the 18‐leaf stage (4.5 wk old at aCO2), as described previously (Pétriacq et al., 2016a). Disease progression was measured as lesion diameters at 13 dpi. Lesion diameters were averaged per plant and treated as one biological replicate. Differences in average lesion diameter per plant between treatments (n = 8) were analysed for statistical significance by ANOVA (R, v.3.1.2). Pathogenicity assays with the jar1‐1, aos1‐1, sid2‐1, npr1‐1, gox1‐2 and haox1‐2 mutants were repeated at least once with similar results. The results of both the Hpa and the Pc assays were verified in independent DC experiments with wild‐type plants (Col‐0), using quantitative PCR (Fig. S3). Shoot material was collected at 6 dpi (n = 4) for quantification of Hpa biomass; fully expanded leaves were collected at 8 dpi (n = 4) for quantification of Pc biomass. The qPCR quantifications of Hpa and Pc biomass were performed with pathogen‐specific primers (Table S1), using the PCR conditions described by Anderson & McDowell (2015) and Sanchez‐Vallet et al. (2010), respectively.
Gene expression analysis by reverse‐transcriptase qPCR
RNA extraction, cDNA synthesis and relative quantification of gene expression by reverse‐transcriptase qPCR (RT‐qPCR) were performed as described previously (Pétriacq et al., 2016a), using gene‐specific primers (Table S1). Basal and hormone‐induced expression of PR1 (AT2G14610) and VSP2 (AT5G24770) were determined in plants of the eight‐leaf stage after spraying shoots with double‐distilled water, 0.1 mM JA (OlChemim, Olomouc, Czech Republic), or 0.5 mM SA, supplemented with 0.01% Silwet L‐77 until imminent runoff. Each biological replicate in these assays consisted of four leaves from four different plants (n = 3). Expression of CAT2 (AT4G35090), GOX1 (AT3G14420) and HAOX1 (AT3G14130) were measured in plants of the eight‐leaf stage, where each biological replicate consisted of shoot material from one plant (n = 5). Differences in relative transcript levels were analysed for statistical significance, using Welch's t‐test (R, v.3.1.2). RT‐qPCR assays to quantify CAT2, GOX1 and HAOX1 gene expression were repeated once with similar results.
Mass spectrometry analyses
SA and JA were quantified by ultra‐pressure liquid chromatography coupled to quadrupole time of flight mass spectrometry (UPLC‐Q‐TOF), using MSE technology to confirm compound‐specific fragmentation patterns, as detailed in Methods S1. Each biological replicate in these assays consisted of four pooled leaves from different plant (n = 5). Untargeted metabolic profiling by UPLC‐Q‐TOF MS and statistical data analysis were performed as detailed in Methods S1.
In situ detection of reactive oxygen species
Extracellular reactive oxygen species (ROS) were analysed by 3,3′‐diaminobenzidine (DAB) staining (Daudi & O'Brien, 2012), whereas intracellular ROS were visualised by 2′,7′‐dichlorofluorescein diacetate (DCFH‐DA), as described previously (Pétriacq et al., 2016b). Each biological replicate in these assays consisted of one individual leaf collected from different plants (n = 10 for DCFH‐DA, n = 5 for DAB). In both cases, mock‐ or Hpa‐treated leaves were sampled at 48 h post‐inoculation (hpi). ROS intensities from DAB or DCFH‐DA images were obtained with an Olympus SZX12 binocular microscope (using an HQ510 1p emission filter for DCFH‐DA fluorescence; excitation/emission: 492–495/517–527 nm) and quantified using Adobe Photoshop (v.CS.5), as described previously (Luna et al., 2011; Pétriacq et al., 2016b).
Results
Plant development biases the assessment of CO2‐dependent disease resistance
To determine the impacts of plant development on CO2‐dependent resistance, we first characterised the growth response of Arabidopsis to CO2 in different atmospheric CO2 concentrations, ranging from 200 ppm (saCO2), 400 ppm (aCO2) to 1200 ppm (e CO2). Using the number of leaves as a marker for developmental stage (Boyes et al., 2001), both 3‐ and 4.5‐wk‐old plants showed enhanced development at e CO2, and reduced development at saCO2, compared to aCO2 (Fig. 1a, upper panel). To determine whether these developmental effects influence disease resistance, we compared resistance phenotypes against biotrophic Hpa and necrotrophic Pc with and without correction for plant developmental stage. This DC was achieved by delaying sowing at e CO2 by 3 d in comparison to plants at aCO2, while starting plant cultivation at saCO2 7 d earlier compared to plants at aCO2 (Fig. S1). DC resulted in equal numbers of leaves at all CO2 regimes at the time of pathogen inoculation (eight‐leaf stage for Hpa and 18‐leaf stage for Pc; Fig. 1a, lower panel). Without DC, 3‐wk‐old plants showed increasing levels of Hpa resistance at rising CO2 concentrations (Fig. 1b, top left), whereas 4.5‐wk‐old plants showed enhanced Pc resistance at both e CO2 and saCO2 (Fig. 1b, top right). This pattern of CO2‐dependent resistance phenotypes changed upon DC application. While eight‐leaf plants showed enhanced Hpa resistance at both saCO2 and e CO2 (Fig. 1b, bottom left), 18‐leaf plants showed increasing levels of Pc resistance with rising CO2 concentrations (Fig. 1b, bottom right). To confirm the development‐independent effects of CO2 on disease resistance, levels of Hpa and Pc colonization were quantified in an independent DC experiment, using qPCR analysis of pathogen‐specific DNA (Fig. S3). The impact of DC on resistance phenotypes at saCO2 and e CO2 indicates that differences in plant development bias the assessment of CO2‐dependent disease resistance against both biotrophic and necrotrophic pathogens. Accordingly, all subsequent experiments were conducted after application of DC.
Figure 1.
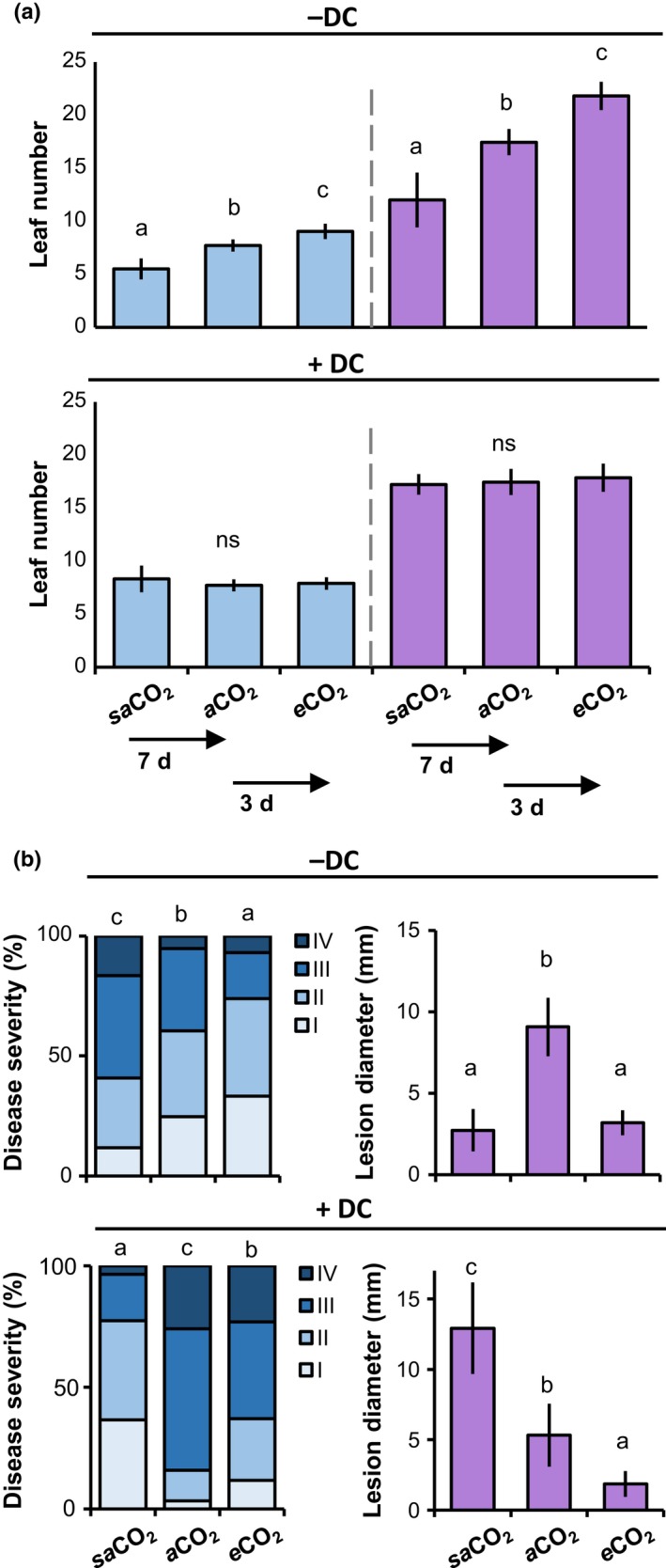
Plant development correction (DC) separates immunological effects of CO 2 from indirect developmental effects on Arabidopsis resistance. (a) Effect of DC on average leaf numbers in Arabidopsis (Col‐0) at sub‐ambient (sa CO 2; 200 ppm), ambient (aCO 2; 400 ppm) and elevated CO 2 (eCO 2; 1200 ppm). DC for sa CO 2 was performed by planting seeds 7 d earlier than at aCO 2; DC for eCO 2 was achieved by planting seeds 3 d later than at aCO 2. Upper panel, leaf numbers of 3‐ (left) and 4.5‐ (right) wk‐old plants without DC. Lower panel, leaf numbers after DC. Data represent mean leaf numbers (± SD, n = 10–18) and are representative of two independent experiments. ns, Not significant. (b) Effect of DC on basal resistance against biotrophic Hyaloperonospora arabidopsidis (Hpa; left) and necrotrophic Plectosphaerella cucumerina (Pc; right). Shown are relative numbers of leaves (n > 50) in Hpa colonization classes of increasing severity (I–IV) at 6 d post‐inoculation (dpi), or average lesion diameters (± SD; n = 8) by Pc at 13 dpi. Different letters indicate statistically significant differences (Fisher's exact test; ANOVA with Tukey honest significant difference post‐hoc analysis; P < 0.05). Pathogenicity assays with Col‐0 were repeated several times with comparable outcomes.
Development‐independent effects of eCO2 on SA‐ and JA‐dependent resistance
SA and JA play important roles in plant defence against biotrophic and necrotrophic pathogens, respectively (Thomma et al., 1998). To examine the direct (development‐independent) effects of e CO2 on defence signalling hormones, we used UPLC coupled to tandem MS to quantify SA and JA levels. In comparison to plants at aCO2, plants at e CO2 showed a 69.3% and 69.4% increase in accumulation of SA and JA, respectively (Fig. 2a). While increases in hormone levels were not sufficient to induce transcription of the SA‐inducible marker gene PR1 and the JA‐inducible marker gene VSP2 directly (Fig. 2b), they were sufficient to prime augmented induction of PR1 and VSP2 after exogenous application of 0.5 mM SA and 0.1 mM JA, respectively (Fig. 2b). To determine the contribution of priming of SA‐dependent defence to e CO2‐induced resistance against Hpa, we analysed resistance phenotypes of Arabidopsis mutants impaired in SA production (sid2‐1) or response (npr1‐1). Although less pronounced than in wild‐type plants (Col‐0), both sid2‐1 and npr1‐1 expressed statistically significant levels of e CO2‐induced resistance against Hpa (Fig. 2c). Hence, priming of SA‐dependent defence is not solely responsible for e CO2‐induced resistance against Hpa. To determine the contribution of priming of JA‐dependent defence to e CO2‐induced resistance against Pc, we analysed resistance phenotypes of mutants in JA production (aos1‐1) or sensitivity (jar1‐1). In contrast to Co1‐0, both aos1‐1 and jar1‐1 failed to express elevated Pc resistance at e CO2 (Fig. 2d), indicating that priming of JA‐inducible defence is critically important for e CO2‐induced resistance against Pc.
Figure 2.
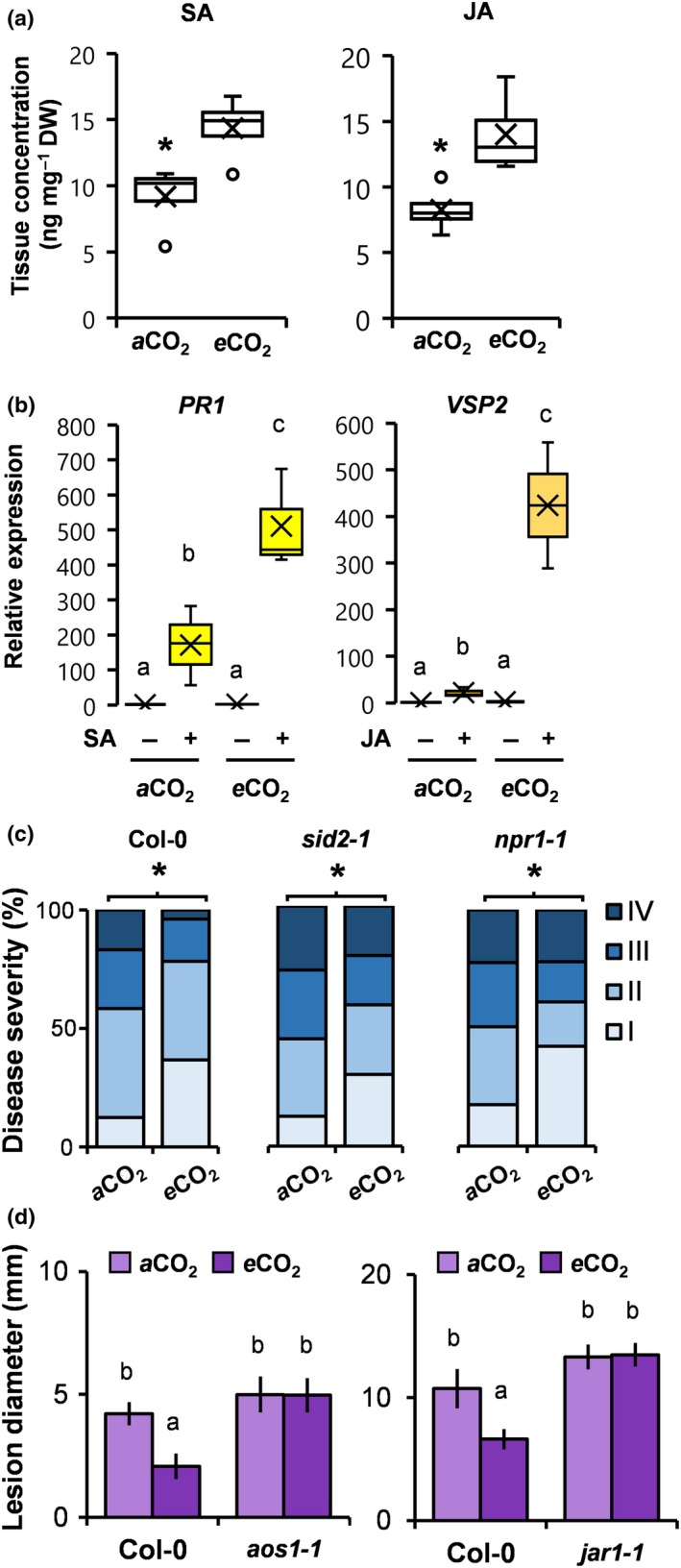
Development‐independent effects of elevated CO 2 (eCO 2) on salicylic acid (SA)‐ and jasmonic acid (JA)‐dependent defence. (a) Accumulation of SA and JA acids in Arabidopsis (Col‐0) of similar developmental stage (eight‐leaf) at ambient CO 2 (aCO 2) (400 ppm) and eCO 2 (1200 ppm). Shown are box plots of replicated metabolite quantifications (n = 5; means are indicated by X; outliers outside the 2.5–97.5 percentile interval are indicated by ○). (b) Responsiveness of SA‐ and JA‐inducible genes (PR1 and VSP2, respectively) in eight‐leaf stage plants (Col‐0) at aCO 2 and eCO 2. Shown are box plots of relative transcript levels at 8 and 24 h after treatment (n = 3; means are indicated by X). (c) Effects of eCO 2 on Hyaloperonospora arabidopsidis (Hpa) resistance in Col‐0, the SA synthesis mutant sid2‐1 and the SA response mutant npr1‐1 at the eight‐leaf stage. Shown are relative numbers of leaves (n > 50) in Hpa colonisation classes of increasing severity (I–IV) at 6 d post‐inoculation (dpi). (d) Effects of eCO 2 on Plectosphaerella cucumerina (Pc) resistance in Col‐0, the JA production mutant aos1‐1 and the jar1‐1 response mutant at the 18‐leaf stage. Shown are average lesion diameters per plant (± SD; n = 8) of Pc at 13 dpi. Asterisks or different letters indicate significant differences between conditions (P < 0.05): (a) Welch's t‐test; (c) Fisher's exact test; (b, d) ANOVA with Tukey honest significant difference post‐hoc analysis. Pathogenicity assays with sid2‐1, npr1‐1, aos1‐1 and jar1‐1 were repeated once with similar results.
Development‐independent resistance at saCO2 relies on photorespiration‐derived ROS
Basal resistance against Hpa was enhanced at both e CO2 and saCO2 (Fig. 1c). This nonlinear relationship between CO2 and Hpa resistance suggests involvement of different defence mechanisms at e CO2 and saCO2. Unlike e CO2 (Fig. 2b), saCO2 did not alter basal and SA‐induced PR1 gene expression (Fig. S4a). Moreover, despite the enhanced disease susceptibility of the SA signalling mutants sid2‐1 and nrp1‐1 in comparison to wild‐type plants, both mutants displayed a statistically significant increase in Hpa resistance at saCO2 in comparison to the same mutant background at aCO2 (Fig. S4b). Hence, the SA‐dependent defence pathway does not have a critical contribution to saCO2‐induced resistance against Hpa. To search for alternative mechanisms, we performed untargeted metabolite profiling of mock‐ and Hpa‐inoculated plants at 24 and 72 hpi, using UPLC‐Q‐TOF MS (Pétriacq et al., 2016b). Unsupervised principal component analysis displayed global metabolic responses, which were affected by both Hpa and CO2 concentration (Fig. S5). To identify ion markers of saCO2‐induced resistance, we applied a stringent pipeline (detailed in Methods S1 and Fig. S6a) to select for ions that are significantly influenced by CO2, Hpa or the interaction thereof (Fig. S6). Subsequent hierarchical clustering identified ion clusters that are either induced by saCO2, or primed by saCO2 for augmented induction after subsequent Hpa inoculation (Fig. 3). Putative ion marker identification by accurate m/z detection revealed enrichment of metabolites involved in cellular redox regulation (NAD metabolism, secondary antioxidant metabolites) and/or defence (glucosinolates, flavonoids, coumarins, alkaloids; Table S2). The cluster containing saCO2‐primed markers also included traces of oxidised amino acids (Stadtman & Levine, 2003). Together, these metabolic profiles suggest that plants at saCO2 are exposed to enhanced oxidative stress due to increased production of ROS.
Figure 3.
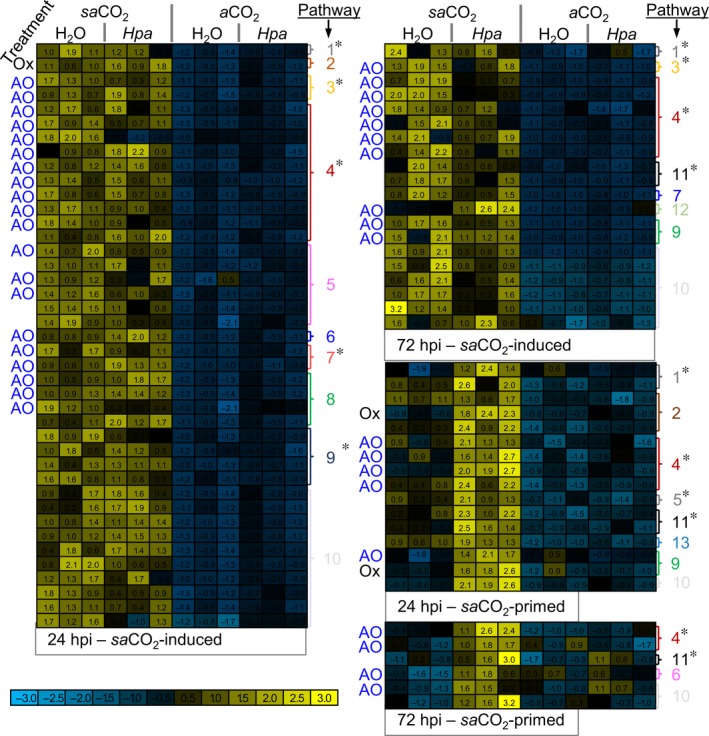
Metabolic profiling of mock‐ and Hyaloperonospora arabidopsidis (Hpa)‐inoculated Arabidopsis leaves of similar developmental stage at sub‐ambient CO 2 (sa CO 2) and ambient CO 2 (aCO 2). Plants of eight‐leaf stage (Col‐0) grown at sa CO 2 (200 ppm) and aCO 2 (400 ppm) were mock‐ or Hpa‐inoculated. Methanol extracts from leaves at 24 and 72 h post‐inoculation (hpi) were analysed by UPLC‐Q‐TOF in negative and positive ionisation mode. Normalised ion intensities were filtered for statistically significant differences between treatments, using ANOVA (P < 0.01 + Benjamini–Hochberg false discovery rate correction), followed by two‐way ANOVA (P < 0.01) to select for ion markers that are significantly influenced by CO 2, Hpa or the interaction thereof, at 24 and 72 hpi. Selected markers were subjected to hierarchical clustering (Pearson's correlation). Shown are subclusters of markers showing either enhanced accumulation at sa CO 2 or priming for augmented induction by Hpa at sa CO 2. Coloured heat‐maps show normalised ion intensities relative to the average and SD across all samples. Pathways corresponding to putative ion identities are shown on the right of the heat‐maps; antioxidant properties of putative metabolites are indicated by ‘AO’ while putative oxidation products are indicated by ‘Ox’. Pathways with defence properties are marked with an asterisk. Pathway designations are as follows: (1) alkaloids; (2) amino acids; (3) coumarins; (4) flavonoids; (5) lipids; (6) photorespiration; (7) polyphenols; (8) redox; (9) terpenoids; (10) unknown; (11) glucosinolates; (12) polyamines; (13) phytohormones.
As ROS can act as defence signals in plants (Torres et al., 2002), we next investigated a possible role for ROS in saCO2‐induced resistance. To this end, mock‐ and Hpa‐inoculated leaves were stained at 48 hpi with DAB, which predominantly marks extracellular ROS production, because most DAB substrate is immediately oxidised after leaf infiltration by apoplastic H2O2 and peroxidases (Daudi & O'Brien, 2012). Although Hpa‐inoculated leaves showed increased DAB staining intensity, there were no statistically significant differences in extracellular ROS intensities between saCO2 and aCO2 conditions (Fig. S7a,b). Furthermore, the respiratory burst oxidase (RBOH) double mutant rbohD/F, which is impaired in stress‐induced production of extracellular ROS (Torres et al., 2002), was unaffected in saCO2‐induced resistance (Fig. S7c). Hence, extracellular ROS do not play a significant role in saCO2‐induced resistance. Subsequently, we stained mock‐ and Hpa‐inoculated leaves with DCFH‐DA, which is hydrolysed by intracellular esterases to generate DCF that reacts with intracellular ROS, yielding a fluorescent signal (Sandalio et al., 2008). Although saCO2 did not increase intracellular ROS accumulation in mock‐inoculated plants, Hpa‐inoculated plants at saCO2 showed augmented ROS accumulation in comparison with Hpa‐inoculated plants at aCO2 (Fig. 4a). Thus, saCO2 primes pathogen‐induced accumulation of intracellular ROS.
Figure 4.
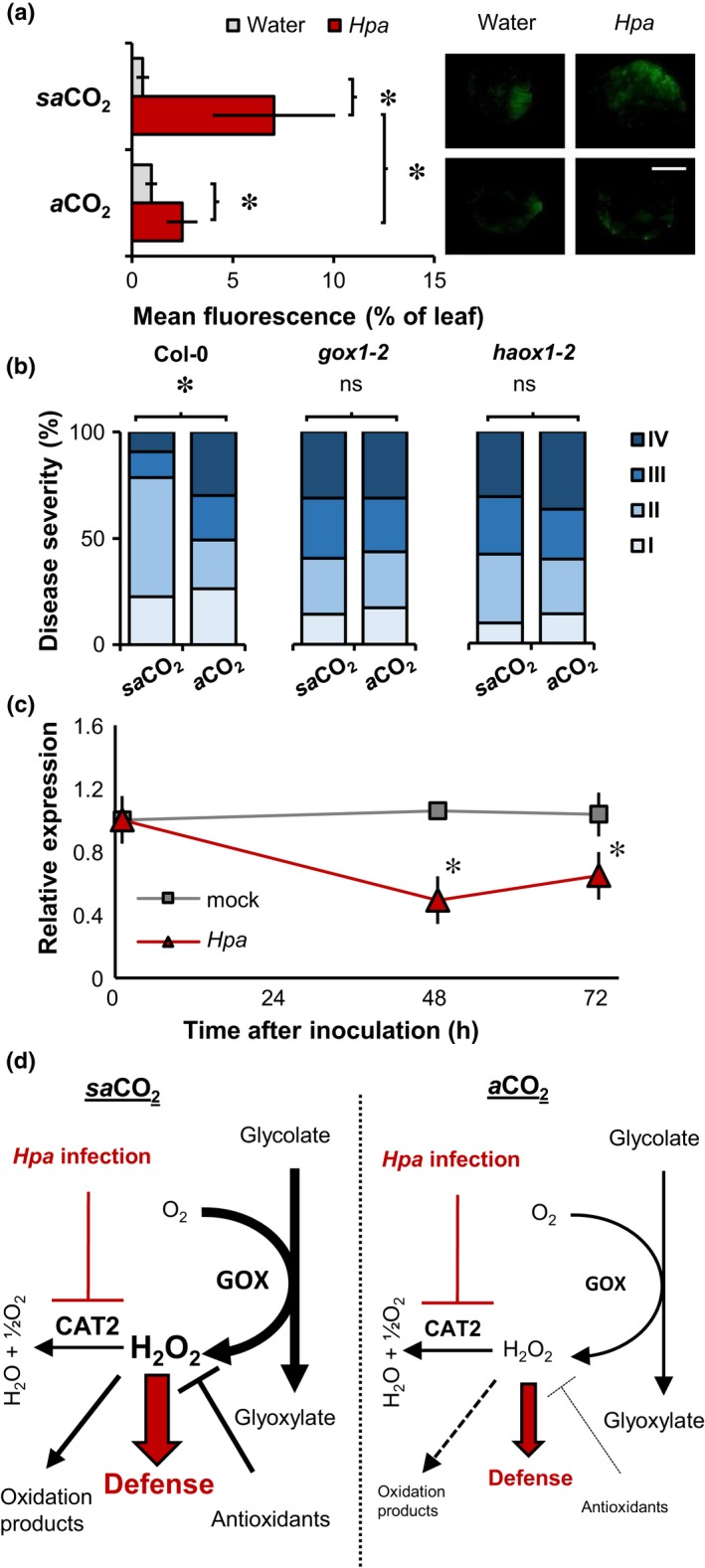
Role of photorespiration in sub‐ambient CO 2 (sa CO 2)‐induced resistance in Arabidopsis against Hyaloperonospora arabidopsidis (Hpa). (a) Quantification of intracellular H2O2 by 2′,7′‐dichlorofluorescein diacetate (DCFH‐DA) staining in plants (Col‐0) of similar developmental stage (eight‐leaf) at sa CO 2 (200 ppm) and aCO 2 (400 ppm). Shown are mean values of the fluorescent proportion of the leaf area (± SD, n = 8–10) at 48 h post‐inoculation (hpi) with water mock or Hpa. Insets show representative staining intensities. Bar, 2 mm. (b) Quantification of Hpa resistance at sa CO 2 and aCO 2 in wild‐type plants (Col‐0) and glycolate oxidase knock‐down mutants gox1‐2 and haox1‐2 at the eight‐leaf stage. Shown are relative numbers of leaves (n > 50) in Hpa colonisation classes of increasing severity (I–IV) at 7 d post‐inoculation (dpi). The experiment was repeated with comparable results. (c) Impacts of Hpa inoculation on CAT2 gene expression in 3‐wk‐old Col‐0 at aCO 2 (eight‐leaf stage). Shown are mean values of relative transcript abundance (± SD, n = 5) at different times after water or Hpa inoculation. Asterisks indicate statistically significant differences (Welch's t‐test; Fisher's exact test; P < 0.05). The experiment was repeated at both sa CO 2 and aCO 2, yielding comparable results (Supporting Information Fig. S9). ns, Not significant. (d) Model explaining the role of photorespiration in priming of reactive oxygen species (ROS)‐dependent defence at sa CO 2. Enhanced photorespiratory activity at sa CO 2 causes increased production of H2O2 by glycolate oxidase (GOX), which is scavenged by CAT2 and antioxidant metabolites in healthy plants. Hpa infection represses transcription of the CAT2 gene, causing augmented accumulation of GOX‐derived H2O2 at sa CO 2. Impacts of photorespiration on intracellular H2O2 are indicated by black arrows. Impacts of Hpa on H2O2‐dependent defence are indicated by red arrows.
A major source of intracellular ROS is photorespiration, which involves production of H2O2 from oxidation of glycolate by glycolate oxidases (GOXs; Chaouch et al., 2010; Rojas et al., 2012). Loss‐of‐function mutations in photorespiration cause dramatic growth reduction or lethality at aCO2 (Timm & Bauwe, 2013), making them unsuitable for evaluation of resistance phenotypes at aCO2 and saCO2. Therefore, we selected single ‘knock‐down’ mutants with T‐DNA insertions in the promotors of GOX or HAOX (gox1‐2 and haox1‐2, Fig. S8a), which have previously been implicated in Arabidopsis resistance (Rojas et al., 2012). Despite the fact that these mutations reduced GOX1 and HAOX1 expression by 42.6% and 75.4%, respectively (Fig. S8b), gox1‐2 and haox1‐2 showed wild‐type growth phenotypes at saCO2 (Fig. S8c). However, unlike wild‐type plants (Col‐0), both mutants failed to express saCO2‐induced resistance against Hpa (Fig. 4b), indicating a critical role for ROS‐generating GOX function.
In unstressed Arabidopsis plants, GOX‐derived ROS are largely scavenged by the peroxisomal catalase enzyme CAT2 (Chaouch et al., 2010). To test whether the augmentation in Hpa‐induced ROS production at saCO2 (Fig. 4a) is related to changes in CAT2 expression, we profiled CAT2 transcript accumulation at different time‐points after mock and Hpa inoculation. At both 48 and 72 hpi, Hpa‐inoculated plants showed a statistically significant reduction in CAT2 expression (Fig. 4c), which was apparent at both aCO2 and saCO2 conditions (Fig. S9). Since saCO2 boosts photorespiration (Li et al., 2014), our results indicate that Hpa‐induced CAT2 repression triggers augmented accumulation of GOX‐derived ROS during infection, which in turn results in enhanced resistance at saCO2 (Fig. 4c).
Discussion
By eliminating bias from indirect developmental effects of CO2 on disease resistance, we have identified distinct mechanisms by which CO2 shapes plant immunity. There is ample evidence that plant development influences immunity through ARR (Kus et al., 2002). ARR in Arabidopsis is effective against (hemi)biotrophic pathogens, including Pseudomonas syringae pv tomato (Pst) and Hpa (Kus et al., 2002; McDowell et al., 2005). When we conducted our experiments without DC, Hpa resistance intensified with increasing CO2 concentrations (Fig. 1b). DC changed this pattern, revealing that plants of similar developmental stage expressed higher levels of Hpa resistance at both e CO2 and saCO2. These results suggest that, in the absence of DC, the resistance‐enhancing effect of saCO2 against Hpa is masked by low ARR of underdeveloped plants. Interestingly, DC had an opposite effect on CO2‐dependent resistance against Pc. Without DC, plants showed enhanced resistance at both saCO2 and e CO2, whereas plants of similar developmental stage (i.e. after DC) displayed increasing levels of Pc resistance with rising CO2 concentrations (Fig. 1b). Thus, without DC, assessment of CO2‐dependent resistance against Pc is biased by defence mechanisms that are more active at earlier developmental stages. Glucosinolates are known to accumulate to higher concentrations in younger plants (Petersen et al., 2002; Brown et al., 2003) and are effective against Pc (Frerigmann et al., 2016). Alternatively, age‐dependent regulation of the JA response could play a role, which is primed in younger plants due to miR156‐dependent repression of JAZ6‐stabilising SPL protein (Mao et al., 2017). Taken together, our results show that DC is an effective method to eliminate bias from developmental effects of CO2 on disease resistance, enabling a more accurate assessment of mechanisms by which CO2 shapes plant immunity.
Previous studies have reported that e CO2 enhances and/or primes phytohormone‐dependent plant defence (Zhang et al., 2015; Mhamdi & Noctor, 2016). However, none of these studies applied DC to eliminate bias from ARR. While some studies transferred plants of similar developmental age from aCO2 to e CO2 before pathogen inoculation (Zhang et al., 2015), we opted against this method, given it can cause abrupt, and potentially confounding, changes in carbon flux. Furthermore, transferring plants from aCO2 to e CO2 before pathogen challenge may neglect the full extent by which e CO2 affects defence hormone production (Mhamdi & Noctor, 2016). Using DC, we confirmed that e CO2 enhances basal production of SA and JA (Fig. 2a), causing priming of JA‐ and SA‐dependent gene expression, respectively (Fig. 2b). The JA signalling mutants aos1‐1 and jar1‐1 were impaired in expression of e CO2‐induced resistance against Pc (Fig. 2d), indicating a critical contribution of JA‐dependent defence signalling. Conversely, the SA signalling mutants sid2‐1 and npr1‐1 were only partially affected in e CO2‐induced resistance against Hpa (Fig. 2c), indicating that priming of SA‐dependent defence is not solely responsible for Hpa resistance at e CO2, which is consistent with previous conclusions regarding e CO2‐induced resistance against hemi‐biotrophic Pst (Zhang et al., 2015; Mhamdi & Noctor, 2016). Furthermore, Mhamdi & Noctor (2016) recently reported that e CO2‐induced resistance to Pst is associated with changes in primary metabolism and increased pools of total and oxidised glutathione, while Arabidopsis mutants in glutathione regulation and NADPH‐generating enzymes were affected in Pst resistance at e CO2. Although it is unclear whether these mutants were similarly affected in basal resistance at aCO2, the study by Mhamdi & Noctor (2016) concluded that oxidative pathways controlling primary metabolism played a role in e CO2‐induced resistance. Since carbohydrate metabolism and signalling can boost SA‐dependent and SA‐independent defence (Tauzin & Giardina, 2014) by augmenting redox signalling (Morkunas & Ratajczak, 2014), we speculate that e CO2‐induced resistance in Hpa resistance is a consequence of changes in carbohydrate metabolism.
So far, the effects of saCO2 on plant disease resistance have received limited attention. Our DC experiments revealed that Arabidopsis expresses enhanced Hpa resistance at saCO2 (Fig. 1b). Untargeted UPLC‐Q‐TOF analysis revealed that this saCO2‐induced resistance was associated with ion clusters displaying constitutively enhanced accumulation and/or primed accumulation after subsequent Hpa infection at saCO2 (Fig. 3). As these ion clusters were enriched with putative metabolites involved in redox regulation, we explored the importance of ROS in saCO2‐induced resistance. While we excluded a role for extracellular ROS (Fig. S7), plants at saCO2 showed augmented production of intracellular ROS after Hpa inoculation (Fig. 4a). Glycolate oxidation by GOX is a major source of intracellular H2O2 (Chaouch et al., 2010), which probably increases at saCO2 due to enhanced photorespiration (Temme et al., 2013; Li et al., 2014). Moreover, GOX‐derived ROS have been linked to resistance against nonhost pathogens in both Arabidopsis and Nicotiana benthamiana (Rojas et al., 2012). Indeed, knockdown mutants with reduced transcription of two separate GOX genes failed to express enhanced Hpa resistance at saCO2, indicating a crucial role for photorespiratory ROS. The peroxisomal catalase enzyme, CAT2, scavenges GOX‐derived H2O2 to mitigate oxidative damage during photorespiration (Chaouch et al., 2010). Interestingly, transcriptional profiling of the CAT2 gene revealed that Arabidopsis reduces CAT2 expression after Hpa inoculation (Figs 4c, S9). Since CAT2 suppresses plant defence (Polidoros et al., 2001; Chaouch et al., 2010), this pathogen‐induced CAT2 repression probably reflects an innate immune response to generate defence‐inducing ROS during infection. In this context, we propose that stimulation of photorespiration‐related GOX activity at saCO2 primes pathogen‐induced accumulation of intracellular ROS. Subsequent repression of CAT2 expression following Hpa attack results in enhanced accumulation of intracellular ROS, mediating enhanced levels of SA‐independent resistance in comparison to aCO2‐exposed plants (Fig. 4d).
It is plausible that photorespiration‐derived ROS were key to survival when plants adapted to glacial periods with low atmospheric CO2. Reduced growth and plant fecundity at glacial CO2 conditions required longer life cycles to maintain reproductive fitness (Ward & Kelly, 2004). Additionally, reduced investment in foliar defence compounds at saCO2 would have put plants at a higher risk of pathogen attack (Quirk et al., 2013), creating selective pressure for a primed immune system. In addition to limiting the toxicity of 2‐phosphoglycolate, we hypothesise that C3 plants benefitted from photorespiration to prime their immune system. This hypothesis may explain why certain C4 plants (e.g. maize) have retained photorespiration and GOX activity (Peterhansel & Maurino, 2011). Our study has uncovered a specific link between saCO2, GOX‐derived ROS and enhanced immunity. This evidence supports the notion that plants have utilised photorespiratory defence signalling over glacial periods to maintain elevated levels of adaptive broad‐spectrum disease resistance. This may be especially pertinent to Arabidopsis, which evolved under the CO2‐limited atmosphere of the Miocene epoch (Beilstein et al., 2010). In this context, future initiatives to replace C3 metabolism with C4 metabolism in major food crops may require careful consideration of the contribution of photorespiration to plant defence.
Author contributions
A.W., P.P., D.J.B. and J.T. planned and conceived the experiments; A.W., R.E.S., P.P. and J.T. performed the experiments; J.T. and D.J.B. provided reagents, equipment and facilities; A.W., P.P. and J.T. analysed the data; A.W., P.P. and J.T. wrote the paper with feedback from all co‐authors.
Supporting information
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Fig. S1 Effects of CO2 on plant development.
Fig. S2 Images of Hpa colonisation classes.
Fig. S3 qPCR‐based quantification of Hpa and Pc biomass.
Fig. S4 Role of SA signalling in saCO2‐induced resistance against Hpa.
Fig. S5 Global metabolic signatures of Hpa‐inoculated Arabidopsis at saCO2 and aCO2.
Fig. S6 Selection of ions that are induced or primed for Hpa‐induced accumulation by saCO2.
Fig. S7 Extracellular H2O2 in saCO2‐induced resistance against Hpa.
Fig. S8 Selection of gox1‐2 and haox1‐2 mutants.
Fig. S9 Impacts of Hpa inoculation on CAT2 gene expression at saCO2 and aCO2.
Table S1 Primers used in this study
Table S2 Putative identification of metabolic markers detected by UPLC‐Q‐TOF
Methods S1 Supplemental materials and methods.
Acknowledgements
We thank David Pardo for practical assistance. The research was supported by a consolidator grant from the European Research Council (ERC; no. 309944 ‘Prime‐A‐Plant’) to J.T., a Research Leadership Award from the Leverhulme Trust (no. RL‐2012‐042) to J.T., a BBSRC‐IPA grant to J.T. (BB/P006698/1), and an advanced grant from ERC (no. 322998 ‘CDREG’) to D.J.B.
Contributor Information
Pierre Pétriacq, Email: pierre.petriacq@inra.fr.
David J. Beerling, Email: d.j.beerling@sheffield.ac.uk.
Jurriaan Ton, Email: j.ton@sheffield.ac.uk.
References
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R et al 2003. Genome‐wide insertional mutagenesis of Arabidopsis thaliana . Science 301: 653–657. [DOI] [PubMed] [Google Scholar]
- Anderson RG, Mcdowell JM. 2015. A PCR assay for the quantification of growth of the oomycete pathogen Hyaloperonospora arabidopsidis in Arabidopsis thaliana . Molecular Plant Pathology 16: 893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilstein M, Nagalingum N, Clements M, Manchesterb S, Mathews S. 2010. Dated molecular phylogenies indicate a Miocene origin for Arabidopsis thaliana . Proceedings of the National Academy of Sciences, USA 107: 18724–18728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes DC, Zayed AM, Ascenzi R, McCaskill AJ, Hoffman NE, Davis KR, Görlach J. 2001. Growth stage‐based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. Plant Cell 13: 1499–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PD, Tokuhisa JG, Reichelt M, Gershenzon J. 2003. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana . Phytochemistry 62: 471–481. [DOI] [PubMed] [Google Scholar]
- Cao H, Glazebrook J, Clarke JD, Volko S, Dong X. 1997. The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 88: 57–63. [DOI] [PubMed] [Google Scholar]
- Chaouch S, Queval G, Vanderauwera S, Mhamdi A, Vandorpe M, Langlois‐Meurinne M, Van Breusegem F, Saindrenan P, Noctor G. 2010. Peroxisomal hydrogen peroxide is coupled to biotic defense responses by ISOCHORISMATE SYNTHASE1 in a daylength‐related manner. Plant Physiology 153: 1692–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daudi A, O'Brien JA. 2012. Detection of hydrogen peroxide by DAB staining in Arabidopsis leaves. Bio‐protocol 2: 1–5. [PMC free article] [PubMed] [Google Scholar]
- Frerigmann H, Piślewska‐Bednarek M, Sánchez‐Vallet A, Molina A, Glawischnig E, Gigolashvili T, Bednarek P. 2016. Regulation of pathogen‐triggered tryptophan metabolism in Arabidopsis thaliana by MYB transcription factors and indole glucosinolate conversion products. Molecular Plant 9: 682–695. [DOI] [PubMed] [Google Scholar]
- Galbraith ED, Eggleston S. 2017. A lower limit to atmospheric CO2 concentrations over the past 800,000 years. Nature Geoscience 10: 295–299. [Google Scholar]
- Kus JV, Zaton K, Sarkar R, Cameron RK. 2002. Age‐related resistance in Arabidopsis is a developmentally regulated defense response to Pseudomonas syringae . Plant Cell 14: 479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake JA, Wade RN. 2009. Plant–pathogen interactions and elevated CO2: morphological changes in favour of pathogens. Journal of Experimental Botany 60: 3123–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xu J, Haq NU, Zhang H, Zhu X‐G. 2014. Was low CO2 a driving force of C4 evolution: Arabidopsis responses to long‐term low CO2 stress. Journal of Experimental Botany 65: 3657–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna E, Bruce TJA, Roberts MR, Flors V, Ton J. 2012. Next‐generation systemic acquired resistance. Plant Physiology 158: 844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna E, Pastor V, Robert J, Flors V, Mauch‐Mani B, Ton J. 2011. Callose deposition: a multifaceted plant defense response. Molecular Plant–Microbe Interactions 24: 183–193. [DOI] [PubMed] [Google Scholar]
- Mao Y‐B, Liu Y‐Q, Chen D‐Y, Chen F‐Y, Fang X, Hong G‐J, Wang L‐J, Wang J‐W, Chen X‐Y. 2017. Jasmonate response decay and defense metabolite accumulation contributes to age‐regulated dynamics of plant insect resistance. Nature Communications 8: 13925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JM, Williams SG, Funderburg NT, Eulgem T, Dangl JL. 2005. Genetic analysis of developmentally regulated resistance to downy mildew (Hyaloperonospora parasitica) in Arabidopsis thaliana . Molecular Plant–Microbe Interactions 18: 1226–1234. [DOI] [PubMed] [Google Scholar]
- Mhamdi A, Noctor G. 2016. High CO2 primes plant biotic stress defences through redox‐linked pathways. Plant Physiology 172: 929–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morkunas I, Ratajczak L. 2014. The role of sugar signaling in plant defense responses against fungal pathogens. Acta Physiologiae Plantarum 36: 1607–1619. [Google Scholar]
- Peterhansel C, Maurino VG. 2011. Photorespiration redesigned. Plant Physiology 155: 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen BL, Chen S, Hansen CH, Olsen CE, Halkier BA. 2002. Composition and content of glucosinolates in developing Arabidopsis thaliana . Planta 214: 562–571. [DOI] [PubMed] [Google Scholar]
- Pétriacq P, Stassen J, Ton J. 2016a. Spore density determines infection strategy by the plant‐pathogenic fungus Plectosphaerella cucumerina . Plant Physiology 170: 2325–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pétriacq P, Ton J, Patrit O, Tcherkez G, Gakière B. 2016b. NAD acts as an integral regulator of multiple defense layers. Plant Physiology 172: 1465–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polidoros AN, Mylona PV, Scandalios JG. 2001. Transgenic tobacco plants expressing the maize Cat2 gene have altered catalase levels that affect plant–pathogen interactions and resistance to oxidative stress. Transgenic Research 10: 555–569. [DOI] [PubMed] [Google Scholar]
- Przybyla D, Göbel C, Imboden A, Hamberg M, Feussner I, Apel K. 2008. Enzymatic, but not non‐enzymatic, 1O2‐mediated peroxidation of polyunsaturated fatty acids forms part of the EXECUTER1‐dependent stress response program in the flu mutant of Arabidopsis thaliana . Plant Journal 54: 236–248. [DOI] [PubMed] [Google Scholar]
- Pugliese M, Liu J, Titone P, Garibaldi A, Gullino ML. 2012. Effects of elevated CO2 and temperature on interactions of zucchini and powdery mildew. Phytopathologia Mediterranea 51: 480–487. [Google Scholar]
- Quirk J, McDowell NG, Leake JR, Hudson PJ, Beerling DJ. 2013. Increased susceptibility to drought‐induced mortality in Sequoia sempervirens (Cupressaceae) trees under Cenozoic atmospheric carbon dioxide starvation. American Journal of Botany 100: 582–591. [DOI] [PubMed] [Google Scholar]
- Riikonen J, Syrjälä L, Tulva I, Mänd P, Oksanen E, Poteri M, Vapaavuori E. 2008. Stomatal characteristics and infection biology of Pyrenopeziza betulicola in Betula pendula trees grown under elevated CO2 and O3 . Environmental Pollution 156: 536–543. [DOI] [PubMed] [Google Scholar]
- Rojas CM, Senthil‐Kumar M, Wang K, Ryu C‐M, Kaundal A, Mysore KS. 2012. Glycolate oxidase modulates reactive oxygen species‐mediated signal transduction during nonhost resistance in Nicotiana benthamiana and Arabidopsis. Plant Cell 24: 336–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez‐Vallet A, Ramos B, Bednarek P, López G, Piślewska‐Bednarek M, Schulze‐Lefert P, Molina A. 2010. Tryptophan‐derived secondary metabolites in Arabidopsis thaliana confer non‐host resistance to necrotrophic Plectosphaerella cucumerina fungi. Plant Journal 63: 115–127. [DOI] [PubMed] [Google Scholar]
- Sandalio LM, Rodríguez‐Serrano M, Romero‐Puertas MC, del Río LA. 2008. Imaging of reactive oxygen species and nitric oxide in vivo in plant tissues. Methods in Enzymology 440: 397–409. [DOI] [PubMed] [Google Scholar]
- Shibata Y, Kawakita K, Takemoto D. 2010. Age‐related resistance of Nicotiana benthamiana against hemibiotrophic pathogen Phytophthora infestans requires both ethylene‐ and salicylic acid‐mediated signaling pathways. Molecular Plant–Microbe Interactions 23: 1130–1142. [DOI] [PubMed] [Google Scholar]
- Sørhagen K, Laxa M, Peterhänsel C, Reumann S. 2013. The emerging role of photorespiration and non‐photorespiratory peroxisomal metabolism in pathogen defence. Plant Biology 15: 723–736. [DOI] [PubMed] [Google Scholar]
- Stadtman ER, Levine RL. 2003. Free radical‐mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 25: 207–218. [DOI] [PubMed] [Google Scholar]
- Staswick PE. 2002. Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole‐3‐acetic acids in an assay for adenylation. Plant Cell 14: 1405–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strengbom J, Reich PB. 2006. Elevated [CO2] and increased N supply reduce leaf disease and related photosynthetic impacts on Solidago rigida . Oecologia 149: 519–525. [DOI] [PubMed] [Google Scholar]
- Tauzin AS, Giardina T. 2014. Sucrose and invertases, a part of the plant defense response to the biotic stresses. Frontiers in Plant Science 5: art293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temme AA, Cornwell WK, Cornelissen JHC, Aerts R. 2013. Meta‐analysis reveals profound responses of plant traits to glacial CO2 levels. Ecology and Evolution 3: 4525–4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temme AA, Liu JC, Cornwell WK, Cornelissen JHC, Aerts R. 2015. Winners always win: growth of a wide range of plant species from low to future high CO2 . Ecology and Evolution 5: 4949–4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomma BP, Eggermont K, Penninckx IA, Mauch‐Mani B, Vogelsang R, Cammue BP, Broekaert WF. 1998. Separate jasmonate‐dependent and salicylate‐dependent defense‐response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proceedings of the National Academy of Sciences, USA 95: 15107–15111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timm S, Bauwe H. 2013. The variety of photorespiratory phenotypes – employing the current status for future research directions on photorespiration. Plant Biology 15: 737–747. [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JDG. 2002. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proceedings of the National Academy of Sciences, USA 99: 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Váry Z, Mullins E, McElwain JC, Doohan FM. 2015. The severity of wheat diseases increases when plants and pathogens are acclimatized to elevated carbon dioxide. Global Change Biology 21: 2661–2669. [DOI] [PubMed] [Google Scholar]
- Vaughan MM, Huffaker A, Schmelz EA, Dafoe NJ, Christensen S, Sims J, Martins VF, Swerbilow J, Romero M, Alborn HT et al 2014. Effects of elevated [CO2] on maize defence against mycotoxigenic Fusarium verticillioides . Plant, Cell & Environment 37: 2691–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JK, Kelly JK. 2004. Scaling up evolutionary responses to elevated CO2: lessons from Arabidopsis. Ecology Letters 7: 427–440. [Google Scholar]
- Wildermuth MC, Dewdney J, Wu G, Ausubel FM. 2001. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414: 562–565. [DOI] [PubMed] [Google Scholar]
- Zhang S, Li X, Sun Z, Shao S, Hu L, Ye M, Zhou Y, Xia X, Yu J, Shi K. 2015. Antagonism between phytohormone signalling underlies the variation in disease susceptibility of tomato plants under elevated CO2 . Journal of Experimental Botany 66: 1951–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Vroegop‐Vos I, Schuurink RC, Pieterse CMJ, Van Wees SCM. 2017. Atmospheric CO2 alters resistance of Arabidopsis to Pseudomonas syringae by affecting abscisic acid accumulation and stomatal responsiveness to coronatine. Frontiers in Plant Science 8: art700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Fig. S1 Effects of CO2 on plant development.
Fig. S2 Images of Hpa colonisation classes.
Fig. S3 qPCR‐based quantification of Hpa and Pc biomass.
Fig. S4 Role of SA signalling in saCO2‐induced resistance against Hpa.
Fig. S5 Global metabolic signatures of Hpa‐inoculated Arabidopsis at saCO2 and aCO2.
Fig. S6 Selection of ions that are induced or primed for Hpa‐induced accumulation by saCO2.
Fig. S7 Extracellular H2O2 in saCO2‐induced resistance against Hpa.
Fig. S8 Selection of gox1‐2 and haox1‐2 mutants.
Fig. S9 Impacts of Hpa inoculation on CAT2 gene expression at saCO2 and aCO2.
Table S1 Primers used in this study
Table S2 Putative identification of metabolic markers detected by UPLC‐Q‐TOF
Methods S1 Supplemental materials and methods.


